Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

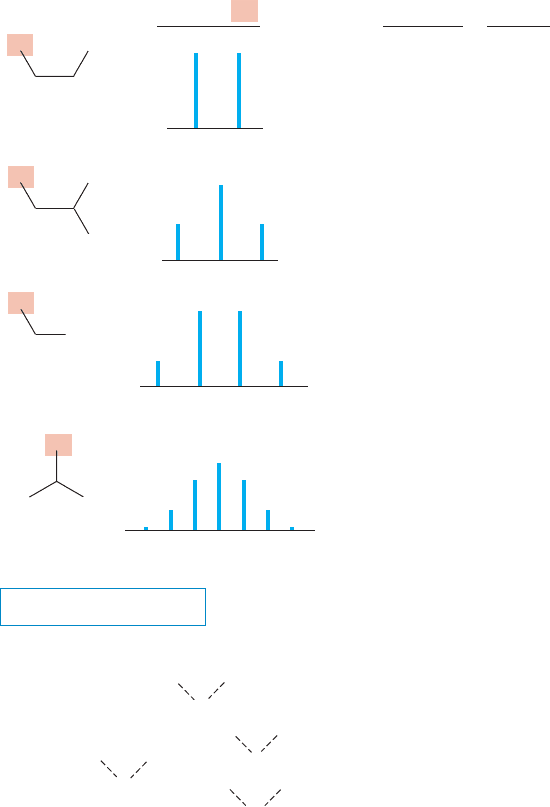
15.6 NMR Measurements 729
In the general situation, for a given hydrogen, H
a
, the NMR signal will consist
of n 1 lines,where n is the number of equivalent adjacent (vicinal) hydrogens, H
b
.
This observation is called the n ⴙ 1 rule.The lines for the signal are separated from
each other by a frequency of J Hz. The intensity of the lines can be determined by
analyzing the various possible spins of the coupled hydrogens as we have been doing,
or by using a device called “Pascal’s triangle.” In Pascal’s triangle, the value (inten-
sity) of a number is the sum of the two numbers above it. Figure 15.39 explains it
better than these words. Pascal’s triangle allows the rapid determination of the
intensities of lines in NMR signals. Note that we are speaking here of the relative
intensities of the lines within a given signal, not of the intensities of signals for dif-
ferent hydrogens in the molecule (the integral).
1133
H
a
CH
3
11206615 15
1
12
23
34
67
12
H
a
H
H
1
Signal for H
a
(n)
Number of
equivalent
adjacent H
(n + 1)
Number
of lines
in signal
1
H
a
H
H
a
H
3
C
CH
3
1
2
33
44
51010 5
15 20 15 6
6
6
1
11
1
1
1
1
1
1
1
1
1
THE GENERAL CASE
Pascal’s triangle: Each number is the sum of the
numbers above (see examples shown with dashed lines);
the intensities of NMR signals follow this pattern
FIGURE 15.39 In the general case, there will be n 1 lines for a hydrogen
adjacent (vicinal) to n equivalent hydrogens.
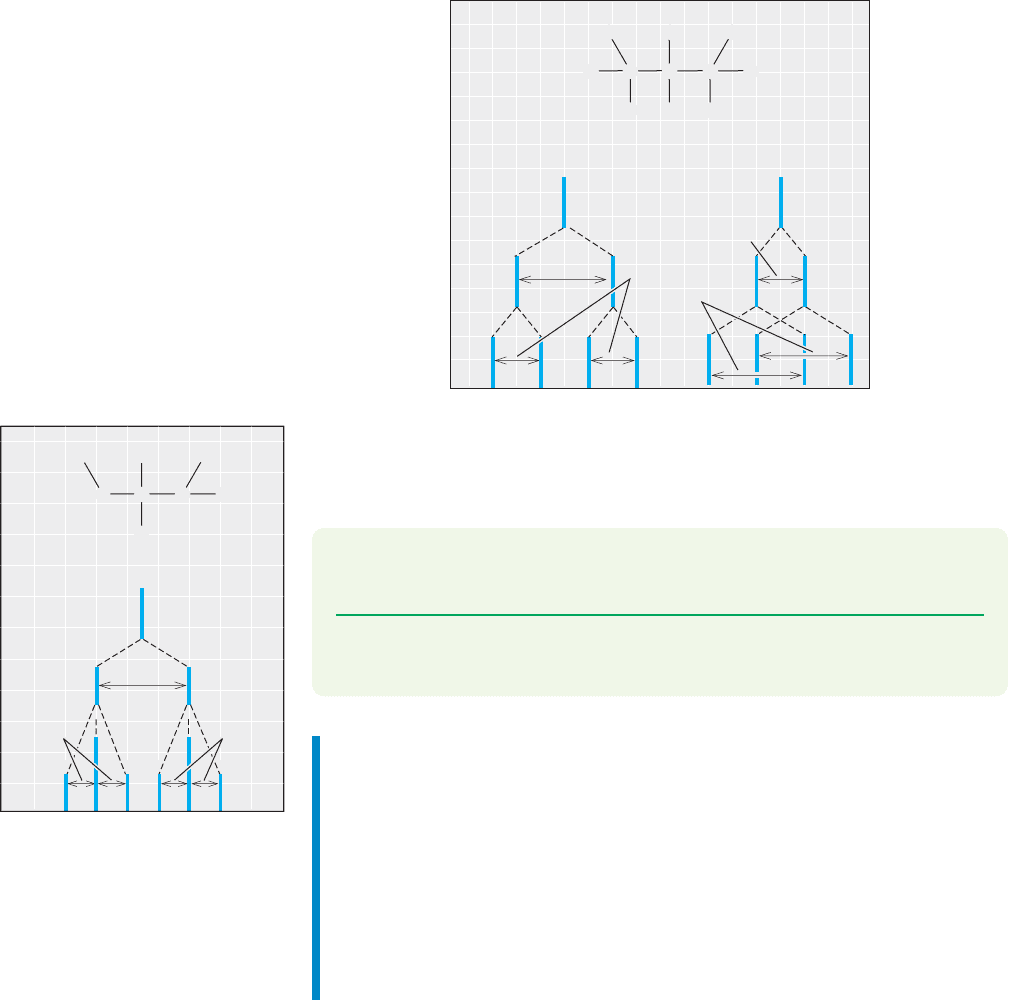
730 CHAPTER 15 Analytical Chemistry: Spectroscopy
Multiple Couplings. What happens when a hydrogen is flanked by more than
one kind of hydrogen, a very common situation in complex organic molecules? The
answer is quite simple—just apply the n 1 rule for each set of equivalent adjacent
hydrogens. Consider the situation in Figure 15.40 in which H
a
is adjacent to two
different hydrogens, H
b
and H
c
.The NMR signal for H
a
will be split into two lines
by H
b
and each of these lines will be further split into two by coupling to H
c
.Let’s
say that J
ab
is equivalent to 4 squares and J
ac
is equivalent to 2 squares.The result is
shown in Figure 15.40a: four lines, a doublet of doublets. But does it matter which
way we do it? In Figure 15.40a we started with J
ab
. In Figure 15.40b, we do it the
other way, starting with J
ac
. As you can see, the order makes no difference, we get
the same four lines either way.
J
ab
= 4
H
a
(a)
H
a
J
ab
= 4
(b)
X
H
b
C
X
H
a
Z
H
c
Y
YC C
J
ac
= 2
J
ac
= 2
FIGURE 15.40 The pattern for a
single hydrogen, H
a
, is determined by
coupling to two different adjacent
single hydrogens, H
b
and H
c
. Note
that the ultimate pattern of lines, a
double doublet, does not depend on
the order in which we write the
coupling constants J
ab
and J
ac
.
Similarly, Figure 15.41 shows what happens if hydrogen H
a
has one adjacent
hydrogen H
b
and two adjacent hydrogens H
c
,with J
ab
3 squares and J
ac
1 square.
A doublet of triplets results.
J
ac
= 1
J
ab
= 3
J
ab
= 3
H
a
H
b
C
H
a
Z
H
c
H
c
C C
J
ac
= 1
J
ac
= 1
FIGURE 15.41 The splitting pattern
for a single hydrogen, H
a
,is
determined by coupling to a single
adjacent hydrogen, H
b
, and two
equivalent adjacent hydrogens, H
c
.
PROBLEM 15.16 What happens in general if hydrogen H
a
is flanked by one hydrogen
H
b
and three hydrogens H
c
?
PROBLEM 15.17 What happens to the NMR spectrum of the molecule in Figure
15.41 if J
ab
2 squares and J
ac
1 square?
Summary
We have learned about the three important NMR phenomena: the integral, the
chemical shift, and the coupling constant. A signal will appear for each set of
equivalent hydrogens. The integration will tell us the relative number of hydro-
gens in that signal. The chemical shift of the signal is dependent on the chemi-
cal environment in which the hydrogen resides.The number of lines in an NMR
signal results from coupling with vicinal hydrogens. Multiple couplings can be
analyzed by constructing “tree”diagrams such as those in Figures 15.40 and 15.41
in which the different couplings are worked out in sequence.The order in which
one applies the different couplings never matters.
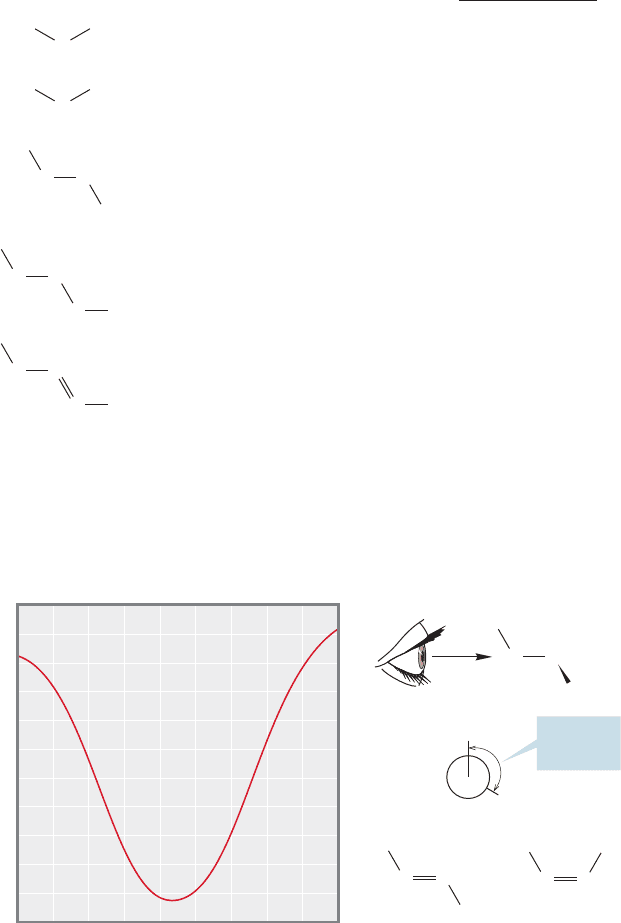
15.6 NMR Measurements 731
The Magnitude of J: The Karplus Curve. There is no observable coupling
between equivalent nuclei. But why is coupling observed only to hydrogens on adja-
cent atoms and not to all other hydrogens in a molecule? Usually, only “two-bond”
or “three-bond coupling” is large enough to be detectable (Fig. 15.42). Two-bond
coupling only occurs when hydrogens on the same carbon are diastereotopic (dif-
ferent).Such coupling can be very small ,and thus hard to detect, or it can
be relatively large (20 Hz). In some cases, long-range coupling can be observed,
especially if the hydrogens are connected in allylic fashion.
16 2 Hz2
0
2–30
6–8
Identical hydrogens
do not couple
Magnitude of J (Hz)
H
a
H
a
C
H
a
is two bonds away from H
b
;
coupling over this distance is
usually observable
H
b
H
a
is three bonds away from H
c
;
coupling over this distance is
usually observable
H
a
H
c
CC
0–1
H
a
is four bonds away from H
d
;
coupling is not usually observable
H
a
H
a
H
d
CC
C
2–3
H is in the allylic position relative
to H
e
; coupling is usually observable
H
e
CC
C
H
a
C
a
FIGURE 15.42 Coupling can be
measured over a three-bond distance,
but is not usually easily detectable
over longer distances.
The coupling constant between hydrogens on adjacent carbons is most sensitive
to the dihedral angle between those hydrogens, and this dependence can often be
used in assigning structure. Figure 15.43a shows the Karplus curve, named for
H
a
H
b
C
Eye
C
H
a
H
b
C
Dihedral
angle
θ
J = 12–18 Hz
θ = 180⬚
C
θ
9
10
7
8
6
5
4
3
2
1
0
–1
0⬚ 20⬚ 40⬚ 60⬚
For two adjacent hydrogens on sp
3
carbons
For two adjacent hydrogens
on sp
2
carbons
80⬚ 100⬚ 120⬚ 140⬚ 160⬚ 180⬚
J (Hz)
H
a
H
b
C
J
= 6–12 Hz
θ = 0⬚
C
H
a
H
b
(a) (b)
FIGURE 15.43 Coupling between
vicinal hydrogens is sensitive to the
dihedral angle between them. (a) The
Karplus curve shows the calculated
relationship between J and the
dihedral angle. (b) Dihedral angles
can be determined by using a
Newman projection and taking the
angle between the bond and
the bond.C
O
H
b
C
O
H
a
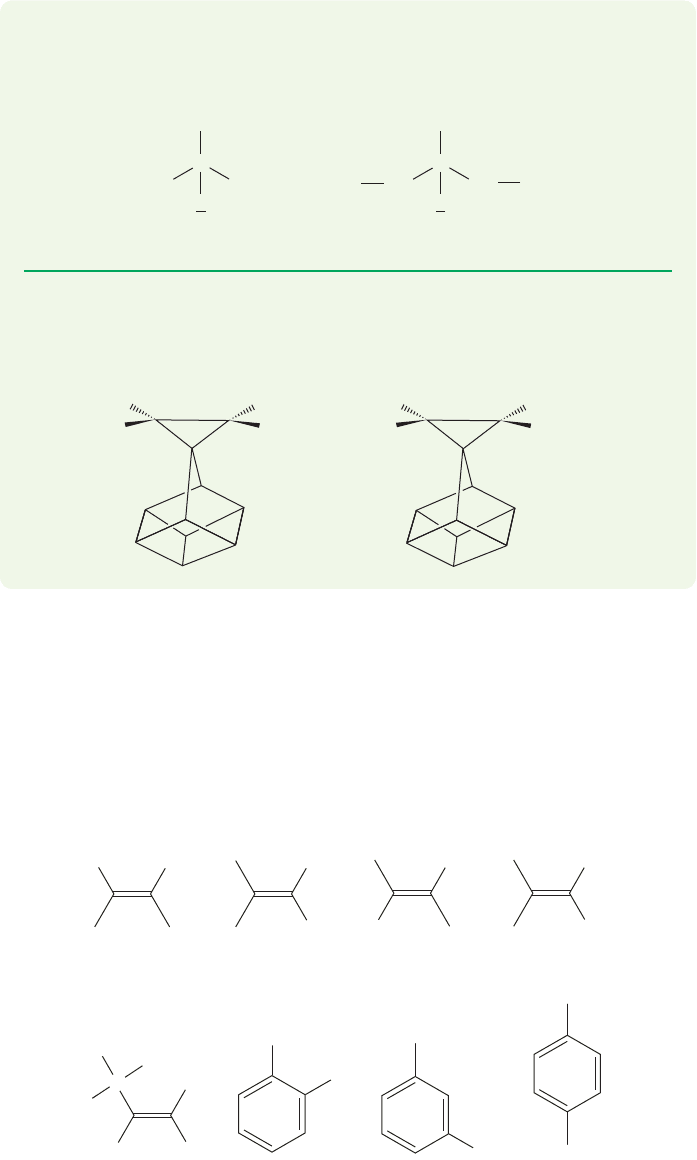
732 CHAPTER 15 Analytical Chemistry: Spectroscopy
PROBLEM 15.18 How many lines do you expect to see in the NMR signal for
the central hydrogen in 2-methoxypropane? How about 1,3-dichloro-2-
methoxypropane? Hint: Be careful! See page 721.
The cis coupling constant, J
cis
,for alkenes and rigid rings is smaller than the trans
coupling constant, J
trans
. In alkenes, J
cis
is in the range 6–12 Hz and J
trans
is in the
range 12–18 Hz. Notice that the ranges slightly overlap. One cannot always assign
stereochemistry from just one stereoisomer. What would you make of a coupling
constant of 12 Hz,for example? But if both isomers are available,the cis stereochem-
istry can be reliably assigned to the one with the smaller J value. Figure 15.44 gives
some typical coupling constants in alkenes and aromatic compounds.
C
Cl Cl
CH
2
CH
2
1,3-Dichloro-2-methoxypropane
H
OCH
3
C
H
3
C
CH
3
2-Methoxypropane
H
OCH
3
H
3
C
CH(CH
3
)
2
H
H
H
H
H
3
C
CH(CH
3
)
2
PROBLEM 15.19 The two molecules shown below have coupling constants for the
hydrogens attached to the three-membered ring of 8.4 and 5.3 Hz. Which
compound has which coupling constant?
J
ab
= 6–12 Hz
J
ab
= 6–8 Hz J
ab
= 1–3 Hz J
ab
= 0–1 Hz
H
a
H
b
J
ab
= 12–18 Hz
H
a
H
a
H
a
H
b
H
b
H
b
H
a
H
b
J
ab
= 0–3 Hz
H
a
H
b
J
ab
= 4–10 Hz
CH
b
H
a
C
J
ab
= 2–3 Hz
H
b
H
a
~~
~
~
~~
~~~
~~~
FIGURE 15.44 Some coupling
constants for hydrogens attached to
double bonds.
Martin Karplus (b. 1930) of Harvard University, which shows the dependence of J
on the dihedral angle for two hydrogens on adjacent carbons. Figure 15.43b shows
the dependence of the coupling constant on the angle between hydrogens attached
to sp
2
hydridized carbons.
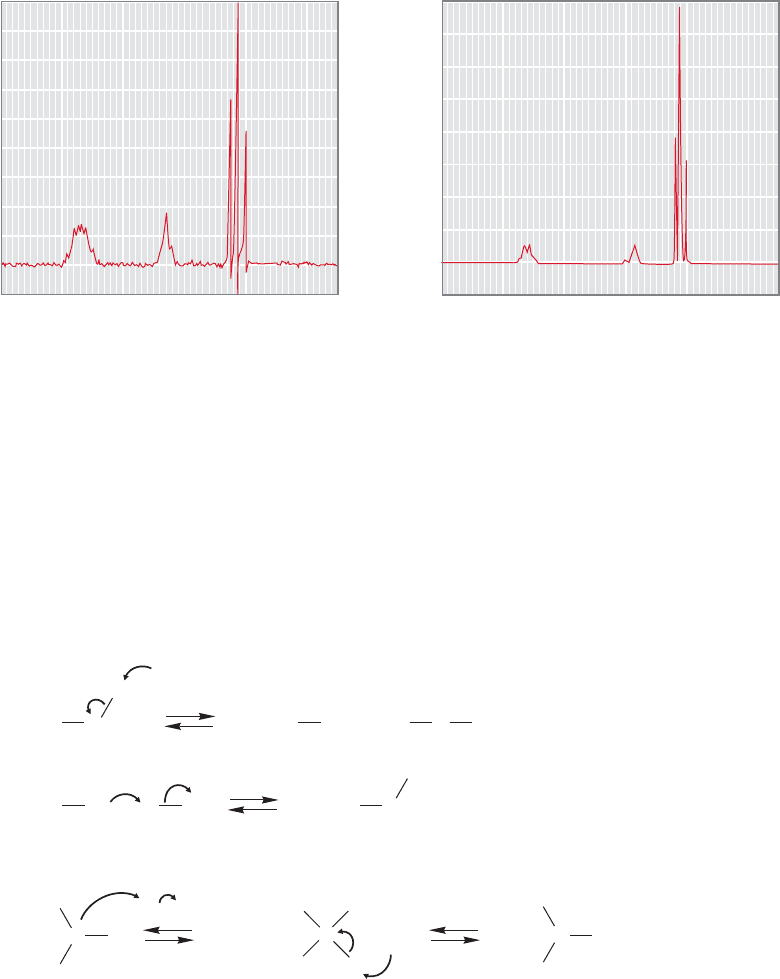
15.6 NMR Measurements 733
Chemical Exchange. The NMR splitting patterns of alcohols and amines are
often not what we would expect. Consider the common alcohol,ethyl alcohol, whose
1
H NMR spectrum is shown twice in Figure 15.45. Notice that these two spectra,
taken from different catalogs in the chemical literature, show a different chemical
shift for the OH signal. Remember there are several factors that can influence the
hydrogen bonding, and thus the chemical shift of a hydrogen on oxygen. These
include concentration, pH, solvent, and temperature.The methyl and methylene sig-
nals are in the same place in the two spectra.The spectra show the expected triplet
for the methyl group, but the methylene and hydroxyl hydrogens do not appear as
our analysis would predict.The most accurate description of them might be “blobs.”
What is going on?
543210
(ppm)
CH
3
CH
2
OH
a
a
bc
543210
(ppm)Chemical shift
(δ)
Chemical shift
(δ)
CH
3
CH
2
OH
abc
a
cb
cb
FIGURE 15.45 Two
1
H NMR spectra of ethyl alcohol show different shifts for the OH signal.
Here the issue is one of chemical exchange. In the presence of either an
acidic or a basic catalyst, or even under neutral conditions, alcohols and amines
exchange the OH or NH hydrogens. Figure 15.46 gives the mechanism for base-
catalyzed exchange of an alcohol and acid-catalyzed exchange of an amine, using
deuterated materials in order to detect the exchange reaction. This exchange
reaction can be used to locate the signals for and groups.
The NMR spectrum is taken, then a drop of D
2
O is added to the sample tube.
N
O
HO
O
H
+
+
–
OD
2
..
..
O
..
..
O
..
..
O
..
..
..
O
..
..
..
+
N
H
N
H
D
CH
3
CH
2
HD
CH
3
CH
2
–
D
–
..
O
..
..
D
–
..
..
O
..
..
O
..
..
..
..
..
+
+
O
..
D
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
N
..
CH
3
CH
2
CH
3
CH
2
DD
H
D
—
OD
2
+
..
HOD
2
CH
3
CH
2
D
FIGURE 15.46 The base-catalyzed
exchange reaction of alcohols, and the
acid-catalyzed exchange reaction of
amines.
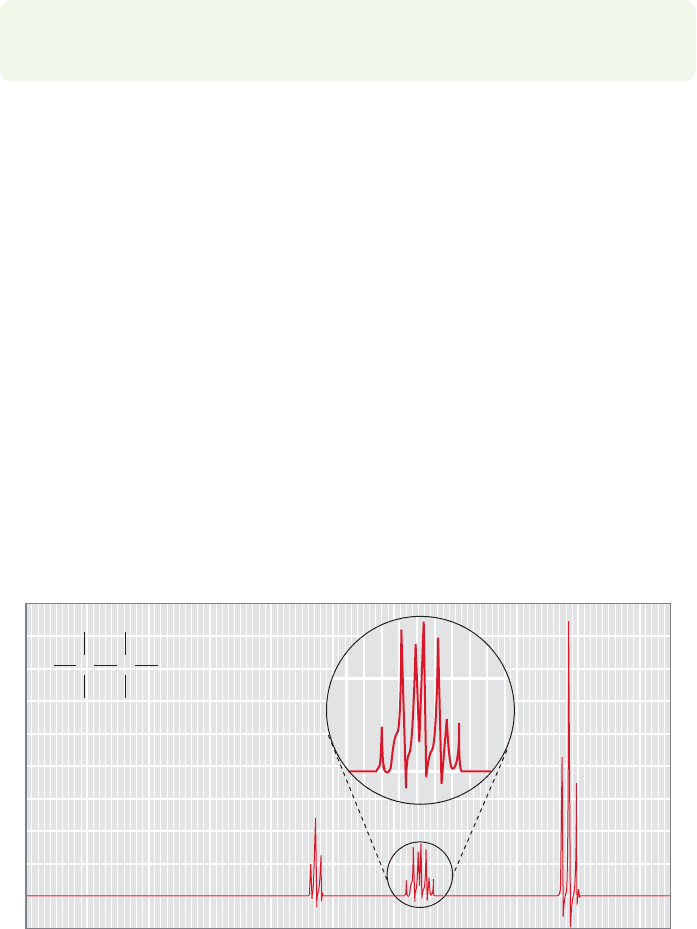
734 CHAPTER 15 Analytical Chemistry: Spectroscopy
The tube is shaken and the spectrum taken again. Hydrogens on carbon will not
exchange in this experiment, so their signals will not shift significantly. However,
the hydrogens exchange and the molecule becomes .The signals
for deuterium, which has a different magnetogyric ratio γ from hydrogen, will not
be observed because they appear in a different NMR range, so the signals for the
exchangeable hydrogens effectively vanish from the spectrum and are replaced by
an HOD signal.
RO
O
DRO
O
H
In IR spectroscopy, the absorption of energy is essentially instantaneous.The IR
spectrometer takes a stop-action picture of the molecule. Nuclear magnetic resonance
spectroscopy is different. Acquisition of the signal is much slower, about 10
3
s, and
the exchange reaction is fast compared to the time it takes to make the NMR meas-
urement. During this time the alcohol oxygen of a given molecule will be bonded
to many different hydrogens.This is why OH and the NH signals are often broad.
In addition, the two methylene hydrogens of ethyl alcohol “see” only an average of
all spins of the hydroxyl hydrogen, not the two different
1
/
2
and
1
/
2
states.
Exchange can be so rapid that there is no coupling with the OH hydrogen.The same
phenomenon serves to remove coupling between alkyl hydrogens that are vicinal to
the NH hydrogens of primary and secondary amines.
If this explanation is correct, the coupling should be detectable in scrupulously
purified ethyl alcohol because the exchange reaction will be stopped or, at least,
greatly slowed. It is very hard to remove the last vestiges of acidic or basic catalysts
from these polar molecules, but if the effort is made, the coupling returns, as it
should. Absolutely pure ethyl alcohol does give the anticipated triplet for the OH
hydrogen and a well-defined doublet of quartets for the methylene signal (Fig.15.47).
10 9 8 7 6 5 4
1H
2H
3H
1H
2H
3H
3210
(ppm)Chemical shift (δ)
a
b
c
H
a
H
a
H
a
C
H
b
H
b
C OH
c
FIGURE 15.47 The
1
H NMR
spectrum of absolutely pure ethyl
alcohol. See Problem 15.60.
This section gives the first hint that NMR spectra are dependent on the rates
of reactions. If the rate of the exchange reaction were slow on the NMR time scale,
we would not see the averaged spectra that we do for alcohols and amines. We will
return to this notion in Section 15.9.
PROBLEM 15.20 Write a mechanism for acid-catalyzed exchange of ethyl alcohol
and the base-catalyzed exchange of diethylamine.
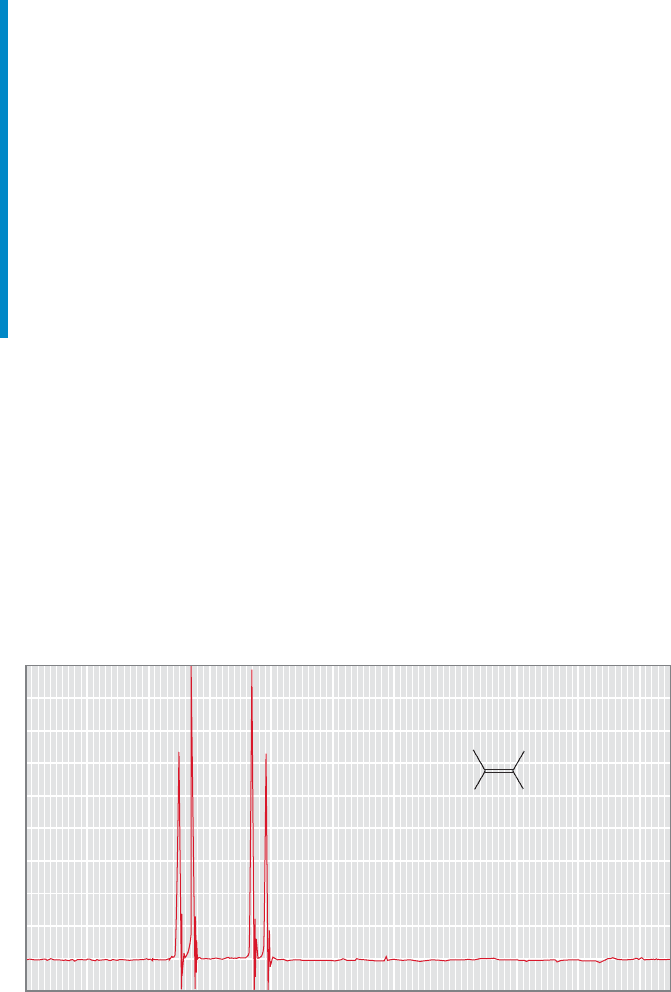
15.6 NMR Measurements 735
Summary
In the best of all worlds (which means in this case that your budget includes
enough money to buy the highest magnetic field spectrometer available), we will
see a different chemical shift for every different hydrogen in our molecule, and
we will be able to determine the number of equivalent adjacent hydrogens from
the n 1 rule. In practice, things may not be quite so simple. We can see some
possible complications right away. Perhaps the signals will not be sufficiently
separated, and the spectrum may consist of complicated overlapping multiplets.
Here is an example that shows why chemists are so anxious to spend taxpayers’
money on ever higher field spectrometers.The higher the field, the better the dis-
persion of the signals (recall Problem 15.9, p. 718).Remember that chemical shifts
(in hertz) are dependent on the strength of the applied field and the higher the
field, the more separated the signals.
109876543210
(ppm)Chemical shift (δ)
H
b
COOH
Cl
H
a
H
a
H
b
FIGURE 15.48 Two different
hydrogens on adjacent carbons will
give rise to a pair of doublets as long
as the hydrogens are very different.
The signal at δ 12 ppm for the
COOH hydrogen is not shown.
Remember that a doublet should appear as two lines in a 1:1 ratio. The sig-
nals in Figure 15.48 are indeed doublets. But the doublets are not simple 1:1 pairs
of lines; the doublet at δ 7.4 ppm is leaning to the right and the doublet at
δ 6.2 ppm is leaning to the left. In fact, we see the simple spectrum of 1:1 dou-
blets only when H
a
and H
b
are very different from each other: when they have
very different chemical shifts. The more alike the two hydrogens, the more the
spectrum deviates from first order, the spectrum predicted by the n 1 rule. As
the chemical environments of the two hydrogens become more similar, the chem-
ical shifts for the corresponding NMR signals of course also become more alike.
15.6d More Complicated NMR Spectra Spectra with coupling that
clearly fits the n 1 rule are called first-order spectra. Even at high field, NMR
spectra are often not the simple first-order collections of lines we might expect.
A classic example of this phenomenon occurs in the two-spin system shown in
Figure 15.48. In the NMR spectrum for this molecule, we expect to see a pair of
doublets because each hydrogen is different, and coupled to only one hydrogen
on the adjacent carbon. The hydrogen is not shown in this spectrum
(it is at δ 12 ppm).
COOH
Predicted spectrum for H
M
, four lines
J
MX
J
MX
J
AM
H
A
O
2-Furoic acid
H
M
H
M
H
X
COOH
WEB 3D
Here is the real spectrum of 2-furoic acid; the COOH hydrogen is off-scale
H
X
H
A
H
M
736 CHAPTER 15 Analytical Chemistry: Spectroscopy
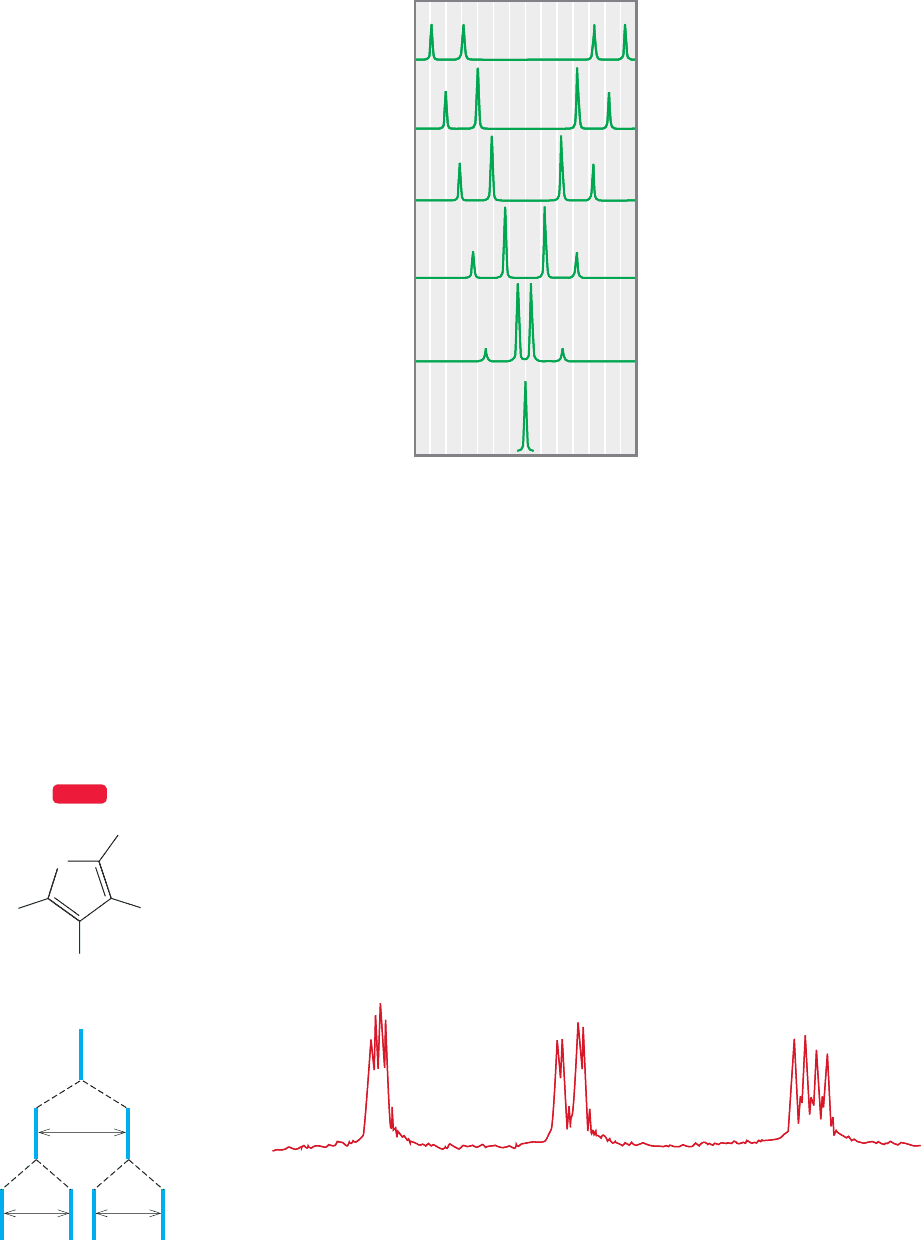
The more alike the hydrogens are,the closer the signals are and the more pronounced
the leaning of the peaks toward the center (Fig. 15.49).
In the limit, when H
a
and H
b
become the same, they have the same chemical
shift. Because there is no coupling between equivalent hydrogens, the signal for them
collapses to a single sharp line. Figure 15.49 shows the gradual transition between
the case in which the two hydrogens are very different and that in which they are
identical. In the language of NMR, the very different system is called AX, the iden-
tical system A
2
, and an intermediate case either AB or AM.
As we saw earlier (p. 730) it is often simple to derive the expected multiplicity of
an NMR signal from the n 1 rule. For example, in a molecule such as 2-furoic acid
(Fig. 15.50), the two different hydrogens on C(3) and C(5) (H
A
and H
X
) are each
coupled to the single hydrogen on C(4) (H
M
). This system of three very different
hydrogens is called an AMX system.We would predict the signals for H
A
and H
X
to
be a pair of doublets. The actual signals are more complicated because H
A
and H
X
are coupled to each other through long-range coupling (Fig. 15.44). Hydrogen H
M
in furoic acid is coupled differently to H
A
and H
X
by the coupling constants J
AM
and J
MX
. How can we figure out the pattern for H
M
that should be observed in the
AX
Two very
different
hydrogens
AM
Intermediate
cases
AB
Two similar
hydrogens
A
2
Two identical
hydrogens
–30 –20 –10 0
2010 30
(Hz)
FIGURE 15.49 As two hydrogens in a
molecule become more similar, the
inner lines grow at the expense of the
outer lines.The signals increasingly
lean toward each other. In the limit,
the two hydrogens become identical,
and there is only a single line.
FIGURE 15.50 In 2-furoic acid, we expect H
A
and H
X
to appear as doublets. Each
is strongly coupled to a single adjacent hydrogen, H
M
. However, both hydrogens
are further split by long-range coupling (J
AX
) into doublets of doublets.
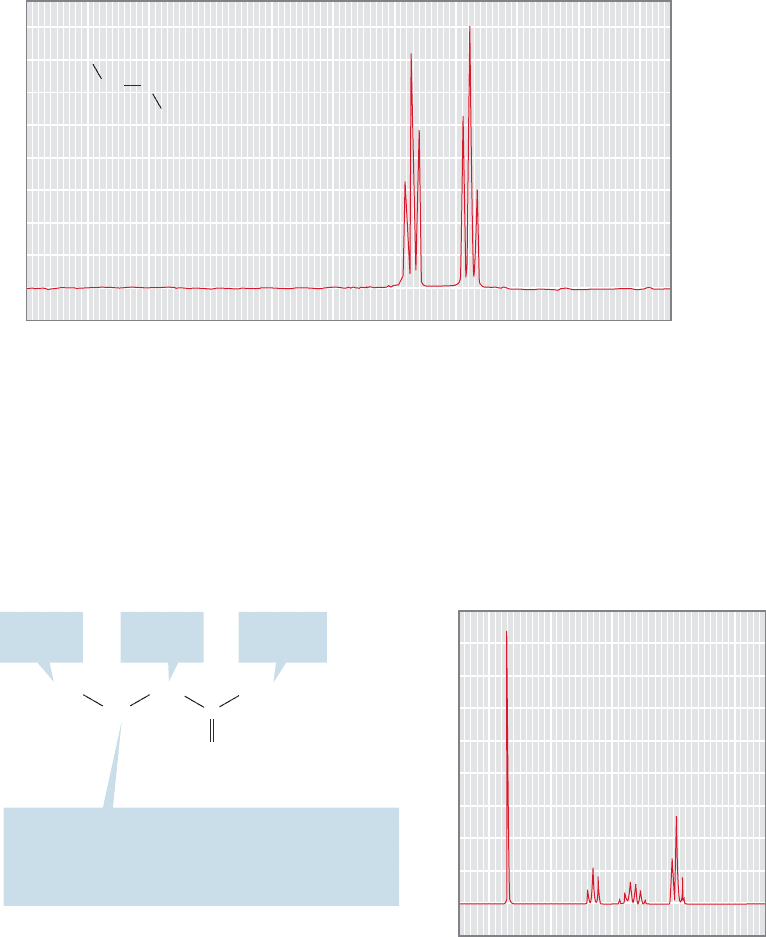
15.6 NMR Measurements 737
spectrum? The first-order pattern can be determined by working out the number of
lines from each coupling one at a time (p. 730). We first determine the number of
lines produced by coupling H
M
to H
A
, and then apply the coupling of H
M
to H
X
to
each of the lines in a “tree diagram.” For example, coupling of H
M
to H
A
produces a
doublet, separated by J
AM
. Each of these two lines is further split into two by cou-
pling to H
X
. The result is four lines, a doublet of doublets. Figure 15.50 shows the
theoretical prediction for H
M
and the real spectrum of 2-furoic acid.
First-order spectra are quite easy to predict. For example, according to the n 1
rule, the two different methylene groups of a generic molecule
should appear as a pair of 1:2:1 triplets. 2-Chloropropanoic acid fits the pattern and, as
Figure 15.51 shows,satisfactorily produces the expected pair of methylene triplets in its
1
H NMR spectrum at δ 2.89 and 3.78 ppm.However,when the molecules become more
complex we can anticipate complicated NMR spectra, even for first-order systems.
X
O
CH
2
O
CH
2
O
Y
109876543210
(ppm)Chemical shift (δ)
COOH
(not shown)
δ~12.0
CH
2
CH
2
Cl
FIGURE 15.51 In 2-chloropropanoic
acid, each methylene group appears
as a simple triplet. Notice that the
signals for these coupled hydrogens
lean toward each other.
O
These hydrogens are split into a triplet by the two
adjacent methylene hydrogens, and into a
quartet
by the three adjacent methyl hydrogens;
the result
is a sextet at δ 1.66, an overlapping set of 12 lines
Methyl butanoate
C
H
3
C
Triplet at
δ 0.94
Triplet at
δ 2.30
Singlet at
δ 3.68
CH
2
CH
2
OCH
3
43210
(ppm)Chemical shift (δ)
3H
2H
2H
3H
3H
2H
2H
3H
FIGURE 15.52 The
multiplet at δ 1.66 ppm
results from the splitting of
one methylene group by the
adjacent methyl and
methylene groups.The
result is 12 lines that overlap
in a complex pattern to give
a sextet.
Methyl butanoate is a simple molecule, and we can predict its first-order spectrum
quite easily (Fig. 15.52). The methyl group attached to oxygen has no neighboring
hydrogens and appears as a singlet at δ 3.68 ppm. The other methyl group is adja-
cent to a pair of methylene hydrogens and appears as a triplet at δ 0.94 ppm. The
methylene group adjacent to the carbon–oxygen double bond is flanked by the same
methylene group. It, too, is a triplet, this time at δ 2.30 ppm. But what about the
other methylene group? It is split by three methyl hydrogens, and then split again
738 CHAPTER 15 Analytical Chemistry: Spectroscopy
by two methylene hydrogens. The result should be a quartet of triplets, or, equiva-
lently, a triplet of quartets. Either way you work it out, the result is a 12-line pat-
tern, a dodectuplet! Fortunately, the coupling constants are nearly identical for a
freely rotating alkane and the signal collapses to a sextet (1:5:10:10:5:1). We can
analyze the coupling by noting that this methylene sees five very nearly equivalent
hydrogens and therefore the n 1 rule correctly predicts the sextet.
First-order spectra are only observed when all the hydrogens in a molecule are very
different from each other. As they become less different, more complicated spectra
appear. If we imagine carrying the process of making the hydrogens more and more
alike to its logical conclusion, we get a single signal for equivalent hydrogens.But when
are hydrogens “very different” and when are they not? In a practical sense, for two
hydrogens H
a
and H
b
to give rise to first-order spectra,the difference in hertz in their
chemical shifts (¢δ) must be at least 10 times greater than their coupling constant, J
ab
(15.5)
This requirement is another reason why high-field NMR spectrometers are so much
more useful than lower field instruments.Although the coupling constant J does not
depend on the field strength, the value (in hertz) of the chemical shifts does (p. 717).
The greater the field strength, the greater the chemical shift difference (¢δ) in hertz,
and therefore the greater the likelihood that the condition of Eq. (15.5) will be sat-
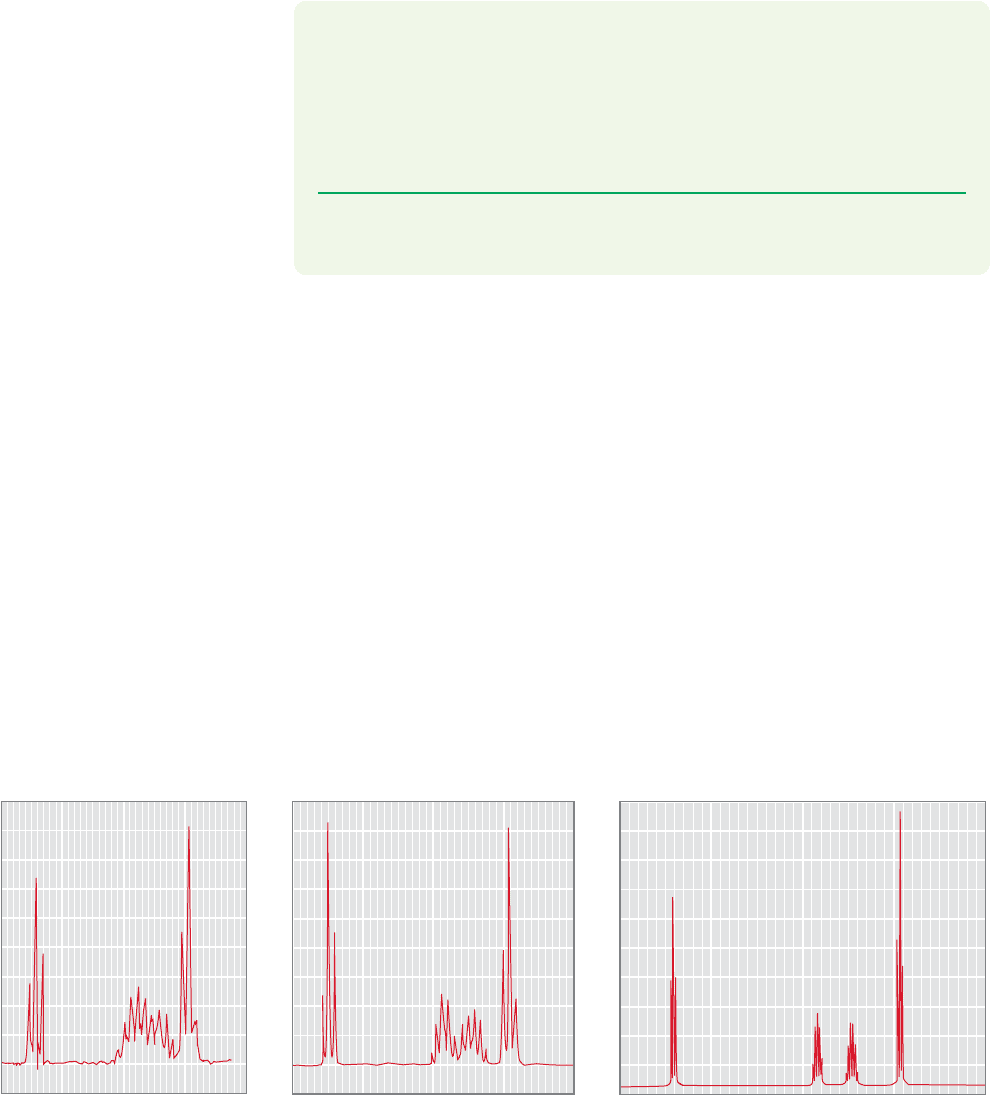
isfied. Figure 15.53 shows the
1
H NMR spectrum of 1-bromobutane at 60, 90, and
300 MHz. Even the modest change from 60 to 90 MHz results in substantial
improvement in the spectrum. You get what you pay for.
¢δ 7 10 J
ab
43210
(ppm)Chemical shift (δ)
43210
(ppm)Chemical shift (δ)
CH
3
CH
2
CH
2
CH
2
Br
60 MHz
43210
(ppm)Chemical shift (δ)
90 MHz
300 MHz
CH
3
CH
2
CH
2
CH
2
Br
CH
3
CH
2
CH
2
CH
2
Br
3H
2H
2H
3H
2H
2H
2H
3H
FIGURE 15.53 The
1
H NMR spectra of 1-bromobutane taken at 60, 90, and 300 MHz.
PROBLEM 15.21 In Figure 15.52, the expected dodectuplet has simplified to six
lines. The methylene group giving rise to that signal is coupled to both the
adjacent methyl and methylene groups. Use a “tree” diagram such as the one in
Figure 15.50 to show that, if the coupling constants J to the methyl group and the
methylene are fortuitously the same, the observed six lines should appear. Graph
paper is very useful!
PROBLEM 15.22 Explain why the methylene group adjacent to the carbonyl group in
methyl butanoate is downfield of the methylene group adjacent to the methyl group.
