Bhushan B. Handbook of Micro/Nano Tribology, Second Edition
Подождите немного. Документ загружается.


© 1999 by CRC Press LLC
bound octadecyl phosphonic acid monolayer on alumina surfaces is shown in Figure 9.20. In this case,
the friction is higher than untreated undamaged α-alumina surfaces, but the bare surfaces easily damage
upon sliding, resulting in an ultimately higher friction system with greater wear rates than the more
robust monolayer-coated surfaces.
Clearly, the mechanism and factors that determine normal friction are quite different from those that
govern interfacial friction. These mechanisms are described in the theoretical section below, but one
should point out that this effect is not general and may only apply to brittle materials. For example, the
friction of ductile surfaces is totally different and involves the continuous plastic deformation of con-
tacting surface asperities during sliding rather than the rolling of two surfaces on hard wear particles
(Bowden and Tabor, 1967). Furthermore, in the case of ductile surfaces, water and other surface-active
components do have an effect on the friction coefficients under normal sliding conditions.
9.11 Theories of Interfacial Friction
9.11.1 Theoretical Modeling of Interfacial Friction: Molecular Tribology
The following friction model, first proposed by Tabor (1982) and developed further by Sutcliffe et al.
(1978), McClelland (1989), and Homola et al. (1989), has been quite successful at explaining the inter-
facial and boundary friction of two solid crystalline surfaces sliding past each other in the absence of
wear. The surfaces may be unlubricated, or they may be separated by a monolayer or more of some
boundary lubricant or liquid molecules. In this model, the values of the critical shear stress S
c
, and the
coefficient of friction µ, of Equation 9.27 are calculated in terms of the energy needed to overcome the
attractive intermolecular forces and compressive externally applied load as one surface is raised and then
slid across the molecular-sized asperities of the other.
This model (variously referred to as the interlocking asperity model, coulomb friction,
or the cobblestone
model) is akin to pushing a cart over a road of cobblestones where the cartwheels (which represent the
molecules of the upper surface or film) must be made to roll over the cobblestones (representing the
molecules of the lower surface) before the cart can move. In the case of the cart, the downward force of
gravity replaces the attractive intermolecular forces between two material surfaces. When at rest the
cartwheels find grooves between the cobblestones where they sit in potential energy minima and so the
cart is at some stable mechanical equilibrium. A certain lateral force (the “push”) is required to raise the
cartwheels against the force of gravity in order to initiate motion. Motion will continue as long as the
cart is pushed, and rapidly stops once it is no longer pushed. Energy is dissipated by the liberation of
heat (phonons, acoustic waves, etc.) every time a wheel hits the next cobblestone. The cobblestone model
is not unlike the old coulomb and interlocking asperity models of friction (Dowson, 1979) except that
it is being applied at the molecular level and where the external load is augmented by attractive inter-
molecular forces.
There are thus two contributions to the force pulling two surfaces together: the externally applied load
or pressure, and the (internal) attractive intermolecular forces which determine the adhesion between
the two surfaces. Each of these contributions affects the friction force in a different way, and we start by
considering the role of the internal adhesion forces.
9.11.2 Adhesion Force Contribution to Interfacial Friction
Consider the case of two surfaces sliding past each other as shown in Figure 9.21. When the two surfaces
are initially in adhesive contact, the surface molecules will adjust themselves to fit snugly together, in an
analogous manner to the self-positioning of the cartwheels on the cobblestone road. A small tangential
force applied to one surface will therefore not result in the sliding of that surface relative to the other.
The attractive van der Waals forces between the surfaces must first be overcome by having the surfaces
separate by a small amount. To initiate motion, let the separation between the two surfaces increase by
a small amount ∆D, while the lateral distance moved is ∆d. These two values will be related via the
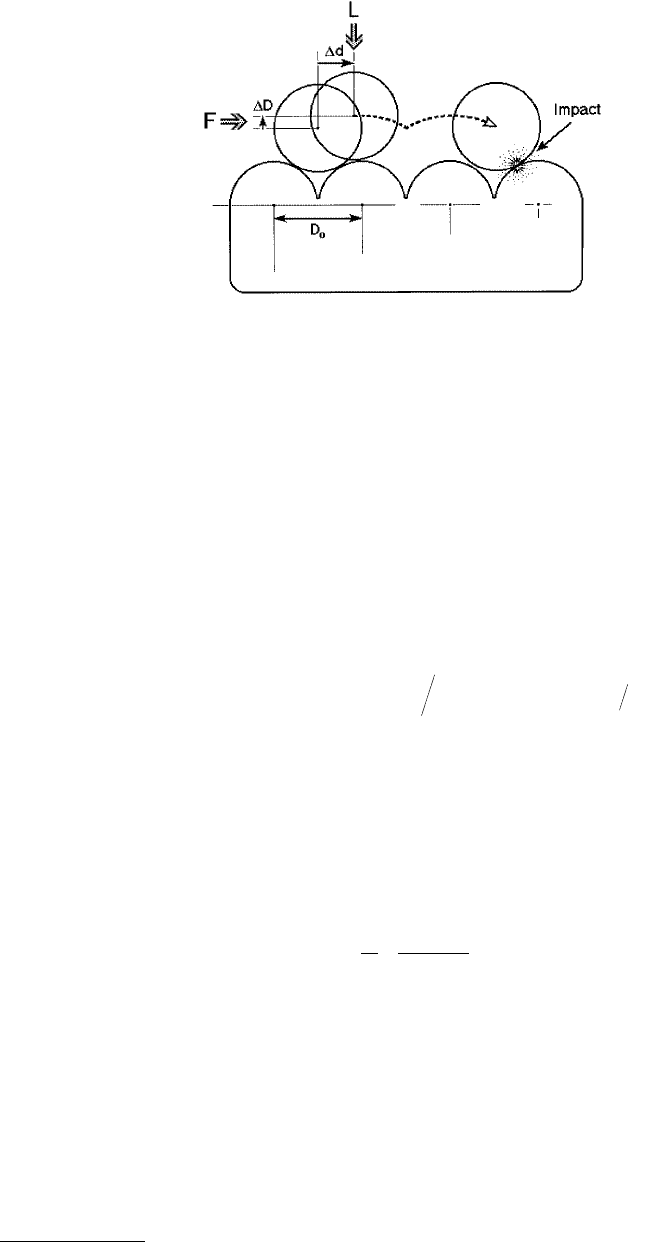
© 1999 by CRC Press LLC
geometry of the two surface lattices. The energy put into the system by the force F acting over a lateral
distance ∆d is
(9.30)
This energy may be equated with the change in interfacial or surface energy associated with separating
the surfaces by ∆D, i.e., from the equilibrium separation D = D
0
to D = (D
0
+∆D). Since γ ∝ D
–2
, the
surface energy cost may be approximated by
(9.31)
where γ is the surface energy, A the contact area, and where D
0
is the surface separation at equilibrium.
During steady-state sliding (kinetic friction), not all of this energy will be lost or absorbed by the lattice
every time the surface molecules move by one lattice spacing: some fraction will be reflected during each
impact of the cartwheel molecules (McClelland, 1989). Assuming that a fraction ε of the above surface
energy is lost every time the surfaces move across the characteristic length ∆d (Figure 9.21), we obtain
after equating the above two equations
(9.32)
For a typical hydrocarbon or a van der Waals surface, γ ≈ 25 × 10
–3
J/m
2
. Other typical values would be
∆D ≈ 0.5 Å, D
0
≈ 2 Å, ∆d ≈ 1 Å, and ε ≈ 0.1 to 0.5. By using the above parameters, Equation 9.32 predicts
S
c
≈ (2.5 to 12.5) × 10
7
N/m
2
for van der Waals surfaces. This range of values compares very well with
typical experimental values of 2 × 10
7
N/m
2
for hydrocarbon or mica surfaces sliding in air or separated
by one molecular layer of cyclohexane (Homola et al., 1989).
The above model may be extended, at least semiquantitatively, to lubricated sliding, where a thin liquid
film is present between the surfaces. With an increase in the number of liquid layers between the surfaces,
D
0
increases while ∆D decreases, hence the lower the friction force. This is precisely what is observed.
But with more than one liquid layer between two surfaces, the situation becomes too complex to analyze
FIGURE 9.21 Schematic illustration of how one molecularly smooth surface moves over another when a lateral
force F is applied. As the upper surface moves laterally by some fraction of the lattice dimension ∆d, it must also
move up by some fraction of an atomic or molecular dimension ∆D, before it can slide across the lower surface. On
impact, some fraction ε of the kinetic energy is “transmitted” to the lower surface, the rest being “reflected” back to
the colliding molecule (upper surface).
Input energy: .Fd×∆
Surface energy change: ,21 4
0
2
0
2
0
γγADDD ADD−+
()
≈
()
∆∆
S
F
A
D
Dd
c
==
⋅
⋅
4
0
γε ∆
∆
.
© 1999 by CRC Press LLC
analytically (actually, even with one or no interfacial layers, the calculation of the fraction of energy
dissipated per molecular collision ε is not a simple matter). Sophisticated modeling based on computer
simulations is now required, as described in the following section.
9.11.3 Relation between Boundary Friction
and Adhesion Energy Hysteresis
While the above equations suggest that there is a direct correlation between friction and adhesion, this
is not the case. The correlation is really between friction and adhesion hysteresis, described in Section 9.8.
In the case of friction, this subtle point is hidden in the factor ε, which is a measure of the amount of
energy absorbed (dissipated, transferred, or lost) by the lower surfaces when it is impacted by a molecule
from the upper surface. If ε = 0, all the energy is reflected and there will be no kinetic friction force, nor
any adhesion hysteresis, but the absolute magnitude of the adhesion force or energy would remain finite
and unchanged. This is illustrated in Figure 9.22.
The following simple model shows how adhesion hysteresis and friction may be quantitatively related.
Let ∆γ = (γ
R
to γ
A
) be the adhesion energy hysteresis per unit area, as measured during a typical load-
ing–unloading cycle (see Figure 9.22C and D). Now consider the same two surfaces sliding past each
other and assume that frictional energy dissipation occurs through the same mechanism as adhesion
energy dissipation, and that both occur over the same characteristic molecular length scale σ. Thus, when
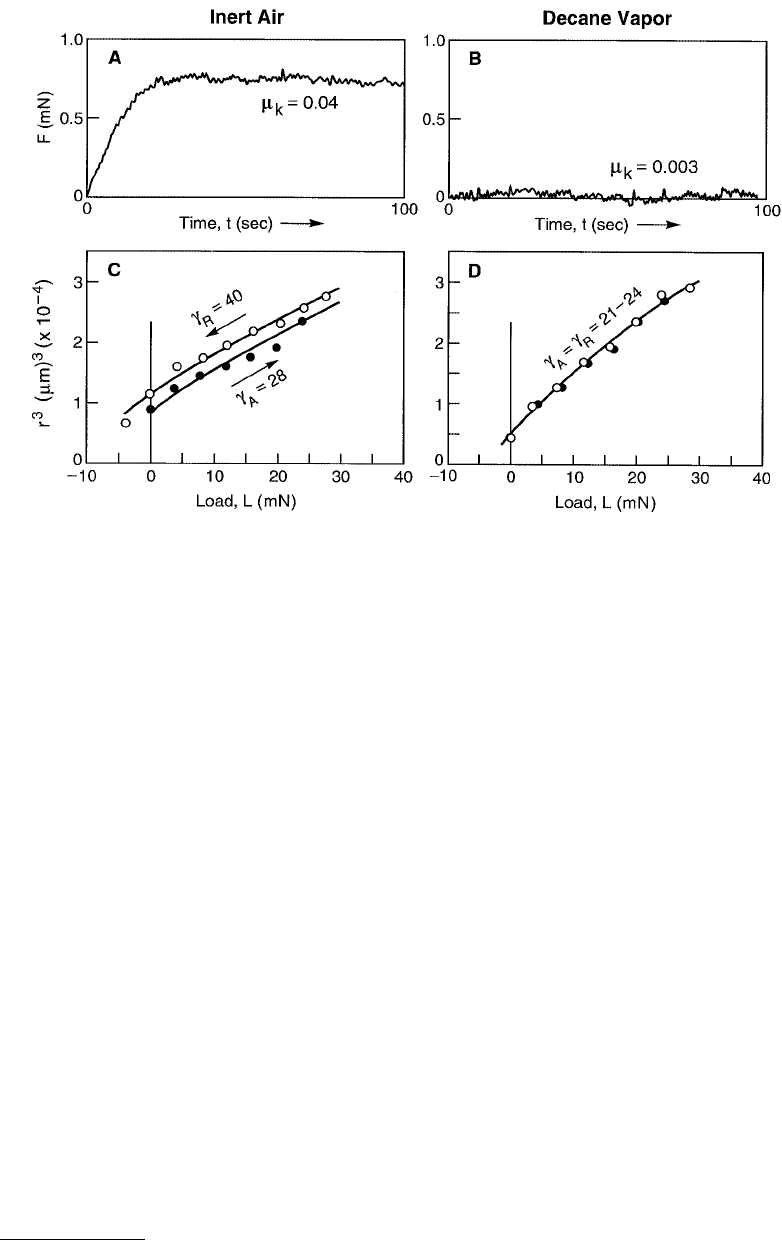
FIGURE 9.22 Top: Friction traces for two fluid-like monolayer-coated surfaces at 25° C showing that the friction
force is much higher between dry monolayers (A) than between monolayers whose fluidity has been enhanced by
hydrocarbon penetration from vapor (B). (From Chen et al., 1991.) Bottom: Contact radius vs. load (r
3
–L) curves
measured for the same two surfaces as above and fitted to the JKR equation (Equation 9.20 — shown by the solid
lines. For dry monolayers (C) the adhesion energy on unloading (γ
R
= 40 mJ/m
2
) is greater than that on loading
(γ
A
= 28 mJ/m
2
), indicative of an adhesion energy hysteresis of ∆γ = γ
R
– γ
A
= 12 mJ/m
2
. For monolayers exposed to
saturated decane vapor (D) their adhesion hysteresis is zero (γ
A
= γ
R
), and both the loading and unloading curves
are well fitted by the thermodynamic value of the surface energy of fluid hydrocarbon chains, γ ≈ 24 mJ/m
2
.

© 1999 by CRC Press LLC
the two surfaces (of contact area A = πr
2
) move a distance σ, equating the frictional energy (F × σ) to
the dissipated adhesion energy (A × ∆γ), we obtain
(9.33)
or
(9.34)
which is the desired expression and which has been found to give order of magnitude agreement between
measured friction forces and adhesion energy hysteresis (Chen et al., 1989). If we equate Equation 9.34
with Equation 9.32, since 4∆D/D
0
∆d ≈ σ, we obtain the intuitive relation:
(9.35)
Figure 9.22 illustrates the relationship between adhesion hysteresis and friction for surfactant-coated
surfaces under different conditions. This effect, however, is much more general, and has been shown to
hold for other surfaces as well (Vigil et al., 1994; Israelachvili et al., 1994, 1995). Direct comparisons
between absolute adhesion energies and friction forces show little correlation. In some cases higher
adhesion energies for the same system under different conditions correspond with lower friction forces.
For example, for hydrophilic silica surfaces it was found that with increasing relative humidity the
adhesion energy increases, but the adhesion energy hysteresis measured in a loading–unloading cycle
decreases, as does the friction force (Vigil et al., 1994). For hydrophobic silica surfaces under dry condi-
tions, the friction at load L = 5.5 mN was F = 75 mN. For the same sample, the adhesion energy hysteresis
was ∆γ = 10 mJ/m
2
, with a contact area of A ≈ 10
–8
m
2
at the same load. Assuming a value for the
characteristic distance σ on the order of one lattice spacing, σ ≈ 1 nm. By inserting these values into
Equation 9.33, the friction force is predicted to be F ≈ 100 mN for the kinetic friction force, which is
close to the measured value of 75 mN. Alternatively, we may conclude that the dissipation factor is ε =
0.75, i.e., that almost all the energy is dissipated as heat at each molecular collision.
9.11.4 External Load Contribution to Interfacial Friction
When there is no interfacial adhesion, S
c
is zero. Thus, in the absence of any adhesive forces between two
surfaces, the only “attractive” force that needs to be overcome for sliding to occur is the externally applied
load or pressure.
For a preliminary discussion of this question, it is instructive to compare the magnitudes of the
externally applied pressure to the internal van der Waals pressure between two smooth surfaces. The
internal van der Waals pressure is given by P = A/6πD
0
3
≈ 10
4
atm (using a typical Hamaker constant of
A = 10
–12
erg, and assuming D
0
≈ 2 Å for the equilibrium interatomic spacing). This implies that we
should not expect the externally applied load to affect the interfacial friction force F, as defined by
Equation 9.27, until the externally applied pressure L/A begins to exceed ~1000 atm. This is in agreement
with experimental data (Briscoe et al., 1977), where the effect of load became dominant at pressures in
excess of 1000 atm.
For a more general semiquantitative analysis, again consider the cobblestone model as used to derive
Equation 9.32 but now include an additional contribution to the surface energy change of Equation 9.31
due to the work done against the external load or pressure, L∆D = P
ext
A · ∆D (this is equivalent to the
work done against gravity in the case of a cart being pushed over cobblestones). Thus,
Friction force: F
A
r
RA
=
×
=
π
−
()
∆γ
σσ
γγ
2
Friction stress: SFA
c
==∆γσ
ε
γ
γ
≈
∆
.

© 1999 by CRC Press LLC
(9.36)
which gives the more general relation:
(9.37)
where P
ext
= L/A and where C
1
and C
2
are constants characteristic of the surfaces and sliding conditions.
The constant C
1
= (4γε · ∆D/D
0
· ∆d) depends on the mutual adhesion of the two surfaces, while both
C
1
and C
2
= ε · ∆D/∆d depend on the topography or atomic bumpiness of the surface groups
(Figure 9.21) — the smoother the surface groups the smaller the ratio ∆D/∆d and hence the lower the
value of C
2
. In addition, both C
1
and C
2
depend on ε — the fraction of energy dissipated per collision
which depends on the relative masses of the shearing molecules, the sliding velocity, the temperature,
and the characteristic molecular relaxation processes of the surfaces. This is by far the most difficult
parameter to compute, and yet it is the most important since it represents the energy transfer mechanism
in any friction process. Further, since ε can vary between 0 and 1, it determines whether a particular
friction force will be large or close to zero. Molecular simulations offer the best way to understand and
predict the magnitude of ε, but the complex multibody nature of the problem makes simple conclusions
difficult to draw. Some of the basic physics of the energy transfer and dissipation of the molecular
collisions can be drawn from simplified models (Urbakh et al., 1995; Rozman et al., 1996, 1997) such as
a one-dimensional three-body system (Israelachvili and Berman, 1995). This system, described in more
detail in Section 9.11.5, offers insight into the mechanisms of energy transfer and relates them back to
the familiar parameter De, the Deborah number.
Finally, the above equation may also be expressed in terms of the friction force F:
(9.38)
Equations similar to Equations 9.37 and 9.38 were previously derived by Derjaguin (1988, 1934) and
by Briscoe and Evans (1982), where the constant C
1
and C
2
were interpreted somewhat differently than
in this model.
In the absence of any attractive interfacial force, we have C
1
≈ 0, and the second term in Equations 9.37
and 9.38 should dominate. Such situations typically arise when surfaces repel each other across the
lubricating liquid film, for example, when two mica surfaces slide across a thin film of water (Figure 9.18).
In such cases the total frictional force should be low and it should increase linearly with the external load
according to
(9.39)
An example of such lubricated sliding occurs when two mica surfaces slide in water or in salt solution,
where the short-range hydration forces between the surfaces are repulsive. Thus, for sliding in 0.5
M KCl
it was found that C
2
= 0.015 (Berman et al., 1998a). Another case where repulsive surfaces eliminate the
adhesive contribution to friction is for tethered polymer chains attached to surfaces at one end and
swollen by a good solvent (Klein et al., 1994). For this class of systems C
2
< 0.001 for a finite range of
polymer layer compressions (normal loads, L). The low friction between the surfaces in this regime is
attributed to the entropic repulsion between the opposing brush layers with a minimum of entanglement
between the two layers. However, with higher normal loads, the brush layers become compressed and
begin to entangle, resulting in higher friction.
It is important to note that Equation 9.39 has exactly the same form as Amontons’ law:
S
F
A
D
Dd
PD
d
c
==
⋅
⋅
+
⋅4
0
γε ε∆
∆
∆
∆
ext
,
SFACCP
c
==+
1 2 ext
,
FSACACL
c
==+
12
.
FCL=
2
.

© 1999 by CRC Press LLC
(9.40)
where µ
is the coefficient of friction. When damage occurs, there is a rapid transition to normal sliding
in the presence of wear debris. As previously described, the mechanisms of interfacial friction and normal
friction are vastly different on the submicroscopic and molecular levels. However, under certain circum-
stances both may appear to follow a similar equation (see Equations 9.39 and 9.40) even though the
friction coefficients C
2
and µ are determined by quite different material properties in each case.
At the molecular level a thermodynamic analog of the coulomb or cobblestone models (see
Section 9.11.1) based on the contact value theorem (Israelachvili, 1991; Berman et al., 1998a; Berman
and Israelachvili, 1997) can explain why F ∝ L also holds at the microscopic or molecular level. In this
analysis we consider the surface molecular groups as being momentarily compressed and decompressed
as the surfaces move along. Under irreversible conditions, which always occur when a cycle is completed
in a finite amount of time, the energy lost in the compression/decompression cycle is dissipated as heat.
For two nonadhering surfaces, the stabilizing pressure P
i
acting locally between any two elemental contact
points i of the surfaces may be expressed by the contact value theorem (Israelachvili, 1992):
(9.41)
where ρ
i
= 1/V
i
is the local number density (per unit volume) or activity of the interacting entities, be
they molecules, atoms, ions, or the electron clouds of atoms. This equation is essentially the osmotic or
entropic pressure of a gas of confined molecules. As one surface moves across the other, as local regions
become compressed and decompressed by a volume ∆V
i
, the work done per cycle can be written as ε P
i
∆V
i
, where ε (ε ≤ 1) is the fraction of energy per cycle lost as heat, as defined earlier. The energy balance
shows that for each compression/decompression cycle, the dissipated energy is related to the friction
force by
(9.42)
where x
i
is the lateral distance moved per cycle, which can be the distance between asperities or the
distance between surface lattice sites. The pressure at each contact junction can be expressed in terms of
the local normal load L
i
and local area of contact A
i
as P
i
= L
i
/A
i
. The volume change over a cycle can
thus be expressed as ∆V
i
= A
i
z
i
, where z
i
is the vertical distance of confinement. Plugging these back into
Equation 9.42, we get
(9.43)
which is independent of the local contact area A
i
. The total friction force is thus
(9.44)
where it is assumed that on average, the local values of L
i
and P
i
are independent of the local “slope”
z
i
/x
i
. Therefore, the friction coefficient µ is a function only of the average surface topography and the
sliding velocity, but is independent of the local (real) or macroscopic (apparent) contact areas.
While this analysis explains nonadhering surfaces, there is still an additional explicit contact area
contribution for the case of adhering surfaces, as in Equation 9.38. The distinction between the two cases
arises because the initial assumption of the contact value theorem, Equation 9.41, is incomplete for
adhering systems. A more appropriate starting equation would reflect the full intermolecular interaction
potential, including the attractive interactions in addition to the purely repulsive contributions of
Equation 9.41, much as the van der Waals equation of state modifies the ideal gas law.
FL=µ ,
PkTkTV
iiB B i
==ρ ,
Fx P V
ii i i
=ε ∆ ,
FLzx
iiii
=
()
ε ,
FF Lzx zxLL
i iii ii i
==
()
==µΣΣ Σεε ,
© 1999 by CRC Press LLC
9.11.5 Simple Molecular Model of Energy Dissipation ε
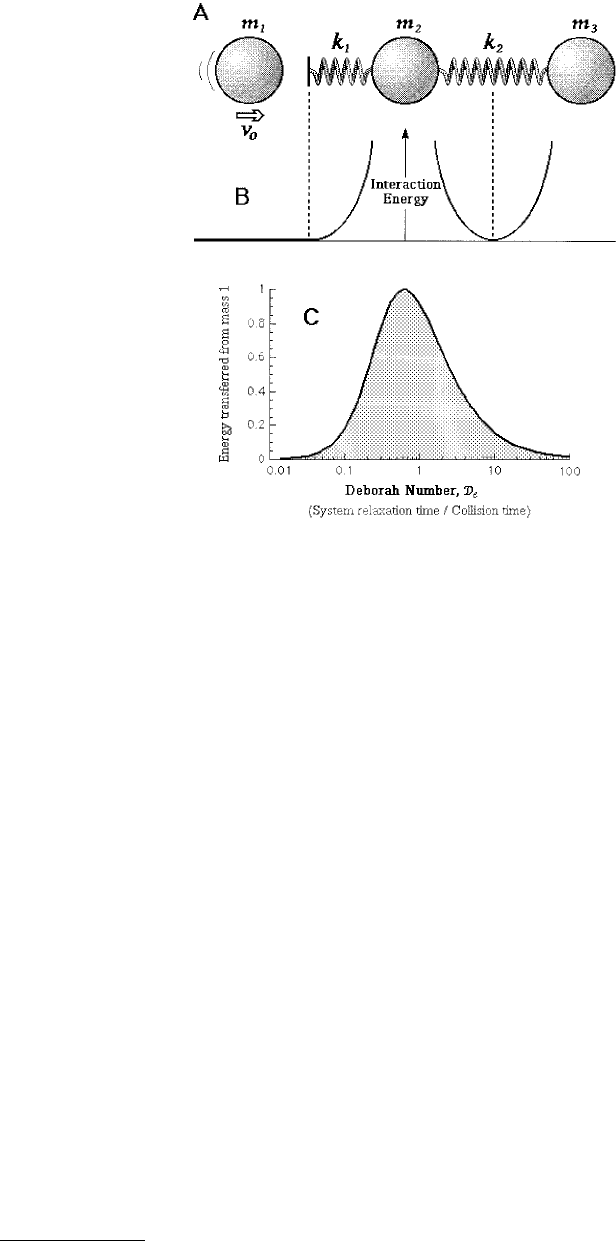
The simple three-body system of balls and springs illustrated in Figure 9.23A provides molecular insight
into dissipative processes such as sliding friction and adhesion hysteresis. Mass m
1
may be considered to
constitute one body or surface that approaches another at velocity v
0
. Masses m
2
and m
3
represent the
other surface. As shown in Figure 9.23B, the masses of the second surface are bound together via a
parabolic (Hookian spring) potential, while masses m
1
and m
2
interact via a nonadhesive repulsion
modeled as a half parabola. Although this system is simple in appearance, it is rich in its physics. Different
relative values of the masses and spring constants lead to very different collision outcomes where the
initial translational or kinetic energy of m
1
is distributed among the final kinetic, translational and
vibrational energies of m
1
and the m
2
–m
3
couple.
The collision is a molecular analogy, and perhaps a good microscopic representation of an adhesive
loading–unloading cycle (see Figure 9.11) or a frictional sliding process. The finer molecular model of
Figure 9.23A allows us to determine exactly how the energy is being dissipated in these cycles. In a
loading–unloading process, m
1
represents the surface molecules of one body approaching the surface
molecules of a second body (m
2
and m
3
). Solutions to the equations of motion show that in the collision
the first mass can be reflected, stopped, or even continue forward at a different velocity. In the case of
lateral sliding, the molecules of the two surfaces can still be considered to interact in this way; the surface
groups from the slider continually collide with those of the opposing surface, with the amount of the
energy transferred during the collisions defining the friction. Analysis of this problem shows that the
FIGURE 9.23 (A) Schematic diagram of a basic three-body collision. Mass 1 approaches the other masses with an
initial velocity, v
0
. The kinetic energy of mass 1 after the collision is compared to its initial kinetic energy. The
characteristic times of the m
1
–m
2
and m
2
–m
3
interactions are functions of the respective masses and the strengths of
the connecting springs k
1
and k
2
(B). The Deborah number for this system is the ratio of the collision time between
masses 1 and 2 to the harmonic oscillating frequency of masses 2 and 3. (C) The energy transferred from mass 1 to
the rest of the system (masses 2 and 3) is plotted as a function of the Deborah number. In this calculation k
1
= 1,
k
2
= 10, m
1
= 1, and masses m
2
= m
3
were varied.

© 1999 by CRC Press LLC
essential determinant of the amount of energy transferred from m
1
is based on the ratio of the collision
time to the characteristic relaxation time of the system, in other words, to the Deborah number:
(9.45)
Thus, it is found that m
1
loses most of its energy to the m
2
–m
3
couple when the collision time is close
to the characteristic vibration time of the m
2
–m
3
harmonic system, which corresponds to De = 1. When
the collision time is much larger or smaller than the system characteristic time, mass m
1
is found to
retain most of its original kinetic energy (Figure 9.23C). In this simple example, the interaction times
are functions only of the three masses, the intermolecular potential between m
1
and m
2
, and the potential
or spring constant of the m
2
–m
3
couple. In more complex systems with more realistic interaction
potentials (i.e., attractive interactions between m
1
and m
2
), the velocity v
0
becomes an important factor
as well, and also affects the Deborah number.
It is important to note that in this simple one-dimensional analysis, additional energy modes and
degrees of freedom of the molecules have not been considered. These modes, when present, will also be
involved in the interaction, affecting the energy transferred from m
1
and sharing in the final distribution
of the energy transferred. In addition, different types of energy modes (e.g., rotational modes) will
generally have different relaxation times, so that their energy peaks will occur at different measuring
times. Such real systems may be considered to have more than one Deborah number.
The three-body system sheds some light on the molecular mechanisms of energy dissipation and the
impact of the Deborah number on the dissipation parameter ε, but because of its simplicity, does not
offer predictive capabilities for real systems. More-sophisticated models have been presented by Urbakh
et al. (1995) and Rozman et al. (1996, 1997).
9.12 Friction and Lubrication of Thin Liquid Films
When a liquid is confined between two surfaces or within any narrow space whose dimensions are less
than five to ten molecular diameters, both the static (equilibrium) and dynamic properties of the liquid,
such as its compressibility and viscosity, can no longer be described even qualitatively in terms of the
bulk properties. The molecules confined within such molecularly thin films become ordered into layers
(“out-of-plane” ordering), and within each layer they can also have lateral order (“in-plane” ordering).
Such films may be thought of as behaving more like a liquid crystal or a solid than a liquid.
As described in Section 9.4, the measured normal forces between two solid surfaces across molecularly
thin films exhibit exponentially decaying oscillations, varying between attraction and repulsion with a
periodicity equal to some molecular dimension of the solvent molecules. Thus, most liquid films can
sustain a finite normal stress, and the adhesion force between two surfaces across such films is quantized,
depending on the thickness (or number of liquid layers) between the surfaces. The structuring of
molecules in thin films and the oscillatory forces it gives rise to are now reasonably well understood,
both experimentally and theoretically, at least for simple liquids.
Work has also recently been done on the dynamic e.g., viscous or shear, forces associated with
molecularly thin films. Both experiments (Israelachvili et al., 1988; Gee et al., 1990; Hirz et al., 1992;
Homola et al., 1993), and theory (Schoen et al., 1989; Thompson and Robbins, 1990; Thompson et al.,
1992) indicate that even when two surfaces are in steady-state sliding they still prefer to remain in one
of their stable potential energy minima, i.e., a sheared film of liquid can retain its basic layered structure.
Thus, even during motion the film does not become totally liquidlike. Indeed, if there is some in-plane
ordering within a film, it will exhibit a yield point before it begins to flow. Such films can therefore sustain
a finite shear stress, in addition to a finite normal stress. The value of the yield stress depends on the
number of layers comprising the film and represents another quantized property of molecularly thin films.
The dynamic properties of a liquid film undergoing shear are very complex. Depending on whether
the film is more liquidlike or solidlike, the motion will be smooth or of the stick-slip type. During sliding,
De Relaxation time Measuring time= .

© 1999 by CRC Press LLC
transitions can occur between n layers and (n – 1) or (n + 1) layers, and the details of the motion depend
critically on the externally applied load, the temperature, the sliding velocity, the twist angle between the
two surface lattices and the sliding direction relative to the lattices.
9.12.1 Smooth and Stick-Slip Sliding
Recent advances in friction-measuring techniques have enabled the interfacial friction of molecularly
thin films to be measured with great accuracy. Some of these advances have involved the SFA technique
(Israelachvili et al., 1988; Gee et al., 1990; Hirz et al., 1992; Homola et al., 1989, 1990, 1993), while others
have involved the AFM (McClelland, 1989; McClelland and Cohen, 1990). In addition, molecular dynam-
ics computer simulations (Schoen et al., 1989; Landman et al., 1990; Thompson and Robbins, 1990;
Robbins and Thompson, 1991) have become sufficiently sophisticated to enable fairly complex tribolog-
ical systems to be studied for the first time. All these advances are necessary if one is to probe such subtle
effects as smooth or stick-slip friction, transient and memory effects, and ultralow friction mechanisms
at the molecular level.
Figure 9.24 shows typical results for the friction traces measured as a function of time (after com-
mencement of sliding) between two molecularly smooth mica surfaces separated by three molecular
layers of the liquid OMCTS, and how the friction increases to higher values in a quantized way when
the number of layers falls from n = 3 to n = 2 and then to n = 1.
With the many added insights provided by recent computer simulations of such systems, a number
of distinct molecular processes have been identified during smooth and stick-slip sliding. These are shown
schematically in Figure 9.25 for the case of spherical liquid molecules between two solid crystalline
surfaces. The following regimes may be identified:
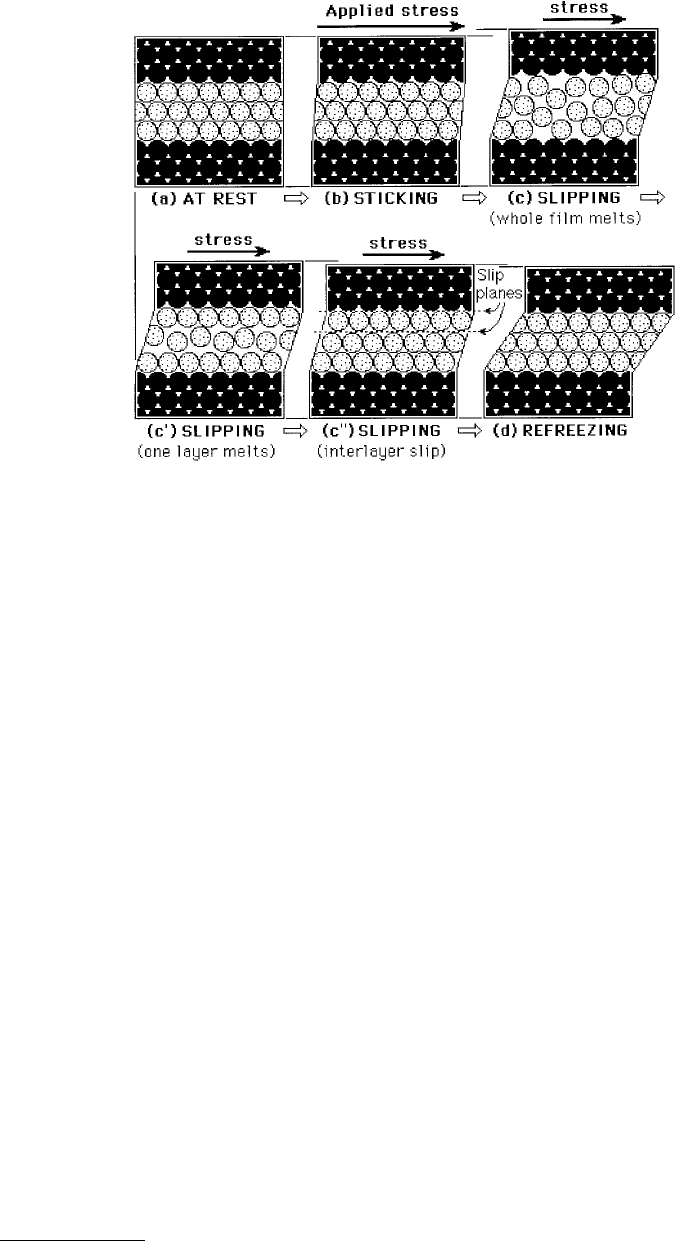
Surfaces at rest — Figure 9.25a: Even with no externally applied load, solvent–surface epitaxial inter-
actions can induce the liquid molecules in the film to solidify. Thus at rest the surfaces are stuck to each
other through the film.
Sticking regime (frozen, solidlike film) — Figure 9.25b: A progressively increasing lateral shear stress is
applied. The film, being solid, responds elastically with a small lateral displacement and a small increase
or “dilatency” in film thickness (less than a lattice spacing or molecular dimension, σ). In this regime
the film retains its frozen, solidlike state — all the strains are elastic and reversible, and the surfaces
remain effectively stuck to each other. However, slow creep may occur over long time periods.
FIGURE 9.24 Measured change in friction during interlayer transitions of the silicone liquid octamethylcyclotet-
rasiloxane (OMCTS, an inert liquid whose quasi-spherical molecules have a diameter of 8 Å) (Gee et al., 1990). In
this system, the shear stress S
c
= F/A was found to be constant so long as the number of layers n remained constant.
Qualitatively similar results have been obtained with other quasi-spherical molecules such as cyclohexane (Israelach-
vili et al., 1988). The shear stresses are only weakly dependent on the sliding velocity v. However, for sliding velocities
above some critical value v
c
, the stick-slip disappears and sliding proceeds smoothly in the purely kinetic value.
© 1999 by CRC Press LLC
Slipping and sliding regimes (molten, liquidlike film) — Figure 9.25c, c
′
, c
″
: When the applied shear
stress or force has reached a certain critical value F
s
, the static friction force, the film suddenly melts
(known as shear melting) or rearranges to allow for wall slip or film-slip to occur, at which point the
two surfaces begin to slip rapidly past each other. If the applied stress is kept at a high value, the upper
surface will continue to slide indefinitely.
Refreezing regime (resolidification of film) — Figure 9.25d: In many practical cases, the rapid slip of the
upper surface relieves some of the applied force, which eventually falls below another critical value F
k
,
the kinetic friction force, at which point the film resolidifies and the whole stick-slip cycle is repeated.
On the other hand, if the slip rate is smaller than the rate at which the external stress is applied, the
surfaces will continue to slide smoothly in the kinetic state and there will be no more stick-slip. (Figure
9.26).The critical velocity at which stick-slip disappears is discussed in more detail in Section 9.13.
Experiments with linear-chain (alkane) molecules show that the film thickness remains quantized
during sliding, so that the structure of such films is probably more like that of a nematic liquid crystal
where the liquid molecules have become shear aligned in some direction enabling shear motion to occur
while retaining some order within the film. Computer simulations for simple spherical molecules (Thomp-
son and Robbins, 1990)
further indicate that during the slip, the film thickness is roughly 15% higher than
at rest (i.e., the film density falls), and that the order parameter within the film drops from 0.85 to about
0.25. Both of these are consistent with a disorganized liquidlike state for the whole film during the slip,
as illustrated schematically in Figure 9.25c. At this stage, we can only speculate on other possible config-
urations of molecules in the slipping and sliding regimes. This probably depends on the shapes of the
molecules (e.g., whether spherical or linear or branched), on the atomic structure of the surfaces, on the
sliding velocity, etc. Figure 9.25c, c′, and c″ show three possible sliding modes wherein the shearing film
either totally melts or where the molecules retain their layered structure and where slip occurs between
two or more layers. Other sliding modes, for example, involving the movement of dislocations or discli-
nations are also possible, and it is unlikely that one single mechanism applies in all cases.
FIGURE 9.25 Idealized schematic illustration of molecular rearrangements occurring in a molecularly thin film of
spherical or simple chain molecules between two solid surfaces during shear. Note that, depending on the system, a
number of different molecular configurations within the film are possible during slipping and sliding, shown here
as stages (c) — total disorder as whole film melts, (c′) — partial disorder, and (c″) — order persists even during
sliding with slip occurring at a single slip plane either within the film or at the walls.
