Mench M.M. Fuel Cell Engines
Подождите немного. Документ загружается.

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
310 Polymer Electrolyte Fuel Cells
COMMENTS: This calculation assumes all water generated goes directly to liquid and
none to vapor, which is unlikely to be true, so that the time scale required for liquid water
accumulation will be even greater than calculated. In a PEFC, this slow time scale, the slow
time scales of evaporation or drainage from the porous media and electrolyte, coupled with
the nearly instantaneous electrochemical and gas-phase response times lead to hysteresis
in a polarization curve and the fuel cell memory effect discussed.
6.2.2 Local Water Balance
We have just discussed the global water balance in a PEFC, but we have also mentioned that
actual flooding loss is a localized phenomenon that can occur as a film resistance and pore
filling in the catalyst layer, DM, and channels. To grasp the localized flooding phenomenon,
it is also important to understand the macroscopic water transport processes which occur
within the fuel cell media.
Water Flux in Polymer Electrolyte Membranes Water flux in the solid electrolyte mem-
brane of the PEFC must be understood to grasp the concept of a local water balance
in the fuel cell. From Chapter 5, we know that the ionic conductivity of perfluorosulfonic
acid–based solid polymer electrolytes is a strong function of water content. Within the elec-
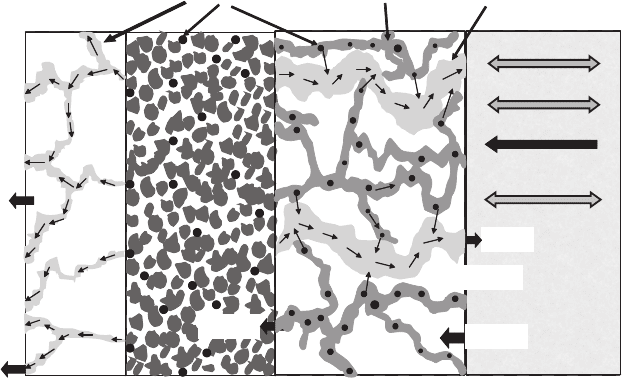
trolyte, there are four basic modes of transport, as schematically illustrated in Figure 6.21:
1. Diffusion—concentration gradient driven flow
2. Electro-osmotic drag—voltage gradient driven flow
3. Hydraulic permeability—gas or capillary pressure gradient driven flow
4. Thermo-osmosis—temperature driven flow
H
2
O
vap
H
2
O
liq
Hydrophilic
Channel
Hydrophobic
Channel
H
2
O
liq
H
2
O
vapor
Diffusion
Medium
Micro-porous Layer Cathode
Catalyst Layer
Membrane
Condensed
Water Droplet
Hydraulic permeation
Diffusion
Electro -osmotic drag
H
2
O
vap
H
2
O
H
2
O
generated
Thermo-osmosis
Figure 6.21 Different modes of water transport inside fuel cell.
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.2 Water Balance in PEFC 311
Diffusion in Nafion Diffusion into Nafion (and other perfluorosulfonic acid–based mem-
branes) can be modeled according to Fick’s law:
˙
n
W
=−D
W
dC
m
W
dx
(6.20)
The water diffusion in the ionomer phase, D
W
, as a function of ionomer water content λ has
been measured by several groups, including Springer et al. [8]. Gong and co-workers [12]
also reported water self-diffusion coefficients for Nafion with pulse field gradient NMR.
Motupally and co-workers expanded the data from [8] to include changes in temperature
[13].
D
W
= 3.10 × 10
−3
λ
−1 + exp
0.28λ
exp
−2436
T (K )
for (0 <λ≤ 3) (6.21)
D
W
= 4.17 × 10
−4
λ
1 + 161 exp
−λ
exp
−2436
T (K )
for (3 ≤ λ<17) (6.22)
where λ is the water content of the membrane per sulfonic acid site:
λ =
H
2
O
SO
3
H
(6.23)
and is defined in Eq. (6.26). The water activity, a, needed to solve for λ is defined as
a =
y
v
P
P
sat
(T )
= RH (6.24)
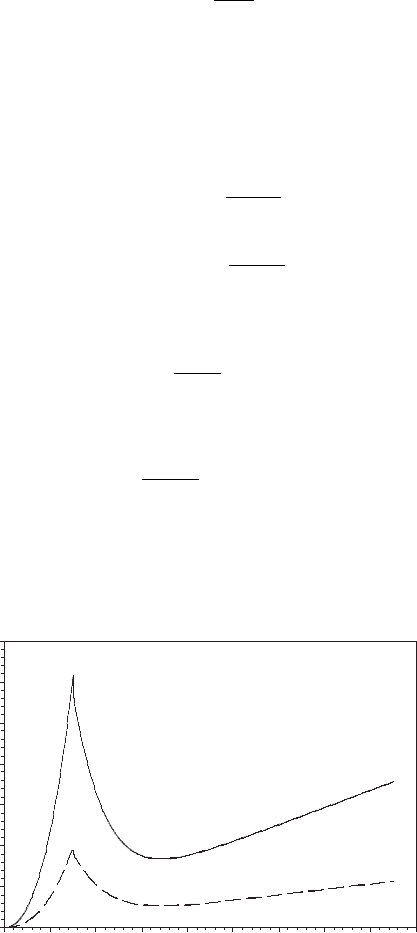
This relationship is shown in Figure 6.22 and is an alternative to the relationship given
in Chapter 5. At low water content, the diffusion coefficient increases with water content
until a peak value around λ = 3, and then decreases with increasing water content until
Membrane water content,
λ
,(H
2
O/SO
3
H)
0 2 4 6 8 1012141618
Diffusivity x 10
-6
(cm
2
/s)
0
2
4
6
8
10
12
14
353 K
303 K
Figure 6.22 Diffusion coefficient as a function of membrane water uptake from Zowadzinski et al.
[14].

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
312 Polymer Electrolyte Fuel Cells
around λ = 6, and finally gradually increases. The diffusivity is a decreasing function of
temperature.
Water Uptake in Nafion In order to determine a relationship between the gas-phase
relative humidity and the ionomer water content, a relation between the ionomer water
concentration C
m
W
and the gas-phase water vapor mole fraction is needed. If we neglect
the volume change of the ionomer caused by water uptake, the water concentration in the
ionomer phase can be expressed by the ionomer water content λ as
C
m
W
=
ρ
dry
EW
λ (6.25)
where ρ
dry
is the density of dry ionomer, EW is the equivalent molecular weight of the
ionomer material, and the water content λ is defined as the ratio of the number of water
molecules to the number of sulfonic acid groups within the ionomer. As discussed in
Chapter 5, Zawodzinski et al. [14] showed
λ = 0.043 + 17.81a
W
− 39.85a
2
W
+ 36.0a
3
W
(6.26)
where a
w
= RH.
For gas-phase contact of vapor with the membrane, the water activity in the gas
phase is equivalent to the gas-phase relative humidity at the cell operating temperature, as
discussed in Chapter 5. For contact with liquid water, λ increases to 22, despite the same
thermodynamic activity as water vapor due to Schroeder’s paradox.
Hydraulic Permeability Hydraulic permeation of water through the membrane occurs as
a result of a pressure difference between the anode and cathode. The molar flow rate of
water from the cathode to the anode can be written from Darcy’s law:
˙
n
H
2
O,cathode
=
kk
r
P
c−a
µl
(6.27)
where k is the effective permeability of the membrane µ is the liquid viscosity, k
r
is
the relative permeability of the membrane, l is the membrane thickness, and P
c−a
is
the gas-phase pressure difference between the cathode and anode. Water flux through the
membrane can occur by gas- and liquid-phase transport, respectively. Transport of water
through the membrane can occur as a result of a gas pressure gradient across the membrane
or the capillary pressure gradient across the membrane. The gas-phase term is typically
small because the anode and cathode pressures are usually similar, so this effect can be
ignored. In the liquid phase, however, a capillary pressure difference between the anode and
cathode can result in a net flux of water via this mode of transport [15]. The DM properties
are often tailored to achieve the desired capillary flux effect, depending on the operating
conditions.
Electro-osmotic Drag Electro-osmotic drag of water is the mass flux resulting from a
polar attraction of the water molecules to the positively charged protons moving from the
anode to the cathode through the electrolyte, as illustrated in Figure 6.23. As each proton

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.2 Water Balance in PEFC 313
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
SO
3
–
H
2
O
H
+
H
2
O
Figure 6.23 Schematic of electro-osmotic drag in electrolyte membrane under a current.
travels through the electrolyte from the anode to the cathode by Grotthuss and vehicular
mechanisms described in Chapter 5, the surrounding cloud of polar water molecules will
be dragged along.
Water transport by electro-osmotic drag is always from the anode to the cathode. Since
the drag is proportional to the current (protons), an expression for the flux of water by
electro-osmotic drag is written as
˙
n
H
2
O
= n
d
iA
F
(6.28)
where n
d
is the electro-osmotic drag coefficient in units of water molecules per proton. Many
studies have been conducted to determine the drag coefficient. A drag coefficient of 1–5
H
2
O/H
+
of Nafion membranes has been shown for a fully hydrated Nafion 117 membrane
[16]. Zawodzinski et al. [17] have investigated the electro-osmotic drag coefficient of
Nafion 117 and other ionomeric polymer electrolyte membranes at 30
◦
C under conditions
relevant to PEFCs. For a Nafion 117 membrane fully hydrated in liquid water (λ = 22),
they obtained n
d
= 2.5. For a nearly fully hydrated membrane with a water content of
λ = 11, a much smaller value, n
d
= 0.9, was obtained. Fuller and Newman [18] obtained
n
d
= 1.4 for λ values of 5–14. From the same kind of measurement, however, Zawodzinski
et al. [17] obtained a convenient value of n
d
=1.0 for λ<14 at 30
◦
C. Ise and Kreuer et al.
[19] also measured the electro-osmotic drag coefficients in Nafion and obtained n
d
= 2.6
for λ =20, which is very close to the value reported by Zawodzinski et al. [17]. In summary,
if equilibrated in moist vapor (0 <λ<14), the value of n
d
is around 1.0–1.5. For Nafion
equilibrated in liquid water, however, the membrane water uptake is much greater, and the
electro-osmotic drag coefficient is around 2–5.
Example 6.4 Comparison of Generated and Drag Water Compare the total water
delivered to the cathode by electro-osmotic drag to that generated by reaction at a given
current density, assuming the membrane is in contact wit vapor-phase water only.
SOLUTION For water drag, the net molar flux of water to the cathode is shown from
Eq. (6.28):
˙
n
H
2
O,drag
= n
d
iA
F

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
314 Polymer Electrolyte Fuel Cells
where n
d
has been shown to be ∼1.0–1.5. For water generation, we have Faraday’s law:
˙
n
H
2
O,gen
=
iA
2F
Comparing the two, we have
˙
n
H
2
O,drag
˙
n
H
2
O,gen
=
n
d
(iA/F)
iA/(2F )
=
(
1–1.5
)
× 2
1
= 2–3
Thus, water transport by electro-osmotic drag is always from the anode to cathode and is
two to three times greater than the water generation at the cathode for vapor-equilibrated
membranes! In terms of flooding at the cathode, the electro-osmotic drag to the cathode
results in more water than the electrochemical generation.
COMMENTS: In hydrogen PEFCs, much of the water driven to the cathode by electro-
osmotic drag is removed by back diffusion to the anode, especially in thin membranes. In
DAFCs with a liquid fuel solution, however, back diffusion does not occur and the cathode
flooding problem can be much more severe.
Temperature and Heat Flux Driven Flow A fourth mode of transport that has been shown
to drive water flux in the membrane is heat flux driven flow. As a general rule, water will
move through the membrane toward a colder location. This occurs in a freezing process
due to capillary forces [20, 21], and nonfreezing processes [22, 23]. The nonfrozen mode
of transport is poorly understood but is likely a result of the combined effects of capillary
pressure change with temperature and thermo-osmosis in membranes.
There may also be a phase change phenomena associated with the motion of water in
the membrane under a temperature gradient, since Bradean et al. [22] showed an exponential
relationship between liquid flux and the temperature gradient and heat flux, but the exact na-
ture of this is not fully understood. This mode of transport has not commonly been included
in the analysis of normal operation, since this effect is obscured by the net diffusive and
electro-osmotic drag transfer. Under startup or shutdown conditions, however, where larger
gradients in temperature can exist, the net water flux from this mode can be significant, and
has been exploited to passively drain the DM on shutdown to a frozen state [22].
Net Transport Coefficient The overall water transport through the membrane can be
written as the combination of the various modes of transport:
˙
n
H
2
O,
net,a−c
=
˙
n
H
2
O,
drag
+
˙
n
H
2
O,
diff
+
˙
n
H
2
O,
perm,gas
+
˙
n
H
2
O,
perm,cap
+
˙
n
H
2
O,
temp
(6.29)
The net drag coefficient α
d
is a parameter used to express the net drag of water from the
anode to the cathode and accounts for the total effect of the modes in Eq. (6.29):
˙
n
H
2
O,
net,a−c
= α
d
iA
F
(6.30)
The net drag coefficient represents the total transport of water to the cathode. In an ideal
situation for water management, α
d
would be uniformly −0.5 along the electrode. In this
case, the exact amount of water generated by reaction is exactly balanced and moved
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.2 Water Balance in PEFC 315
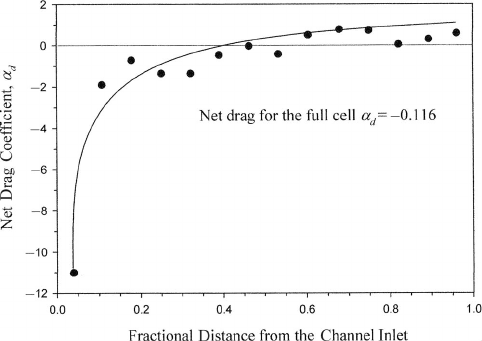
Figure 6.24 Measured local and net effective drag coefficients along gas flow channel of PEFC
operating at 0.7 V, with a dry anode inlet and cathode inlet at 50% RH at 9
◦
C. The net drag is negative,
indicating a net drag of water toward the dry anode. Toward the exit, however, the local effective drag
becomes positive. (Adapted from Ref. [24].)
through the electrolyte from the cathode to the anode. There would be the possibility of
anode flooding, but the membrane would retain maximum moisture content. In practice, for
thethin(∼15–25-µm) membranes used in automotive applications with uniform anode and
cathode inlet humidity, the high back diffusion to the anode nearly completely compensates
for the electro-osmotic drag, and the net drag is nearly zero [25] or slightly negative [24].
For thicker membranes used in stationary applications, the net drag coefficient can be
slightly positive because the back diffusion is limited.
Another point should be made that the assumption of uniform net drag within the fuel
cell is rarely justified. Figure 6.24 shows the measured net drag coefficient distribution
along the flow channel of a PEFC operating at either relatively dry anode or dry cathode
inlet conditions, with a thin 18-µm electrolyte membrane [11]. In this case, even though
the electrolyte is very thin, the net drag coefficient is not near zero because of the initial
imbalance between anode and cathode humidity. For this dry anode inlet case, the overall net
drag coefficient is −0.12, representing a net back-diffusion flow toward the anode. However,
after the initial 40% of the fuel cell flow channel, the net flux is slightly positive, toward
the cathode, since the anode has become humidified, reducing the diffusion concentration
gradient.
As a result of Schroeder’s paradox, there is a very high electro-osmotic drag coefficient
of 2–5 H
2
O/H
+
in applications where the anode is in contact with liquid-phase water such as
the DMFC. This and the lack of back diffusion result in a very positive net drag coefficient
and more severe cathode flooding without special engineering of the DM to provide a strong
capillary pressure gradient toward the anode.
Local Water Balance: Catalyst Layer Mass Balance In this section we discuss the local
water balance, which can be (and usually is) highly nonhomogeneous throughout the fuel
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
316 Polymer Electrolyte Fuel Cells
Cathode
n
in,
c
n
out,
c
CV
dn/dt
iA/2F
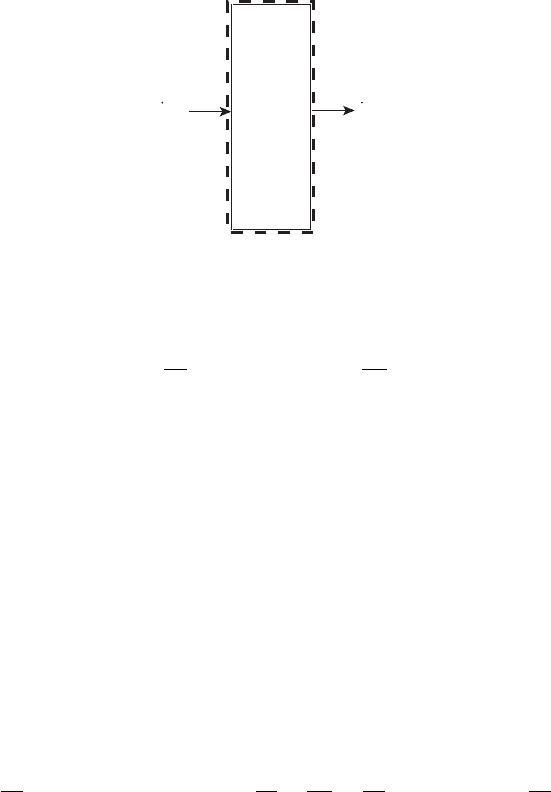
Figure 6.25 Control volume mass balance applied to cathode catalyst layer.
cell. From a mass balance on the cathode catalyst layer (Figure 6.25),
dn
dt
cv
=
˙
n
in,c
−
˙
n
out,c
+
I
2F
(6.31)
In steady state, the water generated will be exactly equivalent to the net water out of
the catalyst layer. The transport in and out of the catalyst layer involves many modes of
transport:
1. Transport into or from the electrolyte by diffusion, electro-osmotic drag, hydraulic
permeation, and temperature effects.
2. Transport of liquid and gas into the porous media between the catalyst layer and the
channel/land locations.
Example 6.5 Calculating Internal Water Balance Given a net drag coefficient of 0.1,
determine the molar rate of water accumulation at the catalyst layer that must be removed
to prevent flooding.
SOLUTION
dn
dt
cv
=
˙
n
H
2
O,
net,a−c
+
˙
n
gen
= α
d
iA
F
+
I
2F
=
iA
F
(
α
d
+ 0.5
)
= 0.6
iA
F
This value must be removed from the cathode exit of the fuel cell to obtain a condi-
tion of water balance. If this value is not removed, saturation of liquid in the catalyst
larger will increase, raising the local capillary pressure until it is pushed into the DM,
or electrolyte, or the active area is sufficienty reduced to flood the electrode and re-
duce the current. The balance can be achieved through a variety of methods already
discussed.
COMMENTS: For thin membranes in fully humidified conditions, we can typically
assume a net drag value close to zero. If there is an imbalance in the inlet RH values
between the anode and cathode, the diffusion flux will change the net drag coefficient. If
the anode flow is relatively underhumidified compared to the cathode flow, the net drag
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.2 Water Balance in PEFC 317
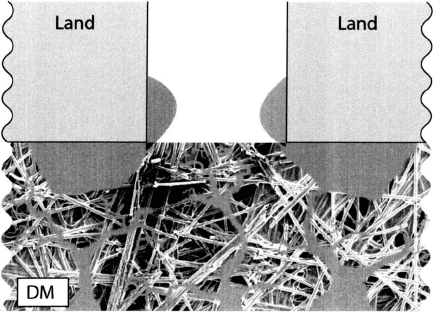
Figure 6.26 Typical liquid water accumulation behavior under lands and channels in PEFC.
coefficient generally becomes slightly negative. In the case of an underhumidified cathode,
the net drag value will become slightly positive.
Liquid Distribution and Transport in Catalyst, Microporous, and Diffusion Layers A
basic question of PEFCs is, where is the flooding occurring? The answer is that it depends
on the porous media properties, flow conditions, and fuel cell geometry. The exact nature
of the liquid water structure in the DM is not precisely known, but it is generally believed
from capillary theory that the liquid water flows through large hydrophobic and hydrophilic
pores, and the gas phase flows through small hydrophobic pores. However, evaporation and
condensation also play a role, so that a unified understanding based on capillary pressure
arguments alone is insufficient.
Figure 6.26 is an illustration of the typical water distribution in the fuel cell porous
media under the lands and channels. Since the coldest location during operation is generally
under the lands, water condenses there first. As the saturation increases, water pushes out
laterally from under the lands and forms connections between the lands under the channels,
resulting in DM flooding and performance degradation. The removal of water from under
the lands into the channels is important to avoid flooding, as lateral connectivity between the
water under adjacent lands in the DM can induce severe performance loss through reactant
blockage. Besides under the lands, there are other locations in a fuel cell where liquid
water tends to accumulate. These locations have been identified primarily using neutron
imaging, which enables a direct nonintrusive quantification of the liquid water content
in the operating fuel cell and is used by several research institutions for this purpose
[26–28].
Liquid accumulation also commonly occurs around channel switchbacks, as shown in
Figure 6.27. This is a result of flow recirculation, stagnation, and pressure drop at locations
of sudden momentum reversal. Additionally, the local flow separation near the corner
accelerates the core flow, promoting annular flow of liquid water.
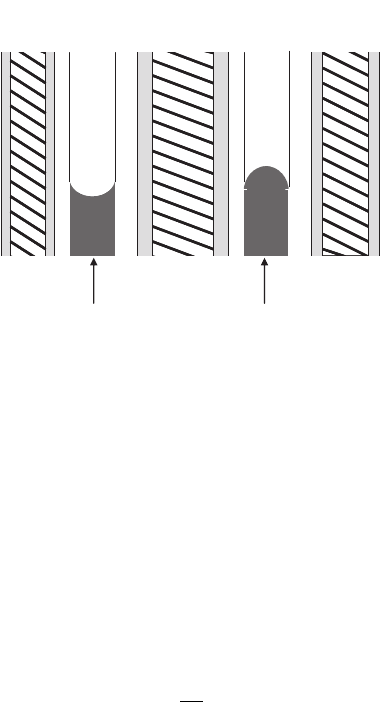
Interestingly, even in very dry operating conditions, there tends to be an accumulation
of liquid water under the lands, as shown in Figure 6.28. This is because the most effi-
cient heat transfer is conduction through the lands, and access to the channel is blocked.

c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
318 Polymer Electrolyte Fuel Cells
Figure 6.27 Neutron radiograph showing liquid water accumulation along corners of 180
◦
switch-
back in operating PEFC. Water is often observed to preferentially accumulate at switchback locations
and along the channel walls. (Adapted from Ref. [9].)
Therefore, during normal operation, there is typically liquid water storage in the gas DM
under lands, since lands are the coolest location.
At low current density, channel-level flooding is prevalent due to slug formation and the
lack of sufficient gas-phase velocity to remove channel droplets via drag force. As the current
density (and channel flow rates) increase, the channels are more efficiently cleared, and the
porous media begin to accumulate liquid from generation. At this point, flooding may occur
in the catalyst layer, DM, or some portion of both depending on the materials and conditions.
From Chapter 5, we know that the capillary pressure liquid saturation relationship depends
on the net hydrophobicity of the DM. Since the carbon materials in the catalyst layer and
DM are hydrophilic and the PTFE additive is hydrophobic, there are regions of mixed
hydrophobic and hydrophilic behavior, and no single surface contact angle describes the
internal structure or multiphase flow within the mixed wettability media. Instead, flow
in these structures takes place along separate hydrophilic–hydrophobic pathways, each
with different behavior for liquids and gases (Figure 6.29). From porosimetry studies, a
typical range of hydrophobic to hydrophilic pores in PEFC diffusion media with PTFE
Land
Channel
Figure 6.28 Neutron image showing modest liquid water accumulation under the lands in a seven
channel parallel flow configuration fuel cell at an underhumidified condition. Lighter areas represent
the accumulation are under lands, while the generally black areas indicate a relatively liquid droplet-
free situation under the channels. The total water mass in the 14.5 cm
2
cell was 79 mg at 0.35 A/cm
2
.
(Adapted from Ref. [29].)
c06 JWPR067-Mench January 26, 2008 20:1 Char Count=
6.2 Water Balance in PEFC 319
air
Water imbibed
automatically
Higher capillary
pressure
air
Hydrophilic
pore
Hydrophobic
pore
Figure 6.29 Schematic of different liquid water imbibitions and transport behavior in hydrophilic
and hydrophobic pores. The catalyst layer and diffusion media are typically mixed wettability media;
thus hydrophilic and hydrophobic pathways exist for transport of liquid and gas phases.
additive is 20–40% hydrophilic and 60–80% hydrophobic [30], so that the net condition of
a PTFE-treated DM is hydrophobic.
It is clear based on experimental studies that, while critical, flooding is not solely a
result of capillary flow, and phase change, morphology, and interfacial properties also play
key roles.
In order to develop a heuristic of the capillary flow related flooding in the catalyst
layer and DM, we should recall from Chapter 5 that the capillary pressure is inversely
proportional to the pore radius through the Laplace equation for capillary tubes:
P
c
=
2γ
r
∗
cos θ (6.32)
For hydrophobic media, the capillary pressure is elevated in the liquid phase and increases
with liquid saturation. In all cases, liquid tends to move along a path toward a lower capillary
pressure, which, for hydrophobic media, means liquid will move toward locations of
(a) lower liquid saturation,
(b) a more hydrophilic location, or
(c) a larger, equally hydrophobic location.
For a hydrophilic media, the liquid phase is nonwetting, a lower liquid-phase pressure exists
and provides a suction force to draw the connected liquid into smaller pores, where it will
remain until removed by convection or evaporation. Capillary flow through a hydrophilic
porous media will flow in the direction of
(a) higher liquid saturation (until fully saturated)
(b) a more hydrophilic surface, or
(c) a smaller hydrophilic pore.
