Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

15.3 Mass Spectrometry (MS) 699
M + e
––
2 e + M
.
+
The in-coming
electron and the
ejected electron
The residual
radical cation
A high-energy
electron
FIGURE 15.3 When bombarded
with high-energy electrons,
molecules can lose an electron to
give a radical cation.
M
.
+
Anode
Slit
70-eV
cathode
Ion
beam
Radical
cation M
.
+
Magnet
Ion detector
High molecular weight
ions are deflected too
little and hit the wall
Low molecular weight
ions are deflected too
much and hit the wall
Sample M
Negatively
charged
plate
Slit
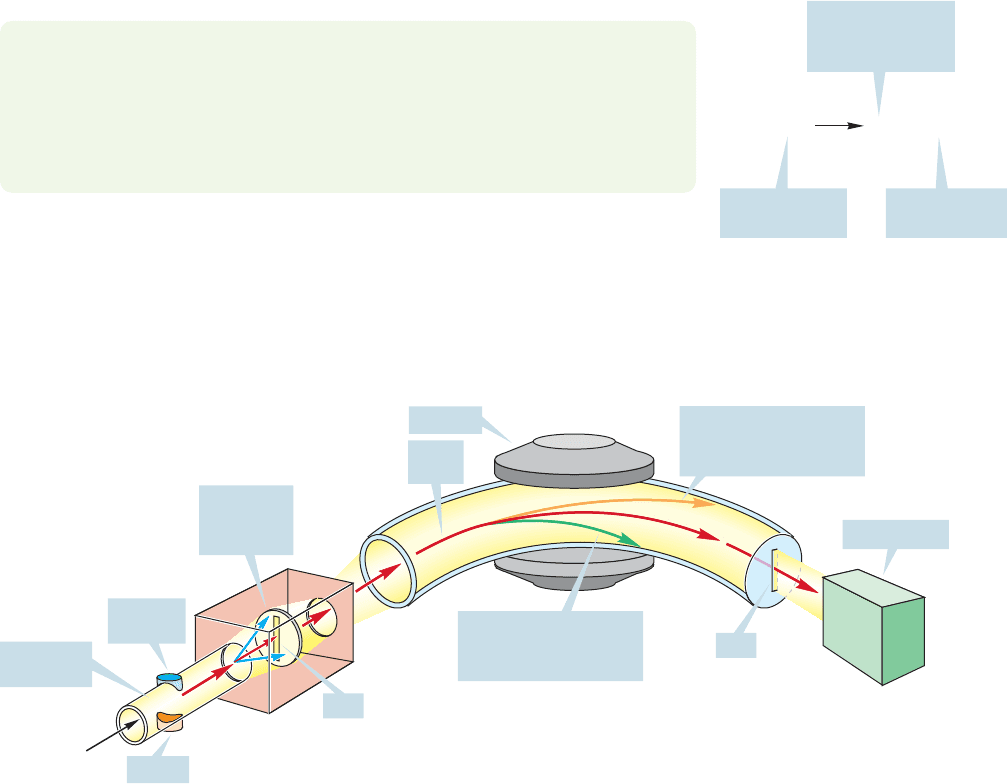
FIGURE 15.4 A schematic view of a
mass spectrometer.
stationary phase coated on the walls. They often have immense separating powers.
Yet, for the practicing chemist, the old-style simple packed GC columns are still
very important because they allow for the collection of the components of the mix-
ture as they pass out of the column. Not only can the mixtures be separated, but
the pure components can be isolated for identification by the simple means of
attaching a cooled receiver to the end of the chromatograph (Fig. 15.2).
A particularly useful modification of a gas chromatograph is to attach it to a mass
spectrometer (GC/MS) or infrared spectrometer (GC/IR) so that the various com-
ponents can be analyzed directly as they emerge from the column. In the following
sections, we will see what kinds of information MS and IR spectroscopy can give
about molecules.
One thing these very high-energy electrons can do is eject an electron from a
molecule, M, to give the corresponding radical cation,M
(Fig. 15.3). Now what
can happen to the newly formed radical cation? In a typical mass spectrometer, it
is first accelerated toward a negatively charged plate (Fig. 15.4). There is a slit in
the plate, and some of the accelerated ions pass through the slit to form a beam.
.
15.3 Mass Spectrometry (MS)
First of all, never make the mistake of calling it “mass spectroscopy.” Spectroscopy
involves the absorption of electromagnetic radiation and as we will see later, mass
spectrometry (MS) is different.The mass spectrometrists sometimes get upset if you
confuse the issue.
15.3a The MS Spectrometer In MS, a compound is bombarded with very
high-energy electrons. Typically, electrons of 70 eV are used.
WORKED PROBLEM 15.2 How many kilocalories per mole does 70 eV represent?
See page 4.
ANSWER One electron volt (eV) is 23.06 kcal/mol, so 70 eV is 1614 kcal/mol.
The point of this problem is to show that the incoming electron is carrying a lot
of energy!

700 CHAPTER 15 Analytical Chemistry: Spectroscopy
The beam of ions then follows a curved path between the poles of a magnet. In a
magnetic field, a moving charged particle, the radical cation in this case, is deflected
by an amount proportional to the strength of the magnetic field. Too little deflec-
tion (too weak a magnetic field) results in the ion hitting the far wall of the tunnel
and in loss of the ion. If there is too much deflection (too strong a magnetic field),
the ion impinges on the near wall and is also lost. If, however, the strength of the
magnetic field is just right, the ion will follow a curved path to the detector and be
“seen.” The magnetic field can be tuned so that ions of different mass are curved
just the right amount to be detected. For ions of low mass, a relatively low magnetic
field will be necessary; for ions of higher mass, the field will have to be stronger to
do the required bending of the path.
Mass spectra are reported as graphs of the mass-to-charge ratio,m/z, versus inten-
sity. As long as we are dealing with singly charged ions, the positions of the signals
will correspond to the molecular weights of the ions detected because the charge,
z, will be 1.
So far, we have a device that can detect M
•
ions formed by bombardment of
a molecule M. This species, M
•
, is called the molecular ion. In our detector, we
should see an ion with the mass m corresponding to the molecular weight of M.
For cyclohexane, we should see a molecular ion of 84 mass units, because the
molecular weight of cyclohexane (C
6
H
12
) is 84 (Fig. 15.5). In the mass spectrum of
cyclohexane, there is a small peak above the molecular ion, at m/z 85. It is only
about 7% of the molecular ion, and therefore one must look closely in order to see it.
Cyclohexane
(C
6
H
12
) M = 84
3,4-Dihydro-
2H-pyran
(C
5
H
8
O) M = 84
70 eV
70 eV
.
+
.
+
O O
M
.
+
= 84
M
.
+
= 84
FIGURE 15.5 Many different ions
can have a molecular weight of 84.
Two possibilities are the molecular
ions of cyclohexane and 3,4-dihydro-
2H-pyran.
This M ⴙ 1 peak is not the result of an impurity, however; it is always there no mat-
ter how carefully the cyclohexane is purified. This M 1 peak exists because the
carbon and hydrogen have naturally occurring isotopes, and the mass spectrometer
can detect molecules containing these heavy isotopes. Table 15.1 gives the relative
natural abundance of some common isotopes.
TABLE 15.1 Some Common Isotopes and Their Abundances
in Nature
Isotope Abundance (%) Isotope Abundance (%)
1
H 99.985
17
O 0.038
2
H (D) 0.015
18
O 0.200
12
C 98.90
35
Cl 75.77
13
C1.10
37
Cl 24.23
14
N 99.63
79
Br 50.69
15
N0.37
81
Br 49.31
16
O 99.762
127
I 100
For cyclohexane, there is a 1.1% probability that each carbon will be a
13
C,and there-
fore about 7% probability that the mass of cyclohexane will be one unit high (M 1).
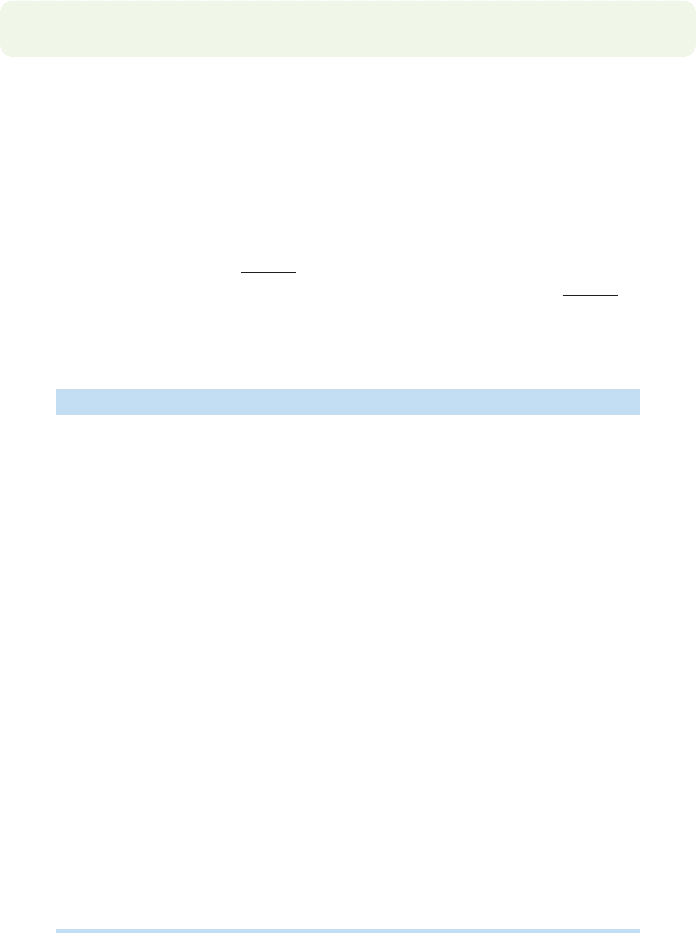
15.3 Mass Spectrometry (MS) 701
Deuterium (
2
H or D) has a natural abundance of only 0.015%,and therefore contributes
relatively little to the M 1 peak. Sometimes the presence of isotopes is useful in the
diagnosis of structure.An analysis of isotope peaks is particularly useful for organic chlo-
rides and organic bromides, as we will see at the end of this section.
But how do we know that a mass spectrum with m/z 84 is really cyclohexane,
C
6
H
12
? If the starting material is of unknown structure, we probably do not know
the formula of the molecule.The detection of a molecular ion of m/z 84 only tells
us that the molecular weight of the unknown is 84, nothing more. Any other
molecule of m 84 could give the same molecular ion. In this case, we might be
looking at 3,4-dihydro-2H-pyran, for example (C
5
H
8
O 84, Fig. 15.5).
C
6
H
12
M of C
6
H
12
6
12
C = 6 12.000 = 72.000
12
1
H = 12 1.008 = 12.096
C
5
H
8
O
M of C
5
H
8
O
5
12
C = 5 12.000 = 60.000
8
1
H = 8 1.008 = 8.064
1
16
O = 15.995 = 15.995
84.096
84.059
FIGURE 15.6 If molecular weights are
measured carefully enough, mass
spectrometry can differentiate
between different molecules of the
nominal molecular weight of 84.
TABLE 15.2 Some Atomic Weights
Element Atomic Weight Element Atomic Weight
1
H 1.008
19
F 19.998
2
H 2.010
28
Si 27.977
H (av) 1.008
29
Si 28.975
10
B 10.013
30
Si 29.974
11
B 11.009 Si (av) 28.086
B (av) 10.810
31
P 30.974
12
C 12.000
32
S 31.972
13
C 13.003
33
S 32.972
C (av) 12.011
34
S 33.968
14
N 14.003
36
S 35.967
15
N 15.000 S (av) 32.066
N (av) 14.007
35
Cl 34.969
16
O 15.995
37
Cl 36.966
17
O 16.999 Cl (av) 35.453
18
O 17.999
79
Br 78.918
O (av) 15.999
81
Br 80.916
Br (av) 79.904
127
I 126.905
These days, mass spectrometers are so accurate that we can actually tell C
6
H
12
from C
5
H
8
O. The two species do not have the same molecular weight if we
measure molecular weights accurately enough (Fig. 15.6). Table 15.2 gives the accurate
atomic weights for a variety of isotopes as well as the average values.
PROBLEM 15.3 Find other possible structures that might fit the observed m/z of 84.
15.3b MS Measurements When a molecular formula is calculated from
combustion data, it is the average values in Table 15.2 that must be used, because
unless there has been a deliberate introduction of an isotope during the synthesis of
702 CHAPTER 15 Analytical Chemistry: Spectroscopy
the material, it will be the natural
isotopic mixture that appears in the
compound. The mass spectrome-
ter, on the other hand, is able to
separate ions containing different
isotopes. Each peak represents a
particular set of isotopes, for exam-
ple,
12
C
6
1
H
12
or
12
C
5
1
H
8
16
O. So, a
good mass spectrometer can give
us the molecular formula of an
unknown, as long as we can detect
the molecular ion. The full mass
spectra of our two m 84 mole-
cules are shown in Figure 15.7.
One can see that the mass spectra
are not simple composites of
molecular ion and M 1 peaks.
There are many additional lower
molecular weight ions detected
that give a characteristic array of
ions known as the fragmentation
pattern for each molecule.
The original radical cation,
M
, can undergo many fragmen-
tation reactions before it is accelerated into the magnetic field and passed on to the
detector. The fragmenting ion is called the parent ion (p). It can react to give many
daughter ions, and these ions appear as peaks in the mass spectrum—the fragmen-
tation pattern. Often the most intense or largest peak is not the molecular ion. In all
cases, the intensities of mass spectral peaks are given as percentages of the largest peak
in the spectrum, which may be the molecular ion, but usually is not.The largest peak
is called the base peak of the spectrum. You can see in Figure 15.7 that the base peak
for cyclohexane is 56 and the base peak for 3,4-dihydro-2H-pyran is 55. Remember
that the molecular ion, M
, is the furthest to the right on the spectrum, except for
the small isotope peaks.
Is there any way to tell how a given molecular ion will fragment? Yes and no.There
is no way to predict the detailed pattern of daughter ions. Yet we can make general
predictions by applying what we know about reaction mechanisms and the stabili-
ties of ions.Fragmentations usually take place to give the most stable possible cations,
and we are now able to make some reasonable guesses as to what these might be.
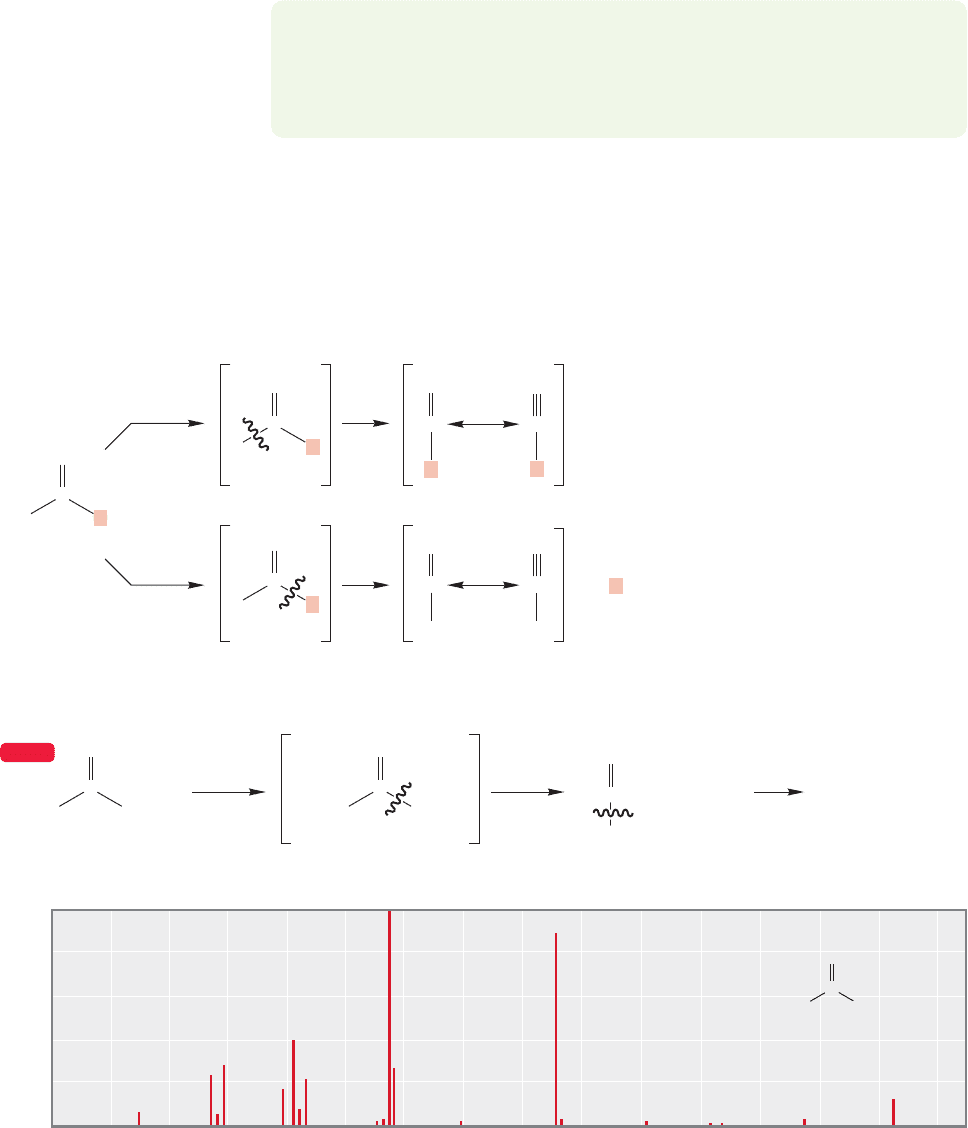
First of all, note that there are two ways for a typical parent radical cation to pro-
duce two fragments (Fig. 15.8). When the radical cation
•
produces A and
B by fragmentation, the charge may go with either A or B. The detector can see
only positively charged ions, and so any neutral species formed are invisible. In the
fragmentation process, the charge will generally wind up where it is more stable,
and we are often able to predict where it will go. For example, tert-butyl chloride
gives large amounts of the tert-butyl cation,m/z 57,in its mass spectrum,not Cl
,
m/z 35 (Fig. 15.8). The charge is more easily borne on the tertiary cation than
on the relatively electronegative chlorine. In fact, the cleavage of tert-butyl chloride
is so facile, the molecular ion is not even detected. Nevertheless, it must be admitted
that predicting is not always a straightforward process. High energies are involved,
and there is plenty of energy available to do a lot of bond breaking. Rearrangements
are also common, especially if a particularly stable ion can be reached.
A
O
B
.
.
0
20
40
60
80
100
Cyclohexane
Relative abundance
m/z
100 110 1202010 30 40 50 60 70 80
m/z
80
90
0
20
40
60
80
100
Relative abundance
100 110 1202010 30 40 50 60 70 90
3,4-Dihydro-
2H-pyran
O
M
.
+
M
.
+
WEB 3D
FIGURE 15.7 The mass spectra of cyclohexane and 3,4-dihydro-2H-pyran.

15.3 Mass Spectrometry (MS) 703
Some other examples will probably be useful. Figure 15.9 shows the mass spectrum
of toluene.The molecular ion is visible as a strong peak at m/z 92, but it is not the base
peak.The largest peak in the spectrum is the M1 peak at m/z 91.What can this be?
+
+
+
THE GENERAL CASE
A SPECIFIC EXAMPLE
.
H
3
C
CH
3
CH
3
ClC
H
3
C
CH
3
CH
3
CCl
H
3
C
CH
3
CH
3
ClC
H
3
C
CH
3
CH
3
C
B
][
A
A
+
B
+
+
.
.
.
.
Cl
+
AB
+
+
+
m/z
80
0
20
40
60
80
100
Relative abundance
100 110 1202010 30 40 50 60 70 90
m/z = 57
m/z = 35m/z = 92
(not observed)
.
H
3
C
CH
3
CH
3
ClC
tert-Butyl chloride
FIGURE 15.8 Two possible
fragmentations for a general
molecule and the mass
spectrum of tert-butyl chloride.
A
O
B
FIGURE 15.9 The mass spectrum
of toluene.
m/z
80
0
20
40
60
80
100
Relative abundance
100 110 1202010 30 40 50 60 70 90
Toluene
CH
3
M
.
+
CH
3
CH
3
H
70 eV
.
.
+
+
CH
2
+
+
+
CH
2
C
7
H
7 ,
m/z = 91 m/z = 92
+
CH
2
–
CH
2
FIGURE 15.10 The molecular ion of
toluene loses a hydrogen atom to give
the benzyl cation.
A little thought brings us quickly to a reasonable answer. If a hydrogen atom (not a
proton,which would be positively charged and leave a neutral fragment behind) is lost
from the parent ion,what is left behind is a resonance-stabilized benzyl ion (Fig. 15.10).
704 CHAPTER 15 Analytical Chemistry: Spectroscopy
Accordingly, loss of the hydrogen atom from the first-formed radical cation is
relatively easy, and the largest peak in the spectrum belongs to the ion C
7
H
7
,
m/z 91, the benzyl cation.
PROBLEM 15.4 There is some evidence that the m/z 91 peak may not be the
benzyl cation but another species. Can you (a) think of a C
7
H
7
species that
might be even more stable than the benzyl cation, and (b) write an arrow formal-
ism for the conversion of the benzyl cation into this new species? Hint: See p. 587.
The mass spectra of compounds containing carbon–oxygen double bonds (car-
bonyl compounds) often have large peaks for the resonance-stabilized acylium ions
(Fig. 15.11). For example, the molecular ion of 2,2,4,4-tetramethylpentan-3-one
(di-tert-butyl ketone) ionizes to give large amounts of the acylium ion of m/z 85.
Acylium ions can break down further to give carbon monoxide and a carbocation.
70 eV
70 eV
.
.
+
C
R
C
R
C
R
+
.
C
R
O
C
+
+
+
.
R
C
R
O
C
+
+
+
R
R
R
R
R
R
O
O
O
O O
70 eV
m/z = 142 m/z = 85 m/z = 57
C
.
.
+
+
++
(CH
3
)
3
C C(CH
3
)
3
C
O
C
(CH
3
)
3
C
(CH
3
)
3
C
C(CH
3
)
3
(CH
3
)
3
C
+
CO
C(CH
3
)
3
OO
m/z
80
0
20
40
60
80
100
Relative abundance
100 110 120 130 140 1502010 30 40 50 60 70 90
(CH
3
)
3
C
C(CH
3
)
3
C
O
M
.
+
WEB 3D
Acylium ion
FIGURE 15.11 Carbonyl compounds usually give large amounts of a resonance-stabilized acylium ion.

15.3 Mass Spectrometry (MS) 705
70 eV
.
.
.
+
+
H
3
C
CH
3
CH
3
CH
3
C
H
3
C
CH
3
CH
3
+
CH
3
C
+
+
H
3
C
CH
3
CH
3
CH
3
C
CH
3
H
3
C
CH
3
CH
3
C
2,2-Dimethylpropane
(neopentane)
m/z = 15
m/z = 57
80
0
20
40
60
80
100
Relative abundance
100 110 1202010 30 40 50 60 70 90
C(CH
3
)
4
m/z
m/z = 72
(not observed)
FIGURE 15.12 As illustrated
by the mass spectrum of
2,2-dimethylpropane, alkanes
fragment to give the most stable
carbocation possible.
Alkanes cleave so as to give the most stable possible carbocation (Fig. 15.12).
For example, 2,2-dimethylpropane (neopentane) fragments in a mass spectrometer
to give an ion of m/z 57, the tert-butyl cation, much more easily than it gives the
far less stable methyl cation, m/z 15.
70 eV
m/z = 56
OH OH
.
+
.
+
+
H
2
O
m/z
80
0
20
40
60
80
100
Relative abundance
100 110 1202010 30 40 50 60 70 90
CH
3
(CH
2
)
3
OH
m/z = 74
(not observed)
FIGURE 15.13 In the mass
spectrometer, alcohols such as
1-butanol can eliminate water to
give alkene radical cations.
There are other fragmentation reactions that resemble solution chemistry. For
example, an important peak in the mass spectrum of 1-butanol is at m/z 56.
High-resolution analysis of this peak shows that its formula is C
4
H
8
. Apparently,
the parent ion eliminates water to give a butene radical cation (Fig. 15.13). This
behavior is typical for alcohols and has clear roots in the solution chemistry
described in our sections on elimination reactions (p. 298).

706 CHAPTER 15 Analytical Chemistry: Spectroscopy
PROBLEM 15.5 Draw a schematic molecular orbital diagram for the π orbitals of
the butene radical cation, C
4
H
8
•
.
WORKED PROBLEM 15.6 What do you suggest as a structure for the ion that cor-
responds to the base peak (m/z 31) in the mass spectrum of 1-butanol shown
in Figure 15.13?
ANSWER The base peak is m/z 31. The mass of 1-butanol (C
4
H
10
O) is 74, so
some quite serious fragmentation must have taken place. A mass of 31 can only
have two heavy (nonhydrogen) atoms, so it must be either a C
2
(m 24) or CO
(m 28) compound. If the ion contained two carbons, it would have to be C
2
H
7
,
an impossible formula, so it can only be CH
3
O. The problem now is to find a
reasonably stable ion of this formula that can be made from a fragmentation of
1-butanol. A simple carbon–carbon bond breaking leads to a resonance-stabilized
cation that certainly is a good candidate.
70 eV
OH
CH
2
OH
CH
2
CH
2
CH
3
O
+
OH
m/z
= 31m/z = 74
Molecular ion
+
+
+
.
.
CH
2
OH
+
As mentioned earlier, molecules containing chlorine or bromine produce easily
characterized spectra. This observation is one of the most useful aspects of mass
spectrometry for organic chemists.Table 15.1 lists the natural isotopic ratio for
35
Cl
to
37
Cl (75.77:24.23), which is essentially a 3:1 ratio. The ratio for
79
Br to
81
Br
(50.69:49.31) is close to a 1:1 ratio. Chlorine and bromine are the only elements
frequently found in organic molecules that have significant M 2 isotopes.There
are other elements (osmium and mercury, for example) that have M 2 isotopes,
but we are less likely to deal with mass spectra of such compounds. Therefore, if a
mass spectrum shows an M 2 peak that is one-third the intensity of the molec-
ular ion (M
•
) as shown in Figure 15.14a, then we know there is a chlorine in the
molecule. If a spectrum has an M 2 peak that is the same intensity as the molec-
ular ion, we can conclude that the molecule has a bromine (see Fig. 15.14b).
(a) (b)
Data from National Institute of Standards and Technology, http://webbook.nist.gov
m/z
0
20
40
60
80
100
Relative abundance
2010 30 40 50 60 70
Chloroethane
m/z
80
100 110 1202010 30 40 50 60 70 90
Bromoethane
Cl Br
64
108
110
66
FIGURE 15.14 (a) The mass spectrum of chloroethane. If there is a chlorine in the molecule, then an M 2 peak with
one-third the height of the M peak will be observed. (b) The mass spectrum of bromoethane. When there is bromine
in a molecule there is a 1:1 ratio of the M and the M 2 peaks.
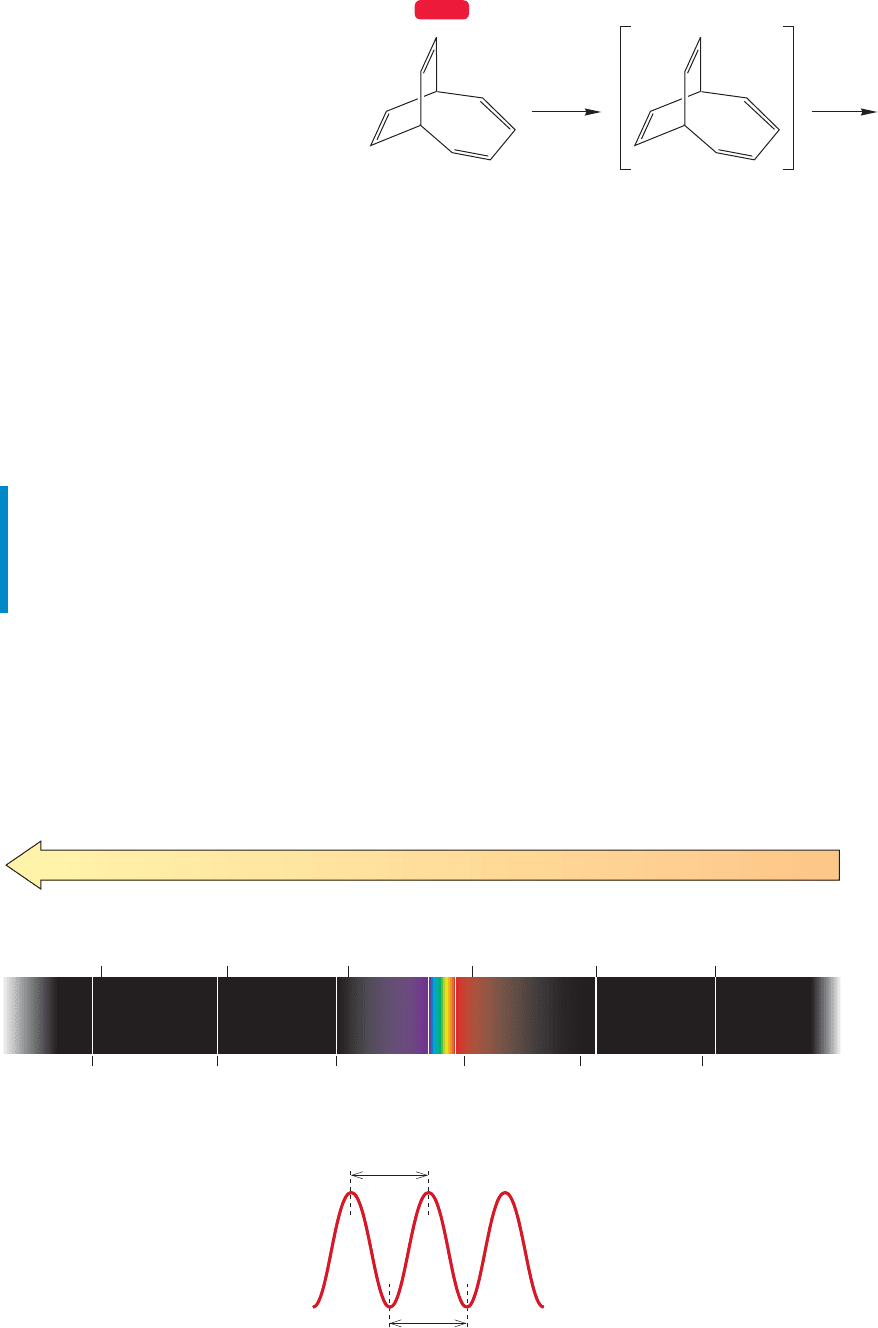
15.4 Infrared Spectroscopy (IR) 707
There are still plenty of mysteries in
mass spectrometry, however. For example,
the Jones research group first made the
molecule bicyclo[4.2.2]deca-2,4,7,9-
tetraene (m 130; Fig. 15.15) over 40
years ago, and were startled then to find
that the base peak in the mass spectrum
was at m/z 115. It seemed likely that
this ion (m 15) represented the loss of
a methyl group from the parent ion. We
have been thinking about this process (not constantly!) over the ensuing three decades
and still have no reasonable explanation for the loss of a methyl group. Any ideas?
Despite occasional mysteries,mass spectrometry is still highly useful,even in the
bare-bones form described here. It gives us a way to determine molecular composi-
tion through detection of a molecular ion, and can give hints as to structure from
the fragment ions produced. Even though it is difficult to predict the fragmenta-
tion patterns in more than a general way, it is possible to compare a fragmentation
pattern with that of a known compound in order to prove identity.
Summary
Mass spectrometry usually provides the molecular weight of an unknown com-
pound. If run at sufficiently high resolution,MS can even allow you to determine
the molecular formula of a compound. Some information can be gathered about
structure from the ions formed on fragmentation.
15.4 Infrared Spectroscopy (IR)
Infrared (IR) spectroscopy is true spectroscopy—it informs us about the electromag-
netic radiation absorbed by a molecule.In Chapter 12,we discussed UV/vis spectroscopy,
and IR spectroscopy is qualitatively similar. A schematic picture of the electromag-
netic spectrum (Fig. 15.16) identifies the regions used for UV/vis, IR, and the spec-
troscopy we will study in the second half of this chapter, nuclear magnetic resonance.
70 eV
Bicyclo[4.2.2]deca-
2,4,7,9-tetraene
.
+
m/z = 115 ???
m/z = 130
WEB 3D
10
20
10
18
10
16
10
14
10
12
10
10
Visible
10
10
10
8
10
6
10
4
10
2
1
Energy (kcal/mol)
Frequency,ν (Hz)
Wavelength, λ (cm)
Cosmic
rays
γ
Rays X Rays Ultraviolet Infrared Microwaves
Radio waves
(NMR)
> 300 300–30 30 –1 ~10
4
~10
6
λ
λ
FIGURE 15.16 The electromagnetic
spectrum.
FIGURE 15.15 Bicyclo[4.2.2]deca-
2,4,7,9-tetraene apparently loses
a methyl group in the mass
spectrometer. How?
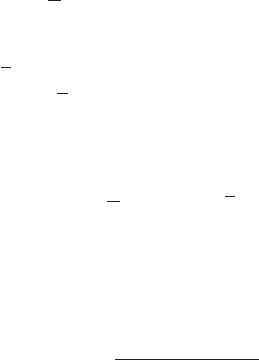
We begin our discussion of IR spectroscopy by recalling certain properties of light
and energy (p. 526). The wavelength of light, λ, is the length of a wave as measured
from peak to peak or from trough to trough (Fig. 15.16). In IR spectroscopy we will
express wavelength in centimeters (cm).Wavelength is related to frequency as shown
in Eq. (15.1). The frequency (ν) is the number of cycles (or waves) passing a given
point per unit time, usually seconds. So, the units of frequency (ν) are cycles per
second (usually written as reciprocal seconds, s
1
), or more simply, hertz (Hz).
the speed of light in
centimeters per second (cm/s)
(15.1)
The quantity is called the wavenumber and its units are reciprocal cen-
timeters (cm
1
). Physically, is the number of wavelengths contained in one cen-
timeter (cm
1
) and is related to the more familiar frequency, ν,by Eq. (15.2). Signals
from IR spectra are reported in wavenumbers (cm
1
).
(15.2)
Absorption of IR light involves energies of roughly 1–10 kcal/mol, and is
associated with molecular vibrations. A chemical bond can be approximated by a
picture of two objects connected by a vibrating spring. Hooke’s law, which is shown
in Eq. (15.3), describes this situation.
(15.3)
The frequency of vibration, ν, is related by a constant k to the masses of the
objects (m
1
and m
2
) and another constant,f, which is the force constant of the spring.
Chemical bonds do behave rather like classical springs. So, like a real spring, the
frequency of vibration in a bond depends on the masses of the two atoms involved
and the strength of the bond between them. Bond strength turns out to be the
dominant feature, although the masses of the atoms do make a difference. We
would expect strong bonds, with higher force constants, to absorb at higher
wavenumbers. Carbon–carbon single bonds ( 90 kcal/mol, 1450 cm
1
), double
bonds ( 168 kcal/mol, 1650 cm
1
),and triple bonds ( 230 kcal/mol, 2180 cm
1
)
illustrate this point well.
Not all molecular vibrations can be detected.For a vibration to be infrared active,
it must produce a net change in the dipole moment of the molecule, which means
that symmetrical vibrations are either weak or invisible in the IR region of the
electromagnetic spectrum.For example, ethylene shows no IR signal for the carbon-
carbon double-bond stretch. However, Raman spectroscopy, which is related to IR
spectroscopy, does detect symmetrical vibrations, and shows a signal at 1623 cm
1
for ethylene. Signals in IR and Raman are usually broad and actually extend over
several wavenumbers. As a result the signal is often referred to as a band.
15.4a The IR Spectrometer In practice, an IR spectrum is obtained by pass-
ing IR radiation (light) through a sample. If no light is absorbed at a given wave-
length, transmission is 100%. If light is absorbed by the sample at a certain
wavelength, transmission is less than 100%. A plot of absorption versus wavenum-
ber (cm
1
) is thus generated and constitutes the IR spectrum of the sample. Spectra
of gases are measured in cells with nonabsorbing windows. For liquids, spectra can
be obtained either as pure samples or in solution.Pure samples are usually described
as “neat.”To measure the spectrum of a solid, the sample is usually compressed into
''''
''
ν = k2f (m
1
+ m
2
)/m
1
m
2
ν =
c
λ
or
ν = cν
ν
1/λ = ν
c =ν =
c
λ
,
708 CHAPTER 15 Analytical Chemistry: Spectroscopy
