Marshall L. Stoller, Maxwell V. Meng-Urinary Stone Disease
Подождите немного. Документ загружается.


348 Lowry and Nakada
stone management and initiation of allopurinol, the renal failure resolved (11). In the
other report, the patient was undiagnosed until after transplanted organ was rejected and
removed; the failure may have been prevented on appropriate therapy (10). In fact, both
patients had multiple previous surgeries for stone recurrence before renal failure and
transplantation. If 2,8-DHA had been properly diagnosed to begin with, these patients
may not have progressed to renal failure. When managed with proper medication, trans-
plantation of these patients is successful (4).
Additionally, the formation of 2,8-DHA stones may be exacerbated during preg-
nancy. A pregnant patient with APRT deficiency, who had stopped allopurinol therapy
on the advice of her obstetrician, presented with a 2,8-DHA steinstrasse. She was man-
aged conservatively with chronic ureteral stent changes until delivery, after which the
stones were removed. She experienced renal insufficiency, and her creatinine never
returned to normal (12). Females with APRT should be adequately counseled about the
potential fetal effects of allopurinol, as well as the possible risks to the mother associated
with discontinuation of allopurinol. Should patients continue through pregnancy, fluid
intake and urine output should be carefully monitored, especially at night when circadian
patterns increase risk of 2,8-DHA crystals in the urine.
Diagnosis may be accomplished with infrared spectroscopy. Quantitative 2,8-DHA
measurement of urine and determination of APRT activity in erythrocytes confirm exist-
ence of APRT deficiency. Quantitative 24 h 2,8-DHA measurements may also be used to
monitor treatment.
Treatment of 2,8-DHA calculi may be accomplished via any surgical modality, including
shock-wave lithotripsy (1). Prevention is accomplished with allopurinol therapy (300 mg/d)
and maintenance of urine output of at least 2.5 L/d (6). Dietary control has shown little
potential benefit (1,6), likely because the majority of adenine does not come from the
diet. However, a reported case described a child presenting in renal failure who was from
a commune where the primary diet was adenine rich lentils, grains, and vegetables. This
report could implicate high adenine intake as detrimental. Consequently, mild purine
restriction may be beneficial (4). Urinary alkalinization has no role; it has been described
as decreasing solubility of 2,8-DHA (11,13), although at physiologic urinary pH ranges,
is probably not detrimental, only ineffective at changing 2,8-DHA solubility (4).
2,8-DHA calculi should be suspected in all children with radiolucent stones, espe-
cially if presumed uric acid stones do not respond appropriately to treatment, or if
coexisting renal insufficiency develops. Brown smudges in diapers and small, yellow-
ish-brown, round urinary crystals suggest APRT deficiency. Suspicion, however, cannot
be limited to the pediatric population, as age presentation ranges from several months
old to the seventh decade (1).
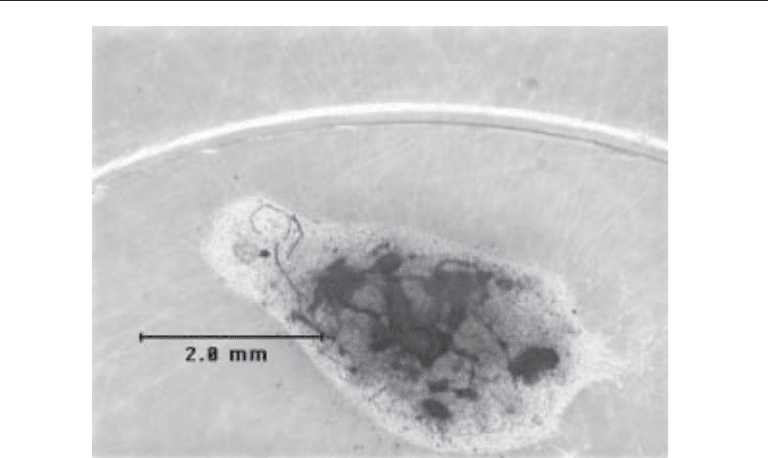
Ammonium Acid Urate Stones (Fig. 2)
Stones composed primarily of ammonium acid urate (AAU) are rarely seen in North
American patients, and account for less than 1% of urinary stones (14). In multiple
studies, the prevalence of predominantly AAU stones has been shown to be in the range
of 0.3 % (14–16). In third world countries, endemic AAU lithiasis, predominantly in the
form of pediatric bladder calculi, is more common. Navajo Indians in the United States
and aboriginal children in Australia have also been identified with AAU calculi (17–19).
Pure AAU stones are radiolucent, but usually these stone types have a secondary com-
ponent which may make them faintly radiopaque.
Chapter 19 / Urinary Stones of Unusual Etiology 349
Risk factors for the formation of AAU stones include decreased urine volume,
hyperuricosuria, stasis (for bladder stones), and the moderate elevation in ammonium
excretion associated with low phosphorus intake or phosphorus malabsorption. Other
risk factors include high urinary ammonium levels (a result of the alkaline urine of
existing infection, or acidic urine owing to diet), hypokalemia, dehydration, or starva-
tion states (14). Commonly, more than one of these risk factors coexist. Dehydration
decreases urine output and can result in acid urine, and a diet with excessive meat causes
hyperuricosuria and acidic urine. Conditions that predispose to uric acid stones and
struvite stones also lead to AAU precipitation.
AAU stones seen in third world countries are postulated to be multifactoral. The
combination of an acidic diet high in purines but low in phosphorus (perhaps owing to
dependence on breast milk), low fluid intake, and frequent diarrhea predisposes to a
urine with elevated concentrations of uric acid and ammonium, leading to AAU precipi-
tation (20,21). The secondary components in AAU calculi are generally uric acid or
calcium oxalate, but in the face of urinary infection with urea splitting organisms, the
urine alkalinization will support struvite formation as the secondary component (see
section on pediatric stones).
Pichette et al. analyzed 1346 stones and found 43 with some percentage of AAU, but
in only 3 of the 43 was AAU the dominant component (>50%) (14). They also observed
that the most common associated components were struvite (41%) and uric acid (35%).
Additionally, it was noted that in 75% of stones, the AAU was found as discrete deposits,
either intercrystalline or peripheral, mixed in with other stone constituents. The remain-
ing 25% of AAU was diffusely mixed with other stone components. Comparatively,
Soble et al. found that in 3400 stones, AAU was present in 44. In 11 of the 44, AAU was
the main component (mean of 44%). The most common secondary components were
uric acid (91%), calcium oxalate (63%), calcium phosphate (50%), and struvite (31%).
Fig. 2. Ammonium acid urate calculus (Courtesy of Mission Pharmacal, San Antonio, Texas).

350 Lowry and Nakada
The cohort was examined for risk factors: 41% were obese (BMI >30), 36% had recur-
rent urinary tract infections, 25% had inflammatory bowel disease, 13% were laxative
abusers, and 9% had a history of recurrent uric acid stones. These population groups of
AAU stone formers are consistent with the risk factors for AAU stone formation.
AAU formation in patients with laxative abuse has been well characterized by Dick
et al. (21). Eleven patients with documented laxative abuse were identified at two insti-
tutions. They were metabolically tested with serum studies and 24-h urine analysis.
Three of the patients had 24-h urine studies while taking laxatives and after a 1-mo hiatus
from laxatives. Serum electrolytes revealed normal parameters, except magnesium and
potassium, which were at the lower limit of normal, and bicarbonate, which was low in
five patients. Twenty-four-hour urine studies showed decrease in volume, sodium, potas-
sium, magnesium, phosphorus, uric acid, and citrate. In the three patients who submitted
a repeat study while not taking laxatives for a month, all values normalized. Laxative
abuse may cause metabolic acidosis, or alkalosis, depending on how long term and
regular the usage occurs and the amount of subsequent bicarbonate loss. Laxative abuse
produces a state similar to that of the endemic stone formation; low urine output, diarrhea
and resulting gastrointestinal (GI) electrolyte losses, sterile urine, and decreased phos-
phorus in the urine. The GI water and electrolyte loss produces a volume-contracted state
with potassium depletion and an intracellular acidosis, leading to low urine volume,
sodium, potassium, and citrate. Increased ammonia in the urine interacts with the urate,
which, because of the low urine sodium, becomes ammonium acid urate instead of the
usual soluble sodium urate (22).
HIV infected patients represent an additional group that is more predisposed to AAU
stone formation (23). In a small series of 11 stones formed by patients taking indinavir,
2 (18%) were composed of AAU. In a manner similar to that of laxative abusers, the
mechanism of AAU formation is thought to be related to the chronic diarrhea and mal-
nutrition.
Although the exact mechanisms for formation of AAU stones are not known, the renal
physiology surrounding AAU stone formation is well characterized. The pH at which a
minimum concentration of AAU will crystallize occurs at 6.2–6.3 (24). As the pH
increases, a higher concentration of AAU is required for stone crystals to form. At the
level of the nephron, ammonia is produced in the proximal convoluted tubule (PCT).
Ammonia is used by the kidney as an acid buffer. Increased amounts of ammonium are
excreted in states of metabolic acidosis, as well as potassium depletion, which is a form
of intracellular acidosis (25). Uric acid, which comes from urate in the serum, is freely
filtered at the glomerulus, and 99% reabsorbed in the PCT. Fifty percent of the original
filtered load is then secreted by the PCT, and all but 10% gets reabsorbed. After leaving
the nephron, the form of urate is pH dependent. At its dissociation constant, a pKa of 5.7,
uric acid and urate exist in a 50:50 ratio. As the pH increases, the urate predominates,
usually in the form of sodium urate.
Uric acid stone formers with urinary tract infections from urea splitting organisms are
at risk for AAU formation. When these patients have sterile urine, normal ammonium,
hyperuricosuria, and any pH, AAU will not form. However, in the face of urinary infec-
tion with urea splitting organisms, the increased pH and elevated ammonium make AAU
formation (with struvite) likely if enough urate is present (14,21). This situation must be
considered in patients with urinary tract infections who are afflicted with the Lesch–
Nyhan syndrome, as these patients are uric acid stone formers. Some may treat Lesch–
Chapter 19 / Urinary Stones of Unusual Etiology 351
Nyhan patients with urinary tract infections with temporary allopurinol to decrease the
amount of urate, lowering the chance of AAU stone formation (26).
Treatment of these stones, after elimination of existing stones, is aimed at prevention.
Acute stones may be successfully treated with SWL, although slow dissolution with
cessation of laxatives and proper fluid intake has been described (21). After initial
treatment, risk factors must be identified for preventive counseling and treatment. Patients
should have follow-up with multiple twenty-four urine studies to ensure adequate treat-
ment and compliance. Patients with diarrhea from ileostomies must maintain proper
urine volume and electrolyte replacement, and must be followed with 24-h urine studies.
Recurrent urinary infections must be eliminated, especially in patients who form uric
acid stones. Uric acid stone patients need treatment with alkalinization, volume and if
hyperuricosuric, dietary restriction and allopurinol. Ammonium acid urate stones are
rare, and occur in discrete situations. Stone analysis provides definitive diagnosis.
Awareness of the conditions that predispose their formation will prevent confusion with
other radiolucent stone types.
Xanthine Stones
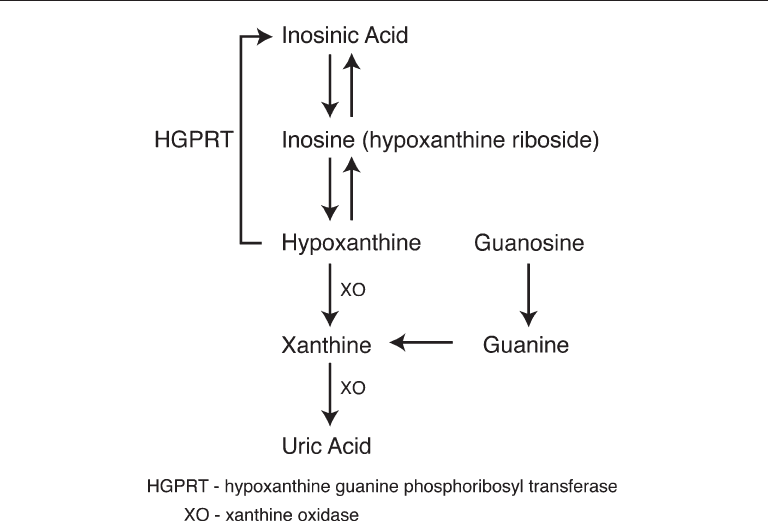
Primary xanthine stones are found only in patients with hereditary xanthinuria.
Xanthinuria is a metabolic disorder of purine metabolism, which is passed on in an
autosomal recessive pattern. This disorder is characterized by a deficiency of xanthine
oxidase, which prevents the conversion of hypoxanthine to xanthine, and further pre-
vents xanthine from being converted into uric acid (Fig. 3). Serum and urine uric acid
levels are extremely low (serum 0.8–1.0) (27,28), and the uric acid precursors, xanthine
Fig. 3. Uric acid metabolism.

352 Lowry and Nakada
and hypoxanthine, are elevated. After being excreted, xanthine precipitates in the urine
to form stones. Hypoxanthine, being more soluble, does not form stones. Stone forma-
tion is the primary manifestation of this disease, and occurs in approximately one-third
of affected patients (27).
Xanthine stones are radiolucent, but can be detected by using computed tomography
(CT) (29). Usual excretion of xanthine and hypoxanthine is between 10 and 20 mg/24 h,
but during allopurinol therapy, these amounts may exceed 150 mg. However, in patients
with excess production of urate, the values can be as high as 700 mg/24 h (30). The
solubility of xanthine increases from a concentration of 5 mg/100 mL at a pH of 5 to
13 mg/100 mL at a pH of 7 (31). This relatively moderate increase in solubility places
more importance on increased urinary output and dietary purine restriction, rather than
on pH modulation as the primary therapy for prevention of xanthine stones. Analysis of
xanthine stones have revealed stones of xanthine mixed with oxypurinol in patients
treated with xanthine oxidase inhibitors (32) and stones made of almost pure xanthine
(27). They are described as faintly radiopaque (27,32).
Secondary xanthine stones are also formed under several specific circumstances. Patients
with Lesch–Nyhan syndrome have a deficiency of the enzyme hypoxanthine guanine
phosphoribosyl transferase (HGPRT), which is important in the purine salvage pathway
(Fig. 1). Without HGPRT, hypoxanthine cannot be taken back into the salvage pathway,
and is consequently metabolized by xanthine oxidase (XO) to uric acid, the end product
of the pathway. As a result, Lesch-Nyhan patients are hyperuricemic and hyperuricosuric.
These metabolic abnormalities are treated with urinary alkalinization and allopurinol,
which inhibits xanthine oxidase, lowering the uric acid production and decreasing the
incidence of uric acid stone formation. However, in rare cases, the inactivation of xan-
thine oxidase by allopurinol may lead to the formation of stones by an excess of xanthine,
the precursor to uric acid (30,32). Attempts at dissolution of allopurinol induced xan-
thine stones with alternating acidic and basic solutions has been described in vitro, but
has not been developed into clinical treatment (32). Some have used high dose allopu-
rinol in the face of urinary tract infection for Lesch–Nyhan patients to decrease the risk
of ammonium acid urate stones, and have reported xanthine stones as an effect of this
therapy(33).To date, the optimal prevention of xanthine stones in Lesch–Nyhan patients
on allopurinol therapy is achieved by continuing allopurinol, but placing added empha-
sis on their hydration and alkalinization.
Another situation in which secondary xanthine stones have been described is with the
use of allopurinol to treat the state of hyperuricemia seen in hematopoetic malignancies.
The urate overproduction seen in hematopoetic malignancies comes from cell break-
down, which result in uric acid excretion up to four-times normal, especially during the
rapid cell lysis associated with chemotherapy or radiotherapy (34). The side effects of
nausea and vomiting can interfere with proper fluid intake, or worse cause dehydration,
further increasing the risk of stone formation in this patient population. Rarely, the use
of allopurinol for inhibition of XO to decrease the uric acid in these patients will result
in excess xanthine accumulation and xanthine stone formation.
The formation of xanthine stones in the hematopoetic malignancy patient population
is through a similar mechanism by which the Lesch–Nyhan patients form xanthine
stones. Unlike Lesch–Nyhan patients, who fail to properly metabolize uric acid precur-
sors via the purine salvage pathway, the metabolic abnormality in patients with
hematopoetic malignancies is urate overproduction. The excess urate overloads the

Chapter 19 / Urinary Stones of Unusual Etiology 353
purine salvage pathway, leading to uric acid production as the method of ridding the
body of the excess by-products released by the malignancy. The massive uric acid
excretion places these patients at risk for uric acid urolithiasis, and to prevent this,
patients are prophylactically treated with allopurinol, alkalinization, and increased
diuresis (3000 mL/d) before the initiation of chemotherapy (35). Paradoxically, the
allopurinol used to prevent uric acid stones can lead to excess accumulation of xanthine,
leading to xanthine urolithiasis. These stones rarely form when patients adhere to rec-
ommended fluid intake.
Formation of xanthine stones has been described in patients with uric acid nephroli-
thiasis or gout who are treated with allopurinol (36). Certainly, with the administration
of allopurinol, the excess xanthine and hypoxanthine will be excreted in the urine. However,
allopurinol does not completely inhibit XO at usual doses. Consequently, one will rarely see
serum urate levels drop below 3 mg/dL or urinary urate levels below 250 mg/d. Xanthine
stones are not likely to form at these levels (28). Patients with uric acid nephrolithiasis
or gout on allopurinol therapy can further decrease their risk by adhering to recom-
mended fluid intake.
Acute stone episodes should be managed in the usual manner to control symptoms.
If operative therapy is required, xanthine stones may be treated endourologically or by
shockwave lithotripsy (SWL)(37). Outside of the patient population with hereditary
xanthinuria, xanthine stones are quite rare, even in the specific instances, described here,
which increase the risk of secondary xanthine stones. Primarily, preventive therapy is
achieved through adequate intake of fluids. Urine alkalinization also increases xanthine
solubility in the urine.
MEDICATION-RELATED STONES
Ephedrine Stones
Ephedrine and its metabolites, pseudoephedrine and norephedrine, have recently
been shown to be capable of forming urinary calculi (38). This initial report resulted from
a patient who chronically abused ephedrine containing medication, and ingested up to
120 ephedrine containing tablets daily (25 mg/tablet). Subsequent reports outline small
series of patients with ephedrine stones, many of whom abuse the medication for stimu-
latory effects (39,40). Often a history of drug or alcohol abuse may be elicited. Claims
of euphoria, increased energy, heightened sexual tension, weight reduction, and addition
of muscle mass are justification given by those abusing ephedrine (39). Some patients
receive ephedrine in drugs made for bronchodilating effects, which become less noticed
after long term use. Patients then increase the dosage, leading to the high doses that cause
calculi (39).
Ephedrine stones are radiolucent, but may be visualized by CT. They are easily
fragmented with SWL (39). To improve diagnosis, attention must be given to obtaining
a proper drug history and social history for previous substance abuse. Treatment is aimed
at reduction of ephedrine intake, and increase of urinary output.
Substance abuse counseling is recommended for abusers of ephedrine or users with
a history of substance abuse not only to prevent further stone episodes, but also to avoid
more serious sequelae of ephedrine abuse. The potential for major complications exist,
including but not limited to death, myocardial infarction, stroke, respiratory depression,
seizure, hypertension, cardiac arrhythmia, and headache. Over 100 ephedrine stones

354 Lowry and Nakada
were identified at a single stone analysis company (L. Herring) between January 1996
and June 1997, out of over 166,000 samples (40). Although the incidence was only
0.064%, one must believe that this represents only a small portion of the ephedrine
abusers who form stones.
Guaifenesin Stones
Approved by the FDA for over the counter use in 1989, guaifenesin is a common compo-
nent of cough/cold medications, which often contain ephedrine (41). Guaifenesin containing
urinary calculi were first noted in 1999 (42,39). Specifically, the guaifenesin metabolite,
β-(2-methoxyphenoxy)-lactic acid, is the component found in the calculi (42). The majority
of the affected patients were abusers of the offending medications (refer to the section on
ephedrine stones). Many were abusing the medication for the effect of the ephedrine. Because
the FDA, in 1994, limited the amount of pure ephedrine that may be contained in medication
(43), abusers take larger amounts of predominantly guaifenesin containing preparations for
the effect of the minor component, ephedrine (42). Some, however, were taking customary
doses of the medications, but were simultaneously taking numerous preparations that con-
tained guaifenesin, resulting in excessive guaifenisin intake (42).
Patients using prescribed amounts as directed by the manufacturer for the treatment
of colds, coughs, or allergies are at almost no risk of forming stones composed of
guaifenesin. Like ephedrine stones, guaifenesin stones are radiolucent, but can be seen
on CT scanning. They are easily fragmented with SWL (39).
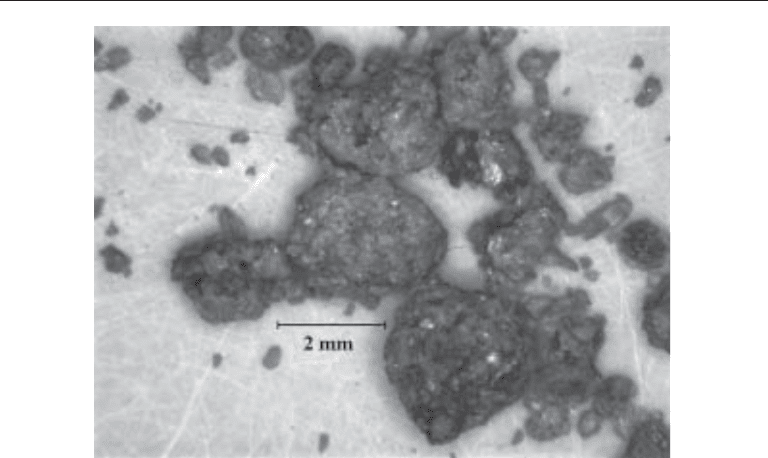
Indinavir Stones (Fig. 4)
Indinavir stones were first described in 1995 (44–46). Used in the treatment of the HIV
virus, indinavir competitively inhibits HIV protease and decreases viral load by 90–99%.
Indinavir is commonly used with reverse transcriptase inhibitors such as nucleoside
analogues and/or nonnucleoside reverse transcriptase inhibitors as part of a multi-drug
antiretroviral regimen. Indications for use of antiretroviral therapy in HIV infected
individuals include acute HIV syndrome, symptomatic disease, and asymptomatic dis-
ease with low CD4+ T cell counts or with elevated HIV titer. Treatment may also be
offered to uninfected individuals for the purpose of postexposure prophylaxis following
an exposure to HIV (47).
Indinavir is predominantly excreted in the feces after hepatic metabolism. Nineteen
percent, however, is excreted in the urine (48). Patients taking this medication may form
indinavir crystals in their urine, leading to stone formation. Indinavir crystals are very
distinctive, with a fan-shaped or starburst type of appearance. They have been described
in 20% of patients on indinavir (49). When examined with microscopy, crystalluria has
been reported to occur in over 30% of patients on indinavir therapy (50). Indinavir stones
were first thought to have an incidence of 2–12 % (51–53); however, much higher rates
of over 40% have also been reported (54). In populations of patients on indinavir therapy,
the average time to stone episodes has varied from 21 wk (range 6–50) (55) to 11 mo
(range 4–19) (56).
The etiology of stones among patients taking indinavir was examined in a group of
11 patients who passed a stone that underwent analysis (23). Of these 11, only 4 patients
(36%) had stones with indinavir as a detected component; the remainder was composed
of calcium oxalate (3), calcium phosphate (1), ammonium acid urate (2), and uric acid
Chapter 19 / Urinary Stones of Unusual Etiology 355
(1). Additionally, nine patients taking indinavir underwent 24-h urine testing, which
revealed hypocitraturia (3), hyperoxaluria (2), hypomagnesuria (2), hypercalciuria (1),
and hyperuricosuria (2). Although previous stone history was not discussed and the
patient numbers were limited, this series reveals abnormal urine testing and the forma-
tion of stones primarily from components other than indinavir. This data suggests that
stone formation by patients on indinavir therapy may have a substantial metabolic com-
ponent, in addition to the effects of urinary indinavir excretion.
Indinavir crystals form at a pH of 7 (57) and are soluble at a pH of 4.5 in vitro (56).
Although such an acidic pH is difficult to obtain in vivo, short term acidification of 2–3 d
for treatment of stone episodes has been suggested (51) to help partially dissolve stones to
facilitate passage. Acidification may result in deposition of uric acid crystals (56), fur-
ther exacerbating episodes, although risk is small in the short term. The metabolic
acidosis and hypocitraturia caused by urinary acidification may also result in calcium
oxalate supersaturation (58). Increasing the intake of citrate containing fluids (lemon-
ade) may decrease the risk of stone calcification. Concern has been raised regarding the
hypothetical risk of virus mutation and subsequent development of resistance during
temporary cessation of indinavir (47), but no evidence exists to support this.
Pure indinavir calculi are radiolucent and are unique in that they are not visualized on
spiral CT scanning (59). On gross appearance, pure indinavir stones are brown and have
a pliable, puttylike consistency.
Conservative management successfully treats most stone episodes. Treatment con-
sists of hydration, analgesics, and temporary withdrawal of indinavir (60–62). Tempo-
rary urinary acidification may be considered. One must keep a low threshold for emergent
stent placement, as these patients may be dangerously immunosuppressed. After reso-
lution of a stone episode, indinavir may be restarted and increased oral hydration may
be initiated (61).
Fig 4. Indinivir calculus (Courtesy of Mission Pharmacal, San Antonio, Texas).

356 Lowry and Nakada
If conservative measures fail, indinavir stones may be fragmented and removed endo-
scopically. Shock wave lithotripsy has been used to successfully treat indinavir stones (56).
It is not understood why some patients taking indinavir form urinary crystals and
stones whereas others do not. Risk factors for the formation of indinavir stones are
unknown. At this time, adequate hydration, 2 L daily, is the best prevention (54,64). To
minimize recurrence, patients with stones of mixed composition or with a history of
stones may benefit from metabolic evaluation. HIV patients need continuation of
indinavir for optimal treatment of their disease, and because almost 10% of patients have
discontinued the use of indinavir because of nephrolithiasis, maximal effort must be
given to prevention of stones in this patient population.
Nelfinavir Stones
The most recent medication to cause stone formation is nelfinavir, a protease inhibitor
used for the treatment of the HIV virus. Before 2002, nephrolithiasis owing to protease
inhibitors as an etiology had only been described with indinavir. However, stones com-
posed of nelfinavir have now been reported (65).
Similar to indinavir stones, those composed of nelfinavir are radiolucent. However,
in contrast to indinavir calculi, nelfinavir stones are visible on CT scan (65). Nelfinavir
stones are successfully fragmented with SWL, or may be treated with other forms of
stone ablation.
In one report, a patient had a short course of indinavir therapy and changed to nelfinavir
because of intolerable side effects. Subsequently, the patient developed a large renal
pelvis calculus. The stone composition was 99% nelfinavir and 1% indinavir. As only
1–2% on nelfinavir is excreted in the urine, compared to 20% of indinavir, it was pos-
tulated that indinavir crystals may have provided the nidus for nelfinavir stone formation
(65,66).
Patients on other lithogenic medications and patients with a history of nephrolithiasis
who also have a need for nelfinavir may benefit from metabolic evaluation and surveil-
lance to keep stone episodes minimized.
Oxypurinol Stones
Oxypurinol was an early xanthine oxidase inhibitor, but was abandoned in favor of
allopurinol, in part owing to the improved urinary solubility of allopurinol. Oxypurinol
is the primary metabolite of allopurinol and is excreted via the kidneys. Although
allopurinol is broken down into an oxypurinol component, about half is excreted as
allopurinol, keeping the oxypurinol excretion lower than if oxypurinol were the only
drug administered (67). The presence of oxypurinol has been detected in stones pro-
duced by patients with the Lesch–Nyhan syndrome who were on allopurinol therapy
(68). Oxypurinol as a primary stone constituent, mixed with xanthine (increased excre-
tion owing to the xanthine oxidase inhibitor) and uric acid, has also been described in
patients on high dose allopurinol (600 mg/d) for prophylaxis of uric acid stones (69).
These stones are described as radiolucent (69). Oxypurinol has not been described as a
constituent with calcium stones. In fact, there is no interaction between oxypurinol and
either calcium or oxalate at oxypurinol concentrations expected with therapeutic doses
of allopurinol (70).
The presence of oxypurinol as a stone constituent is primarily seen in patients using
high dose (600 mg/d) allopurinol. Patients with oxypurinol as a stone constituent may

Chapter 19 / Urinary Stones of Unusual Etiology 357
benefit from a decrease in the allopurinol dosing, although, because these stones are
uncommonly rare, the risks and benefits in modification of allopurinol dosing should be
carefully considered. Metabolic studies of 24-h uric acid excretion will reveal how much
of an advantage, if any, exists with increased doses of allopurinol. This can be compared
to the increased oxypurinol excretion resulting from increased allopurinol dosing;
approximately half of an allopurinol dose is excreted as oxypurinol. One report (69)
showed, with constant diet, uric acid excretion falling from 481 to 346 to 334 mg/d on
0, 300, and 900 mg allopurinol daily. On the same respective allopurinol doses,
oxypurinol excretion increased from 0 to 243 to 440 mg/d, and xanthine excretion
increased from 6 to 47 to 145 mg/d. The patient identified in this report showed only a
minute benefit in uric acid excretion with an allopurinol increase of 300 to 900 mg/d, yet
the daily excretion of oxypurinol and xanthine increased by over 80% and 200% respec-
tively. In this instance, the 3% decrease in uric acid excretion must be weighed against
the 80% increase in oxypurinol excretion in this patient who produced stones made of
oxypurinol (major constituent) and xanthine.
If high allopurinol doses reveal a treatment advantage as seen with 24-h urine analy-
sis, a decrease of the allopurinol dose might place the patient at risk for other clinical
problems. Increasing urine output to dilute urinary oxypurinol concentration may better
serve the patient. Twenty-four hour urine studies will help guide therapy on an individual
basis. Continued radiolucent stone formation while on allopurinol therapy should raise
suspicion to other types of stones, and oxypurinol must be considered as a possibility.
For accurate stone analysis, special notation should be made on analysis requests to
reflect this suspicion.
Silicate Stones
Silicate urinary calculi occur exclusively in patients taking large amounts of magne-
sium silicate antacids. In 1912, silica stones were first found in cattle living in sandy
areas (71). The first human patient with silicate stones was reported in 1953 (72). This
patient had gastric ulcers and ingested 2 g daily of magnesium trisilicate, an antacid.
Subsequently, other reports of silicate stones have been presented, all involving patients
with a history of silicate abuse (73–76). Invariably, sufferers of silicate stones also have
associated upper gastrointestinal disease such as esophagitis, gastric ulcers, gastritis, or
hiatal hernia, prompting them to abuse magnesium trisilicate antacids.
No report exists linking an episode of silicate stones to any source other than silicate
antacids. The only report suggesting another etiology was regarding a patient who died
in the months before establishment of silicate antacids as an etiology. This patient’s
family reported no knowledge of abdominal pain or antacid use; this is the only reported
case of silicate stones in a patient not on silicate antacid therapy (77). It has also been
postulated, although never reported, that methyl polysiloxane, or dimethicone, used for
prevention of flatulence, may be a source of urinary silica (77).
As in other unusual stones, failure to suspect silicate stones leads to a delay in diag-
nosis and prevention. A 1973 report outlines a 5-yr delay in diagnosis in a patient with
multiple stone passages; part of the explanation for the delay was poor quality of stone
analysis (74). The gross appearance of silica stones can resemble the gross appearance
of calcium oxalate jackstones. Electron microscopy, however, reveals the subtle differ-
ence of the spikes blending into the body in silica, whereas they appear more demarcated
from the body on calcium oxalate jackstones (78). This reinforces the value of quality
