Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

15.4 Infrared Spectroscopy (IR) 709
a disk with KBr and placed in the beam of IR radiation; alternatively, it can be dis-
solved in Nujol®, which is a petroleum oil.
Spectrometers are routinely attached to computers that can search for matches
between the spectrum of an unknown and a library of known spectra. As with mass
spectrometry, gas chromatographs can be attached to IR spectrometers and spectra
can be determined as the individual components of a mixture elute from a column.
As noted in Section 15.2, this technique is called gas chromatography/infrared spec-
troscopy, or GC/IR.
15.4b Characteristic Infrared Absorptions From the foregoing discussion,
one might expect IR spectroscopy to be a simple matter of noting what characteris-
tic absorptions appear in the spectrum.An IR spectrum might be a very simple phe-
nomenon, composed only of signals for each kind of bond present in the sample
molecule. There certainly are characteristic regions of absorption for certain bonds,
but the practical situation is not quite so simple because connected vibrating springs
interact.Two springs attached to the same object (or atom) do not vibrate independ-
ently—try it. If you set one spring in motion, the other moves as well. In fact, this
phenomenon occurs even if the two springs (bonds) are attached to different atoms
within the same molecule. The closer in energy the two vibrations are, the more
strongly they affect each other. A molecule is like a collection of interacting springs,
and it is often not possible to make a simple assignment of all the bands in an IR
spectrum, although computational methods are becoming more proficient at this
task. Most IR spectra are complicated series of bands from which we can extract
information, but which we cannot completely rationalize in more than a general way.
Figure 15.17 shows the IR spectrum of a typical organic molecule and includes a
few band assignments.
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
Cyclohexanone
O
Overtone of C O
at 1710 cm
–1
C C
C O
C H
FIGURE 15.17 A typical IR spectrum.
Although this complexity may seem confusing at first,it is usually possible to gain
a lot of information about the functional groups present in a molecule, even if we
cannot assign all the bands, or draw a complete structure of the sample molecule. It
is a great help in structure assignment to know which types of bonds are present in
a molecule. There is also an important side benefit to these complicated IR spectra.
Their very complexity means that the IR spectrum of every molecule is different.
Each spectrum serves as a fingerprint of the molecule. If two IR spectra are identical
710 CHAPTER 15 Analytical Chemistry: Spectroscopy
(not similar—MJ’s research advisor insisted on there being no difference greater than
the width of the pen line drawn by the recorder) the compounds must be the same.
Table 15.3 gives the general positions of absorptions of a variety of functional
groups.
TABLE 15.3 Typical Infrared Absorptions of Functional Groups
a
Functional Group Position (cm
–1
) Intensity
b
2980–2850 m–s ( stretch)
1480–1420
H
C
m
( bend)
C
C
H
C
C
C
Alkanes
3350–3300
2260–2100
C
C
C
C
H s
( stretch)
H
C
m–w ( stretch)
C
C
Alkynes
free 3650–3580
3550–3300
1260–1000
1150–1050
H
RO
hydrogen bonded
H
RO
OH
R
s
( stretches)
O
C
m
( stretch)
H
O
br, s ( stretch)
H
O
Alcohols
RNH
2
3500–3100 (two bands)
R
2
NH 3500–3100 (one band)
RNH
2
, R
2
NH, or R
3
N ~1200
br, m ( stretches)
H
N
br, m ( stretch)
HN
m
( stretch)
N
C
Amines
3150–3000
1680–1620
1630–1600(conj)
995–985
915–905
980–960
730–665
895–885
840–790
C
CH
CC
C
C
m
( stretch)
H
C
m–w ( stretch)
C
C
m–w ( stretch)
C
C
s
( out-of-plane bends)
H
C
s
( out-of-plane bend)
H
C
s
( out-of-plane bend)
H
C
m
( out-of-plane bend)
H
C
s
( out-of-plane bend)
(br, variable)
H
C
Alkenes
(conjugation generally lowers C“ C double-bond stretching vibrations by about 20 cm
–1
)
R
H
CH
2
C
R
R
CH
2
C
H
R
R
H
CC
R
H
R
H
CC
R
H
R
R
CC
15.4 Infrared Spectroscopy (IR) 711
TABLE 15.3 Typical Infrared Absorptions of Functional Groups
a
(continued)
Functional Group Position (cm
–1
) Intensity
b
3080–3020
1600–1580
Monosubstituted 770–730
710–690
Ortho disubstituted 770–735
Meta disubstituted 900–860
810–750
725–680
Para disubstituted 860–800
s
( out-of-plane bends)
H
C
s
( out-of-plane bend)
H
C
s
( out-of-plane bend)
H
C
s
( out-of-plane bend)
H
C
m
( out-of-plane bend)
H
C
m
( out-of-plane bend)
H
C
Aromatic Compounds
H
Ar
m–w ( stretch)
H
C
m–w ( stretch)
C
C
~2250
Nitriles
RC
N
s ( stretch)
N
C
a
CAUTION! ere certainly is some subjectivity in this table, and the values represent average positions for “normal” compounds.
b
Medium = m, strong = s, weak = w, broad = br.
1680–1640
m
( stretch)
N
C
Imines
or
R
2
C
NR
R
2
C
NH
RCHO
R
2
C
2900–2700 (two bands)
1730–1700 (higher in strained cyclic molecules)
1750–1735
1300–1000
1730–1700
3200–2800
1820–1770
1820–1750 (two bands)
1150–1000
Carbonyl Compounds
O
s ( stretch)
O
C
s ( stretch)
O
C
s ( stretch)
O
C
s ( stretch)
O
C
s ( stretch)
O
C
s ( stretch)
O
C
s ( stretch)
O
C
br, s ( stretch)
H
O
w
( stretch)
H
C
(subtract 20–30 cm
–1
for a conjugated carbonyl)
O
ORR
O
OHR
O
ClR
O O
RR
O
Once again, we come to the question of memorization. Should one learn this
chart by heart? We think not. It is important to know that this kind of
general correlation exists, and you should have a rough idea of where some impor-
tant functional groups absorb. If you come to use IR often, you will automati-
cally learn the relevant details of the chart, as you work out what the signals in
your IR spectra tell you.
712 CHAPTER 15 Analytical Chemistry: Spectroscopy
ANSWER (a) In the stretch region (3350–2850 cm
1
) almost all the bands
are below 3000 cm
1
, indicating that there are only alkane carbon–hydrogen bonds.
No hydrogens are attached to double or triple bonds, because such carbon–hydrogen
C
O
H
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
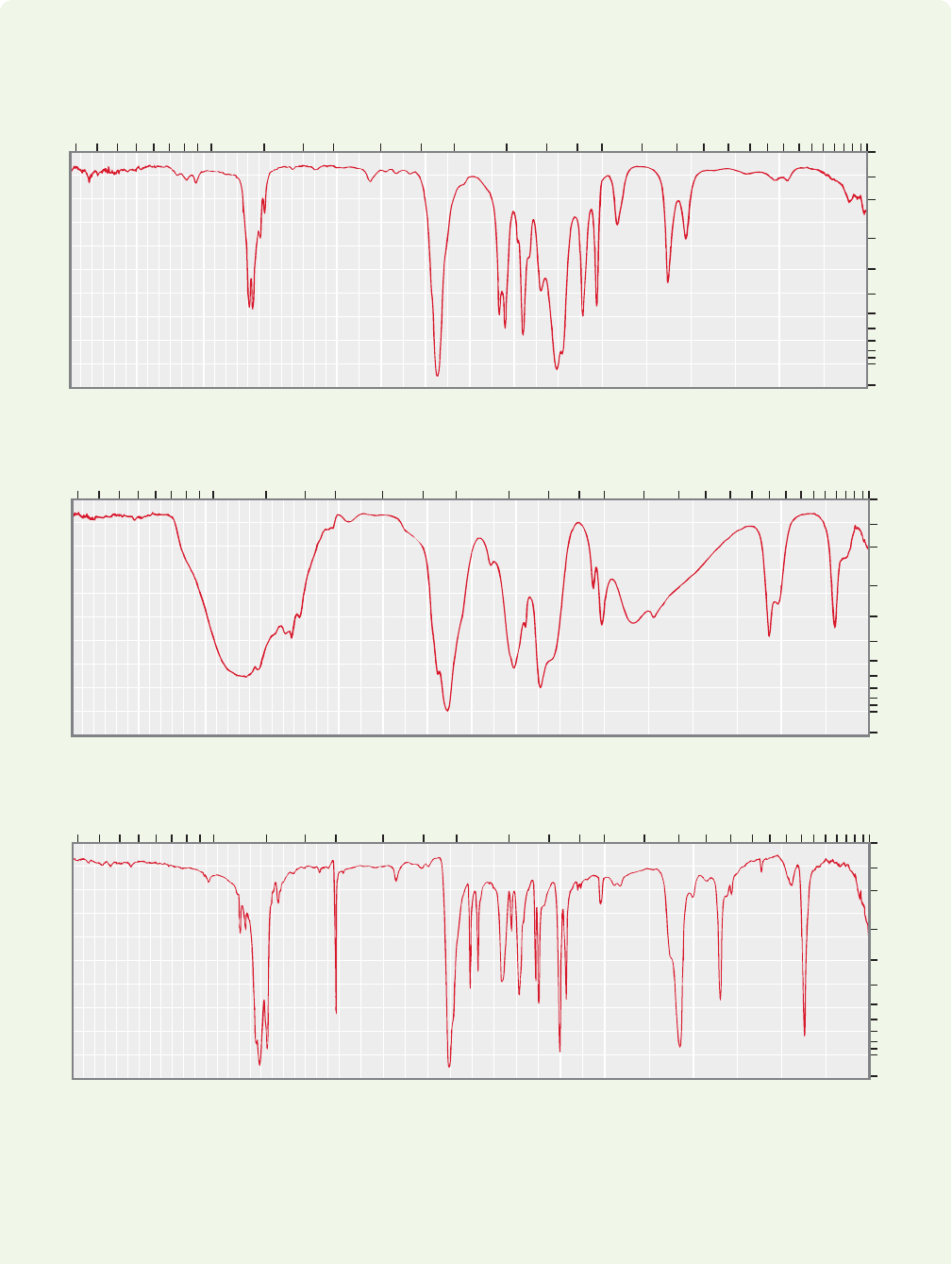
0
100
*(a)
(b)
(c)
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
C
4
H
8
O
2
C
2
H
4
O
2
C
8
H
5
NO
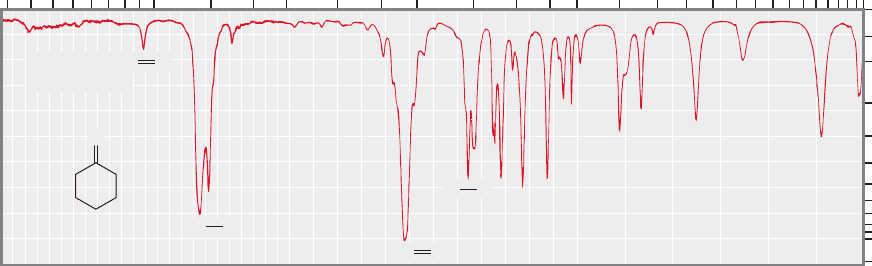
WORKED PROBLEM 15.7 Use the data in Table 15.3 to identify the functional
groups in the IR spectra below. Perhaps, given the molecular formulas, you can
make some guesses as to structure.
(continued)

15.5
1
H Nuclear Magnetic Resonance Spectroscopy (NMR) 713
bonds would absorb above 3000 cm
1
.There is a strong absorption in the carbonyl
region at about 1740 cm
1
. A 1740 cm
1
band is too high to be a ketone or an
acid. The compound must be an ester, or perhaps an aldehyde. Finally, there is a
strong band at 1200 cm
1
, appropriate for the stretch for an ester. Given
the formula of C
4
H
8
O
2
, one can make two guesses at the structure.
C
O
O
OCH
3
C
O
Methyl propanoate
H
3
C
OCH
2
CH
3
C
O
Ethyl acetate
H
3
C
or
CH
2
This problem tries to show that without further information there is no reli-
able way to choose between these two possibilities. Given the information so far,
either answer is possible. We could either compare the IR spectrum with those of
known samples, or obtain further spectra. An NMR spectrum would distinguish
the two possibilities easily, as you will soon see. In fact, this compound is methyl
propanoate. For (b) and (c), see the Study Guide.
Summary
The IR spectrum of a compound provides information on the
kinds of functional groups in the molecule. In addition, if
standard spectra of known compounds are available, compar-
ison of the highly detailed IR spectra can be used as a proof
of identity—two identical IR spectra can only come from
identical compounds.
15.5
1
H Nuclear Magnetic Resonance
Spectroscopy (NMR)
In contrast to IR spectroscopy, which gives an overall view of the
functional groups in a molecule, nuclear magnetic resonance
(NMR) spectra can give a wealth of detail. It is the general feel-
ing among organic chemists that given good NMR spectra of a
molecule, the structure must follow. Today, the frontier of struc-
ture determination by NMR lies in immense biomolecules, and
even these structures are yielding to an increasing variety of
sophisticated NMR techniques.
Nuclear magnetic resonance was introduced very early on in
Section 2.14 (p. 88). There we only developed a bare-bones
outline—just enough to let you work out the numbers of differ-
ent carbon or hydrogen atoms in a molecule and to have you get
used to working with symmetry. Here, we go on to many of the
gory details of this incredibly powerful spectroscopic technique.
We will start with
1
H NMR (hydrogen nuclear magnetic reso-
nance), and then pick up
13
C NMR (carbon nuclear magnetic
resonance) again.
An increasingly useful and powerful tool in the medical
field is NMR spectroscopy, called Magnetic Resonance
Imaging (MRI) in this setting.
714 CHAPTER 15 Analytical Chemistry: Spectroscopy
As you can see from Figure 15.16, NMR spectroscopy involves energies much
smaller than those in IR, UV, or visible spectroscopy. What happens when radia-
tion that has a wavelength of 1–100 m ( 10
6
kcal/mol) is absorbed? What change
can such a tiny amount of energy induce? Molecular vibrations require much
higher energies (1–10 kcal/mol). Molecular rotations demand much less energy than
vibrations ( 10
4
kcal/mol), but even these motions require about 100 times more
energy than that of the radio waves used for NMR.To see what happens when radio
waves are absorbed by molecules, we must first go back to the beginning of our
discussion of atomic structure, and learn a bit more about the structure of the
atomic nucleus.
Like the electron,the nucleus has spin.For some nuclei (
1
H,
13
C,
15
N,
19
F,
29
Si),
the value of the nuclear spin (I ) is
1
/
2
. For others, the spin can take different val-
ues, such as . A nonzero spin is
a requirement for the NMR phenomenon. It is convenient that some very com-
mon isotopes have zero spin (
12
C,
16
O) and therefore are NMR inactive. However,
the most common nucleus in organic chemistry, hydrogen, is one of the nuclei
with a spin of
1
/
2
and is NMR active. Keep in mind that hydrogens in molecules
are sometimes referred to as protons. The NMR effect arises as follows: Any
spinning charged particle generates a magnetic field, and therefore we can think
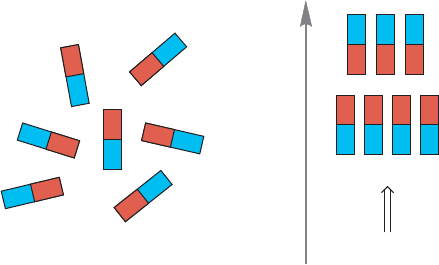
of the proton as a bar magnet. In the absence of a magnetic field, these bar mag-
nets will be oriented randomly (Fig. 15.18a). However, when we apply a magnetic
field (B
0
), the proton can align either with the field or against it (Fig. 15.18b).
0
(
12
C,
16
O), 1 (
2
H,
14
N),
3
/
2
(
11
B,
35
Cl), or
5
/
2
(
17
O)
'
'
In the absence of an applied
magnetic field
In the presence of an applied
magnetic field B
0
–
+
–
+
–
+
–
+
–
+
–
+
–
+
Energy
Aligned against
the field: higher
energy hydrogens
Aligned with the
field: lower energy
α hydrogens
Applied magnetic field
+ + +
–
+
–
+
–
+
–
+
–––
–Pole
+Pole
B
0
(b)(a)
FIGURE 15.18 (a) In the absence of an applied magnetic field, nuclear spins will
be randomly oriented. (b) When an external magnetic field is applied, alignment
with the field will be slightly favored energetically over alignment against the
field; there will be a small excess of molecules aligned with the field.
Alignment with the field (I
1
/
2
, α hydrogen) will be slightly more favor-
able energetically than alignment against the field (I
1
/
2
, β hydrogen), and
there will be an excess of nuclei in the lower energy, more favorable orienta-
tion. Because the energy difference between the two orientations is very small,
there will only be a slight excess, but it will exist, and there is the possibility of
inducing transitions between the two orientations if the proper amount of energy
is supplied. The function of the radio waves is to supply the energy necessary
to change the orientation of the nuclear spin (often called “flipping” the spin).
15.5
1
H Nuclear Magnetic Resonance Spectroscopy (NMR) 715
Energy
–Pole
+Pole
+ + +
–
+
–
+
–
+
–
+
B
0
–––
Aligned against
the field: higher
energy β hydrogens
Aligned with the
field: lower energy
α hydrogens
β β
αααα
β
+ + +
–
+
–
+
–
+
–
+
–––
β β
αααα
β
+ + +
–
+
–
+
–
+
B
0
–––
β β
+
–
β
ααα
β
Radiowave
energy absorbed
Energy
released
B
0
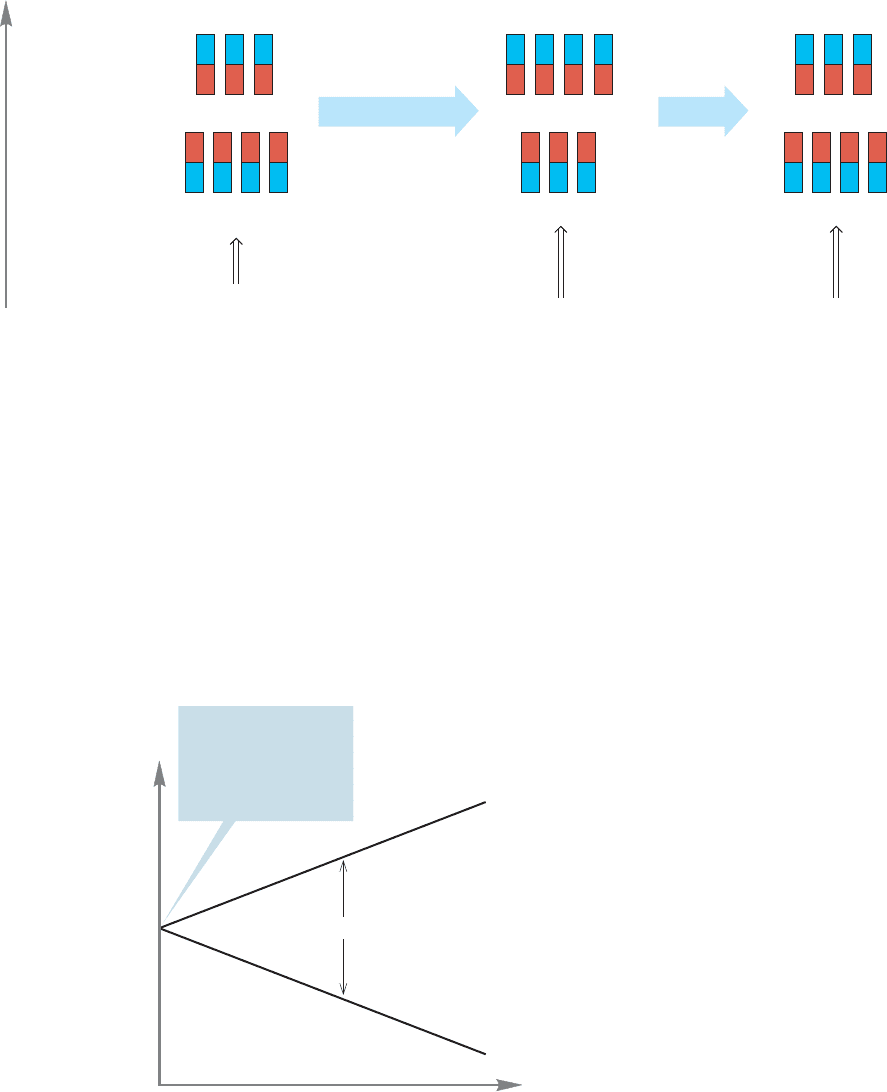
FIGURE 15.19 Absorption of energy can “flip” a nuclear spin, moving the low energy proton into the higher
energy level.
The disturbed equilibrium is reestablished when this absorbed energy is returned
to the environment as heat (Fig. 15.19).
The magnitude of the separation of the two states—alignment with and against
the applied magnetic field—depends on the strength of that field, B
0
, as shown in
Eq. (15.4) and Figure 15.20. The higher the field strength, the greater the energy
difference between hydrogens aligned with and opposed to the applied mag-
netic field. For values of B
0
in the range commonly used, the frequencies ν for
typically studied isotopes are in the range of 60–750 MHz.The gyromagnetic ratio,
γ, is characteristic of a given nucleus, which means there is a different γ for every
NMR active nucleus.
(15.4)ν = γB
0
/2π
Energy
B
0
β
α
ΔE is a function of B
0
In the absence of
an applied magnetic
field (B
0
= 0), the
nuclear spin states
are of equal energy
FIGURE 15.20 The energy difference
between the two spin states of
hydrogen depends on the strength
of the applied magnetic field.
If we can build an instrument capable of delivering the required radio waves and
of detecting the absorption of tiny amounts of energy, we should be able to find a
signal from any hydrogen-containing material. But won’t there be problems from

716 CHAPTER 15 Analytical Chemistry: Spectroscopy
WORKED PROBLEM 15.8 The magnetic field strength is measured in units of
Tesla (T). Calculate the energy involved in an NMR transition at a field
strength of 4.7 T. For H, γ is 2.7 10
8
T
1
s
1
, and Planck’s constant is
9.5 10
14
(kcal/mol)(s).
ANSWER Energy equals Planck’s constant (h) frequency (ν)or E hν. The
frequency is given by Equation (15.4), ν γB
0
/2π, so the useful relationship for
this problem is
h
1.9 10
5
whats? Figure out the units using the same equation.
E (kcal/mol)(s)(T
1
s
1
)(T) kcal/mol
The answer is 1.9 10
5
kcal/mol or 0.019 cal/mol. Notice that this last value
is calories per mole! These NMR transitions involve tiny energies.
E = (9.5 * 10
-14
)(2.7 * 10
8
)(4.7)/2(3.14) = 1.9 * 10
-5
γB
0
/2π E =
the other nuclei? Many common nuclei are NMR active, and there might be too
many overlapping signals for us to make sense of the spectrum. We are saved from
this fate by the gyromagnetic ratios γ, which are sufficiently different so that for the
usual field strengths, there is a wide separation of signals from different nuclei. For
example, signals from
13
C or
19
F nuclei appear far from those for
1
H.
In the early NMR spectrometers, the magnetic field was varied, while the
frequency of the radio wave was held constant.The magnetic field was swept over
the appropriate range for the nucleus in question, most often hydrogen or carbon.
As the chemically different nuclei absorbed energy (came into resonance) they
would be detected one by one. It took several minutes to acquire the spectrum and
rather large samples—several milligrams—were necessary. Today a different and
more sensitive spectrometer is used. These days, superconducting magnets supply
far higher magnetic fields than did the old permanent magnets. The magnetic
field is not varied; instead, the sample is irradiated with a very short pulse of
radio frequency that extends over the entire range for the nucleus in question.
All hydrogen atoms or all carbon atoms absorb energy at once. They return to
equilibrium over time, and a computer transforms the intensity versus time data
into intensity versus resonance frequency data through what is called a Fourier
transform (FT). An FT spectrum can be obtained in a few seconds on very small
samples, and the results from many pulses are averaged to increase the signal-to-
noise ratio.
Resonance frequencies are always referenced to the resonance frequency of a
standard signal, and given in parts per million (ppm) of the magnetic field. The
standard chosen for
1
H NMR is tetramethylsilane (TMS), which shows a sharp
signal at a frequency somewhat removed from resonance frequencies of hydrogens
in typical organic molecules.The position of TMS is defined as the zero point of
the ppm scale. Why is the somewhat odd ppm scale used? Let’s assume that we
are using a 60-MHz spectrometer and that the resonance frequency of the sam-
ple molecule appears at 120 Hz from TMS. If we measured the NMR spectrum
of the same sample on one of the higher field instruments common today, say,
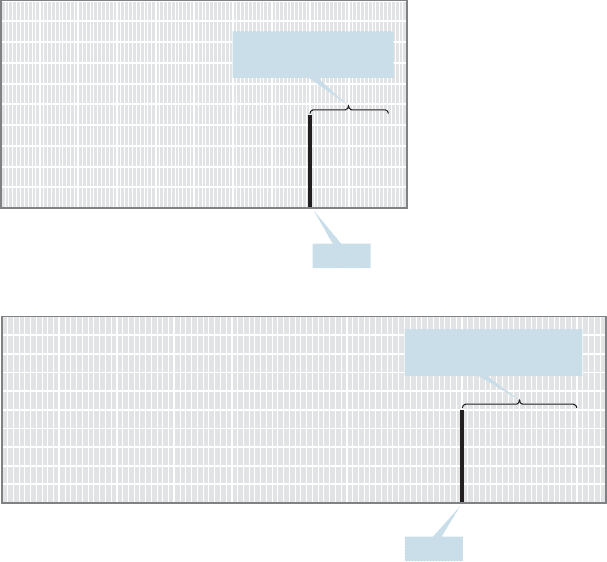
15.6 NMR Measurements 717
300 MHz, the resonance frequency would be different because the gap between
the two nuclear spin states depends on the strength of the applied magnetic field
(Fig. 15.20). At 300 MHz, our sample hydrogen signal would appear at 600 Hz
from TMS.Although the measured resonance position is different for every applied
magnetic field, the position is always the same in ppm. In the example above, it
appears at 2.0 ppm each time: 120 Hz/(60 10
6
Hz), or 600 Hz/(300 10
6
Hz)
(Fig. 15.21). The ppm scale was devised so that chemists could report the same
values for resonance positions regardless of the magnetic field strength of their
spectrometer.
Spectrum taken at 60 MHz
Intensity
109876543210
(ppm)
Chemical shift (δ)
Signal
120 Hz from TMS or
2.0 ppm from TMS
Spectrum taken at 300 MHz
Intensity
109876543210
Chemical shift (δ)
(ppm)
Signal
600 Hz from TMS but
still 2.0 ppm from TMS
FIGURE 15.21 The ppm scale ensures
that peak positions will be the same
regardless of external field strength.
15.6 NMR Measurements
Up to this point, NMR spectroscopy might seem to be merely an expensive way of
seeing if a compound contained hydrogen. We pay some hundreds of thousands of
dollars for a 300-MHz machine, put a sample of an organic compound in it, and if
we see absorption of radio waves at an appropriate frequency, we know the sample
contains hydrogen. That’s usually not very surprising if we are dealing with organic
molecules. So what’s the big deal? It turns out that we don’t see just one big signal
from a hydrogen-containing molecule. Instead, there is a signal at a different posi-
tion for every different hydrogen in the molecule.The resonance frequency for each
hydrogen in the molecule is critically dependent on its precise local environment—
any difference in environment will product a different resonance frequency.
The NMR spectrum is obtained by dissolving approximately 10 mg of the
compound to be analyzed in about 1 mL of a deuterated solvent,usually deuteriochlo-
roform (CDCl
3
), with 0.05% TMS added.The spectrum is then taken and the sig-
nals analyzed.
718 CHAPTER 15 Analytical Chemistry: Spectroscopy
WORKED PROBLEM 15.9 The higher the external magnetic field of the NMR
spectrometer, the greater the separation between the different signals, and
therefore the greater the resolving power of the instrument. This resolution
is one of the reasons that chemists are willing to spend extra thousands of
dollars to get extra megahertz. For example, two signals appearing at 120
and 126 Hz in a spectrum taken on a 60-MHz machine will be much better
separated if the spectrum is obtained at 300 MHz. Show that this last statement
is true.
ANSWER At 60 MHz, the signals at 120 and 126 Hz are at 2.0 and 2.1 ppm,
respectively, [120 Hz/(60 10
6
Hz) and 126 Hz/(60 10
6
Hz)]. At 300 MHz,
these signals are at 600 and 630 Hz, respectively. So the two signals are separated
by 6 Hz at 60 MHz, but by 30 Hz at 300 MHz. That’s quite an improvement in
separation.
There are three pieces of data associated with every NMR signal. The first is
called the integral. One can determine the relative number of hydrogens giving rise
to the signal by measuring the integral, or the area under the peak.The second piece
of data is the chemical shift, ␦.The chemical shift is the location of the NMR sig-
nal along the x-axis (see Fig.15.21). It is reported in ppm; for example,as δ 4ppm.
The chemical shift of most organic molecules lies between 0.8 and 10 ppm. The
third piece of data is the coupling, or multiplicity—the number of lines in each sig-
nal. Coupling changes the signal from a single line into multiple lines.The coupled
signals are called “doublets”(d, two lines),“triplets”(t, three lines),“quartets”(q, four
lines), and so on. The distance between the lines of the multiplet (the coupling
constant, J ) can be measured in Hz. A typical coupling constant J is about 7 Hz,
but the range is 1 to 16 Hz.
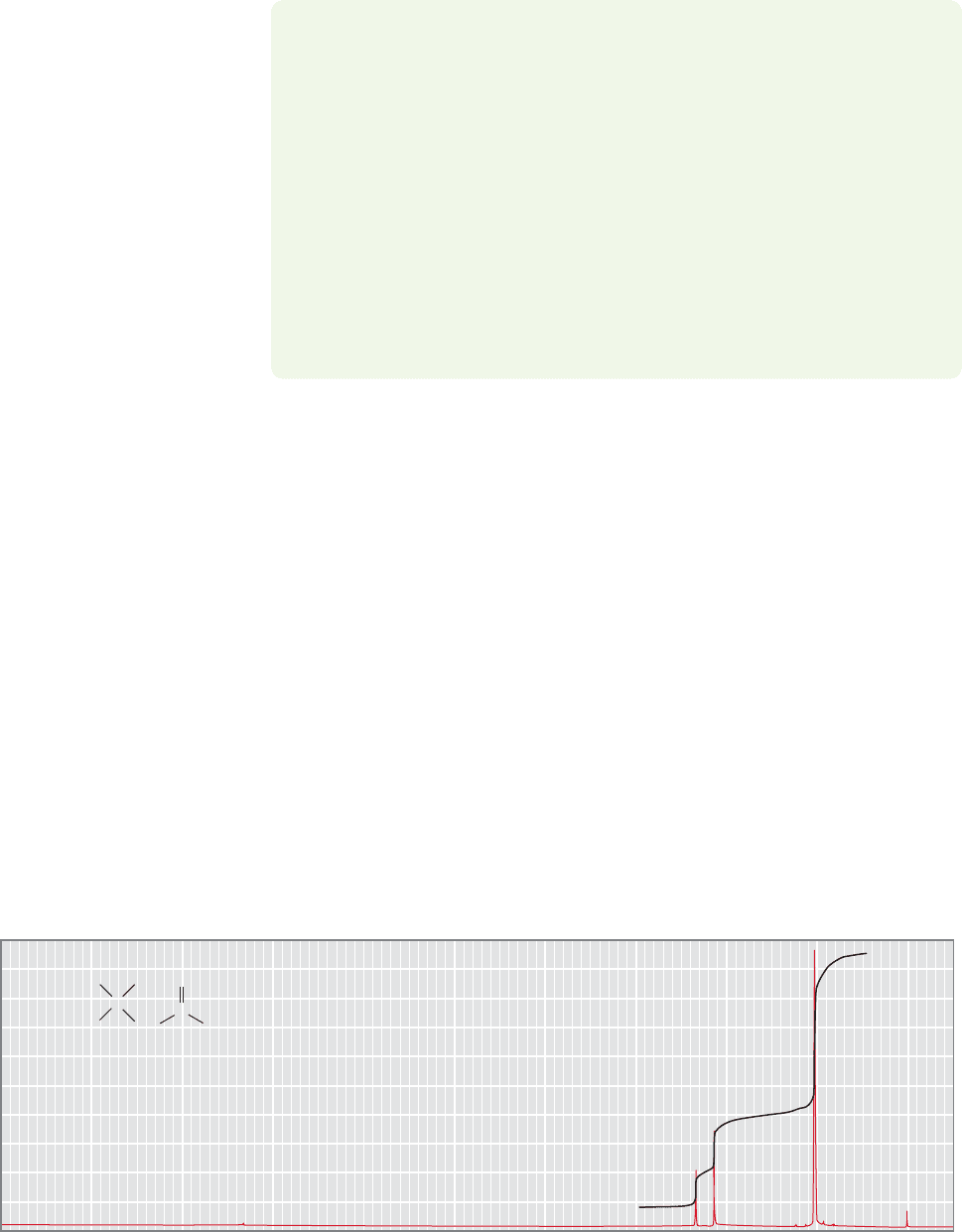
15.6a The Integral The
1
H NMR spectrum of 4,4-dimethyl-2-pentanone is
shown in Figure 15.22. Three signals appear, and the integration shows that they
are in the ratio 2:3:9. These days, integrations are calculated electronically and
printed out as numbers below or next to the signal. In older spectra, one sees a line
traced by a pen,as in Figure 15.22.The integral is determined by measuring the ver-
tical (y-axis) distance the pen traverses as it moves across the signal along the x-axis.
10 9 8 7 6 5 4 3 2 1 0 (ppm)
Chemical shift (δ)
4,4-Dimethyl-2-pentanone
C
O
CH
2
CH
3
H
3
C
H
3
C
CH
3
C
9H
3H
2H
9H
3H
2H
FIGURE 15.22 The
1
H NMR spectrum of 4,4-dimethyl-2-pentanone.
