Marshall L. Stoller, Maxwell V. Meng-Urinary Stone Disease
Подождите немного. Документ загружается.


Chapter 20 / Imaging of Urinary Stone Disease 369
III
PRESENTATION AND EVALUATION
Chapter 20 / Imaging of Urinary Stone Disease 371
371
From: Current Clinical Urology, Urinary Stone Disease:
A Practical Guide to Medical and Surgical Management
Edited by: M. L. Stoller and M. V. Meng © Humana Press Inc., Totowa, NJ
20
Imaging of Urinary Stone Disease
Richard S. Breiman, MD and Fergus V. Coakley, MD
CONTENTS
BACKGROUND
GENERAL CONSIDERATIONS IN THE IMAGING
OF
URINARY STONE DISEASE
SPECIFIC IMAGING MODALITIES IN URINARY STONE DISEASE
FUTURE TRENDS
SUMMARY
REFERENCES
Key Words: Computed tomography; urogram; radiograph; obstruction.
BACKGROUND
Acute flank pain, with or without hematuria, is a common complaint and urolithiasis
is the primary consideration in many of these patients. Clinical findings are often non-
specific and may overlap other conditions. Imaging plays an important role in both
diagnosis and subsequent management of urinary stone disease. Radiological imaging
of urinary stones dates back to 1897, the year after Roentgen’s discovery of X-rays. Early
attempts at opacification of the urinary tract included retrograde placement of ureteral
intraluminal wires and opaque catheters, air, colloidal silver, and sodium iodide (1).
Iodinated contrast agents that were excreted by the kidneys and could be administered
intravenously were developed in the 1920s. For the next 70 yr, intravenous pyelography
or excretory urography, including a preliminary noncontrast scout view, was the primary
modality for imaging urinary stones. Computed tomography (CT) was introduced in the
mid-1970s. Early CT scanners could sometimes visualize urinary calculi, but CT was not
a reliable method to confidently exclude stones because these slower nonhelical scan-
ners were plagued by misregistration between sequential images. Stones present in these
nonvisualized gaps could escape detection. If seen, stone size was frequently under-
estimated if only the top or bottom edge of the stone was included in the slice. For these

372 Breiman and Coakley
reasons, nonhelical CT was unsuitable for the primary work-up of suspected urolithiasis.
The introduction of helical CT scanners in the early 1990s revolutionized the imaging
of urinary stone disease. With these more rapid helical CT scanners, large anatomic
regions could be scanned during a single breath hold with thin slices and no misregis-
tration. Multislice helical scanners, introduced in the late 1990s, led to the ability to
obtain even thinner slices in less time, allowing the detection of smaller, less dense
calculi and reducing the likelihood of false-negative scans. In most centers, nonenhanced
CT has replaced the intravenous urography (IVU) as the modality of choice for the
imaging of urinary stones.
The selection of the appropriate imaging modalities remains complex, with patient-
specific issues influencing the imaging work-up of urinary calculi. Currently, radio-
graphs and noncontrast CT are the cornerstones of the imaging assessment of most
patients with suspected stone disease. IVU, ultrasonography (US), magnetic resonance
imaging (MR), scan projection radiography (SPR), radionuclide scintigraphy, retro-
grade and antegrade pyelography, cystography, and tomography are available as alter-
native or supplemental modalities as needed.
GENERAL CONSIDERATIONS
IN THE IMAGING OF URINARY STONE DISEASE
Goals of Imaging
The primary goal of imaging in urolithiasis is the detection of all urinary stones in the
urinary tract. The accuracy of stone detection by the various modalities is discussed in
the subsequent section. Other secondary goals include stone characterization, surveil-
lance of stone evolution or migration, guidance and monitoring of therapy, such as
endoscopy and lithotripsy, diagnosis of underlying anatomic variants predisposing to
stone formation, such as ureteropelvic junction obstruction or caliceal diverticulum,
detection of obstruction and other complications, such as atrophy and pyelonephritis,
evaluation of the opposite kidney and collecting system, and detection of other condi-
tions that may present with flank pain and mimic renal colic such as appendicitis or
diverticulitis. As all imaging techniques have some limitations or may be contra-indi-
cated in certain patients, a combination of examinations is often necessary.
Stone Characterization by Imaging
Imaging features that reflect stone composition and formation include morphology,
internal structure, and density.
STONE MORPHOLOGY
Most stones are small and round, which is relatively nonspecific. Pure calcium oxalate
stones are often homogenous, dense, and smooth. Mixed calcium oxalate stones may be
irregular in shape, inhomogeneous, and may have dendritic projections. Staghorn calculi
are so called because they develop in the pelvicaliceal system, and in advanced cases
have a branching configuration that resembles the antlers of a stag (Fig. 1). Staghorn
calculi are typically composed of magnesium ammonium phosphate (struvite), which
forms in urine that has abnormally high pH (above 7.2). However, large uric acid and
cystine stones also can have a staghorn configuration, and staghorn calculi in children
or young adults with no history of infection are frequently composed of cystine. Radio-
Chapter 20 / Imaging of Urinary Stone Disease 373
graphically, struvite stones are relatively low density. Low-density struvite stones may
not be appreciated on plain radiographs, but can be readily detected by US or CT. IVU
or retrograde pyelography may also be used to demonstrate the typical branching appear-
ance of staghorn calculi. Faceted stones are usually multiple and have arisen in a small
cavity, such as a renal pelvis above an ureteropelvic junction obstruction or in a caliceal
diverticulum (Fig. 2), where the stones are minimally mobile but in contact with each
other.
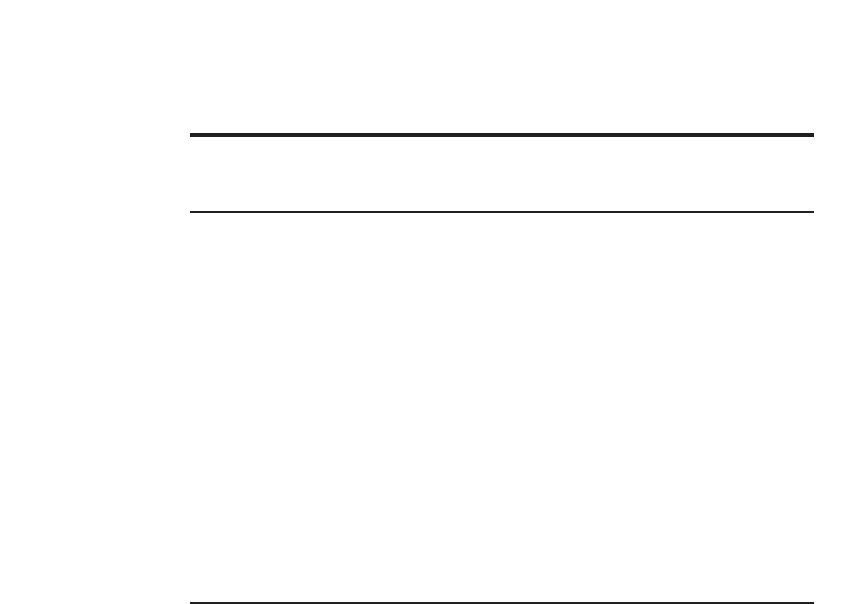
Fig. 1. Plain abdominal radiograph showing a staghorn calculus (arrow) in the right kidney. Such
calculi are so called because they develop within the pelvicaliceal system, and advanced cases
have a branching configuration that conforms to the shape of the pelvicaliceal system and there-
fore resembles the antlers of a stag.
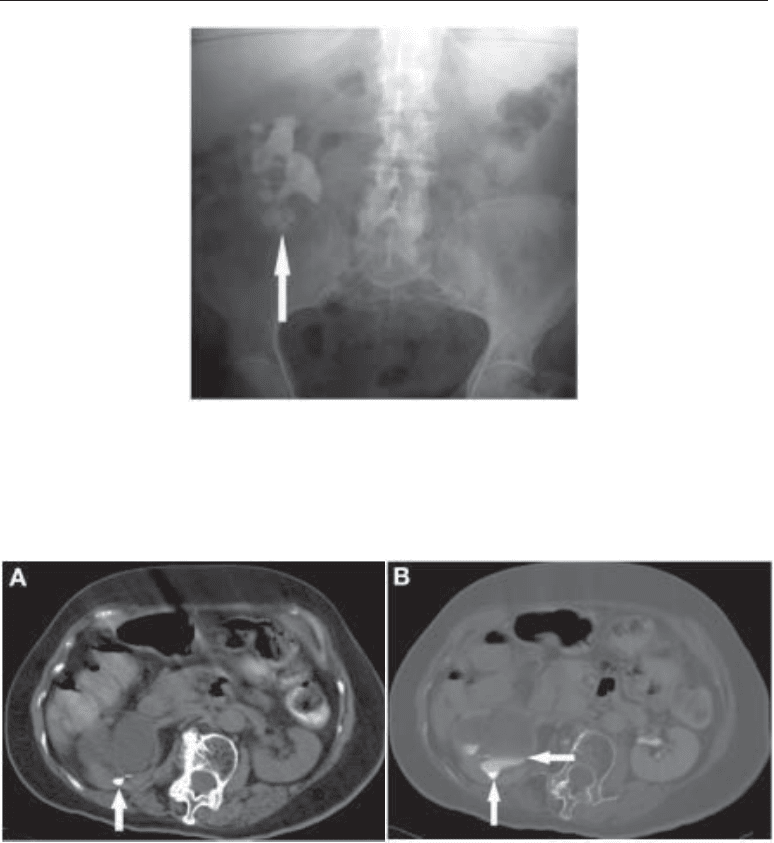
Fig. 2. (A) Nonenhanced CT in a patient with longstanding right-sided ureteropelvic junction
obstruction. A small faceted stone (arrow) is seen within the dilated pelvicaliceal system. (B)
Delayed post-contrast CT (shown at bone windows) confirms the stone (vertical arrow) is within
a dilated pelvicaliceal system, because layering contrast (horizontal arrow) can be seen superior
to the stone.
374 Breiman and Coakley
INTERNAL STRUCTURE
Most stones are homogeneously dense. Struvite stones may have a laminated appear-
ance, with alternating dense and relative lucent layers (Fig. 3).
D
ENSITY
In the IVU era, stones were traditionally divided into radiopaque (calcium and struvite
stones) and radiolucent (pure cystine, urate, and matrix or mucoprotein stones) (Fig. 4).
This division was not precise, because so-called lucent stones could become secondarily
calcified and become at least partially opaque. More importantly, the distinction between
radiopaque and radiolucent stones has become obsolete in the CT era. Because of the
exquisite sensitivity of CT to even small amounts of calcium, all stones appear opaque
(i.e., white) on CT. The only common exception to this statement is the occurrence of
indinavir stones in HIV-infected patients. These “lucent” stones may still be detectable
due to secondary calcification (2–5) or to signs of ureteral obstruction in the setting of
an HIV patient with flank pain. Stone density affects detectability on plain radiographs;
a 1–2 mm pure calcium stone may be detectable, whereas pure cystine or urate stones
may not be detectable until they are 3–10 mm in size (6,7). On CT, the threshold size for
stone detection varies with composition but ranges from 0.8 to 1.3 mm. These threshold
sizes increase by 8–17% if low radiation dose parameters are selected (8).
The ability to predict stone fragility is of great interest to urologists, as lithotripsy could
be avoided in patients with shock-wave resistant stones. Stones composed of a greater
amount of calcium are harder and denser. These stones create more X-ray attenuation, and
are therefore associated with a higher CT number or Hounsfield unit (HU). Uric acid and
cystine stones create relatively less X-ray attenuation compared with stones composed of
other materials, with attenuation coefficients ranging from 100 to 300 HU. Stones that
contain significant calcium are often associated with CT numbers of 600 HU or greater,
occasionally exceeding 1000 HU. Several studies have assessed stone density based on
CT attenuation coefficients and have suggested that denser stones are more resistant to
shock wave, particularly when CT numbers are greater than 1000 HU. However, stone
density characterization is currently more accurate in vitro than in vivo (9–11).
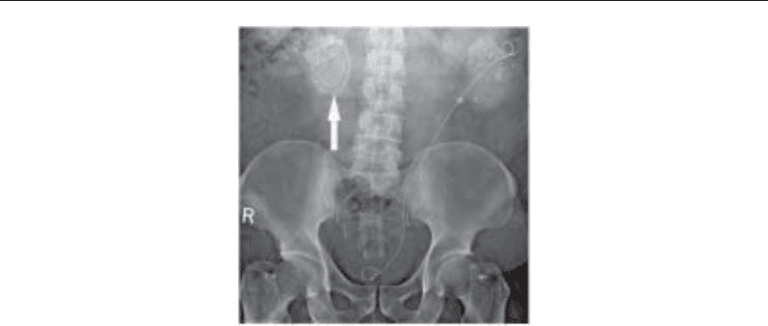
Fig. 3. Plain abdominal radiograph in a patient with bilateral partial staghorn calculi. Such stones
are often composed of struvite, and may have a laminated appearance as demonstrated in the
stone (arrow) in the right kidney.
Chapter 20 / Imaging of Urinary Stone Disease 375
Noncalculous Renal Calcifications
It is important to be aware that not all calcifications in the kidney are urinary stones.
Arterial calcification is frequently seen in the renal hilum of older or diabetic patients,
and has a characteristic curvilinear and perivascular appearance (Fig. 5). Dystrophic
renal calcification may be a result of infection, tumors, or prior hemorrhage. Nephrocal-
cinosis (deposition of calcium in the renal parenchyma) is commonly a result of renal
tubular acidosis, hyperparathyroidism, or medullary sponge kidney (Figs. 6 and 7).
Fig. 5. Nonenhanced CT showing the typical curvilinear and perivascular appearance of arterial
calcification (arrow), which is frequently seen in the renal hilum of older or diabetic patients.
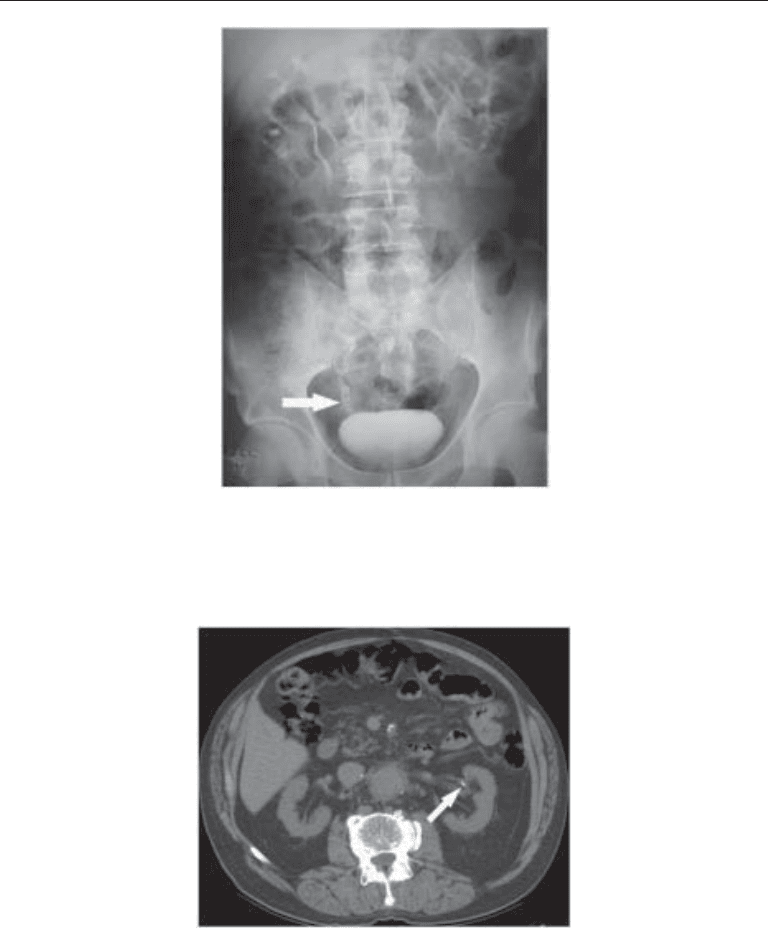
Fig. 4. Twenty-minute film from an IVU demonstrating radiolucent stones (arrow) in the distal
right ureter of a patient with cystinuria. In the IVU era, stones were traditionally divided into
radiopaque and radiolucent stones. Cystine stones, as in this patient, were among the stone types
that could be radiolucent.
376 Breiman and Coakley
Other causes include hypercalciuria, primary hyperoxaluria (oxalosis, Fig. 8), acute
cortical necrosis, chronic glomerulonephritis, and renal transplant rejection. It should
be noted that some of these conditions, such as hypercalciuria and hyperoxaluria, can
cause both urinary calculi and nephrocalcinosis. It has been suggested that stones may
begin as calcified deposits in the subepithelial portion of the renal papilla, with subse-
quent extrusion into and growth within the collecting system. This hypothesis is sup-
ported by the finding that papillary calcifications are common at high-resolution
radiography of pathology specimens. These so-called Randall’s plaques have also been
shown to be more common in stone formers than nonstone formers, particularly in
association with calcium oxalate and phosphate stones (12). In vivo, these plaques are
too small to be detected by any imaging examination except high-resolution noncontrast
CT, where they may be mistaken for renal calculi (Fig. 9). Detection of these papillary
calcifications may be important in identifying individuals at increased risk for stone
disease (13).
Fig. 7. Bilateral nephrocalcinosis in a patient with medullary sponge kidney. Note that in this case
the calcifications are more discreet and correspond to the locations of the medullary pyramids,
where these calcifications form.
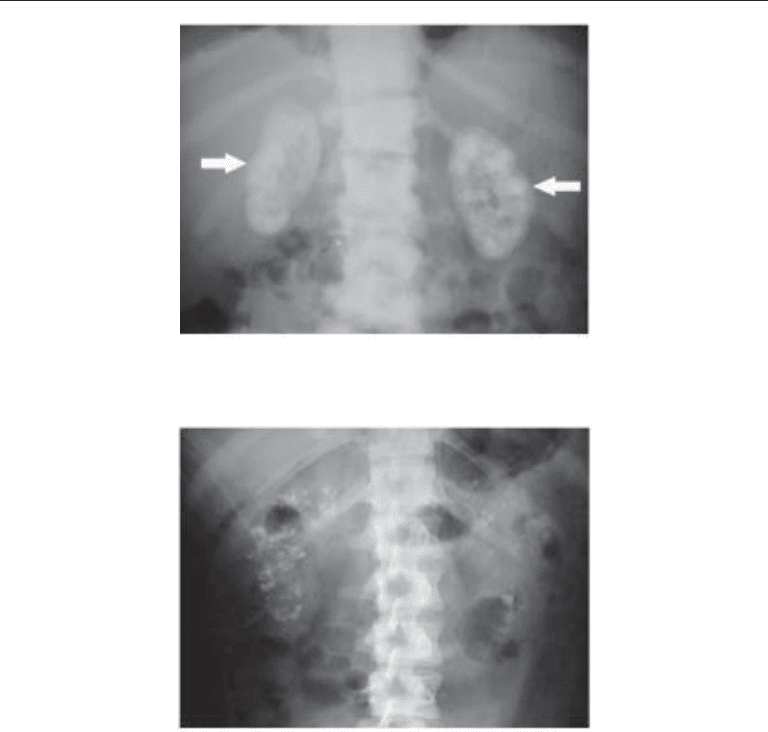
Fig. 6. Plain abdominal radiograph showing widespread nephrocalcinosis in both kidneys
(arrows), which in this patient was caused by renal tubular acidosis.
Chapter 20 / Imaging of Urinary Stone Disease 377
Impact of Stone Location on Management
The location of a stone within the urinary tract is an important factor in determining
management, because it is related to the likelihood of spontaneous passage and to the
choice of therapeutic approach. Coll et al. found the rate of spontaneous stone passage
to be 48% for stones in the proximal ureter, 60% for mid ureteral stones, 75% for distal
stones, and 79% for ureterovesical junction stones (14). For example, lithotripsy may not
be as successful in treating stones in the lower pole calyx if the angle between the lower
pole infundibulum and renal pelvis is acute, because this anatomic arrangement could
impede the passage of small stone fragments. Ultrasound or fluoroscopy can be used for
guidance during percutaneous nephrostomy, needed to relieve obstruction caused by
stone impaction or for the placement of an endoscopic lithotripsy device. Imaging,
particularly CT, may be helpful in planning of a safe route for nephrostomy, in an effort
to avoid injury to adjacent organs, such as the spleen or bowel (15). CT, with the patient
in the prone position used for percutaneous nephrostomy, can demonstrate the effect of
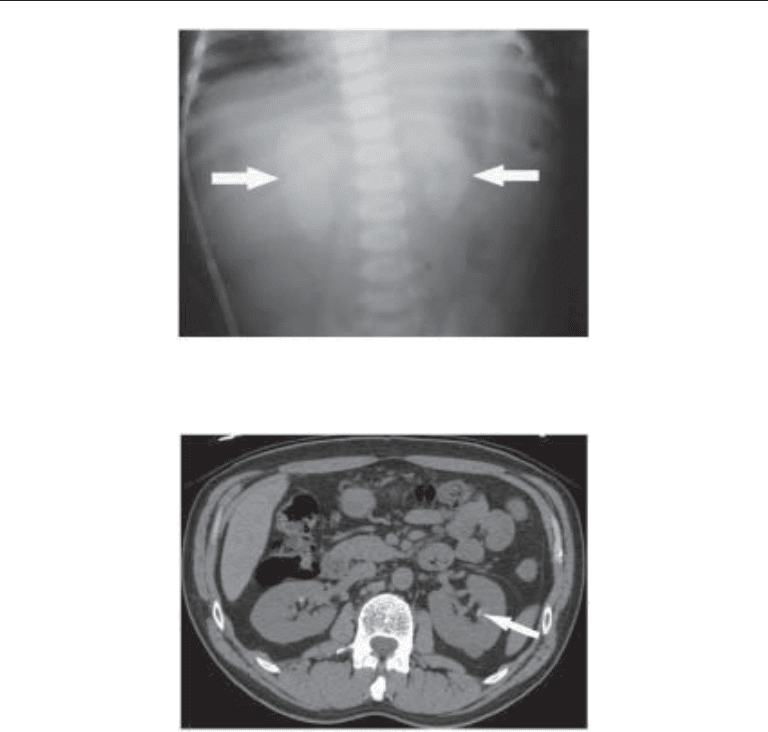
Fig. 9. Nonenhanced CT showing a thin curvilinear calcification at the expected location of a
medullary pyramid in the left kidney. The appearance suggests this may be a Randall’s plaque
in the parenchyma rather than a true stone in the collecting system.
Fig. 8. Plain abdominal radiograph in a child demonstrating the characteristic nephrocalcinosis
(arrows) seen in children with primary hyperoxaluria (oxalosis).
378 Breiman and Coakley
gravity on the position of bowel or spleen relative to the kidney in the patient position
used during intervention. Imaging demonstrating the presence of ureteropelvic junction
obstruction may also influence the choice of therapy, as stone fragments created by
lithotripsy may not pass.
Urinary Tract Anomalies Associated With Stone Formation
Most urinary calculi form in the collecting system as a consequence of metabolic
disturbances. Occasionally, stones form because of anatomic anomalies in the urinary
tract that result in stasis or infection. Caliceal diverticula are associated with stasis and
often result in stone formation. The resultant stones are frequently small and seed-like
or faceted. Diverticula may be associated with milk of calcium, a fine colloidal suspen-
sion of calcium carbonate (Fig. 10). Diverticula occur in 0.2–0.4% of the population but
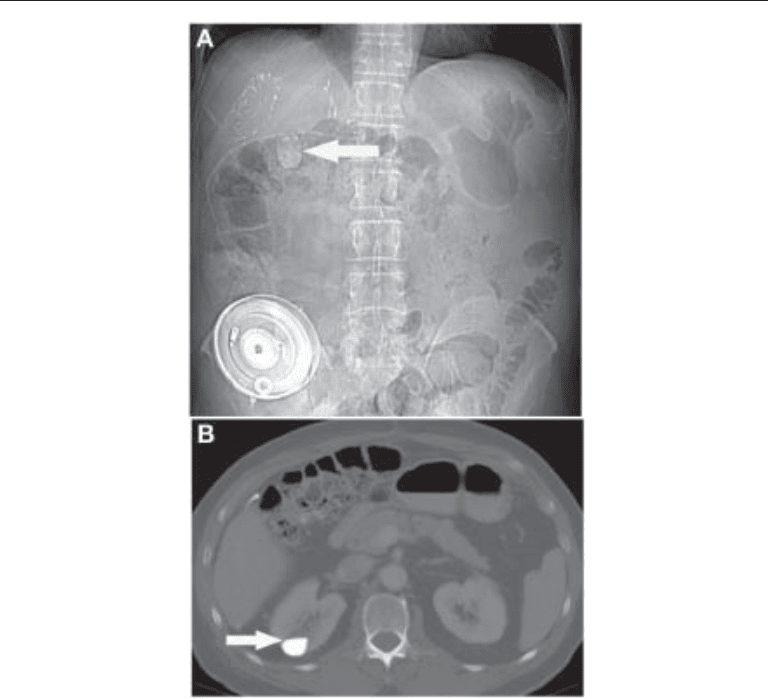
Fig. 10. (A) Plain abdominal radiograph showing a 2-cm calcific density (arrow) projected over the
right kidney. The radiographic appearances are suggestive of a stone. A hepatic artery chemo-
therapy infusion reservoir and catheter are also evident, as are surgical clips related to prior right
hepatectomy, in this patient being treated for metastatic colorectal cancer. (B) Axial CT image
through the level of the calcific density demonstrates that it is caused by milk of calcium within a
caliceal diverticulum. Layering of milk of calcium results in a characteristic fluid level (arrow).

Chapter 20 / Imaging of Urinary Stone Disease 379
stones occur in 9.5–39% of diverticula (16,17). Diverticular stones may be related to
scarring, frequently associated with adjacent focal atrophy. Calculi rarely escape diver-
ticula through their narrow neck. Milk of calcium may appear to represent a stone on a
supine frontal film. The margins of a collection of milk of calcium may fade gradually
rather than demonstrate the sharp defined margins of a stone. A fluid-calcium level may
be visible on upright or decubitus views, or on CT and US. On post intravenous contrast
excretory films, the diverticulum fills with contrast and the milk of calcium is no longer
visible. No filling defect is seen within the opacified diverticulum. High echogenicity is
often seen on US with milk of calcium and occasionally shadowing, with layering and
shifting of the material with changes in patient position. Milk of calcium can develop in
less than 12 mo. Although it is most frequently seen in calyceal diverticula, milk of
calcium may be encountered with chronic dilation of a renal pelvis or calyx, as well as
within cysts. Anatomic anomalies that may result in primary ureteral calculi include
acquired or congenital megaloureter, ureteral stricture or obstruction, ureteral stump,
blind ended or bifid ureter, or ureteral foreign body. Stones occur in a higher frequency
with anatomic variations such as a horseshoe kidney, an ectopic kidney with a high
insertion of ureters in the bladder, congenital UPJ stenosis with stasis and in polycystic
kidneys. There is an increased incidence of stone formation within megalocalyces or
megaloureters. Ureteral reflux is often associated with stasis and infection leading to an
increased frequency of stone formation.
Medullary sponge kidney is an anatomic condition associated with stone formation.
Dilatation of the collecting ducts of Bellini in the renal papilla leads to ductal urine stasis,
increasing the risk of stone formation. Half of patients with medullary sponge kidney
develop calcifications in the medulla and 12% develop urinary calculi (18). Calcifica-
tions as well as calculi are often bilateral in patients with medullary sponge kidneys, but
may be unilateral, frequently segmental or even localized to a single papilla. Calcifica-
tions related to most other entities are usually diffuse. Stones associated with medullary
sponge kidney may vary from tiny to large. They are often clustered in a triangular shape
corresponding to the configuration of a papilla (Fig. 7).
Pathophysiology of Ureteral Obstruction
Calculous obstruction of the ureter results in an abrupt rise in intraluminal pressure
from the usual 6–12 mmHg to 50–70 mmHg or more (19–21). The luminal pressure
depends on the rate of urine flow and the degree of spasm. Ureteral spasm or more active
peristalsis may be associated with an increase in colicky pain. In the first sixty to ninety
minutes, a paradoxical increase in renal blood flow occurs associated with an increase
in afferent arteriolar dilatation, in an attempt to prolong glomerular filtration. One and
a half to five hours following ureteral obstruction, there is a decrease in renal blood flow
secondary to vascular constriction of afferent arterioles. Ureteral pressure is main-
tained as tubular filtration continues. Edema occurs in the perinephric soft tissues with
increased lymphatic resorption, resulting in prominent perinephric lymphatics (22,23).
Edema and distended lymphatics contribute to thin wispy opacities in perinephric fat
on CT, referred to as fat stranding, usually associated with a loss of definition of renal
contours (Fig. 11). Perinephric edema appears as high T2-weighted signal intensity
on MRI. Increased volume of urine is associated with collecting system and ureteral
dilatation. Persistent high luminal pressure may result in calyceal or forniceal rupture
with perinephric fluid collections representing extravasated urine (Fig. 12). Prolonged
elevated luminal pressures may eventually result in a decrease in collecting system
