Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

15.6 NMR Measurements 719
In this spectrum, the assignment is easy; the nine hydrogens of the tert-butyl
group give rise to a signal around δ 1 ppm, the methyl group produces the signal
for three hydrogens, and the methylene group gives the peak for two hydrogens.
From now on, integrations in the figures will often be indicated by a number
followed by H, as in 1H, 2H, and so on.
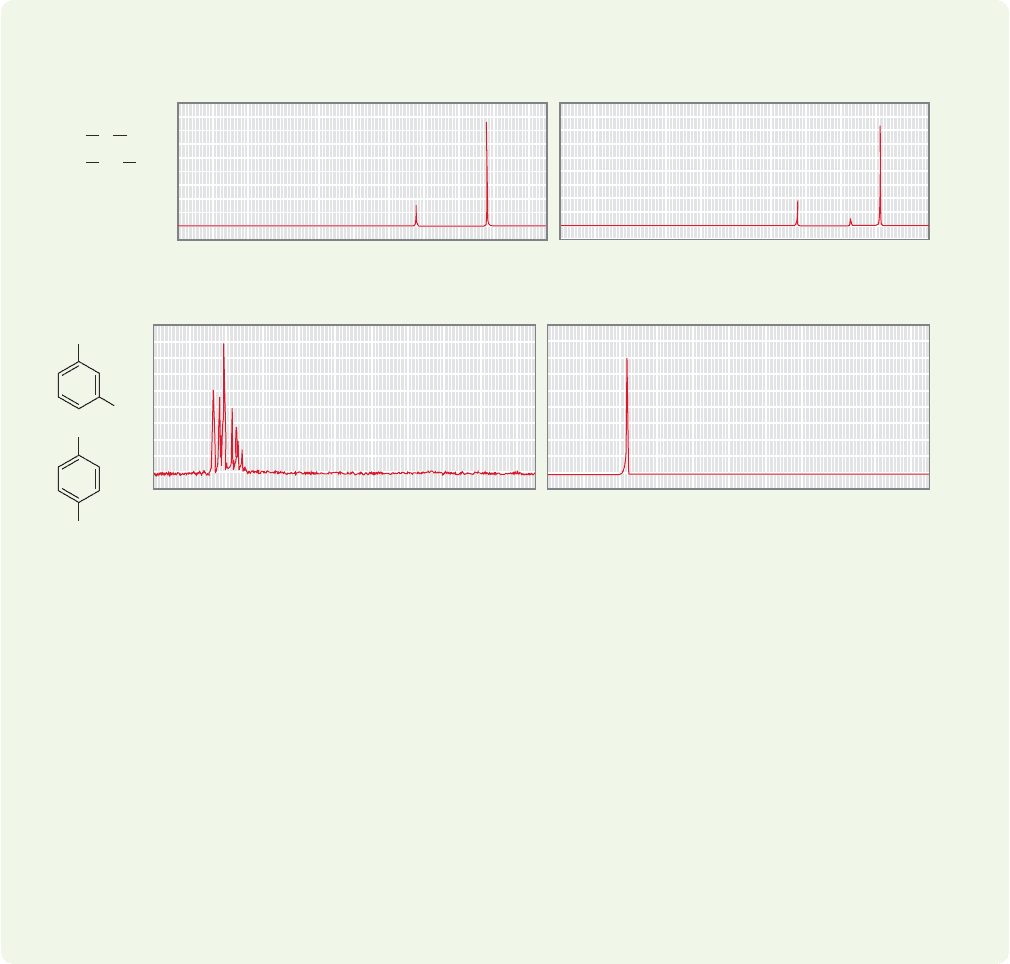
ANSWER (a) First of all, notice that this problem can be solved just by counting
signals. tert-Butyl methyl ether has only two kinds of hydrogen whereas
neopentyl alcohol (2,2-dimethylpropanol) has three. Thus, spectrum A1
belongs to the ether and spectrum A2 belongs to the alcohol. That’s all there is
to it. Why is the ratio of hydrogens in spectrum A1 1:3 not 3:9? Remember that
integration only gives the relative number of hydrogens—it is not an absolute
measure. There are other clues here. A nine-hydrogen signal? That’s certain to
be a tert-butyl group. In spectrum A1 there is a signal at approximately δ 3.3
ppm. That region is something of a desert in
1
H NMR spectra, and almost
always indicates a hydrogen attached to a carbon that is also attached to an
oxygen.
(b) Here symmetry also allows a quick assignment. 1,4-Dicyanobenzene has only
one kind of hydrogen and must give rise to the spectrum B2. 1,3-Dicyanobenzene
has three different hydrogens and has the far less symmetrical spectrum B1.
109876543210
(ppm)
Chemical shift (δ)
109876543210
(ppm)
Chemical shift (δ)
(b)
CN
CN
CN
CN
1H
2H
1H
3H 9H
Spectrum B1 Spectrum B2
109876543210
(ppm)
Chemical shift (δ)
109876543210
(ppm)
Chemical shift (δ)
(a)
CH
3
O(CH
3
)
3
C
OHCH
2
(CH
3
)
3
C
1H
2H
1H
3H 9H
1H
2H
1H
3H 9H
Spectrum A1 Spectrum A2
The examples in Problem 15.10 are straightforward, and are designed to ease
you into analyzing NMR spectra. In no area of organic chemistry is practice so
necessary in the development of skill.You simply cannot become proficient at this
kind of structure determination by reading about how it is done and studying
WORKED PROBLEM 15.10 Which of the molecules below give rise to which
spectra?

15.6b The Chemical Shift We need to know how the dependence of the
chemical shift (δ) on the local environment arises. In a sense, this is a nonquestion.
If two hydrogens are different, they must give different NMR signals. It is in fact
just a question of whether our ability to detect the difference is great
enough, because the difference must be there. Electrons in molecules occu-
py regions of space defined by the molecular orbitals of the molecule. The
chemical shift depends on the local environment because, when an external
magnetic field (B
0
) is applied, the electrons will circulate within those
regions of space so as to create an induced magnetic field (B
i
) that opposes
the applied field B
0
(Fig. 15.23).
The net magnetic field (B
net
B
0
B
i
) must be slightly different at every
different hydrogen in the molecule,and therefore the resonance frequency will
also be different for every different hydrogen.Relative to the hydrogens in TMS,
for most (not quite all) hydrogens in a sample molecule,the circulation of elec-
trons produces an induced magnetic field that augments the applied magnet-
ic field.The net magnetic field (B
net
) felt by most hydrogens is actually greater
than the applied magnetic field. Such hydrogens are said to be “deshielded”by
the induced field and they appear downfield of TMS, that is, to the left of
TMS, in the NMR spectrum. In a very few instances, we find that hydrogens
are “shielded” and appear upfield of TMS. In these cases, the magnetic field,
B
0
,is opposed by the induced field,B
i
,and it takes less energy to flip the nuclear
720 CHAPTER 15 Analytical Chemistry: Spectroscopy
Table 15.4, which gives approximate values of δ for many kinds of hydrogen.
Working problems is an absolute must. Do the problems at the end of this chapter
and seek out others. Other textbooks have good ones, and NMR problems can also
be found on-line; don’t be reluctant to dig them out.
TABLE 15.4 Chemical Shifts of Various Hydrogens
a
Hydrogen ␦ (ppm)
CH
3
(methyl hydrogens) 0.8–1.0
CH
2
(methylene hydrogens) 1.2–1.5
CH (methine hydrogen) 1.4–1.7
(allylic hydrogens, x 1, 2, or 3) 1.7–2.3
(α to carbonyl hydrogens, x 1, 2, or 3) 2.0–2.7
(benzylic hydrogens, x 1, 2, or 3) 2.3–3.0
(alkynyl hydrogen) 2.5
(α to amine hydrogens, x 1, 2, or 3) 2.0–2.7
(α to iodine, x 1, 2, or 3 ) 3.2
(α to bromine, x 1, 2, or 3) 3.4
(α to chlorine, x 1, 2, or 3) 3.5
(β to chlorine) 1.8
(α to fluorine, x 1, 2, or 3) 4.4
(α to ether or alcohol oxygen, x 1, 2, or 3) 3.2–3.8
(vinylic hydrogens) 4.5–7.5
(aromatic hydrogens) 6.5–8.5
(aldehydic hydrogen) 9.0–10.0
ROH (hydroxyl hydrogens) 1.0–5.5
ArOH (phenolic hydrogens) 4.0–12.0
RNH
x
(amine hydrogens, x 1 or 2) 0.5–5.0
CONH
x
(amide hydrogens, x 1 or 2) 5.0–10.0
RCOOH (carboxylic hydrogens) 10–13
a
These values are approximate. There will surely be examples that lie outside the ranges indicated.
Use them as guidelines, not “etched in stone”inviolable numbers.
O
P
CH
Ar
O
H
C
P
CH
O
O
CH
x
F
O
CH
x
Cl
O
C
O
CH
2
Cl
O
CH
x
Br
O
CH
x
I
O
CH
x
R
2
N
O
CH
x
q
C
O
H
Ph
O
CH
x
O
P
C
O
CH
x
C
P
C
O
CH
x
B
0
(the applied
magnetic field)
B
i
(the induced
magnetic field)
A hydrogen
nucleus at this
position “feels”
B
net
= B
0
– B
i
A hydrogen
nucleus at this
position “feels”
B
net
= B
0
+ B
i
Electron
cloud
FIGURE 15.23 In an applied magnetic field
B
0
, electrons circulate so as to generate an
induced magnetic field (B
i
) that opposes
the applied field.
15.6 NMR Measurements 721
spin. Because there are so few molecules that have signals
upfield of TMS, we can set δ for TMS as 0, with the spec-
trum usually appearing entirely to the left of TMS.
We can expect to see a signal for each different hydro-
gen in a molecule,and this idea leads to the another some-
times thorny question, What constitutes different
hydrogens? There is a simple scheme for finding identi-
cal (chemically and magnetically equivalent) hydrogens.
First, carry out mental substitutions of the hydrogens in
question with a phantom group X one at a time. If the
resulting molecules are identical, the hydrogens are also
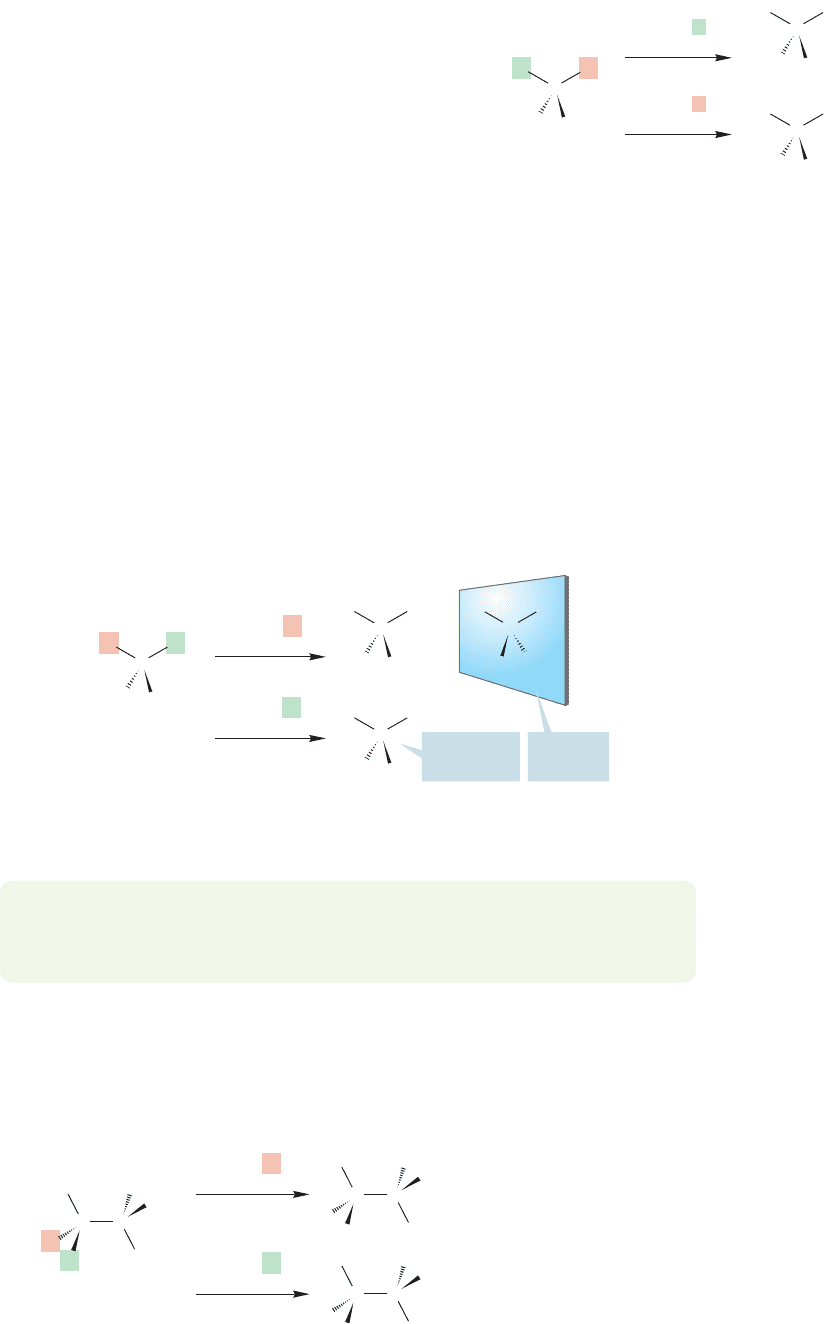
identical, or homotopic. Figure 15.24 gives an example using methylene chloride.
Replacement of either hydrogen (red or green in the figure) with X gives the same
CHCl
2
X. The two hydrogens of methylene chloride are homotopic and equivalent
in all chemical and spectroscopic operations.
Hydrogens in more complicated molecules are usually not homotopic.
Chlorofluoromethane is a good example. Replacement of the two hydrogens by the
phantom X in this case does not yield identical molecules (Fig. 15.25). The result-
ing compounds are enantiomers.When this mental replacement exercise gives enan-
tiomers, the hydrogens are said to be enantiotopic. Enantiotopic hydrogens are
equivalent in chemical reactions and NMR spectra, as long as chiral reagents or sol-
vents are not involved.
PROBLEM 15.11 Explain why the enantiotopic hydrogens of chlorofluoromethane
will show different signals if the NMR spectrum is obtained with an optically
active additive (often the solvent).
C
Cl
C
H
H
C
Cl
C
H
X
C
Cl
C
X
H
replace H
with X
replace H
with X
These molecules are
identical—the two
hydrogens are
homotopic, or
“NMR equivalent”
Cl
Cl
Cl
FIGURE 15.24 Substituting either of the two hydrogens gives
identical molecules; therefore the hydrogens in methylene
chloride are homotopic. Homotopic hydrogens are equivalent.
C
Cl
C
H
H
C
Cl
C
H
X
Mirror
C
C
X
H
C
Cl
C
X
H
replace H
with X
replace H
with X
These molecules are enantiomers;
the two hydrogens in chlorofluoro-
methane are enantiotopic
Chlorofluoro-
methane
Slide this
molecule…
…to this
position
F
F
Cl
F
F
FIGURE 15.25 The two molecules
obtained by substituting one of the
two hydrogens are enantiomers;
therefore the hydrogens are
enantiotopic. Enantiotopic hydrogens
are equivalent in typical NMR
experiments.
CC
H
H
Cl
Cl
H
H
H
Cl
Cl
X
replace H
with X
replace H
with X
These molecules are
diastereomers; the two
methylene hydrogens are
diastereotopic and not
“NMR equivalent”
1,2-Dichloro-
fluoroethane
F
F
CC
H
H
Cl
Cl
X
F
CC
FIGURE 15.26 The two molecules
obtained by substituting one of the
two hydrogens are diastereomers;
therefore the hydrogens are
diastereotopic. Diastereotopic
hydrogens are not equivalent in
NMR analysis.
Finally, consider 1,2-dichlorofluoroethane (Fig. 15.26). Now replacement of
the two methylene hydrogens gives neither identical molecules nor enantiomers,
but diastereomers (p. 164). These hydrogens are diastereotopic. Diastereotopic
hydrogens are chemically different and will always give different signals in the NMR
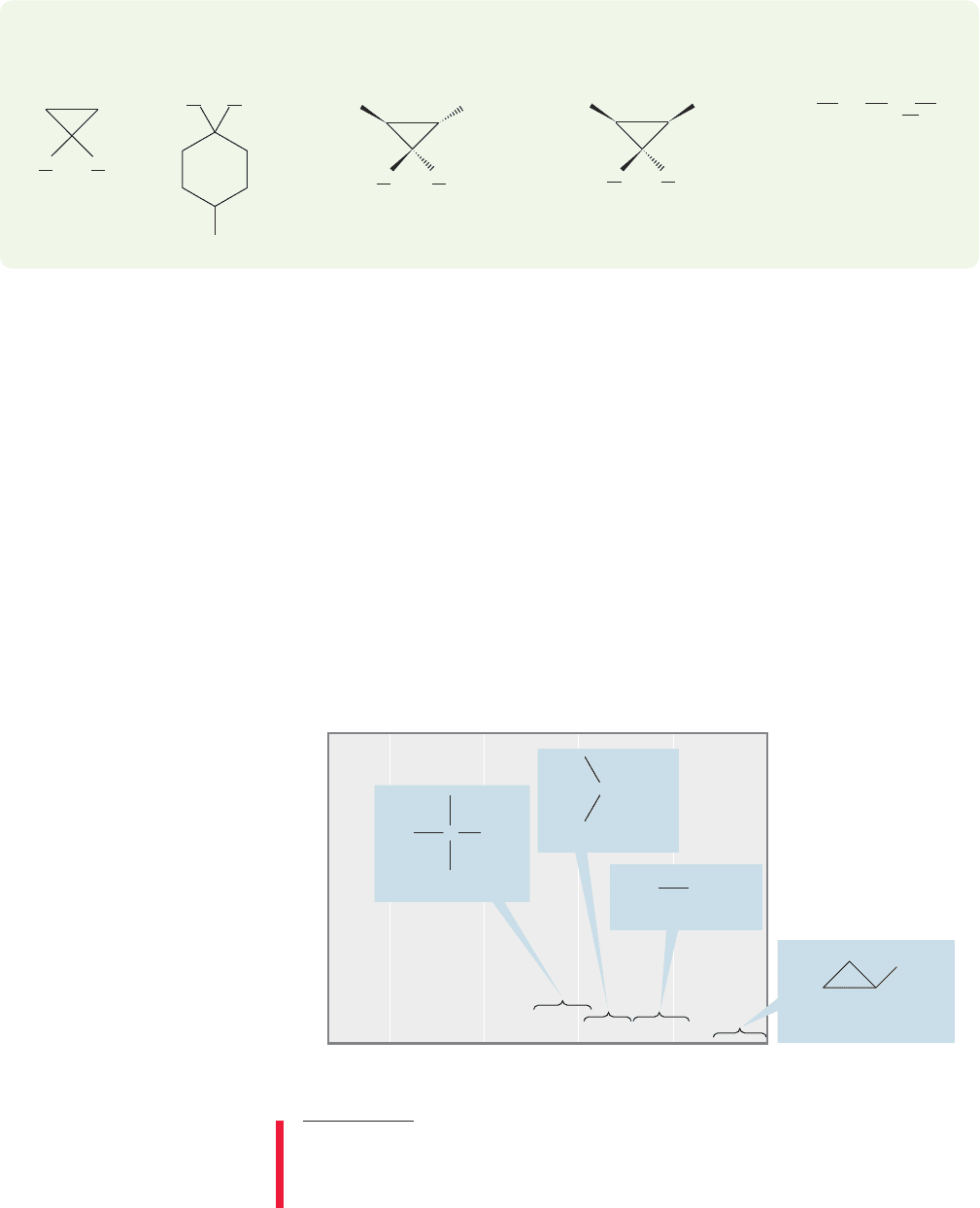
(a) (b)
H
H
H
H
HH
C(CH
3
)
3
H
3
C
CH
3
(c)
HH
H
3
C
CH
3
CH
3
(d) (e)
CH
2
CH
2
OH
722 CHAPTER 15 Analytical Chemistry: Spectroscopy
spectrum. Of course, diastereotopic hydrogens can be so similar that the spectrom-
eter cannot resolve the signals. There are many such examples, especially in old
spectra in which low-field spectrometers were used.
Now let’s return to the issue of chemical shifts. There is a general correlation
between the resonance frequency in an NMR spectrum of hydrogens that are in
similar enviroments in different molecules. For example, all methyl groups attached
to double bonds appear at roughly the same position, all aromatic hydrogens at
another, and so on.
3
Table 15.4 gives a correlation chart for the peak positions of
many different kinds of hydrogen. Use this table when working problems and you
will gradually become familiar with the chemical shifts of commonly encountered
hydrogens. Now we will discuss several of the factors that influence the chemical
shift in more detail.
Alkanes. NMR signals from saturated alkanes (and the simplest cycloalkanes)
are typically the furthest upfield. Because alkane hydrogens are unperturbed by any
substituents, we can think of the region where they appear (around 1 ppm) as a
beginning point. Signals for all organic compounds would appear at about 1 ppm if
there were no functional groups.There are exceptions of course.For example, hydro-
gens on a cyclopropane ring are at unusually high field, sometimes even appearing
upfield (to the right) of TMS (Fig. 15.27). A useful trend for analyzing NMR
2.5 2 1.5 1
(ppm)
0
CH
2
CH
3
HC
Methine
Methyl
Methylene
Cyclopropyl hydrogens
(very high field)
H
FIGURE 15.27 The
1
H NMR
chemical shifts of alkanes.
3
To say “aromatic hydrogen”is certainly wrong; the hydrogen isn’t aromatic, it is the ring to which it is attached
that is. Nonetheless, one must admit that it is slightly awkward to say “hydrogens attached to aromatic sys-
tems,” and this kind of loose talk is common. One refers to vinylic hydrogens, allylic hydrogens, cyclopropyl
hydrogens, for example.
CONVENTION ALERT
PROBLEM 15.12 Identify the underlined hydrogens in each of the following
molecules as homotopic, enantiotopic, or diastereotopic.
15.6 NMR Measurements 723
spectra of alkanes is that in any given environment a methyl group will be about
0.3 ppm upfield from a methylene group and a methylene group will be about 0.3 ppm
upfield of a methine. This trend is the reason why several entries of Table 5.4
have a range of chemical shift values that depends on the number of hydrogens on
the carbon.
Electronegative Groups. Electron-withdrawing groups will reduce the electron
density at neighboring hydrogens through an inductive effect (p.237), which decreas-
es electron-shielding of the nucleus. As a result of this deshielding,the chemical shifts
appear further downfield than those of alkanes.This effect is directly related to the
electronegativity of the substituent. Notice, for example, that because fluorine is
the most electronegative halogen,alkyl fluorides appear furthest downfield of all the
halogen-substituted molecules (Fig. 15.28). Chlorine and oxygen have almost the
same electronegativity and, as a result, the signals for the hydrogens of
and both appear at about 3.5 ppm (Table 5.4).CH
2
O
Cl
CH
2
O
O
6 5.5 5 4.5 4 3.5 3 2.5 2
1.5
CHCl
CHBr
CHI
CHF
(RO)
2
C
H
CHRO
CHR
2
N
(ppm)
X = F X = Cl X = Br X = I
CH
3
X
CH
2
X
2
CHX
3
4.26 3.05
5.31
7.28
2.68
4.96
6.86
2.16
3.88
5.36
Cl
3.5 1.4
1.8 0.9
FIGURE 15.28 The
1
H NMR chemical shifts of alkanes substituted with electron-withdrawing groups.
The effect of electronegative groups is understandably additive. Thus, increas-
ing the number of electronegative groups in the neighborhood of a hydrogen increas-
es the overall electron-withdrawing effect, and the resonance position shifts further
downfield. A clear example of this additivity is the comparison between CH
3
Cl
(δ 3.1 ppm), CH
2
Cl
2
(δ 5.3 ppm), and CHCl
3
(δ 7.3 ppm). The chlorine has a
deshielding effect of about 2.2 ppm (remember that unperturbed methyl hydrogens
appear about 0.9 ppm).The effect of the second chlorine in CH
2
Cl
2
is an addition-
al 2.2 ppm, which produces the observed chemical shift of 5.3 ppm. To predict the
chemical shift of CHCl
3
, one adds the effect of another chlorine (2.2 ppm), giving
7.5 ppm,which is close to its observed chemical shift of 7.3 ppm.The inductive effect
is also felt to a small extent by the hydrogens on the β carbon. The α carbon is the
carbon that is directly attached to the electronegative group. The hydrogens on the
α carbon are significantly shifted as shown in Table 5.4. The β carbon is attached
to the α carbon (there can be more than one β carbon). The hydrogens on the β
carbon in 1-chlorobutane (see Fig. 15.28) appear at δ 1.8 ppm. If the chlorine were
not present, then the chemical shift would be about 1.3 ppm. So the effect of the
chlorine on the β position is about 0.5 ppm, compared to the 2.2 ppm effect on the
α position. As we move further away from the electronegative group there is little
to no impact on the chemical shift.
724 CHAPTER 15 Analytical Chemistry: Spectroscopy
Allylic Hydrogens. A carbon–carbon double or triple bond is electron-withdrawing,
and therefore exerts a deshielding effect, shifting the signal downfield for hydrogens
in the allylic position. The carbon–oxygen double bond exerts a similar, but greater
effect (Fig. 15.29).
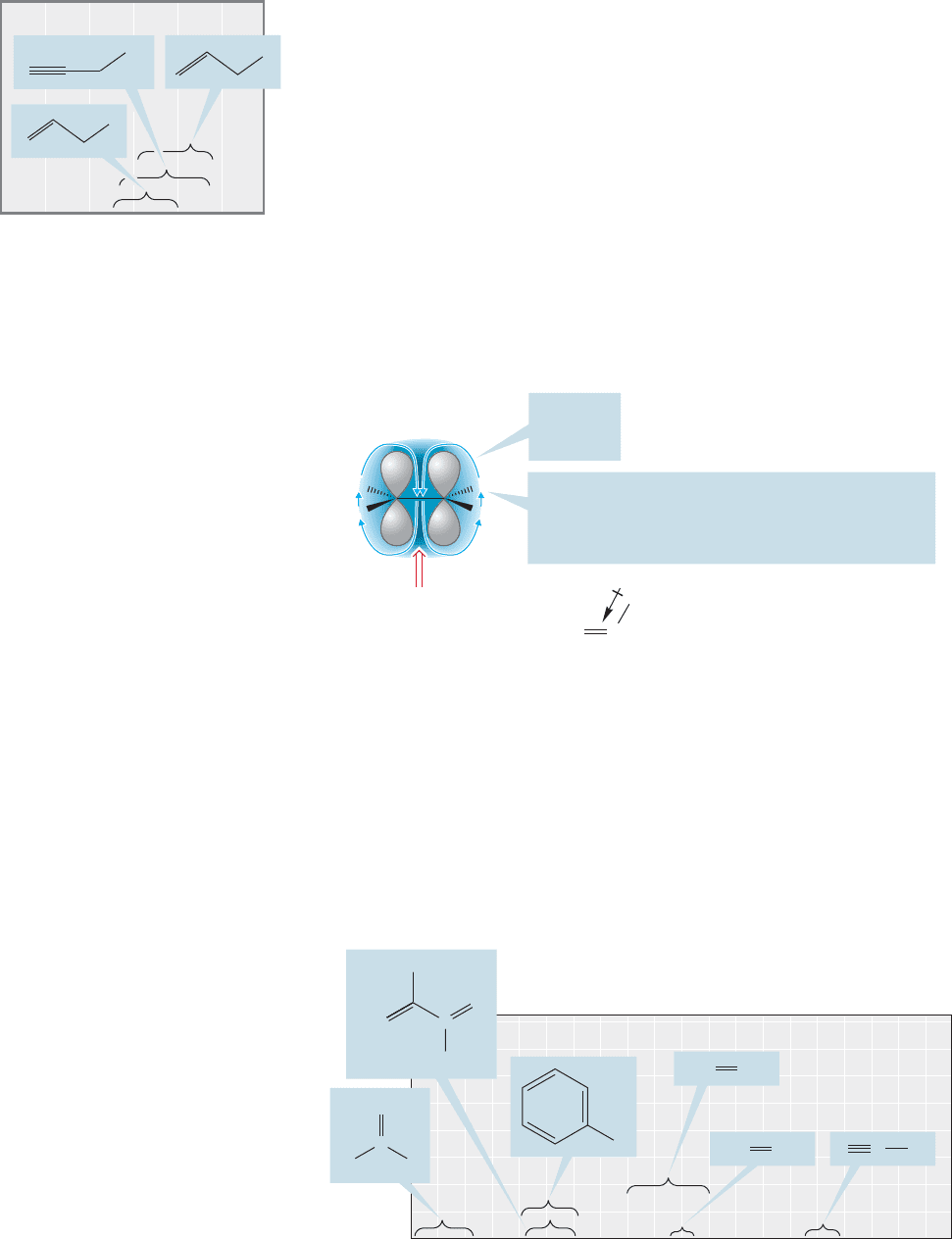
Hybridization. Hydrogens attached directly to a carbon–carbon double bond are
called vinylic hydrogens. Vinylic hydrogens appear downfield from hydrogens on
sp
3
hybridized carbons. Two effects are operating to deshield such hydrogens and
produce the downfield signals. In an external magnetic field, B
0
, the electrons in the
π bond will circulate so as to create a magnetic field B
i
that adds to B
0
in the region
where the hydrogens reside (Fig. 15.30). In addition, the sp
2
carbon of the double
bond has high s character and attracts electrons, thereby removing electrons from
the vicinity of the hydrogen and deshielding it. As a result of these two phenome-
na, vinylic hydrogens appear around δ 5.5 ppm.
There are important differences between the various kinds of hydrogens
attached to double bonds. For example, terminal methylene hydrogens appear
further upfield than most vinylic hydrogens the same way that a methylene
group appears upfield of a methine. Based on the hybridization trend, a hydro-
gen directly attached to a triply bonded carbon (alkynyl hydrogen) ought to
have a chemical shift around δ 9 ppm. However, it appears at δ 2.2–2.8, at
substantially higher field than hydrogens attached to double bonds (Fig. 15.31).
The sp
2
carbon has high
s character and withdraws
electrons, deshielding the
hydrogen
H
H
H
B
0
H
H
H
2
CCH
Induced
magnetic
field, B
i
At this point, B
0
is augmented by B
i
. The hydrogen
will “feel” a net B
net
= B
0
+ B
i
, and require a reduced
B
0
to
come into resonance. It appears at relatively
low field (downfield)
FIGURE 15.30 A carbon–carbon
double bond acts in two ways to
deshield attached hydrogens. Such
deshielded hydrogens appear
relatively downfield.
109876
(ppm)
5432
10
H
RCH CHR
R = alkyl
R
2
C
CH
CH
2
β
H
2
C
C
R
O
R
C
O
R H
FIGURE 15.31 The
1
H NMR
chemical shifts of alkenes and
alkynes.
4 3.5 3 2.5 2 1.5
O
H
H
(ppm)
H
FIGURE 15.29 The
1
H NMR
chemical shifts of hydrogens adjacent
to double and triple bonds.
15.6 NMR Measurements 725
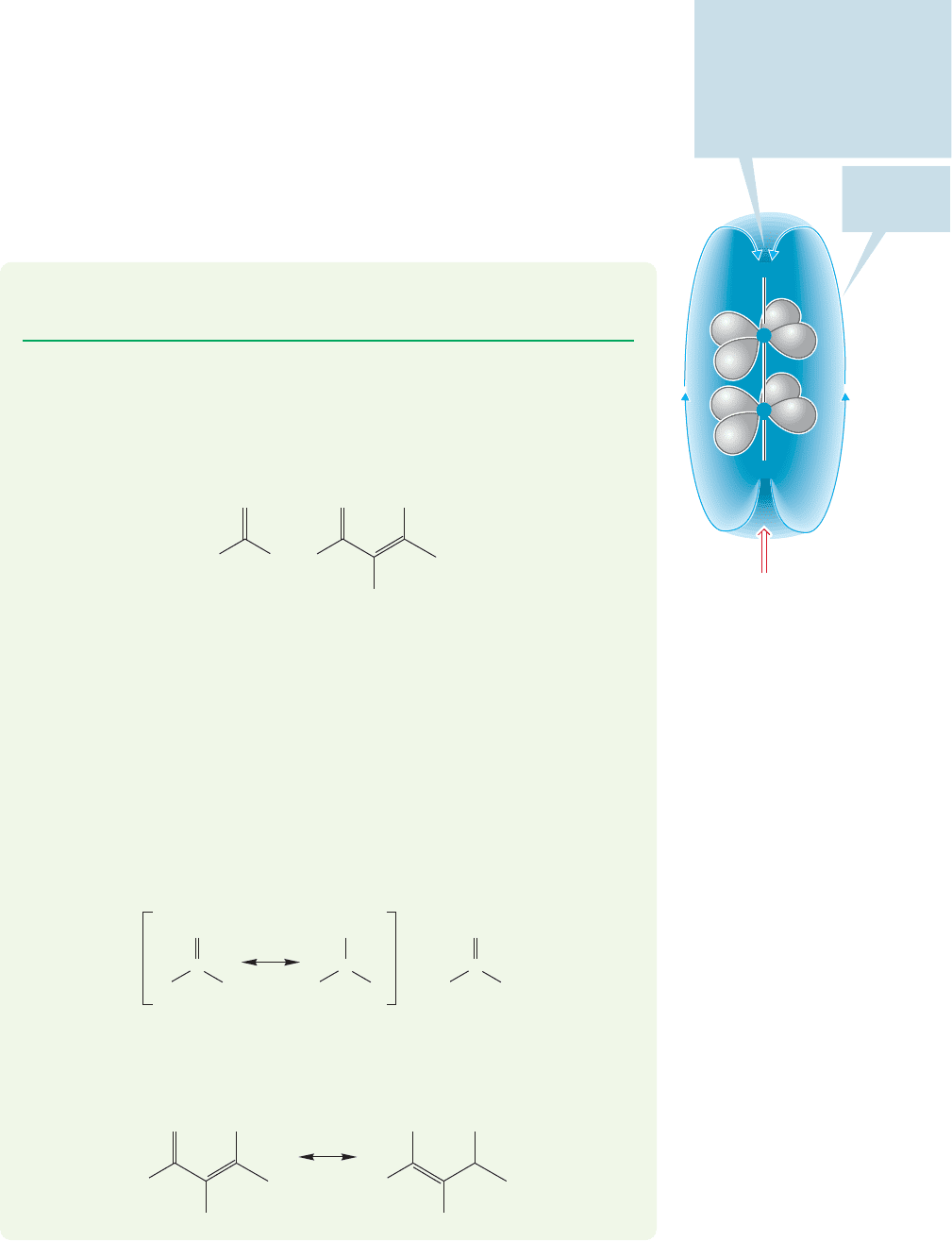
The induced
magnetic
field B
i
C
C
H
H
B
0
At this point the induced magnetic
field B
i
opposes the applied field
B
0
; a hydrogen here “feels” a net
magnetic field, B
net
= B
0
– B
i
. The
alkynyl hydrogen is shielded, and
a relatively high B
0
will have to be
applied to bring this shielded
hydrogen into resonance
FIGURE 15.32 Acetylenic hydrogens
are strongly shielded by the induced
magnetic field.
The upfield shift results from a strong shielding effect induced by circulation of
electrons in the triple bond (Fig. 15.32).
Delocalization. Hydrogens attached to a double bond, and β to a carbonyl group,
have a chemical shift surprisingly far downfield, and the hydrogens of aldehydes are
even further to the left (Fig. 15.31). These two examples show the effect that delo-
calization can have on chemical shifts.Groups that withdraw electrons by resonance
significantly deshield hydrogens on carbons at the β position. Alternatively, a
group that can donate electrons by resonance shifts hydrogens on the adjacent
carbon upfield (Problem 15.13). Such hydrogens are the furthest upfield of all
vinylic hydrogens.
O
R H
O
R
H
H
H
α
β
(a) (b)
O
C
R
H
..
..
O
..
..
..
–
+
C
=
R
H
O
..
..
C
R
H
δ
+
δ
–
O
R
H
H
H
α
β
R
H
H
H
α
β
+
O
..
..
..
..
..
–
ANSWER (a) In the absence of other effects, we would expect to see the hydro-
gen of an aldehyde group at about the position of hydrogens attached to
carbon–carbon double bonds. However, the carbonyl group is very polar. The
oxygen atom is much more electronegative than the carbon and will attract elec-
trons strongly, becoming partially negative (δ
) and leaving the carbon partially
positive (δ
). The same effect can be seen from a resonance formulation. The
electron density around carbon is depleted and the attached hydrogen is the most
deshielded hydrogen in neutral organic molecules. Accordingly, a weaker mag-
netic field is necessary to reach the resonance frequency, and the hydrogen
appears downfield.
(b) Conjugated carbonyl compounds have important resonance forms in which
the β carbon is positively charged. The resulting deshielding leads to an especially
downfield chemical shift for the β hydrogens.
PROBLEM 15.13 Predict the chemical shifts for the hydrogens in methoxyethene
(methyl vinyl ether).
WORKED PROBLEM 15.14 (a) A hydrogen attached directly to the carbon of a car-
bonyl group (aldehyde hydrogen) appears at δ 9–10 ppm, and must be strongly
deshielded. Explain why. (b) Vinylic hydrogens that are on the β position of a
conjugated carbonyl compound are also substantially downfield. Use resonance
structures to explain why.
H
H
2
C
δ
–3.0
(these negative chemical shifts mean
that the signals are to the right of TMS)
δ –0.5 δ –0.01
H
H
H
H
H
CH
2
WEB 3D
726 CHAPTER 15 Analytical Chemistry: Spectroscopy
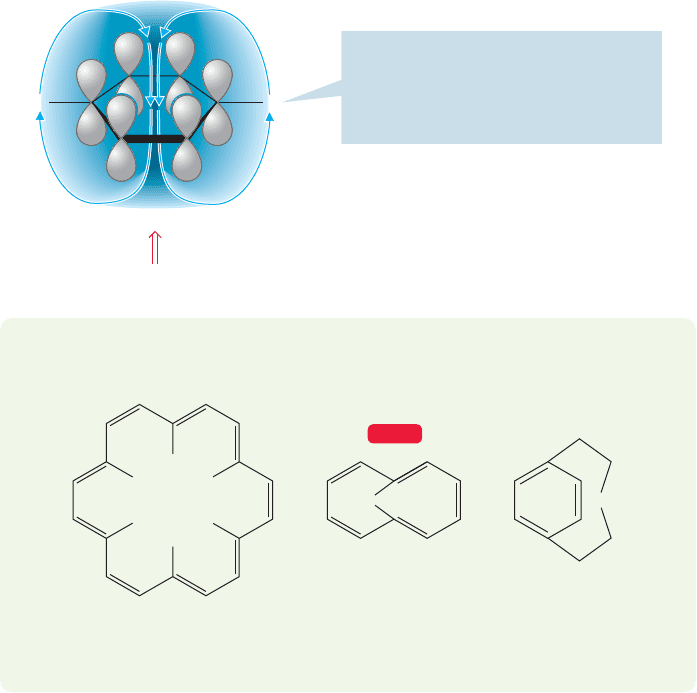
Aromaticity. Hydrogens on aromatic rings are found downfield from most
hydrogens attached to double bonds. In fact, so-called aromatic hydrogens can be
quite reliably diagnosed by the existence of signals in the range δ 6.5–8.0 ppm.The
reason for the downfield chemical shift is similar to that for hydrogens attached to
double bonds. The external magnetic field induces a circulation of electrons in the
ring (a “ring current”) that creates its own magnetic field B
i
opposing B
0
in the cen-
ter of the ring. At the edge of the ring where the hydrogens are, the induced mag-
netic field augments B
0
.Therefore, such hydrogens require a weaker applied magnetic
field to bring them into resonance (Figs. 15.31 and 15.33).
PROBLEM 15.15 Explain the unusual position (far upfield, above TMS) of the
hydrogens indicated in the following molecules:
HH
B
0
At this point the induced magnetic field,
B
i
augments the applied field B
0
; the
hydrogen will “feel” a net magnetic field,
B
net
= B
0
+ B
i
, and require a reduced
applied field
to
come into resonance
FIGURE 15.33 The field induced (B
i
)
by the ring current of an aromatic
ring in an applied magnetic field (B
0
).
Hydrogens Attached to Oxygen or Nitrogen. Hydrogens attached to a carbon
bearing the electronegative oxygen or nitrogen atom of an alcohol or a primary or
secondary amine absorb, as we might expect,in the same region as the related hydro-
gens in ethers and tertiary amines (Fig. 15.28). But what about the OH and NH
hydrogens themselves? Here we do not see what a simple analysis would lead us to
expect. First of all, the chemical shifts of OH and NH vary greatly from sample to
sample.The problem is that these molecules are extensively hydrogen bonded. The
extent of hydrogen bonding depends on the concentration of the alcohol or amine,
the nature of the solvent, the pH of the solution, and the temperature. The chemi-
cal shift depends strongly on the chemical environment and thus on the extent of
hydrogen bonding.As a result,it is very difficult to predict where a hydrogen attached
to oxygen or nitrogen will appear. Generally, the less hydrogen bonding, the further
upfield the resonance appears. In the gas phase, where hydrogen bonding does not
occur, the chemical shifts of these hydrogens are about 1 ppm.
15.6 NMR Measurements 727
15.6c Spin–Spin Coupling: The Coupling Constant, J The utility of
NMR spectroscopy goes far beyond that of a simple hydrogen-detecting machine.
We can determine the number of different kinds of hydrogen in a molecule, and
gain a general idea of what chemical environments those hydrogens might occupy.
But there is much more information available.
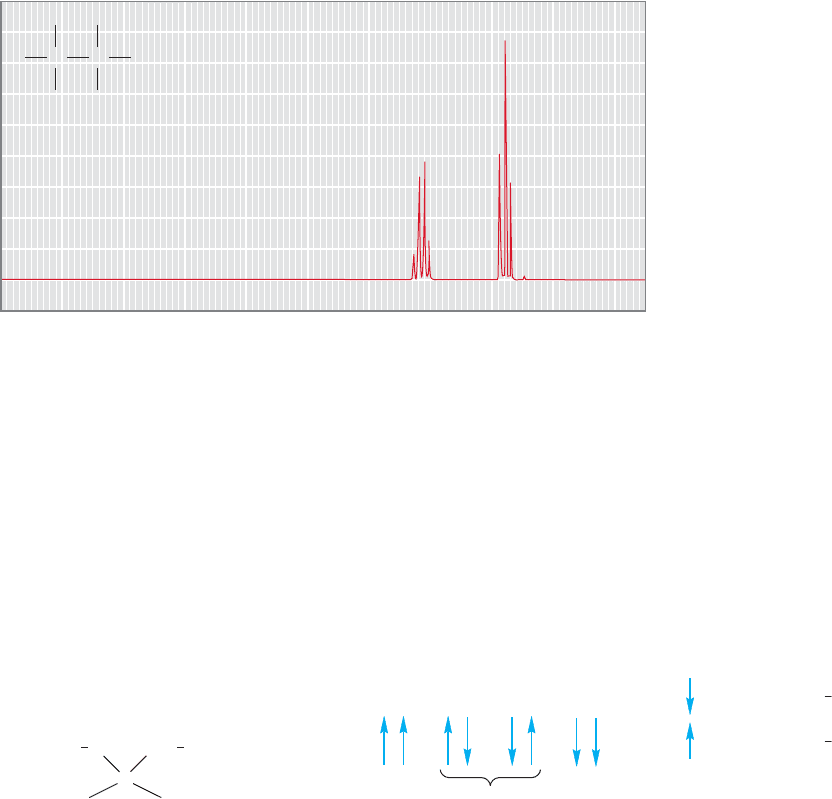
Most NMR spectra are not as simple as those of Figure 15.22 or Problem 15.10.
The signals in typical NMR spectra are not all single peaks, but are often multiplets
containing much fine structure. Ethyl iodide makes a nice example. Our initial expec-
tation might be that the spectrum would contain only two signals, one for the three
equivalent hydrogens of the methyl group and another for the two hydrogens of the
methylene group. A look at Table 15.4 would allow us to estimate the general posi-
tion for each peak. What we see, however, is somewhat different (Fig. 15.34). There
are signals at the positions we estimated, but each signal is composed of several lines.
They are not singlets as were most of the signals of the molecules in Problem 15.10.
The signal for the methyl hydrogens shows three lines (a triplet) in a 1:2:1 ratio cen-
tered at δ 1.85 ppm, and the methylene appears as four lines (a quartet) in the ratio
1:3:3:1, centered at δ 3.2 ppm.The spacings between the lines are all the same.
10 9 8 7 6 5 4 3 2
2H
3H
2H
3H
10
(ppm)
Chemical shift (δ)
H
H
H
C
H
H
C
I
FIGURE 15.34 The
1
H NMR
spectrum of ethyl iodide.The methyl
signal is a triplet and the methylene
signal is a quartet.
Our job is first to see how these multiple signals arise, and then to see how we
can use that information.
The (n ⴙ 1) Rule. The net magnetic field experienced by a hydrogen will be sig-
nificantly modified by adjacent (vicinal) hydrogens. Let’s start by examining the
triplet for the methyl hydrogens in Figure 15.34. To do this, we look first at the
neighboring methylene hydrogens.Each of the two equivalent methylene hydrogens
has a spin of either
1
/
2
or
1
/
2
.Therefore, there are four different combinations of
these spins, (
1
/
2
1
/
2
), (
1
/
2
1
/
2
), (
1
/
2
1
/
2
), and (
1
/
2
1
/
2
) (Fig. 15.35). The
methyl hydrogens will experience an applied magnetic field (B
0
) modified by each
C
I
HH
H
3
C
Equivalent
Means spin = –
Means spin
= +
To explain the three-line signal for the
methyl hydrogens of ethyl iodide, look
first at the neighboring methylene hydrogens
The possible combinations for the
methylene hydrogens are
+
–
1
2
1
2
1
2
+
–
1
2
FIGURE 15.35 For two hydrogens there are four possible combinations of the nuclear spins.Two of
these combinations are equivalent.
728 CHAPTER 15 Analytical Chemistry: Spectroscopy
of the different spin combinations of the neighboring methylene hydrogens. So we
now expect to see four lines,one for each possible spin combination. But two of these
combinations,(
1
/
2
1
/
2
) and (
1
/
2
1
/
2
),are equivalent and will give rise to the same
net magnetic field, which is the reason we see only a three-line signal in approxi-
mately a 1:2:1 ratio for the methyl hydrogens (Fig. 15.36). The methyl hydrogens
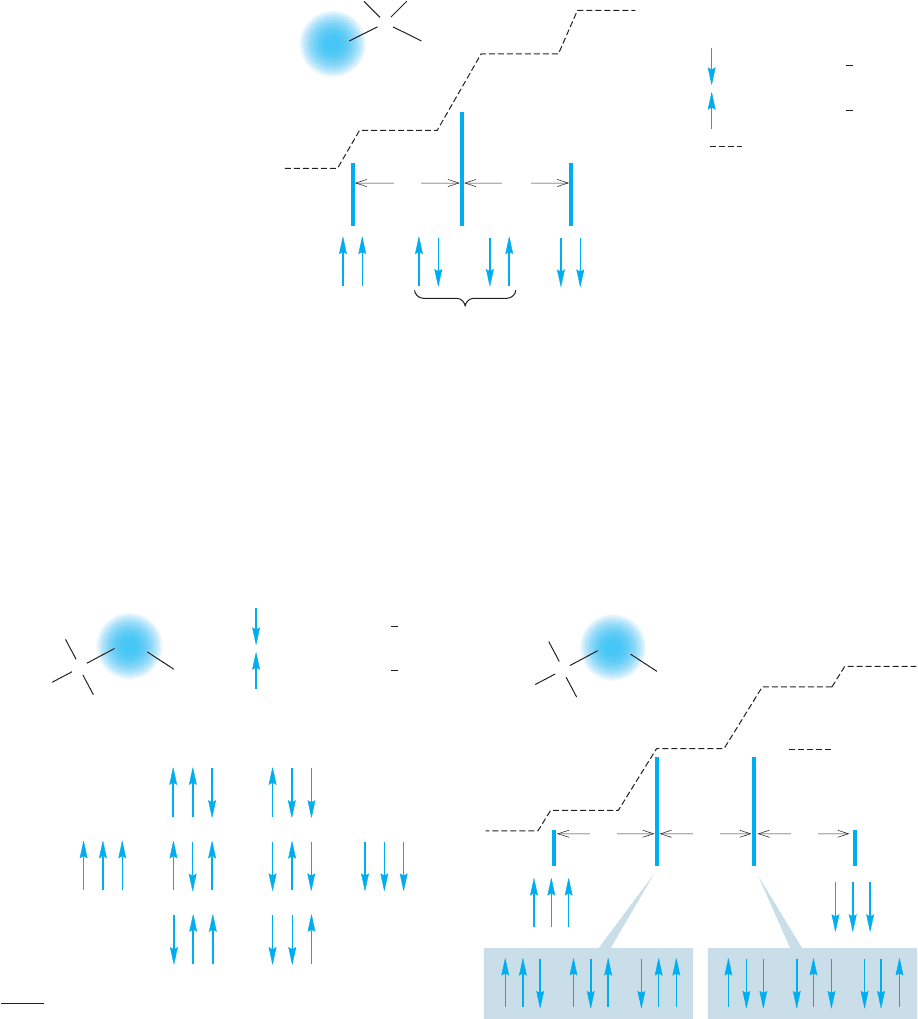
are said to be coupled to the adjacent methylene hydrogens. The distance between
any two of the lines, as mentioned earlier, is the coupling constant ( J ), and is
measured in hertz. In ethyl iodide the methyl signal is split by 7.6 Hz (Fig. 15.36).
Now let’s rationalize the four-line signal for the methylene hydrogens of ethyl iodide.
There are eight possible combinations of the nuclear spins of the three equivalent hydro-
gens that are vicinal to the methylene group (Fig. 15.37). Note that there are now two
sets of three equivalent spin combinations (two up and one down or one up and two
down).So,the methylene hydrogens feel an applied magnetic field modified by the four
different spin combinations of the adjacent methyl hydrogens. Accordingly, the spec-
trum consists of four lines in a 1:3:3:1 ratio, a quartet. The methylene hydrogens are
coupled to the methyl hydrogens with the same coupling constant, J 7.6 Hz,
by which the methyl hydrogens are coupled to the methylene hydrogens (Fig. 15.38).
C
1
2
1
HH
7.6 Hz
7.6 Hz
Equivalent
JJ
Means spin = –
Means spin
= +
Integral
1
2
1
2
H
3
C
I
FIGURE 15.36 The methyl group
adjacent to the two methylene
hydrogens will be split into three
lines in the ratio 1:2:1.The splitting
between the lines is called the
coupling constant, J.
I
All three
up
Two up,
one down
One up,
two down
All three
down
C
H
H
H
Spins
Means spin = –
Means spin
= +
1
2
1
2
CH
2
FIGURE 15.37 For the three methyl hydrogens in ethyl
iodide there are eight possible spin combinations, but
only four different energy combinations.
1
1
3
3
7.6 Hz
J
7.6 Hz
J
7.6 Hz
J
Integral
I
C
H
H
H
CH
2
FIGURE 15.38 The signal for the methylene group of
ethyl iodide will be split into four lines in the ratio
1:3:3:1.
