Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
320 Polymer Electrolyte Fuel Cells
ChannelGDLMiPLCL
S
W
0
S
MiPL
S
GDL
at SS
iA
2F
in
iA
2F
out
Figure 6.30 Typical water distribution in fuel cell porous media under steady state after sufficient
time to allow equilibrium in porous media between stored and flowing liquid. The discontinuities in
the saturation level are a result of the changing pore size and hydrophobicity between the different
layers. Hydrophobic layers are shown, and all water generated is assumed to leave through a single
channel.
It should be emphasized that the capillary motion will be induced only for droplets along
a connected fluid path. That is, isolated droplets which have condensed on a surface will
feel no force other than that from the droplet interaction with the surface. Therefore, some
fraction of liquid can remain as isolated droplets despite capillary effects in the continuous
liquid phase. At equilibrium conditions, a pressure balance in the gas and liquid phases
across interfaces must exist. Therefore, we can predict the water distribution and flooding
locations depending on the hydrophobicity of the surfaces and the pore size distribution.
Figure 6.30 shows the liquid water saturation distribution in the porous media assuming
an isothermal condition with no phase change and hydrophobic surfaces at steady state.
In steady state, there is a flow of water from the catalyst to the channel and out of the
fuel cell as condensed slugs and vapor. Discontinuities in the liquid saturation due to the
pore size variations result in a saturation jump for the same capillary pressure. At each
interface, the liquid-phase capillary pressure is balanced (in steady state). The typical pore
size distribution in the fuel cell soft goods (membrane, electrode, and DM) is as follows:
catalyst layer (∼50 nm) < microporous layer (∼100 nm) < DM (∼100–500 µm).
For the same interfacial liquid pressure and hydrophobicity, the media with the smallest
hydrophobic pores (catalyst layer) will have the lowest liquid saturation. If there were
similar levels of hydrophobicity in each larger, the capillary pressure gradient will drive
the liquid flow from the catalyst layer through the microporous layer and DM into the flow
channel. Recall however that the microporous layer is typically much more hydrophobic.
Therefore, the MPL can act as a barrier for liquid flow unless the break though pressure is
reached. Typical MPLs have a break through pressure around 5–10 kPa.
In hydrophilic pores of the CL and DM, liquid will accumulate until removed by
evaporative or convective forces and will not spontaneously drain into channels.
In our discussion so far, we have not included the possibility of phase change and
temperature gradients which play a large role as well. While isothermal conditions may be
true at low current density, higher current densities include phase change and temperature

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.2 Water Balance in PEFC 321
gradients. Under current, the polarization losses will generate a majority of the heat dis-
sipated by the fuel cell. Since most of the entropy change and activation polarization are
generated at the cathode, this is typically the hottest location in the fuel cell, by up to 5
◦
C
or more under high current depending on the thermal properties of the DM. At the cathode
catalyst layer, vapor-phase water will be transported into the electrolyte and back to the
anode or outward to the cathode flow channel. As the temperature cools, there will be a
saturation plane that develops where liquid water condensation occurs and fills the porous
media. The location of condensation will have a tremendous effect on the flooding. For the
mixed wettability catalyst layer, the condensation would tend to take place in the predomi-
nantly hydrophilic pores first, flooding them, while the hydrophobic pores remained mostly
liquid free. If the condensation plane is beyond the catalyst layer and in the microporous
or diffusion layer, the highly hydrophobic nature of the MPL will prevent backflow into
the catalyst layer. Some have also suggested the MPL acts to force liquid water toward
the anode, preventing dryout [31, 32]. This can be true of filled hydrophilic connected
networks in the catalyst layer. When the CL is flooded and cannot overcome the break-
through pressure into the cathode MPL, then back flow to the anode by pressure-driven flow
can occur.
Gas-Phase Transport in Catalyst, Microporous, and Diffusion Layers The role of the
different porous media in the fuel cell (catalyst layer, microporous layer, and diffusion
media) in liquid transport can be grasped by understanding capillary flow behavior in mixed
wettability porous media. The role of these porous media in controlling gas-phase flow can
be simply understood through gas-phase transport relationships. Recall from Chapter 5 that
the diffusivity in porous media must be modified to account for the tortuosity and porosity
of the porous media:
D
eff
= D
φ
τ
(6.33)
Thus, the higher the tortuosity and the lower the porosity of the porous media, the more
the diffusion will be restricted. Therefore, from a gas-phase transport perspective the
microporous layer in the fuel cell restricts transport from the DM to and from the catalyst
layer. Lands also play an important role in restricting gas-phase transport to the catalyst
layer. The proper landing width is a balance beween reactant flow restriction and electrical
contact limitation.
6.2.3 Overall Role of Materials in Maintaining High-Performance
Electrode
Based on our understanding of the gas- and liquid-phase transport in the fuel cell, a unified
view of the role of the various porous media can be constructed and is shown in Figure
6.31. It should be noted that due to the lack of direct experimental observation of the very
small microporous and catalyst layers, there exist several different theories to resolve the
observed influences of the material properties on fuel cell performance.
Microporous Layer: Cathode Side A MPL on the cathode side has been experimentally
determined by many to enhance performance under high-current-density and high humidity
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
322 Polymer Electrolyte Fuel Cells
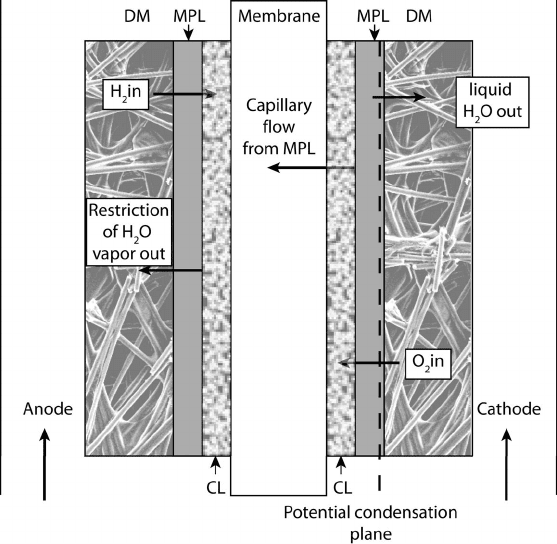
Figure 6.31 Schematic of roles of different porous media in PEFC.
conditions, where catalyst layer and DM flooding is prevalent. Since the performance in
this situation is generally limited by the flooding, and not the gas-phase oxygen transport,
the additional diffusion resistance of the MPL does not significantly reduce performance.
Based on the schematic shown in Figure 6.31, it is believed that the MPL serves as
a highly hydrophobic boundary to prevent water flow back into the catalyst layer after
condensation and can also force water back to the anode by capillary pressure forces.
The capillary pressure gradient through the hydrophobic microporous and macroporous
layers also favors flow toward the lower pressure channel, draining the DM and preventing
catalyst layer flooding. The DM also serves as an intermediate transition between the very
small pore catalyst layer and the macroporous layer. With no MPL, small water droplets
emerging from the catalyst layer into a macroporous layer will see the equivalent of an open
channel, and capillary flow will stop, pooling liquid in this location. The MPL therefore
provides a continuous, connected flow of liquid emerging from the catalyst layer to flow to
the macroporous DM and into the channel.
In low RH conditions, the cathode MPL can force flow through the membrane into
the anode by capillary pressure forces, reducing dryout and increasing performance. The
backflow of water would occur primarily through the hydrophilic pore network in the
catalyst layer, since complete pore saturation in the catalyst layer would result in nearly
total performance loss, and high saturation in the hydrophobic pores of the CL would likely
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.2 Water Balance in PEFC 323
overcome the breakthrough pressure of the MPL, resulting in sporadic liquid slug emission,
a phenomenon which has been observed to occur experimentally.
Microporous Layer: Anode Side The role of the MPL on the anode side is completely
different from that on the cathode side, although use of a MPL on the anode side has also
been observed to enhance performance in low-humidity environments where the water in
the anode ionomer can be easily lost to the dry hydrogen stream. The mass diffusivity of
water vapor into hydrogen is up to four times greater than into air, as shown in Chapter 5.
Thus, combined with electro-osmotic drag of water from the anode to the cathode,
local anode dryout is a common reason for low performance and reduced longevity in
low-humidity conditons. The use of an anode MPL limits the moisture removal from
the anode by acting as a diffusion barrier, reducing this dryout effect, as illustrated in
Figure 6.31. Under high-humidity conditions, the use of an anode MPL does not normally
have much of an impact on performance, which has been validated experimentally [33].
Mechanically, the MPL has also been suggested to serve to protect the electrolyte from
puncture from protruding fibers from the macroporous layer.
Diffusion Media: Anode and Cathode Sides In terms of optimizing gas-phase trans-
port in the macro–diffusion layers or the MPLs, there is a key engineering trade-off to
consider. Obviously, high diffusivity of reactant to the catalyst is desired to promote re-
action and limit concentration polarization. However, high moisture in the electrolyte is
also desired. High-temperature and low-humidity conditions simplify system design but
can lead to anode dryout with accelerated degradation and poor performance. For high
reactant diffusivity, liquid saturation must be minimized, and there must be a high hy-
drophobic porosity. On the cathode side, oxygen transport is already limited by a low
initial mole fraction, high water saturation, and reduced diffusivity coefficient, compared
to hydrogen. Here, the focus is generally on prevention of flooding. On the anode, how-
ever, water vapor diffusivity loss into hydrogen can be severe, there is very little con-
centration polarization limitation at the anode, and hence a flow-restricting structure is
preferred. The dominating role of the DM depends on the operating conditions. Under high
current conditions, the oxygen transfer to the electrode is limiting performance, and an
open hydrophobic structure promoting gas-phase transport with good liquid water removal
on the cathode is necessary. In low-humidity environments, the lack of moisture domi-
nates, and a closed-pore, less hydrophobic structure is needed to restrict vapor loss to the
flow channel.
From an overall porous media design perspective, the various porous media should be
tailored to achieve the desired liquid- and gas-phase transport behavior. In the examples
mentioned, and in most common materials, the properties are mostly uniform. However,
the potential for enhanced vapor and liquid flow with hydrophobicity or pore size gradients
exists and has been exploited in some specialized materials. Overall, different membrane
electrode assembly configurations and materials are preferred by different manufacturers,
and the exact nature of transport in these regions is not yet perfectly understood. Complicat-
ing factors that must be considered include the tightly coupled heat transport phenomena,
nonisotropic material transport properties, and highly nonhomogeneous current density
along the electrode. Since phase-change plays a key role, the thermal conductivity is another

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
324 Polymer Electrolyte Fuel Cells
Figure 6.32 SEM of PEFC catalyst layer with relatively large scale macro cracking. (Image by
Soowhan Kim, Penn State Fuel Cell Dynamics and Diagnostics Lab.)
design parameter of interest. Other factors include assembly sag into gas channels, which
has been linked to flow maldistribution. Thus, a stiff DM is generally preferred for case of
assembly and prevention of interfacial gaps.
Mud Cracking, Interfacial and Morphological Effects Although it is easy to think of
pore size as a constant in the various porous media, there is a distribution of pores in the
DM and MPLs, and the catalyst layer can have significantly large cracks and gaps between
interfaces in the catalyst surface, as shown in Figure 6.32. Catalyst layer surface cracking
is typically present from manufacture and is a result of the presence of volatile compounds
in the catalyst slurry. The viscous catalyst slurry is heated in an oven after application,
removing these volatiles and shrinking the catalyst layer, causing what some have termed
“mud cracks” to appear. From a multiphase flow perspective, this situation is very different
than a continuous homogeneous phase, and some larger gaps and cracks may dominate flow
physics in these regions. These cracks and gaps are orders of magnitude larger (they can be
upto ∼10 µm wide) than the normal pore size in the catalyst layer, and these cracks would
be regions of reduced capillary pressure, promoting liquid pooling. In terms of gas-phase
transport, these macrocracks may enhance reactant transport to catalyst regions by reducing
flow resistance. These cracks also increase the effective catalyst layer porosity, enabling
high reactant species flux to the catalyst surface. From a durability perspective, however,
these cracks have been linked to enhanced degration.
In PEFCs, the interface between the channel, land, and DM, and the interface between
the DM and catalyst layer can also affect transport of liquid. If there is a gap, this can
serve as a pooling location for water. At the interface between the land and the DM, water
stored in the DM can be drawn out with a hydrophilic surface or retained in the DM by a
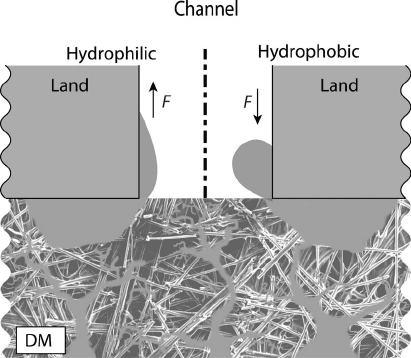
hydrophobic surface (Figure 6.33).
Neutron imaging has also been used to confirm the important role the land interface
has on the liquid water content stored at equilibrium. For fuel cells with otherwise identical
operating conditions, the greater the number of hydrophilic land interfaces, the less liquid
water content there is in the DM [34]. On the other hand, hydrophobic land surfaces have
been shown to retain water under the lands, and promote flooding by restricting drainage
from the DM.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.3 PEFC Flow Field Configurations and Stack Design 325
Figure 6.33 Liquid water removal from under land location. With hydrophilic channel wall sur-
faces, there is a net suction from the DM to the channel. If the wall is hydrophobic, however, liquid
is retained in the diffusion media, causing earlier flooding.
6.3 PEFC FLOW FIELD CONFIGURATIONS
AND STACK DESIGN
Design Considerations There are many different flow field configurations that have been
used for fuel cells in general and PEFCs in particular. The design of a flow field is a complex
balance between many coupled constraints, many with opposing functional dependencies
on the fuel cell performance, so that trade-offs must be made. The design constraints include
the following:
1. Gas-Phase Transport and Parasitic Pressure Loss The channel and stack manifold
design directly affects the flow and mass transport to the catalyst surfaces as well
as the parasitic pressure drop. The flow through the DM and to the catalyst surface
must be adequate to limit mass transport losses, and the pressure drop must be
minimized.
2. Electron Transport For acceptable fuel cell performance, the electrical resistance
must be low. This is provided by adequate contact area and material choices between
the land and DM material.
3. Heat Transport Since a vast majority of the heat generated by fuel cell operation
must be removed by the fuel cell and not through the exhaust gas, the landing–DM
interface area carries much of the heat into the coolant. Local hot spots caused by
poor design can cause dryout and lead to premature membrane failure. The coolant
and reactant flow channels should be designed in tandem to minimize flooding and
hot spots.
4. Liquid Water Storage and Transport The particular cell design has an impact on
the amount of liquid water stored in the PEFC during normal operation and the ease
with which it is removed. The presence of liquid water has a critical impact on the
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
326 Polymer Electrolyte Fuel Cells
operating performance, pressure loss, degradation via ionic contaminants, and time
to start and degradation from a frozen condition in a fuel cell stack.
5. Gas-Phase Vapor Transport For low humidity operation, dryout of the ionomer
into the channel flow can severely limit performance. Large channel widths pro-
mote dryout, while limiting dead zones under thinner lands. A channel to land
ratio must be found to balance the oxygen restrictions with dryout for low RH
operation.
6. Degradation Mitigation There are many different modes of degradation in PEFC
performance, as discussed in more detail in Section 6.5. The fuel cell system must
be designed to avoid unacceptably high performance loss rate from irrecoverable
losses (e.g., a pinhole in the membrane) and be capable of sensing and mitigating
recoverable losses (e.g., a flooded electrode).
7. Freeze–Thaw Damage Mitigation/Rapid Start-up If the fuel cell will be operated
in ambient environments, survivability at subfreezing temperatures and rapid power
availability at startup are required.
8. Other Constraints Many other factors can affect the ultimate performance of the
PEFC stack. In this context, the term “performance” is meant to be a general measure
of the overall effectiveness of the fuel cell design, including power density, cost,
and durability.
It should be emphasized that the constraints listed are not independent. In many cases,
design to satisfy one constraint will impact others. An excellent example is the channel to
land width ratio, discussed below.
Design Constraints: Using Extremes to Illustrate Trade-offs Often in engineering sys-
tems the basic trade-offs can be best illustrated by consideration of extreme cases. Consider
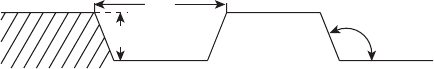
the generic fuel cell channel design with typical geometric parameters shown in Figure
6.34. The lands are an obstruction for mass transport that can potentially cause dead zones
where reactant is unavailable. In the extreme case, the entire flow channel would be land,
and no reaction could take place.
At the other extreme, as shown in Figure 6.35, the entire fuel cell is open in design,
with no mass transfer obstruction from lands. However, the performance of this design is
poor, resulting from lack of electron conduction contacts provided by lands, and DM sag
into the channel volume is likely. The best case is a balance between the two constraints that
d
L
W
θ
L = land width
W = channel width
= draft angle
d = depth
W:L
θ
(typically 0.2–2.5 mm)
(typically 0.5–2.5 mm)
(typically 0–15
o
)
(typically 0.5–2.5 mm)
(typically 3:1–1:1)
Figure 6.34 Typical PEFC channel/land design features. The draft angle can be used to tailor the
channel cross-sectional area without affecting the depth (stack volume) or land contact area.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.3 PEFC Flow Field Configurations and Stack Design 327
Open
Current collector
Figure 6.35 Extreme-case design-maximum channel width current collector along boundary and
otherwise open. This design has the least obstructed mass transfer but the worst electrical contact and
would suffer severe DM tenting.
minimizes the presence of both dead zones and electrical contact resistance losses. Under
low humidity conditions, a balance between vaper loss and heat transfer is also required.
Another common trade-off is channel velocity versus parasitic pressure drop. As dis-
cussed in Chapter 5, flow in channels is typically laminar, so that pressure drop, ignoring
entrance and turbulence effects, can be written as
P =
32µLV
d
2
h
(laminar flow) (6.34)
Some turbulence and minor losses from channel switchbacks and entrance effects are
common. For a given cell design, an empirically derived relationship that combines the
main laminar losses and minor losses can be used as
P = av + bv
2
(6.35)
On one extreme, a high channel velocity (and stoichiometry) ensures nearly uniform reactant
concentration from inlet to outlet and helps to remove any liquid droplets by shear force.
However, very high flow rate results in higher parasitic pressure losses and can also lead
to unintended maldistribution of flow throughout the stack. At the other extreme of very
low flow rates, liquid water slugs will not be removed by channel shear forces, and reactant
concentration losses will be severe near the fuel cell exit. The normal range of balance
in H
2
PEFCs is laminar flow with a stoichiometry of 1.2–1.5 in the anode and 2.0–2.5
in the cathode. Many more trade-offs exist and involve complex interactions and multiple
considerations, so that no true optimal channel design exists. However, it is clear that a
prespective involving understanding of the coupled capillary, phase-change, heat transfer,
and gas phase flows must be used to arrive at an ideal design.
PEFC Flow Field Designs The ideal fuel cell flow field design results in the following
qualities:
1. Excellent mass transport of the reactants to and products from the catalyst layer
with a proper water balance to achieve a moist electrolyte with minimal flooding of
the DM and channels under a wide range of operating conditions.
2. Excellent electron transport to and from the catalyst layers.
3. Adequate heat transport to the coolant channels.
4. Low pressure drop from inlet to exit.
5. Low cost of manufacture.
6. Compact design.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
328 Polymer Electrolyte Fuel Cells
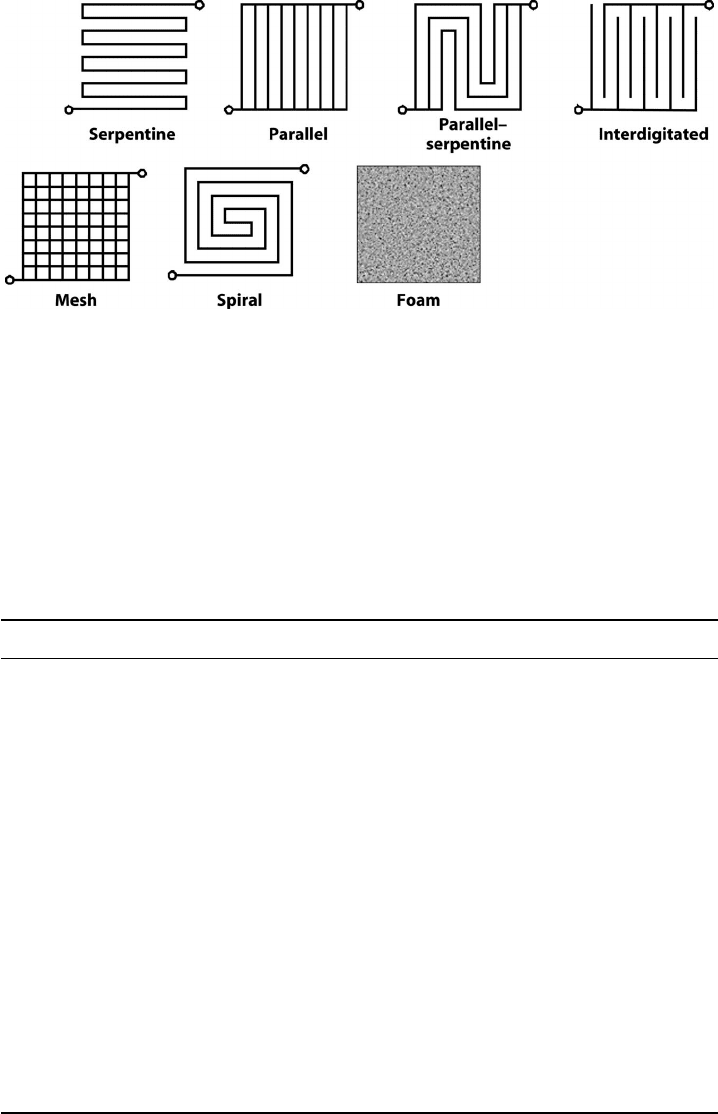
Figure 6.36 Schematics of some common flow field design patterns. Many other basic design
features have also been tried as well, including fractal and spotted post designs. (Image by Soowhan
Kim.)
The particular fuel cell design has a great deal of influence on the transport of reactants
to the electrode. Some basic fuel cell flow channel designs are illustrated in Figure 6.36, with
additional details on the inherent trade-offs in Table 6.3. Most standard fuel cell designs are
a combination of parallel and serpentine designs, like that shown in Figure 6.37. By tailoring
the channel size and parallelization, the channel velocity and pressure drop can be balanced.
Table 6.3 Channel Designs in PEFCs
Flow Field Design Advantages Disadvantages
Serpentine High channel velocity, good water
removal, high performance
High pressure drop, uneven species
concentration distribution
Parallel Low pressure drop, more even
concentration distribution
Low channel velocity, poor liquid
water removal
Parallel–serpentine
combination
Can balance advantages of
serpentine and parallel design
Combination of parallel and
serpentine disadvantages
Interdigitated High performance, excellent water
removal from under landings
High pressure drop for forced flow
through DM, possible long-term
damage to structure
Porous plate Excellent water uptake capabilities Expensive design, coolant used
must be freezable water
Mesh Low pressure loss, controllable
total contact area
Dead zones and water accumulation
away from path of least resistance
Spiral Lower humidity load since exit
channels run by inlet channels
Unintentional reactant bypass
between low-pressure exit can
high-pressure inlet channels
Metal foam plate Uniform compression on DM
surface, ease of manufacture
Higher pressure drop
Radial Lowest diffusion path length for
passive designs
Only used in passive system
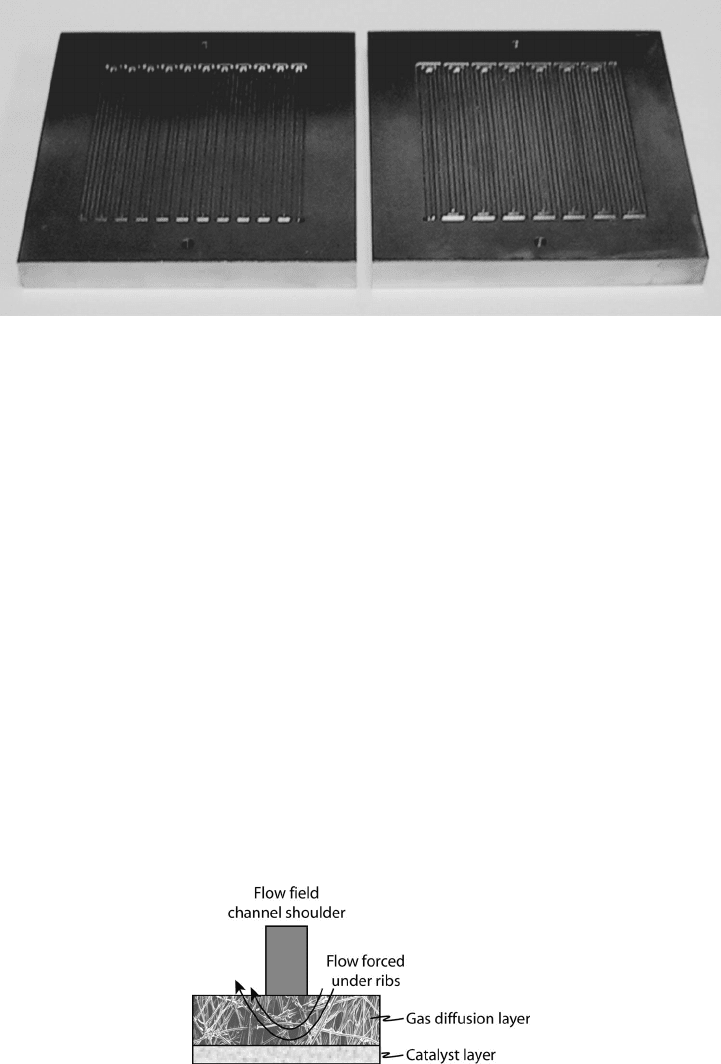
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.3 PEFC Flow Field Configurations and Stack Design 329
Figure 6.37 Photographs of graphite 50 cm
2
active area parallel–serpentine flow field used for
laboratory studies. The laboratory flow field plates are over 1 cm thick to improve durability in a
laboratory environment. Actual fuel cell stack flow field plates are considerably thinner to improve
power density.
The interdigitated design is a unique design that has no continuous channels. Through
a series of dead-end channel inlet fingers, as shown in Figure 6.36, flow is forced into the
DM and under the lands into the outlet channel fingers. A crosssection of the interdigitated
design is shown in Figure 6.38. In the process, liquid water is removed from the DM, and
the reactant transport to the surface is greatly facilitated by forced convection. Performance
with the interdigitated design is very good, but the parasitic pressure drop is also increased.
United Technologies has developed a unique porous plate flow field technology,
illustrated in Figure 6.39. The flow channels have a typical parallel and serpentine de-
sign, but the landings have many small hydrophilic capillary columns, which allow the
cell to wick water from the DM surface by capillary action. When the DM becomes sat-
urated, the porous plate wicks water away and into the coolant channels, maintaining a
low saturation level and improving high-current-density performance. This design is a
passive method to manage water transport and flooding using capillary forces. Naturally
occurring capillary forces are quite effective to transport water (consider that trees have no
pumps). Interestingly, natural forces are very rarely the first possible solution considered
by engineers, although they can often be exploited, as in this unique design.
Figure 6.38 Schematic of cross section of interdigitated design to force flow convectively to catalyst
surface under land locations.
