Bhushan B. Handbook of Micro/Nano Tribology, Second Edition
Подождите немного. Документ загружается.

© 1999 by CRC Press LLC
9.12.2 Role of Molecular Shape and Liquid Structure
The above scenario is already quite complicated, and yet this is the situation for the simplest type of
experimental system. The factors that appear to determine the critical velocity v
c
depend on the type of
liquid between the surfaces (as well as on the surface lattice structure). Small spherical molecules such
as cyclohexane and OMCTS have been found to have very high v
c
, which indicates that these molecules
can rearrange relatively quickly in thin films. Chain molecules and especially branched chain molecules
have been found to have much lower v
c
, which is to be expected, and such liquids tend to slide smoothly
rather than in a stick-slip fashion (see Table 9.4). With highly asymmetric molecules, such as multiply
branched isoparaffins and polymer melts, no regular spikes or stick-slip behavior occurs at any speed
since these molecules can never order themselves sufficiently to “solidify.” Examples of such liquids are
perfluoropolyethers and polydimethylsiloxanes (PDMS).
Table 9.4 shows the trends observed with some organic and polymeric liquid between smooth mica
surfaces. Also listed are the bulk viscosities of the liquids. From the data of Table 9.4 it appears that there
is a direct correlation between the shapes of molecules and their coefficient of friction or effectiveness
as lubricants (at least at low shear rates). Small spherical or chain molecules have high friction with stick-
slip because they can pack into ordered solidlike layers. In contrast, longer-chained and irregularly shaped
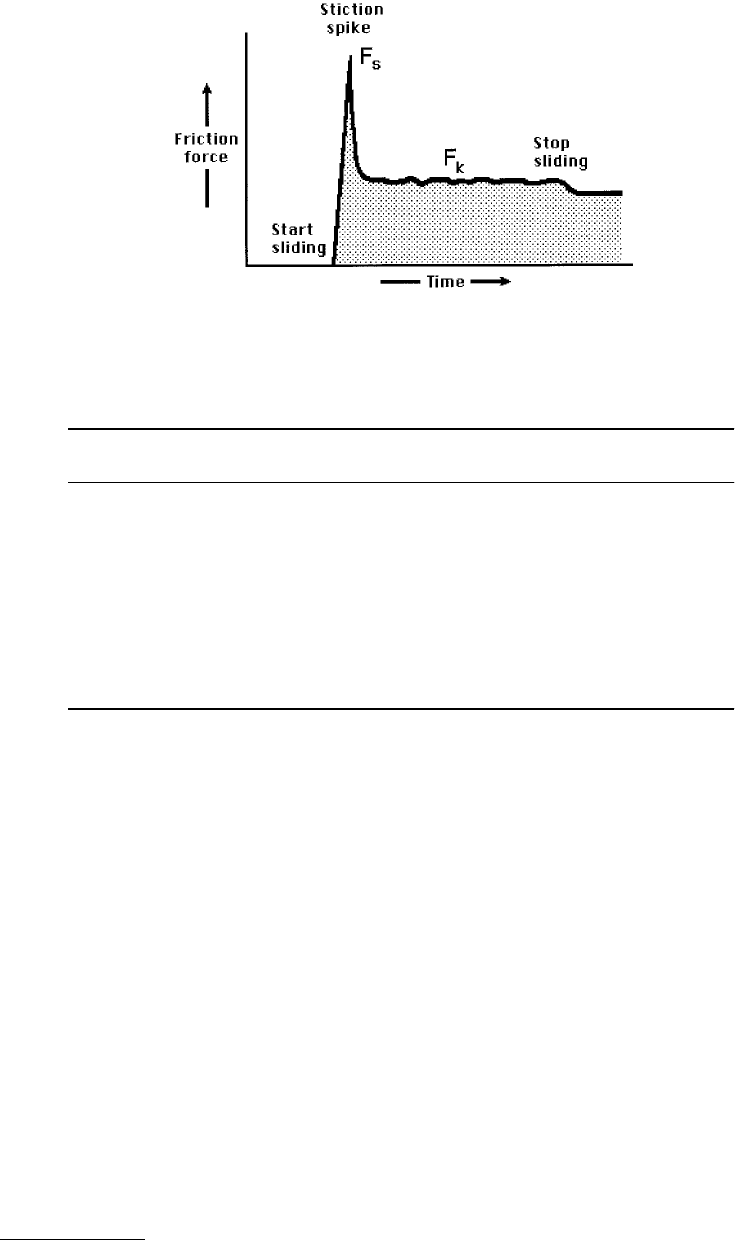
FIGURE 9.26 Stiction is the high starting frictional force F
s
experienced by two moving surfaces which causes them
to jerk forward rather than accelerate smoothly from rest. It is a major cause of surface damage and wear. The figure
shows a typical “stiction spike” or “starting spike,” followed by smooth sliding in the kinetic state.
TABLE 9.4 Effect of Molecular Shape and Short-Range Forces on Tribological Properties
a
Short-Range Type of Friction Bulk Liquid
Liquid (Dry) Force Friction Coefficient Viscosity (cP)
Organic
Cyclohexane Oscillatory Quantized stick-slip »1 0.6
Octane Oscillatory Quantized stick-slip 1.5 0.5
Tetradecane Oscil↔smooth Stick-slip↔smooth 1.0 2.3
Octadecane (branched) Oscil↔smooth Stick-slip↔smooth 0.3 5.5
PDMS (M = 3700, melt) Oscil↔smooth Smooth 0.4 50
PBD (M = 3500, branched) Smooth Smooth 0.03 800.0
Wat er
Water (KCl solution) Smooth Smooth 0.01–0.03 1.0
a
For molecularly thin liquid films between two shearing mica surfaces at 20°C.
Note:
PDMS: Polydimethylsiloxane, PBD: Polybutadiene.

© 1999 by CRC Press LLC
molecules remain in an entangled, disordered, fluidlike state even in very thin films and these give low
friction and smoother sliding. It is probably for this reason that irregularly shaped branched chain
molecules are usually better lubricants. It is interesting to note that the friction coefficient generally
decreases as the bulk viscosity of the liquids increases. This unexpected trends occurs because the factors
that are conducive to low friction are generally conducive to high viscosity. Thus, molecules with side
groups such as branched alkanes and polymer melts usually have higher bulk viscosities than their linear
homologues for obvious reasons. However, in thin films the linear molecules have higher shear stresses
because of their ability to become ordered. The only exception to the above correlations is water, which
has been found to exhibit both low viscosity and low friction (see Figure 9.19). In addition, the presence
of water can drastically lower the friction and eliminate the stick-slip of hydrocarbon liquids when the
sliding surfaces are hydrophilic.
If an effective viscosity η
eff
were to be calculated for the liquids of Table 9.1, the values would be many
orders of magnitude higher than those of the bulk liquids. This can be demonstrated by the following
simple calculation based on the usual equation for Couette flow (see Equation 9.24):
(9.46)
where F
k
is the kinetic friction force, D is the film thickness, A the contact area, and v the sliding velocity.
By using typical values for experiments with hexadecane (Yoshizawa and Israelachvili, 1993) — F
k
=
5 mN, D = 1 nm, A = 3 × 10
–9
m
2
and v = 1 µm/s — yields η
eff
≈ 2000 N m
–2
s, or 20,000 P, which is
~10
6
times higher than the bulk viscosity η
bulk
of the liquid. It is instructive to consider that this very
high effective viscosity nevertheless still produces a low friction force or friction coefficient µ of about
0.25. It is interesting to speculate that if a 1-nm film were to exhibit bulk viscous behavior, the friction
coefficient under the same sliding conditions would be as low as 0.000001. While such a low value has
never been reported for any tribological system, one may consider it as a theoretical lower limit that
could, conceivably, be attained under certain experimental conditions.
Various studies (Van Alsten and Granick, 1990a,b, 1991; Granick, 1991; Hu and Granick, 1992) have
shown that confinement and load generally increase the effective viscosity and/or relaxation times of
molecules, suggestive of an increased glassiness or solidlike behavior. This is in marked contrast to studies
of liquids in small confining capillaries where the opposite effects have been observed (Warnock et al.,
1986; Awschalom and Warnock, 1987). The reason for this is probably because the two modes of
confinement are different. In the former case (confinement of molecules between two structured solid
surfaces) there is generally little opposition to any lateral or vertical displacement of the two surface
lattices relative to each other. This means that the two lattices can shift in the x-y-z plane (Figure 9.27A)
to accommodate the trapped molecules in the most crystallographically commensurate or “epitaxial”
way, which would favor an ordered, solidlike state. In contrast, the walls of capillaries are rigid and cannot
easily move or adjust to accommodate the confined molecules (Figure 9.27B), which will therefore be
forced into a more disordered, liquidlike state (unless the capillary wall geometry and lattice is exactly
commensurate with the liquid molecules, as occurs in certain zeolites).
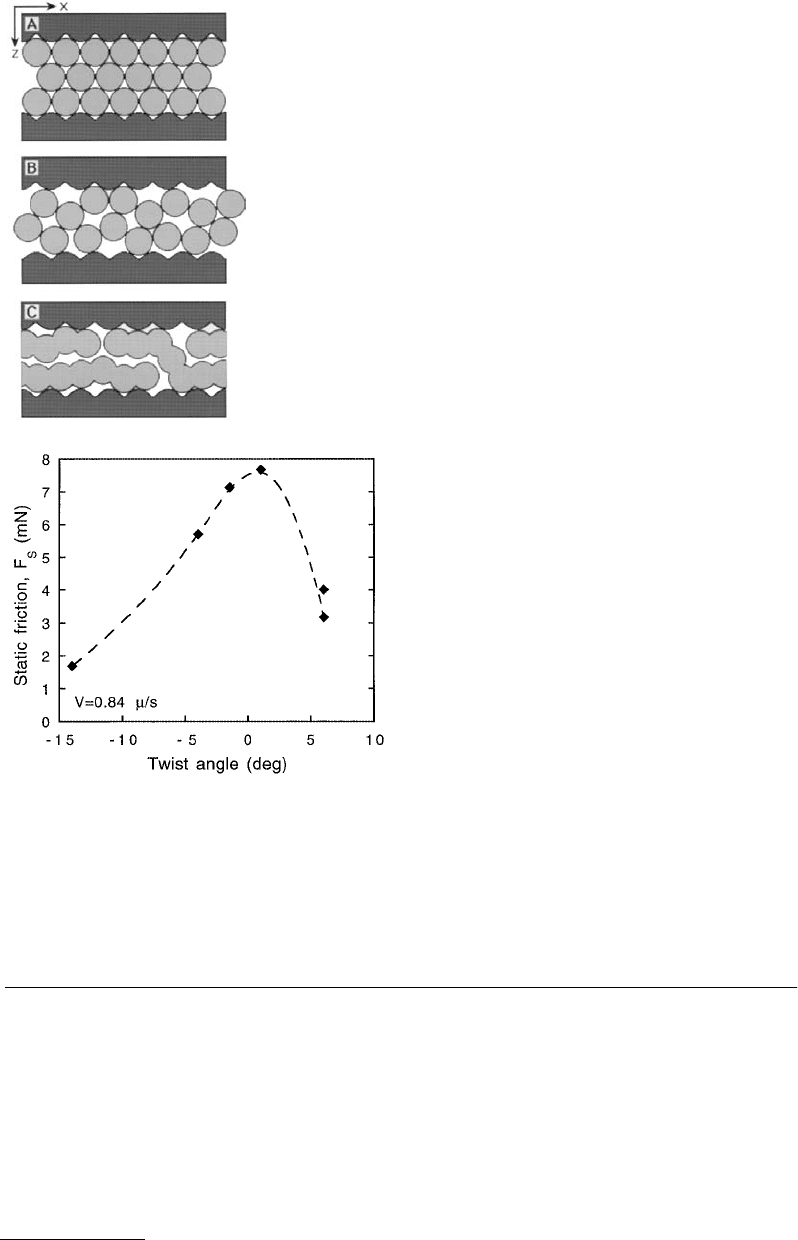
Experiments have demonstrated the effects of surface lattice mismatch on the friction between surfaces
(Hirano et al., 1991; Berman, 1996). Similar to the effects of lattice mismatch on adhesion (Figure 9.5,
Section 9.4.1), the static friction of a confined liquid film is maximum when the lattices of the confining
surfaces are aligned. For OMCTS confined between mica surfaces (Berman, 1996) the static friction was
found to vary by more than a factor of 4 (Figure 9.28), while for bare mica surfaces the variation was by
a factor of 3.5 (Hirano et al., 1991). In contrast to the sharp variations in adhesion energy over small
twist angles, the variations in friction as a function of twist angle were much more broad — both in
magnitude and angular spread. Similar variations in friction as a function of twist or misfit angles have
also been observed in computer simulations (Gyalog and Thomas, 1997).
With rough surfaces, i.e., those that have random protrusions rather than being periodically structured,
we expect a smearing out of the correlated intermolecular interactions that are involved in film freezing
and melting (and in phase transitions in general). This should effectively eliminate the highly regular
η
eff
= FD Av
k
,
© 1999 by CRC Press LLC
stick-slip and may also affect the location of the slipping planes. The stick-slip friction of “real” surfaces,
which are generally rough, may therefore be quite different from those of perfectly smooth surfaces
composed of the same material (see next section). We should note, however, that even between rough
surfaces, most of the contacts occur between the tips of microscopic asperities, which may be smooth
over their microscopic contact area.
9.13 Stick-Slip Friction
An understanding of stick-slip is of great practical importance in tribology (Rabinowicz, 1965) since
these spikes are the major cause of damage and wear of moving parts. But stick-slip motion is a much
more common phenomenon and is also the cause of sound generation (the sound of a violin string, a
squeaking door, or the chatter of machinery), sensory perception (taste, texture, and feel), earthquakes,
granular flow, nonuniform fluid flow, such as the “spurting” flow of polymeric liquids, etc. In the previous
section the stick-slip motion arising from freezing–melting transitions in thin interfacial films was
described. But there are other mechanisms that can give rise to stick-slip friction, which will now be
considered. However, before proceeding with this, it is important to clarify exactly what one is measuring
during a friction experiment.
FIGURE 9.27 Schematic view of interfacial film composed of spherical
molecules under a compressive pressure between two solid crystalline sur-
faces. If the two surface lattices are free to move in the XYZ directions so as
to attain the lowest energy state, they could equilibrate at values of X, Y, and
Z which induce the trapped molecules to become “epitaxially” ordered into
a solidlike film. (B) Similar view of trapped molecules between two solid
surfaces that are not free to adjust their positions, for example, as occurs in
capillary pores or in brittle cracks. (C) Similar to (A) but with chain mole-
cules replacing the spherical molecules in the gap. These may not be able to
order as easily as do spherical molecules even if X, Y, and Z can adjust,
resulting in a situation that is more akin to (B).
FIGURE 9.28 Static friction of a 2-nm-thick OMCTS
film as a function of the lattice twist angle between the
two confining crystalline mica surfaces. The variation
in friction is comparable to variations in an unlubri-
cated mica–mica system (Hirano et al., 1991).
© 1999 by CRC Press LLC
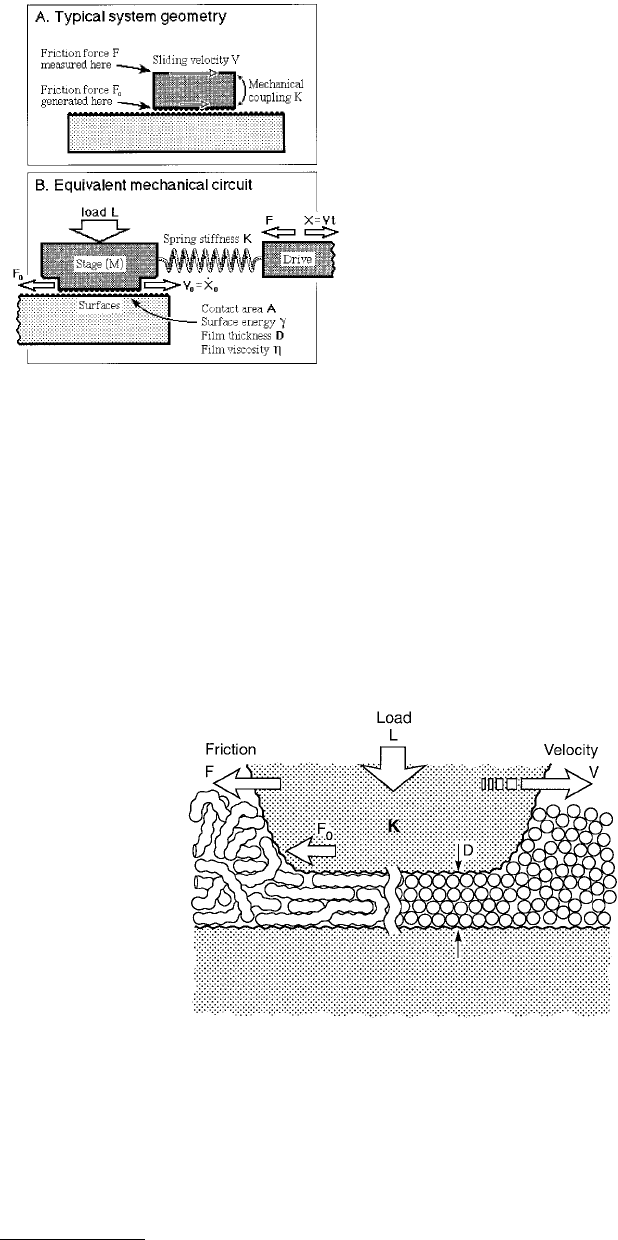
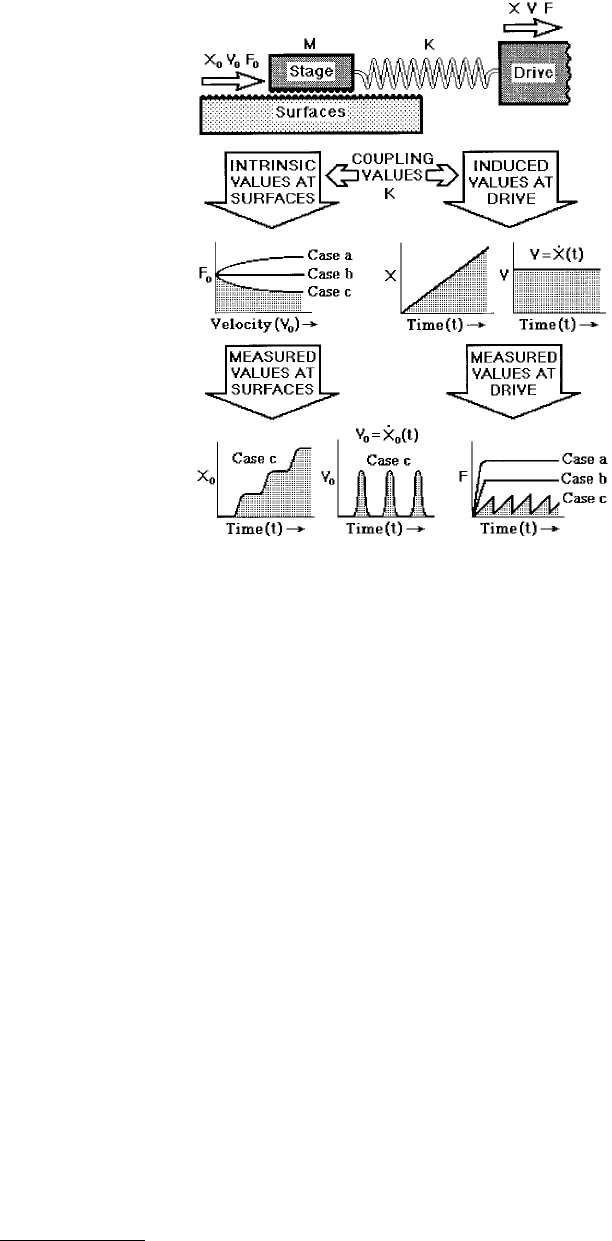
Figure 9.29 shows the basic mechanical coupling and equivalent mechanical circuit characteristic of
most tribological systems and experiments. The distinction between F and F
0
is important because in
almost all practical cases, the applied, measured, or detected force, F, is not the same as the “real” or
“intrinsic” friction force, F
0
, generated at the surfaces. F and F
0
are coupled in a way that depends on
the mechanical construction of the system, for example, the axle of a car wheel which connects it to the
engine. In Figure 9.29A, this coupling is shown to act via the material supporting the upper surface,
which can be modeled (Figure 9.29B) as an elastic spring of stiffness K and mass M. This is the simplest
type of mechanical coupling and is also the same as in SFA and AFM experiments, illustrated in
Figure 9.30. More-complicated real systems can be reduced to a system of springs and dashpots as
described by Luengo et al. (1997).
We now consider four different models of stick-slip friction. These are illustrated in Figures 9.31
through 9.34, where the mechanical couplings are assumed to be of the simple elastic spring type as
FIGURE 9.29 (A) Schematic geometry of two shearing sur-
faces illustrating how the friction force, F
0
, which is generated
at the surfaces, is generally measured as F at some other place.
The mechanical coupling between the two may be described
in terms of a simple elastic stiffness or compliance, K, or in
terms of more complex nonelastic coefficients, depending on
the system. Here, the mechanical coupling is simply via the
backing material supporting one of the surfaces. (B) Equiva-
lent mechanical circuit for the above setup applicable to most
tribological systems. Note that F
0
is the force generated at the
surfaces, but that the measured or detected force is F = (X –
X
o
)K. The differences between the forces, the velocities and
the displacements at the surfaces and at the drive (or detector)
are illustrated graphically in Figures 9.31 through 9.34.
FIGURE 9.30 (A) Schematic geometry of two contacting asperities separated by a thin liquid film. This is also the
geometry adopted in most pin-on-disk, SFA, and AFM experiments. In the SFA experiments described here, typical
experimental values were: undeformed radius of surfaces, R ≈ 1 cm; radius of contact area, r = 10 to 40 µm; film
thickness, D ≈ 10Å; externally applied load, L = –10 to +100 mN; measured friction forces, F = 0.001 to 100 mN;
sliding or driving velocity, V = 0.001 to 100 µm/s; surface or interfacial energy, γ = 0 to 30 mJ/m
2
(erg/cm
2
); effective
elastic constant of supporting material, K = 10
8
N/m; elastic constant of friction-measuring spring, K = 500 N/m;
temperature, T = 15 to 40°C.

© 1999 by CRC Press LLC
shown in Figures 9.29 and 9.30. The first two mechanisms (Figures 9.31 and 9.33) may be considered as
the “traditional” or “classical” mechanisms or models (Rabinowicz, 1965), the third (Figure 9.34) is
essentially the same as the freezing–melting phase-transition model described in Section 9.12.
9.13.1 Rough Surfaces Model
Rapid slips can occur whenever an asperity on one surface goes over the top of an asperity on the other
surface. As shown in Figure 9.31, the extent of the “slip” will depend on asperity heights and slopes, on
the speed of sliding, and on the elastic compliance of the surfaces and the moving stage. We may note
that, as in all cases of stick-slip motion, the driving velocity (V) may be constant but the resulting motion
at the surfaces (V
0
) will display large slips as shown in the inset. This type of stick-slip has been described
by Rabinowicz (1965). It will not be of much concern here since it is essentially a noise-type fluctuation,
resulting from surface imperfections rather than from the intrinsic interaction between two surfaces.
Actually, at the atomic level, the regular atomic-scale corrugations of surfaces can lead to periodic stick-
slip motion of the type shown here. This is what is sometimes measured by AFM tips (McClelland, 1989;
McClelland and Cohen, 1990).
9.13.2 Distance-Dependent Model
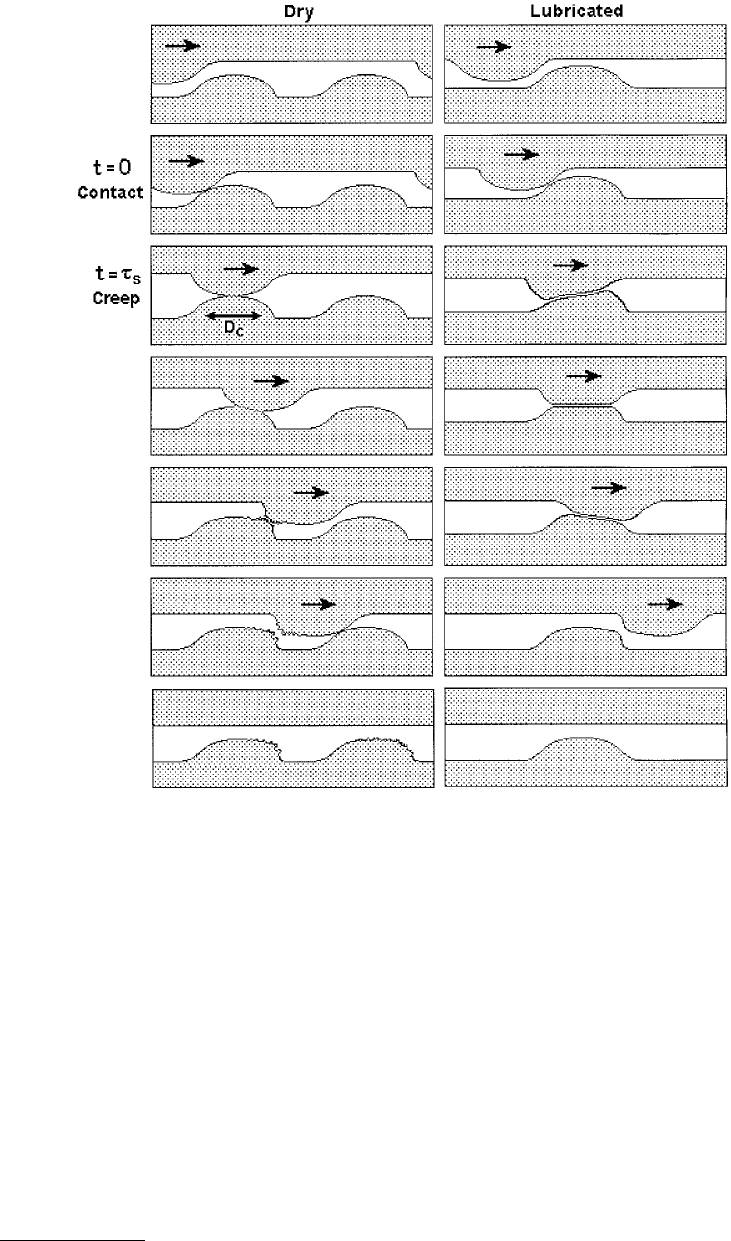
Another theory of stick-slip, observed in solid-on-solid sliding, is one that involves a characteristic distance
(but also a characteristic time, τ
s
, this being the characteristic time required for two asperities to increase
their adhesion strength after coming into contact). Originally proposed by Rabinowicz (1958, 1965), this
model suggests that two rough macroscopic surfaces adhere through their microscopic asperities of
characteristic length D
c
. During shearing, each surface must first creep a distance D
c
— the size of the
contacting junctions — after which the surfaces continue to slide, but with a lower (kinetic) friction force
than the original (static) value. The reason for the decrease in the friction force is that even though, on
average, new asperity junctions should form as rapidly as the old ones break, the time-dependent adhesion
and friction of the new ones will be lower than the old ones. This is illustrated in Figure 9.32.
The friction force therefore remains high during the creep stage of the slip, but once the surfaces have
moved the characteristic distance D
c
, the friction rapidly drops to the kinetic value. For any system where
the kinetic friction is less than the static force (or one that has a negative slope over some part of its
F
0
–V
0
curve) will exhibit regular stick-slip sliding motion for certain values of K, m, and driving velocity, V.
This type of friction has been observed in a variety of dry (unlubricated) systems such as paper-on-
paper (Baumberger et al., 1994; Heslot et al., 1994) and steel-on-steel (Sampson et al., 1943; Heymann
et al., 1954; Rabinowicz, 1958). This model is also used extensively in geologic systems to analyze rock-
on-rock sliding (Dieterich, 1978, 1979).
FIGURE 9.31 Figures 9.31 through 9.34 show three different models of friction and the stick-slip friction force vs.
time traces they give rise to. Figure 9.31 shows the Rabinowicz model (Rabinowicz, 1965) for rough surfaces which
produces irregular stick-slip (inset) when the elastic stiffness of the system (reflected by the slopes of the SLIP lines)
is not too high.
© 1999 by CRC Press LLC
While originally described for adhering macroscopic asperity junctions, the distance-dependent model
may also apply to molecularly smooth surfaces. For example, for polymer lubricant films, the character-
istic length D
c
would now be the chain–chain entanglement length, which could be much larger in a
confined geometry than in the bulk.
9.13.3 Velocity-Dependent Friction Model
This is the most-studied mechanism of stick-slip and, until recently, was considered to be the only cause
of intrinsic stick-slip. If a friction force decreases with increasing sliding velocity, as occurs with boundary
films exhibiting shear thinning, the force (F
s
) needed to initiate motion will be higher than the force (F
k
)
needed to maintain motion. Such a situation is depicted in Figure 9.33 (Case c), where a decreasing
intrinsic friction force F
0
with sliding velocity V
0
results in the sliding surface or stage moving in a periodic
fashion where during each cycle rapid acceleration is followed by rapid deceleration (see curves for X
0
FIGURE 9.32 Distance-dependent friction model (also known as the creep model) in which a characteristic distance
D
c
has to be moved to break adhesive junctions. The model also has a characteristic, τ
s
, this being the time needed
for the adhesion and friction forces per junction to equilibrate after each contact is made.
© 1999 by CRC Press LLC
and V
0
in Figure 9.33). So long as the drive continues to move at a fixed velocity V, the surfaces will
continue to move in a periodic fashion punctuated by abrupt stops and starts whose frequency and
amplitude depend not only on the function F
0
(V
0
) but also on the stiffness K and mass M of the moving
stage, and on the starting conditions at t = 0.
More precisely, referring to Figures 9.29 and 9.33, the motion of the sliding surface or stage can be
determined by solving the following differential equation:
(9.47)
or
(9.48)
where F
0
= F
0
(X
0
, V
0
, t) is the intrinsic or real friction force at the shearing surfaces which is generally a
function of X
0
, V
0
=
·
X
0
and t, F is the force on the spring (the externally applied or measured force), and
F
s
= (F
0
– F) is the force on the stage. To solve fully Equation 9.47, one must also know the initial (starting)
conditions at t = 0, and the driving or steady-state conditions at finite t. For example, in the present
experiments, the driving condition is
FIGURE 9.33 The lower part of the figure shows the stage or surface displacement X
0
, surface velocity V
0
( = dX
0
/dt
or X
0
), and measured friction force F, as functions of time t for surfaces whose intrinsic friction force is F
0
. In general,
F
0
is a function of X
0
, V
0
, and t. Three specific examples are shown here corresponding to F
0
either increasing
monotonically (Case a), remaining constant (Case b), or decreasing monotonically (Case c) with V
0.
The latter case
corresponds to a film that exhibits “shear thinning.” Only when F
0
(V
0
) has a negative slope is the resulting motion
of the stick-slip type, characterized by very different motions and friction forces being detected at the surfaces (the
Stage) and the detector (the Drive).
MX F F F X X K
˙˙
00 0 0
=−
()
=− −
()
MX X X K F
˙˙
,
00 0
0+−
()
−=

(9.49)
In other systems, the appropriate driving condition may be F = constant.
Various forms for F
0
= F
0
(X
0
,V
0
,t) have been proposed, mainly phenomenological, to explain various
kinds of stick-slip phenomena. These models generally assume a particular functional form for the friction
as a function of velocity only, F
0
= F
0
(V
0
), and they may also contain a number of mechanically coupled
elements comprising the stage (Tomlinson, 1929; Carlson and Langer, 1989).
One version is a two-state
model characterized by two friction forces, F
s
and F
k
, which is a simplified version of the phase-transitions
model (next section). More complicated versions can have a rich F–v spectrum, as proposed by Persson
(1994). Unless the experimental data is very detailed and extensive, these models cannot generally
distinguish between different types of mechanisms. Neither do they address the basic question of the
origin of the friction force, since this is assumed to begin with.
Experimental data have been used to calculate the friction force as a function of velocity within an
individual stick-slip cycle (Nasuno et al., 1997). For a macroscopic granular material confined between
solid surfaces, the data show a velocity-weakening friction force during the first half of the slip. However,
the data also show a hysteresis loop in the friction–velocity plot, with a different behavior in the
deceleration half of the slip phase. Similar results were observed for a 1 to 2 nm liquid lubricant film
between mica surfaces (Berman, Carlson and Ducker, unpublished results). These results indicate that a
purely velocity-dependent friction law is insufficient to describe such systems, and an additional element
such as the state of the confined material must be considered (next section).
9.13.3 Phase Transitions Model
Recent molecular dynamics computer simulations have found that thin interfacial films undergo first-
order phase transitions between solidlike and liquidlike states during sliding (Thompson and Robbins,
1990; Robbins and Thompson, 1991) and have suggested this is responsible for the observed stick-slip
behavior of simple isotropic liquids between two solid crystalline surfaces. With this interpretation, stick-
slip is seen to arise because of the abrupt change in the flow properties of a film at a transition (Israelachvili
et al., 1990; Thompson et al., 1992) rather than the gradual or continuous change as occurs in the previous
example. Such simulations have accounted for many of the observed properties of shearing liquids in
ultrathin films between molecularly smooth surfaces, and have so far offered the most likely explanation
for experimental data on stick-slip friction, such as shown in Figure 9.36.
A novel interpretation of the well-known phenomenon of decreasing coefficient of friction with
increasing sliding velocity has been proposed by Thompson and Robbins (1990) based on their computer
simulation. This postulates that it is not the friction that changes with sliding speed v, but rather the
time various parts of the system spend in the sticking and sliding modes. In other words, at any instant
during sliding, the friction at any local region is always F
s
or F
k
, corresponding to the static or kinetic
values. The measured frictional force, however, is the sum of all these discrete values averaged over the
whole contact area. Since as v increases, each local region spends more time in the sliding regime (F
k
)
and less in the sticking regime (F
s
) the overall friction coefficient falls. One may note that this interpre-
tation reverses the traditional way that stick-slip has been explained, for rather than invoking a decreasing
friction with velocity to explain stick-slip, it is now the more fundamental stick-slip phenomenon that
is producing the apparent decrease in the friction force with increasing sliding velocity. This approach
has been studied analytically by Carlson and Batista (1996), with a comprehensive rate- and state-
dependent friction force law. This model includes an analytic description of the freezing–melting tran-
sitions of a film, resulting in a friction force that is a function of sliding velocity in a natural way. This
model predicts a full range of stick-slip behavior observed experimentally.
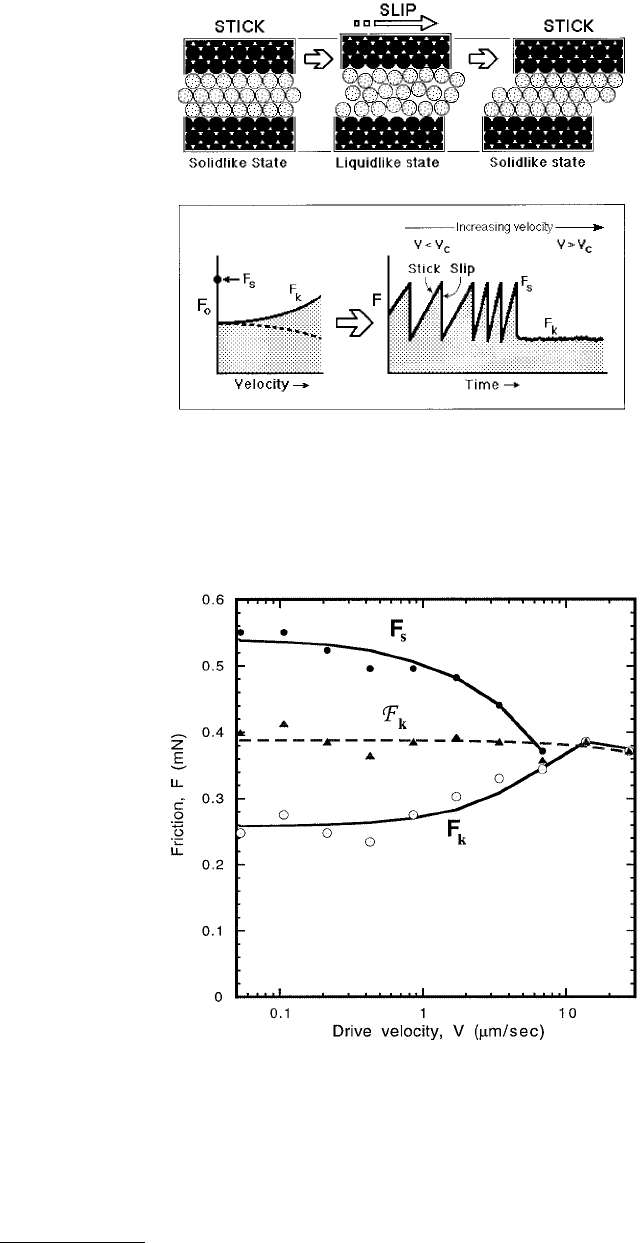
An example of the rate- and state-dependent model is observed when shearing thin films of OMCTS
between mica surfaces (Berman et al., 1996a,b). In this case the static friction between the surfaces is
dependent on the time that the surfaces are at rest with respect to each other, while the intrinsic kinetic
friction F
k0
is relatively constant over the range of velocities (Figure 9.35). At slow driving velocities, the
Xt
XVt t V
=<
=> =
00
0
for
for where constant.
,
,
© 1999 by CRC Press LLC
© 1999 by CRC Press LLC
FIGURE 9.34 Phase-transition model of stick-slip, for example, where a thin liquid film alternately freezes and
melts as it shears, shown here for 22 spherical molecules confined between two solid crystalline surfaces. This model
differs from that of Figure 9.33 in that the intrinsic friction force F
0
is here assumed to change abruptly (at the
transitions) rather than smoothly or continuously. The resulting stick-slip is also different, for example, the peaks
are sharper and the stick-slip disappears above some critical velocity, V
c
. Note that while the slip displacement is here
shown to be only two lattice spacings; in most practical situations it is much larger.
FIGURE 9.35 Measured friction F
s
and F
k
for increasing drive velocity V with OMCTS confined between two mica
surfaces. F
s
(solid circles) decreases with increasing velocity because at higher drive speeds the sticking time is shorter,
resulting in less complete freezing of the lubricant layer. The observed F
k
(open circles) increases as F
s
decreases,
resulting in a nearly constant intrinsic friction force F
k0
(triangles) ≈ (F
s
+ F
k
)/2 for these underdamped conditions.
The critical velocity is reached when F
s
decreases to the intrinsic friction F
k0
.

© 1999 by CRC Press LLC
system responds with stick-slip sliding with the surfaces reaching maximum static friction before each
slip event, and the amplitude of the stick-slip, F
s
– F
k
, is relatively constant. As the driving velocity
increases, the static friction decreases as the time at relative rest becomes shorter with respect to the
characteristic time of the lubricant film. As the static friction decreases with increasing drive velocity, it
eventually equals the intrinsic kinetic friction F
k0
, which defines the critical velocity V
c
, above which the
surfaces slide smoothly, without the jerky stick-slip motion.
The above classifications of stick-slip are not exclusive, and molecular mechanisms of real systems may
exhibit aspects of different models simultaneously. They do, however, provide a convenient classification
of existing models and indicate which experimental parameters should be varied to test the different
models.
9.13.4 Critical Velocity for Stick-Slip
For any given set of conditions, stick-slip disappears above some critical sliding velocity V
c
, above which
the motion continues smoothly in the liquidlike or kinetic state. The critical velocity is found to be well
described by two simple equations. Both are based on the phase-transition model, and both include some
parameter associated with the inertia of the measuring instrument. The first equation is based on both
experiments and simple theoretical modeling (Yoshizawa and Israelachvili, 1993):
(9.50)
where τ
ο
is the characteristic nucleation time or freezing time of the film. For example, inserting the
following typically measured values for a ~ 10 Å thick hexadecane film between mica: (F
s
– F
k
) ≈ 5 mN,
spring constant K ≈ 500 N/m, and nucleation time (Yoshizawa and Israelachvili, 1993) τ
ο
≈ 5 s, we obtain
V
c
≈ 0.4 µm/s, which is close to typically measured values (Figure 9.36). The second equation is based
on computer simulations (Robbins and Thompson, 1991):
(9.51)
where σ is a molecular dimension and M is the mass of the stage. Again, inserting typical experimental
values into this equation, specifically, M ≈ 20 gm, σ ≈ 0.5 nm, and (F
s
– F
k
) ≈ 5 mN as before, we obtain
V
c
≈ 0.3 ∝µm/s, which is also close to measured values.
Stick-slip also disappears above some critical temperature T
c
, which is not the same as the melting
temperature of the bulk fluid. Certain correlations have been found between V
c
and T
c
, and between
various other tribological parameters, that appear to be consistent with the principle of “time-temperature
superposition,” similar to that occurring in viscoelastic polymer fluids (Ferry, 1980).
We end by consid-
ering these correlations.
9.13.5 Dynamic Phase Diagram Representation
of Tribological Parameters
Both friction and adhesion hysteresis vary nonlinearly with temperature, often peaking at some particular
temperature, T
0
. The temperature dependence of these forces can therefore be represented on a dynamic
phase diagram such as shown in Figure 9.37. Experiments have shown that T
0
, and the whole bell-shaped
curve, are shifted along the temperature axis (as well as in the vertical direction) in a systematic way
when the load, sliding velocity, etc. are varied. These shifts also appear to be highly correlated with one
another, for example, an increase in temperature producing effects that are similar to decreasing the
sliding speed or load.
V
FF
K
c
sk
≈
−
()
5
0
τ
,
V
F
M
c
≈
⋅
01.,
∆σ
