Biermann Ch. Handbook of Pulping and Papermaking
Подождите немного. Документ загружается.

424 21. COLLOro AND SURFACE CHEMISTRY
Most colloids have charged surfaces from
anionic or cationic functional groups, which play
central roles in their behaviors. Vegetable fibers
have negative charges on their surfaces due to the
carboxylic acid groups of hemicellulose and
cellulose. Mineral fillers and many polymers used
in colloidal systems also have charges. These
charges may be positive from cationic groups
(from polymers with amine groups or metal
cations) or negative (from phosphate groups in
potato starch, carboxylate groups in many anionic
polymers, sulfate groups, and carbonate groups, to
name several examples). The amount and type of
surface charges are critical to the stability and
solubility properties of colloids. The type and
amount of electrolytes (especially the amount and
valence of metallic cations), pH, and temperature
play critical roles in the solution properties of
charged colloids. These factors will be considered
in detail presently.
Microscopy of
colloids
The resolution of optical microscopy is
limited by the wavelength of light. The resolution
is about 0.15 iim under the best of conditions and
perhaps double this for routine work (about 500 to
1000 magnification since the unaided human eye
can distinguish objects about 0.1 mm apart). A
technique known as dark field microscopy can be
used to detect small particles, but with a low level
of detail. In this technique, light is directed per-
pendicular to the direction of viewing. With no
particles present, the light does not reach the
objective. Lyophobic (insoluble and of different
index of refraction as the solution) particles (0.02
/xm and larger) can scatter enough light that some
of it will be visible. Although detail is lacking,
the motion of colloids can be followed to study
flocculation, sedimentation, and electrophoretic
mobility. Zsigmondy used dark field microscopy
extensively in his classic work on colloidal gold
for which he received the 1925 Nobel Prize.
Light scattering is an important property of col-
loids and will be considered in detail. Scanning
electron microscopy (SEM) can be applied to
colloid particles but not colloid solutions.
Ionic interactions and
(external)
electric fields
There are four electrokinetic phenomena of
charged surfaces in solution; the first is much
more important than the others. Electrophoresis is
the movement of charged surfaces (with associated
ions and water) in the stationary liquid induced by
an external field. Sedimentation potential is the
charged field generated by charged particles
moving in a stationary liquid. Streaming potential
is the generation of an electric field by movement
of the liquid along stationary charged surfaces.
Finally,
electroosmosis
is the movement of liquid
relative to stationary charged particles induced by
an external electric field.
The net charge on particles in aqueous solu-
tions can be measured by the process of electro-
phoresis, which is the basis of some wet end
chemistry sensors. When an electric field is
applied to two metal plates across a solution of
charged particles, the particles will move in re-
sponse to the force set up by electrostatic attrac-
tion (between oppositely charged objects) or repul-
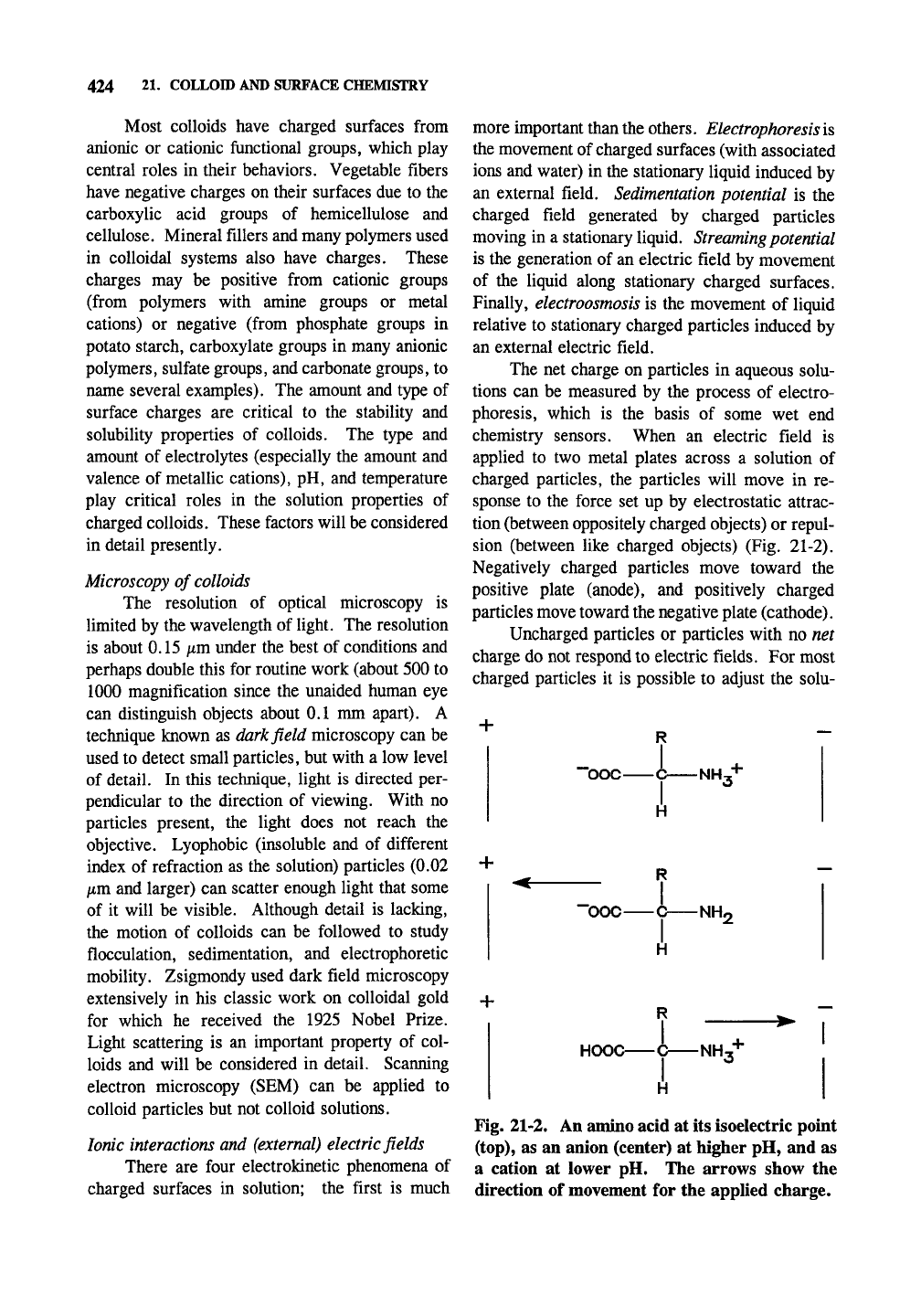
sion (between like charged objects) (Fig. 21-2).
Negatively charged particles move toward the
positive plate (anode), and positively charged
particles move toward the negative plate (cathode).
Uncharged particles or particles with no net
charge do not respond to electric fields. For most
charged particles it is possible to adjust the solu-
"ooc-
-NH,
H
"OOC HHr
HOOC
r\
C NH,
H
Fig. 21-2. An amino acid at its isoelectric point
(top),
as an anion (center) at higher pH, and as
a cation at lower pH. The arrows show the
direction of movement for the applied charge.
COLLOID CHEMISTRY 425
tion so that the particle has no net charge. This is
accomplished by changing the pH or changing the
composition of electrolytes in solution. When a
particle has no net charge it is at its isoelectric
point and it will not move in the electric field.
Electrophoresis is an important tool of biochemis-
try used to separate groups of proteins and other
molecules; it is also one method of measuring
zeta potential (effective surface charge).
Many amino acids are zwitterions, dipolar
ions or inner salts, and represent structures with
no net charge. Figure 21-2 (top) shows a repre-
sentative amino acid at its isoelectric point (around
pH 8). Although it is has two opposite charges,
there is no net charge. With increasing pH (mid-
dle),
the amine spends more and more of its time
deprotonated (since this is an equilibrium reaction)
and the molecule has a slight to moderate negative
charge. With decreasing pH the carboxylate salt
is protonated (a hydrogen atom is bound to it) a
higher and higher percentage of the time to give
the molecule a positive charge. The solubility of
proteins (and other materials) is usually lowest at
their isoelectric point.
Electrical double layer
Most materials involved in papermaking have
charged surfaces. For example, wood fibers in
aqueous suspensions have a net negative surface at
pH above 3.5 due to the carboxylic acid groups of
cellulose and hemicellulose, which exist as their
carboxylate salts. We will consider the behavior
of such a fiber in the development of surface
charge properties.
If metallic salts are added to a suspension of
wood fibers, the wood fibers will absorb (bind) a
certain portion of them (this is an equilibrium
reaction) and become less negatively charged or
even positively charged depending on how many
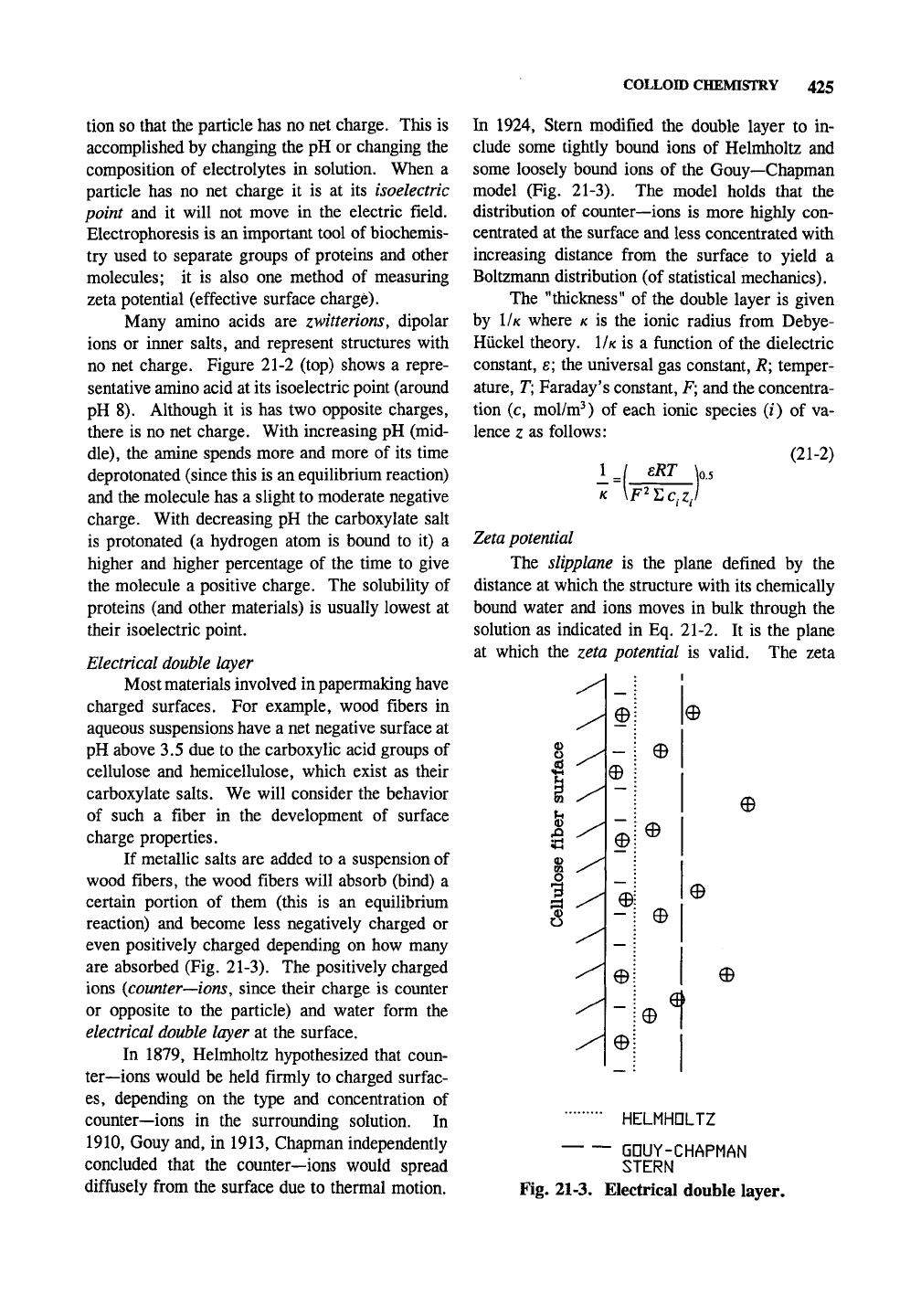
are absorbed (Fig. 21-3). The positively charged
ions {counter—ions, since their charge is counter
or opposite to the particle) and water form the
electrical double layer at the surface.
In 1879, Helmholtz hypothesized that coun-
ter—ions would be held firmly to charged surfac-
es,
depending on the type and concentration of
counter—ions in the surrounding solution. In
1910,
Gouy and, in 1913, Chapman independently
concluded that the counter—ions would spread
diffusely from the surface due to thermal motion.
In 1924, Stern modified the double layer to in-
clude some tightly bound ions of Helmholtz and
some loosely bound ions of the Gouy—Chapman
model (Fig. 21-3). The model holds that the
distribution of counter—ions is more highly con-
centrated at the surface and less concentrated with
increasing distance from the surface to yield a
Boltzmann distribution (of statistical mechanics).
The "thickness" of the double layer is given
by \IK where K is the ionic radius from Debye-
Huckel theory. 11
K
is a function of the dielectric
constant, e\ the universal gas constant, R\ temper-
ature, T\ Faraday's constant, F; and the concentra-
tion (c, mol/m^) of each ionic species (z) of va-
lence z as follows:
(21-2)
\0.5
1
ERT
K XF^YsC.z.
Zeta potential
The slipplane is the plane defined by the
distance at which the structure with its chemically
bound water and ions moves in bulk through the
solution as indicated in Eq. 21-2. It is the plane
at which the zeta potential is valid. The zeta
I
Si
O
I
§
e
e
e
e
®
®
0
®
©
e
HELMHOLTZ
GDUY-CHAPMAN
STERN
Fig. 21-3. Electrical double layer.

426
21.
COLLOID AND SURFACE CHEMISTRY
potential
is the
"modified"
or
"effective" surface
charge.
The
equation shows that with increasing
concentration
of
electrolyte,
the
slipplane will
contract toward
the
particle surface
and the
zeta
potential will become less negative, until
the
isoelectric point
is
achieved,
and may
even
be-
come positive. Each type
of
particle (softwood
fibers,
hardwood fibers, parenchyma, TiOj filler,
clay filler, etc.) will have
its
own zeta potential
or
even
a
zeta potential distribution.
The valence
of the
cation
is of
exponential
importance
in
decreasing
the
negative charge
on
anionic surfaces
(as
determined
by
precipitation).
This
was
independently observed
by
Schultze
in
1882
and
Hardy
in 1900 and is
referred
to as the
Schultze—Hardy rule. Later work showed that
charge neutralization
is
proportional
to the
sixth
power
of z
(more detail
is
found below). Other
factors such
as the
size
of the ion and the
size
of
the hydration layer modify these numbers some-
what. Despite this enormous influence other
factors
may
have strong influences
as
well, such
as
the
formation
of
coordinate complexes
or
insoluble precipitates.
For
example,
Cu^"^
ions
form strong complexes with amines
and may
impart more
of a
charge
to
polyamines than
AP"*"
ions because
the
copper—amine interaction
is
much stronger than ionic interactions. In ammonia
solution, copper ions exist
as
Cu(NH3)6^'^. High
ionic strength (corresponding
to
conductivity
> 1000 /xmho) suppresses the zeta potential and
its
useftilness as
a
control parameter
in
papermaking.
Coagulation
Under many conditions, colloidal particles
can clump together
to
form large particles that
are
no longer stable
to
suspension; this
is the
process
of coagulation. Coagulation
is
analogous
to the
formation
of a
liquid from
a gas or the
precipita-
tion
of a
solid from solution.
In all
three cases
it
is largely
a
matter
of
the thermodynamic stability
of
the
products
and
activation energies
to be
overcome.
The
stability
of a
suspension depends
on whether
the
repulsive forces between particles
are larger than
the
attractive forces between
particles
and the
energy
of
the various states.
In
some cases
the
solution
is
unstable
to
begin with,
and
it is
only
a
matter
of
time before coagulation
occurs. Conversely,
an
unstable
or
stable colloid
can sometimes
be
formed from
two
continuous
phases
by a
large amount
of
agitation.
Attractive forces (Levine,
1978) are due to
any
of the
normal secondary chemical forces
(as
opposed
to
covalent bonds) that hold materials
together.
The
forces between identical particles
are usually induced dipole-dipole interactions such
as
the
weak
van der
Waals
force (based
on the
work
of
1873), which
is
proportional
to 1/r^;
they
depend (only
to a
small degree)
on the
properties
of
the
solvent. Even
a
material like argon
is
subject
to van der
Waals forces, since these
are
the attractive forces between atoms
of
argon
in its
liquid
or
solid form.
The
energy
of
this interac-
tion
is
the London (based
on
the work
in
1930)
or
dispersion
energy and is proportional
to 1/r^. The
magnitude
of
this interaction
at the
most favorable
distance
is
typically
0.5 to 2
kcal/mol. These
forces are very dependent
on
distance but
act
only
at short range. Hydrogen bonding may be another
important attractive force
at
higher consistencies
with energies
of
2—8 kcal/mol.
Two molecules
are
prevented from being
"sucked into each other"
by
repulsive forces, Born
repulsion, predicted
by the
Pauli exclusion princi-
ple,
which
act at
shorter range than van
der
Waals
forces
and are
proportional
to 1/r^—1/r^^.
The bottom curve
of Fig. 21-4
shows
how
these forces
act in the
gas-liquid equilibrium
of a
pure material.
The
bottom curve
is in
the form
of
the
Lennard—Jones
6—12
intermolecular potential
(for
Ar or Nj),
where
v =
4e[(a/r)^^
- {alrf\. {v
is the sum
of
the very short range repulsive poten-
tial
and
short—range attractive potential,
e is the
depth
of the
minimum potential,
and a is the
intermolecular distance where
v = 0.) At a
given
intermolecular distance
two
molecules form
a
(somewhat) stable dimer
(or
liquid
or
solid)
de-
pending
on the
temperature (average kinetic
energy
of
the species).
At
high temperatures
the
atoms
or
molecules overcome
the
binding energy
and exist
as a gas.
Liquids
are
incompressible
because
of
the high dependence
on
distance
of
the
short—range Pauli exclusion repulsive forces.
In colloids with charged surfaces, relatively
long range repulsive forces have
two
origins:
stabilization
of
the particles
by the
solvent (hydro-
gen bonds
and
London energy)
and
double-layer
ionic interactions.
In
the former,
if
particles have
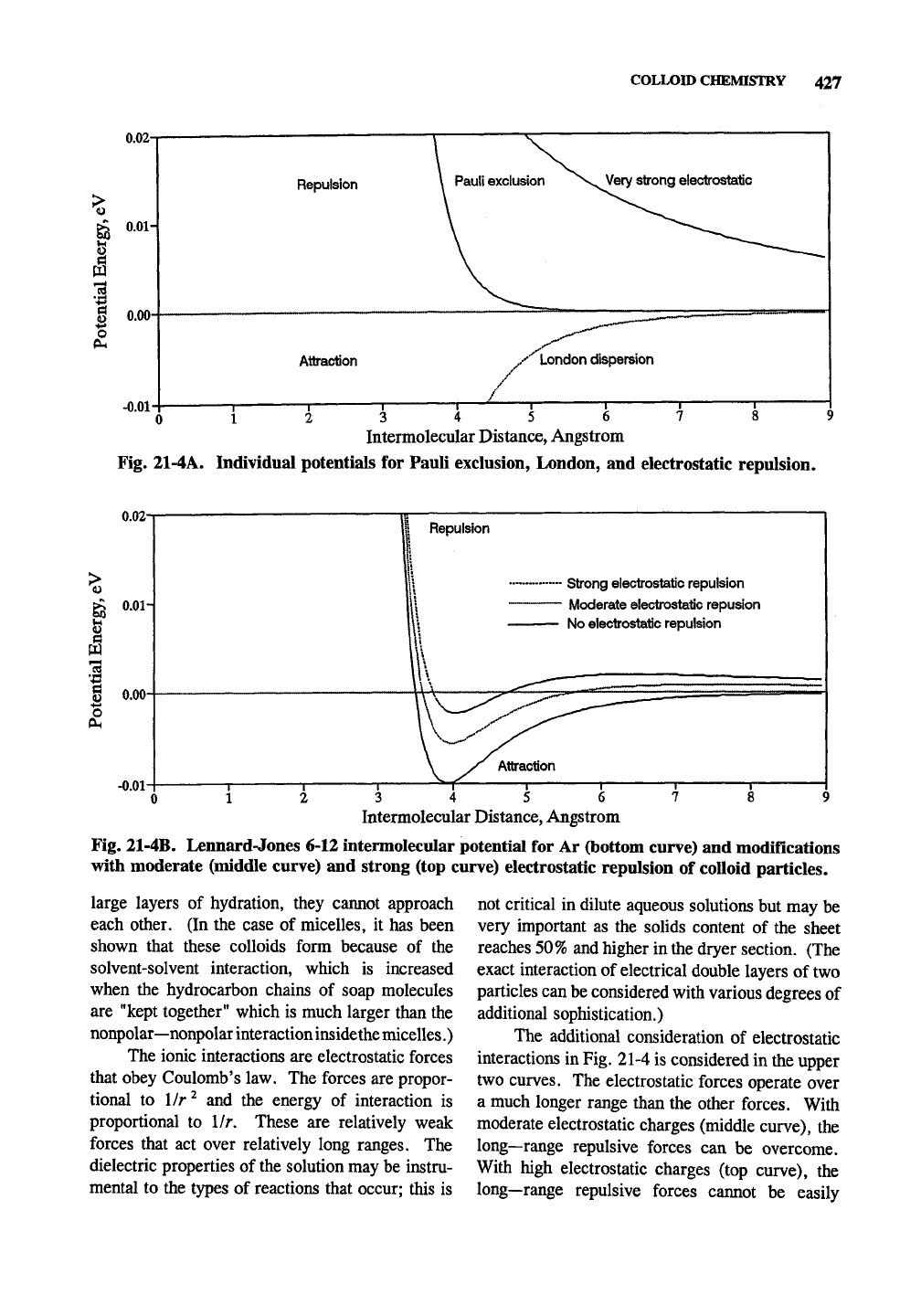
COLLOID CHEMISTRY 427
0.02-
I
I
S
o
0.01-
0.00-
-0.01
Repulsion
Very strong electrostatic
Attraction
12 3 4 5 6
Intermolecular Distance, Angstrom
Fig. 21-4A. Individual potentials for Pauli exclusion, London, and electrostatic repulsion.
I
1
PL,
0.02-
0.01-
0.00-
-0.01-
Repulsion
Strong electrostatic repulsion
Moderate electrostatic repusion
No electrostatic repulsion
3 4 5
Intermolecular Distance, Angstrom
Fig. 21-4B. Lennard-Jones 6-12 intermolecular potential for Ar (bottom curve) and modifications
with moderate (middle curve) and strong (top curve) electrostatic repulsion of colloid particles.
large layers of hydration, they cannot approach
each other. (In the case of micelles, it has been
shown that these colloids form because of the
solvent-solvent interaction, which is increased
when the hydrocarbon chains of soap molecules
are "kept together" which is much larger than the
nonpolar—nonpolar interaction insidethe micelles.)
The ionic interactions are electrostatic forces
that obey Coulomb's law. The forces are propor-
tional to 1/r
^
and the energy of interaction is
proportional to 1/r. These are relatively weak
forces that act over relatively long ranges. The
dielectric properties of the solution may be instru-
mental to the types of reactions that occur; this is
not critical in dilute aqueous solutions but may be
very important as the solids content of the sheet
reaches 50% and higher in the dryer section. (The
exact interaction of electrical double layers of two
particles can be considered with various degrees of
additional sophistication.)
The additional consideration of electrostatic
interactions in
Fig.
21-4 is considered in the upper
two curves. The electrostatic forces operate over
a much longer range than the other forces. With
moderate electrostatic charges (middle curve), the
long—range repulsive forces can be overcome.
With high electrostatic charges (top curve), the
long—range repulsive forces cannot be easily

428 21. COLLOro AND SURFACE CHEMISTRY
overcome. When two particles combine, the
electrostatic charges rearrange, because they must
occur at the surface of particles. This rearrange-
ment will mean that the entire short—range attrac-
tive potential will be realized.
In fact, it is not necessary to consider the
Pauli exclusion forces when considering the
stability of colloids (since these act only at short
ranges after the weak chemical attraction forces
have caused coagulation or coalescence). In the
DLVO (from Deryaguin, Landau, Verway, and
Overbeek) theory, colloid stability is considered in
terms of the long—range repulsive forces and the
short—range attractive forces.
With increasing temperature, pure materials
go from solid to liquid to gas (decreasing particle
size).
With increasing temperature, colloids often
go from solutions to solids (protein coagulation,
such as occurs when cooking an egg) or from
small particles to large particles. The apparent
contradiction lies in the fact that increasing tem-
perature in colloidal reactions is overcoming
repulsive forces, whereas increasing temperature
in the vaporization of a pure material is overcom-
ing attractive forces. Increased agitation or in-
creased temperature may cause colloids to form
from larger particles if the London potential is
weak, just as in a liquid.
At great distances there is no interaction
between identical colloid particles. As they
approach each other, the electrostatic repulsive
forces act at relatively long distances. Pushing the
particles together causes the van der Waals force
to increase until the particles attract each other and
combine. (The Pauli exclusion repulsive forces
act to prevent the particles from approaching
closer than in normal solids and liquids.)
Therefore, coagulation
depends on
decreasing
the electrostatic repulsive forces and/or forcing
particles to be in close proximity to each other.
Small amounts of polymers can sometimes be used
to prevent coagulation. For example, gelatine
prevents coagulation of gold particles by steric
stabilization, whereby particles are prevented from
getting close to each other by adsorbed layers of
gelatin. Work by Neuman, Berg, and Claesson
(1993) indicates that steric stabilization of cellu-
losic surfaces (that does not behave according to
DLVO theory) may be much more important
than ionic interactions. The contribution of this
phenomenon in papermaking chemistry
needs
more
consideration in fixture work. This finding ex-
plains why the use of the zeta potential is not
always helpftil in papermaking chemistry and why
too much polymer can reverse flocculation.
The electrostatic potential depends on the
usual factors of electrolyte concentration and
composition, pH, temperature, and degree of
agitation. For example, when the zeta potential
approaches zero, the electrostatic forces are
decreased; even with a zeta potential of zero there
may be electrostatic repulsion as the particles tend
to polarize each other and the double layers affect
each other; therefore, the maximum coagulation
often occurs within + 5 mV of
the
isoelectric point
(i.e.,
where the zeta potential is zero). A com-
pressed double layer (resulting from higher levels
of electrolytes and/or high—valence cations) also
decreases electrostatic repulsion; generally the
effectiveness toward coagulation of M^rM^'^iM^'^
is
1:75:625
according to the Schulze-Hardy rule.
Within a given valence, a large ionic radius is a
little more effective toward coagulation than one
of small ionic radius.
Overbeek (1952) demonstrated these concepts
in his work (reprinted in Hunter, 1987 and Shaw,
1980).
For example, the critical coagulation
concentrationsy ccc, for a sol of AS2S3 is 58
mmol/L of LiCl, 51 mmol/L of NaCl, and 50
mmol/L of KNO3 for monovalent ions; 0.72
mmol/L of MgClj (10% higher for the sulfate salt)
and 0.69 mmol/L of
ZnCl2
for divalent ions; 0.093
mmol/L of AICI3 (barely higher for the sulfate
salt) and 0.08 mmol/L for Ce(N03)3. The same
approximate ratios are realized with two other sols
as well in this work, with one of the sols positive-
ly charged and precipitated by CI", 804^', and
other negative ions. [Theoretically the cmc de-
creases to the sixth power of z (Hunter, 1987);
for z = 1, 2, 3 this is
1:64:729.]
If too much
electrolyte is present, charge reversal is possible
and coagulation may be reversed. It is interesting
to note that aluminum behaves according to this
rule despite its complex chemistry with water.
One wonders how polyelectrolytes behave since
they may have numerous charges per molecule.
Up to this point this section has considered
identical colloidal particles. With different parti-

COLLOID CHEMISTRY 429
cles there can be very strong attractive electrostat-
ic forces if some particle are negatively charged
and others are positively charged. In this case
coagulation is very quick.
Flocculation in the papermaking system
Flocculation in the papermaking system is
analogous to coagulation except that a charged
polymer is used either to decrease the repulsive
forces or to act with several particles at one time
(bridging) to achieve aggregation. Consider the
use of cationic polymers in a suspension of wood
fiber fines with negative surface charges. Without
polymers, the fines repel each other due to the like
charges. The addition of a low molecular weight
cationic polymer will decrease the zeta potential so
that fines may flocculate.
Flocculation can also occur by the bridging
mechanism, whereby small amounts of cationic
polymer attach to the particles and create local
neutral charges (a patch), although the overall
particle may have a net negative charge. Two
patches, each on a different particle, may form a
bridge even though the overall zeta potential is still
appreciably negative.
The molecular weights of polymers are
important to their
use.
Usually a minimum molec-
ular weight is required for a particular application.
High molecular weights allow direct bridging of
particles. Sometimes two component polymer
systems are used, with one polymer having a low
molecular weight (perhaps a few tens of thou-
sands) to provide suitable charge on a part of the
surface and a second, high molecular weight
molecule used to provide bridging between patch-
es.
High shear forces in solution must be avoided
if high molecular weight is required in an applica-
tion since shear forces will cleave large molecules.
The use of branched polymers (for a given overall
molecular weight) decreases their effectiveness
toward bridging particles.
Like coagulation, flocculation can be revers-
ible with shear forces (mixing or agitation) espe-
cially if the flocculation is weak such as when
barely enough polymer is added to initiate floccu-
lation. If too much polymer is added, charge
reversal or steric stabilization may occur.
One area that has been overlooked by the
paper industry, but offers great potential, is the
use of diblock and other block polymers. This
approach has the potential of allowing fillers to
attach directly to fibers and fines to increase
opacity. Too, fillers (of different indexes of
refraction) could be made to attach to each other
to increase light scattering.
[Strictly speaking, coagulation and floccula-
tion are both the clumping of small particles (like
or not) together into groups; the terms are usually
used interchangeably by colloid chemists, although
coagulation implies a stronger interaction that is
generally not reversible. (Admittedly, this is
contradictory in terms of how these behave to
shear forces in the papermaking system.) Coales-
cence is the fusion of two small particles into one
large particle where the original particles are
indistinguishable. Oil droplets of an oil in water
emulsion may coalesce into larger oil droplets.
The large materials may settle from solution, if
they are more dense, by sedimentation or rise to
the top, if they are less dense, by creaming.}
21.3 POLYELECTROLYTES
Polymers with charged groups may be called
poly electrolytes. Anionic groups are usually
carboxylate salts and occasionally sulfonate or
phosphate salts; cationic groups are usually
amines or quaternary ammonium salts (quats).
The charge density (often measured as percentage
of monomers containing a charge) is an important
criterion for polyelectrolytes. A polymer with a
high charge density (above 30%) tends to be com-
pletely "unraveled" in solution.
21.4 SURFACE TENSION AND SIZING
Mechanism of
sizing
action
The Lucas (1918) equation [also referred to
as the Washburn (1921) equation] shows that the
liquid penetration (depth of penetration, /) into a
material is a fimction of the size of capillaries or
pores in a material, r; the contact angle of the
liquid with the solid,
Q\
the surface tension of the
liquid, 7; the viscosity of the liquid, ly; and time,
t. The equation is:
l^ = rtycos6/2ri
(21-3)
Lucas used this equation to calculate pore
sizes in paper based on the speed of the rise of
430 21. COLLOID AND SURFACE CHEMISTRY
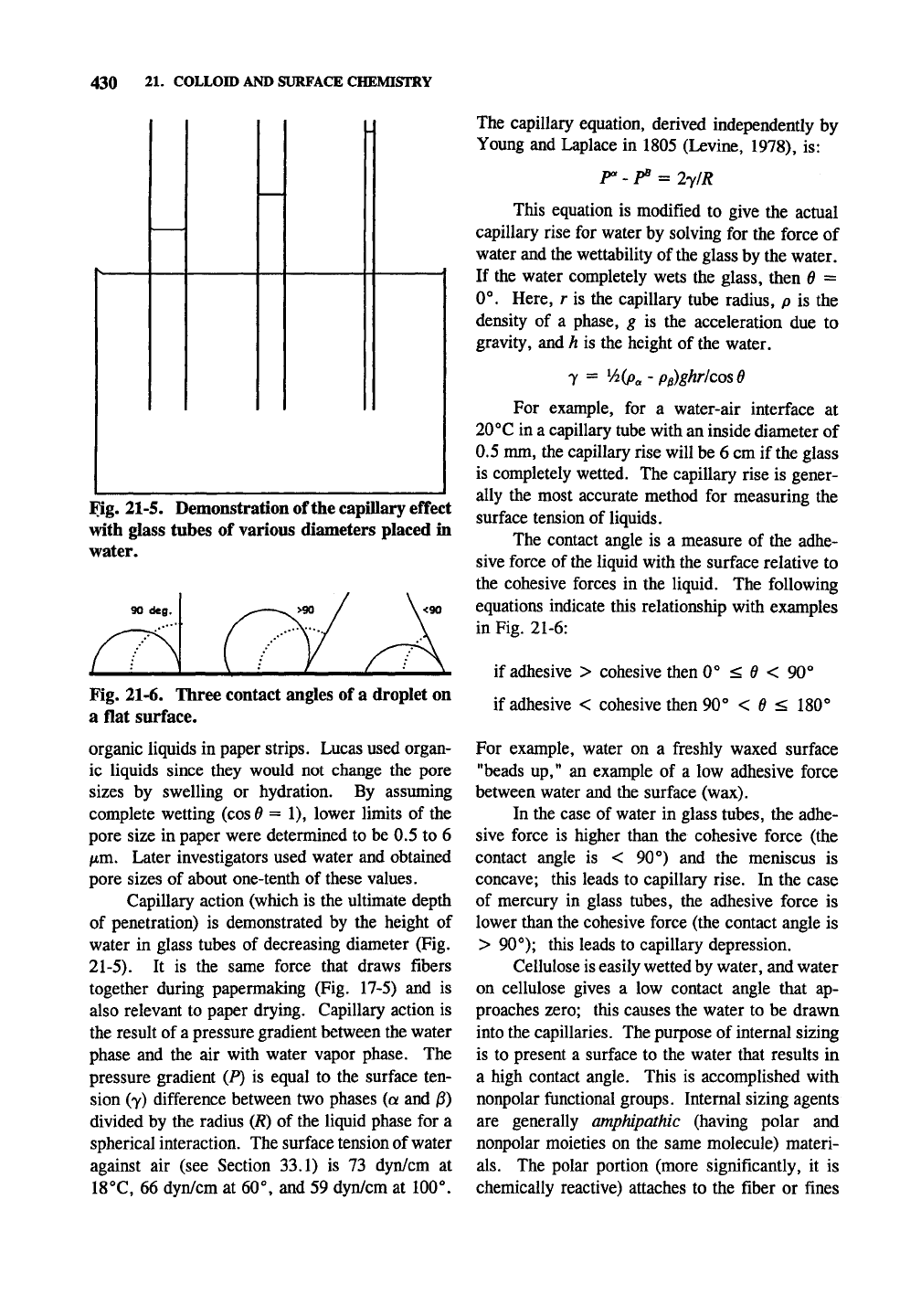
Fig. 21-5. Demonstration of the capillary effect
with glass tubes of various diameters placed in
water.
Fig. 21-6. Three contact angles of a droplet on
a flat surface.
organic liquids in paper strips. Lucas used organ-
ic liquids since they would not change the pore
sizes by swelling or hydration. By assuming
complete wetting (cos 6 = 1), lower limits of the
pore size in paper were determined to be 0.5 to 6
/xm. Later investigators used water and obtained
pore sizes of about one-tenth of these values.
Capillary action (which is the ultimate depth
of penetration) is demonstrated by the height of
water in glass tubes of decreasing diameter (Fig.
21-5).
It is the same force that draws fibers
together during papermaking (Fig. 17-5) and is
also relevant to paper drying. Capillary action is
the result of a pressure gradient between the water
phase and the air with water vapor phase. The
pressure gradient (P) is equal to the surface ten-
sion (7) difference between two phases (a and p)
divided by the radius (R) of the liquid phase for a
spherical interaction. The surface tension of water
against air (see Section 33.1) is 73 dyn/cm at
18°C,
66 dyn/cm at 60°, and 59 dyn/cm at 100°.
The capillary equation, derived independently by
Young and Laplace in 1805 (Levine, 1978), is:
p« -
/>»
= 2y/R
This equation is modified to give the actual
capillary rise for water by solving for the force of
water and the wettability of
the
glass by the water.
If the water completely wets the glass, then 6 =
0°.
Here, r is the capillary tube radius, p is the
density of a phase, g is the acceleration due to
gravity, and h is the height of the water.
7 =
V2(p„-
pp)ghr/cose
For example, for a water-air interface at
20°C in a capillary tube with an inside diameter of
0.5 mm, the capillary rise will be 6 cm if the glass
is completely wetted. The capillary rise is gener-
ally the most accurate method for measuring the
surface tension of liquids.
The contact angle is a measure of the adhe-
sive force of
the
liquid with the surface relative to
the cohesive forces in the liquid. The following
equations indicate this relationship with examples
in Fig. 21-6:
if adhesive > cohesive then 0° < 0 < 90°
if adhesive < cohesive then 90° < ^ < 180°
For example, water on a freshly waxed surface
"beads up," an example of a low adhesive force
between water and the surface (wax).
In the case of water in glass tubes, the adhe-
sive force is higher than the cohesive force (the
contact angle is < 90°) and the meniscus is
concave; this leads to capillary rise. In the case
of mercury in glass tubes, the adhesive force is
lower than the cohesive force (the contact angle is
> 90°); this leads to capillary depression.
Cellulose is easily wetted by water, and water
on cellulose gives a low contact angle that ap-
proaches zero; this causes the water to be drawn
into the capillaries. The purpose of internal sizing
is to present a surface to the water that results in
a high contact angle. This is accomplished with
nonpolar functional groups. Internal sizing agents
are generally amphipathic (having polar and
nonpolar moieties on the same molecule) materi-
als.
The polar portion (more significantly, it is
chemically reactive) attaches to the fiber or fines
SURFACE TENSION AND SIZING 431
directly through a covalent bond or indirectly
through a mordant such as alum. Since the adhe-
sive strength of water to the sized surface is now
less than the cohesive strength of water, the water
does not penetrate the pores of the fibers. (Sur-
face sizing with starch physically plugs the surface
capillaries of paper, which is its chief mechanism
of sizing even though starch is hydrophilic.)
Some observations will be considered in light
of this theory. Hexane has very little cohesive
strength, that is, a low surface tension, and easily
penetrates paper, whether sized or unsized.
Lowering the surface tension of water (by use of
a surfactant) will cause a decreased capillary
action (sizing would be observed to improve)
directly as indicated in Eq. 21-3; however, this
will cause the contact angle to decrease, which has
the effect of decreasing the observed sizing.
Usually the latter effect induces a larger relative
change, so that the use of surfactants will decrease
the observed sizing to a modest degree (see Chen
and Biermann, 1995, in Chapter 22).
Inverse gas chromatography has been used to
characterize surface energy; in pulp and paper
this has been accomplished with treated and
untreated pulps (Pyda et al., 1993). Another
technique that has been used is dynamic contact
angle analysis (Huang et al., 1995)
The contact angle of buffer solutions at
various pH values on individual wood fibers was
studied by Jacob and Berg (1993). Their study
indicates that the change in surface ionizable
groups brought about by variation of pH affects
the wettability of the fibers. More recent work of
some workers refutes this approach, however.
Capillary condensation (Stamm, 1962)
Above 90% relative humidity, wood and
paper can adsorb small amounts of water vapor as
free water due to surface tension effects of water.
The relative humidity must reach 99.5% to fill pit
chambers and the tips of fibers and 99.9% to fill
fiber lumens of wood.
A water droplet with a positive radius (con-
cave surface) has a slightly higher vapor pressure
than a flat surface of water. A water droplet with
a negative radius (convex) has a lower vapor
pressure, which allows condensation to occur. The
radius of the pores in which water will condense
is calculated by a variation of the Kelvin equation:
r
=
.
27y
RTlnip/p)
where r is the radius in meters, y is the surface
tension of the liquid (0.073 J/m^ for water at
20°C),
y is the molar mass of the liquid (1.8-10"^
mol/m^ for water), R is 8.314 J/(mol-K), T is
temperature in Kelvin (293), and p/p^ is the
relative vapor pressure of the liquid.
At 20 °C and 50% relative humidity the pore
size is
1.6-10"^
(1.6 nm); at 90% RH the pore
size is
1.02-10*
m or 0.01 /xm; at 99% RH the
pore size is 0.1 fim; at 99.9% RH the pore size
is 1 iim. Possibly, pocket ventilation at the last
few dryer cans must be particularly effective (to
give a sufficiently low vapor pressure of water) to
get the last water out of paper during drying.
[There is an equation (derived by Oswald in
1907) that is analogous to the Kelvin equation but
applies to the solubility of particles in solution. A
small particle actually has a slightly higher solubil-
ity than a larger particle. If the solubility is suffi-
ciently high, with aging, small particles decrease
in size while larger particles increase in
size.
This
is the basis of aging precipitates in some analytical
chemistry experiments. The surface energy of
solid-solution interfaces is about 1
]/w?.]
21.5 SURFACTANTS
Introduction
Surfactants are surface—active agents that
aggregate near or have a strong effect on modify-
ing the interface between two materials. This
occurs because of their dual nature: hydrophobic
and hydrophilic. The hydrophilic moiety may be
anionic (carboxylate, sulfate, or phosphate),
cationic (quaternary ammonium salt), ampholytic
(cationic and anionic), or nonionic (Span®,
Tween®, Triton®) depending on the type of
charge(s) carried, if any. Performance of
surfactants depends strongly on their surface
activity and micelle formation.
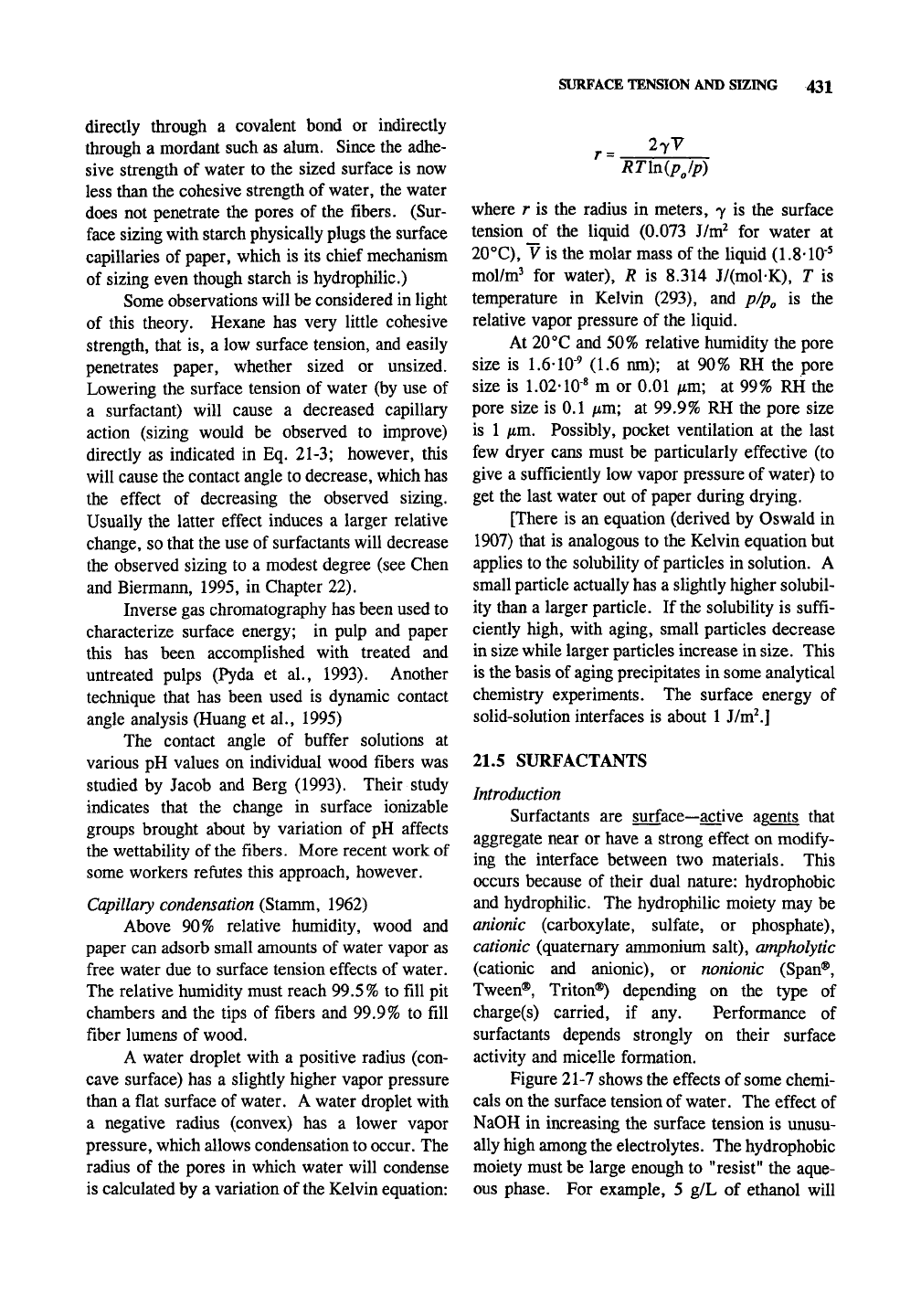
Figure 21-7 shows the effects of some chemi-
cals on the surface tension of water. The effect of
NaOH in increasing the surface tension is unusu-
ally high among the electrolytes. The hydrophobic
moiety must be large enough to "resist" the aque-
ous phase. For example, 5 g/L of ethanol will
432 21. COLLOID AND SURFACE CHEMISTRY
lower the surface tension of water at 20°C (73
mJ/w?) by about 2 mJ/m^, 5 g/L propanol by 5
mJ/m^, 5 g/L butanol by 7 mJ/m^ 5 g/L pentanol
by 22 mJ/m^, and 5 g/L hexanol by 35 mJ/m^
Anionic surfactants are generally less expen-
sive than cationic ones, though the latter some-
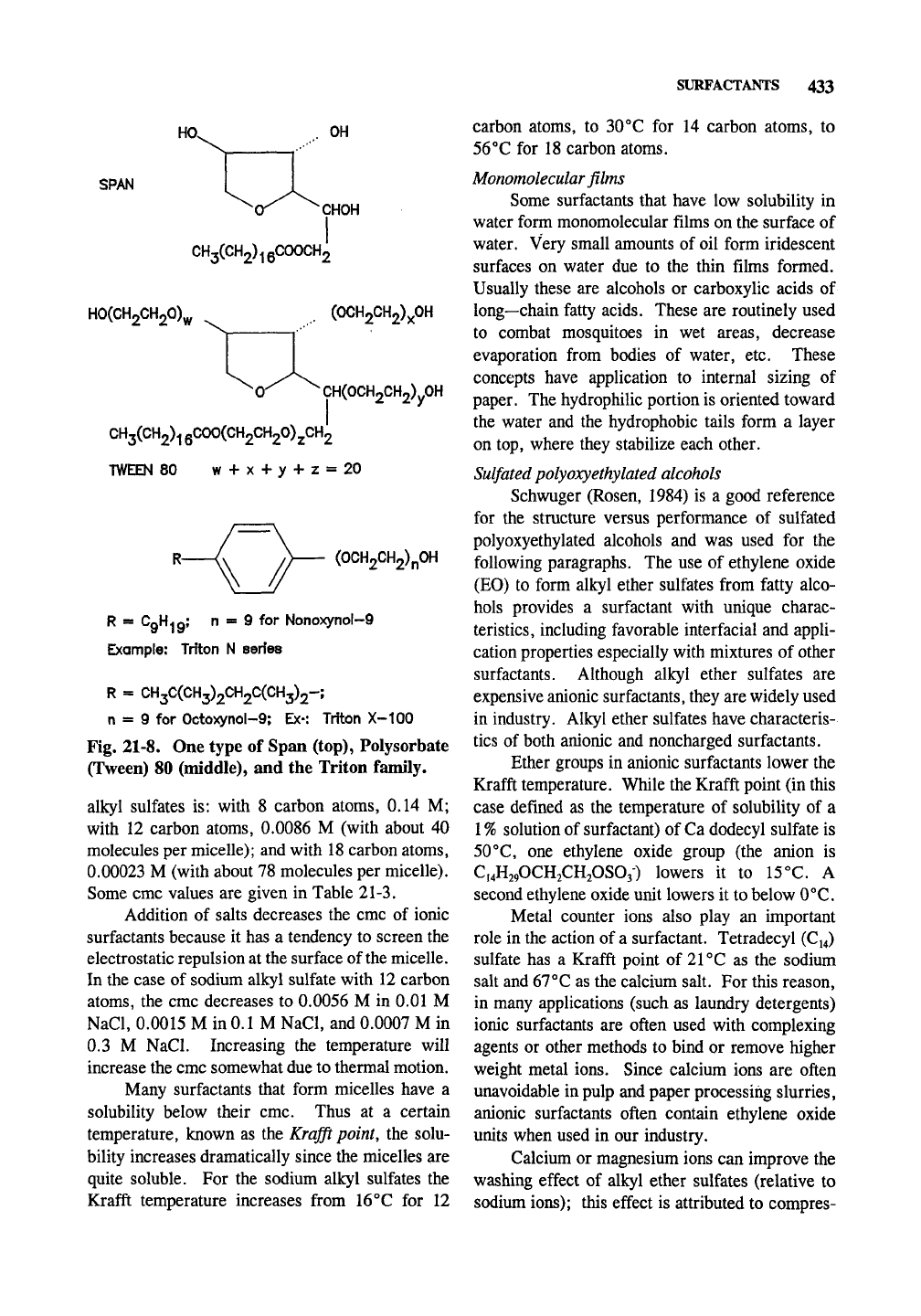
times have biocidal action. Span and Tween
materials (Fig. 21-8) are formed from hexahydric
alcohols (Span, derived from sorbitol), fatty acids,
and alkene oxides (Tween). Tween 80 is called
Polysorbate 80 when used in pharmaceutical and
food products. The Span materials tend to be less
water soluble than the Tween materials. The
Triton family is shown in Fig. 21-8. Surfactants
are often named according to their use. The same
material could be a detergent, a wetting agent, an
emulsifier, or a dispersant. The majority of all
•surfactants are detergents. Detergents are often
alkyl sulfates. Alkyl aryl sulfates used to have
widespread use, but they are not readily biode-
gradable. Detergents are used with silicates to
help bind interfering metal ions, phosphates to
help prevent flocculation (scum formation), and
other materials to keep them free-flowing.
Critical
micelle
concentration,
cmc
The cmc of surfactants depends on several
factors (Shaw, 1980). The most important factor
is the strong water—water interaction (the hydro-
phobic effect) that is allowed when water does not
interact with individual fatty acid chains in solu-
tion; the nonpolar—nonpolar interaction in the
center of the micelle does not provide the thermo-
dynamic energy required for micelle formation.
Most ionic surfactants have a cmc that depends on
the hydrocarbon chain. In Table 21-3 all of the
C-12 ionic surfactants have a similar cmc. Non-
ionic surfactants usually have lower cmc values.
Longer chain fatty acid portions increase the
hydrophobic effect; an example was already given
with the lower chain alcohols. After about 18
CH2 groups, an additional effect is not noted since
these would coil on their own in solution by
themselves. For example, the cmc of sodium
100-
90-
« 80-
CD
CO
C/3
Q
70H
60H
.An
53
50H
(D
O
en
40H
30-
20-
X
X
X NaOHlSC
Sucrose 25 C
>K
•
^ nPiOH 25 C
n Phenol 20 C
+ HAc30 C
EtOH 40 C H-
y^^
T
1 1 \—I I I I I
1
n—I
i I I I—
10
1—I I I I
0.1 1 10 100
Concentration of "surfactant", %
Fig. 21-7. The effect of various chemicals on the surface tension of water. Data from CRC
Handbook of
Chemistry
and Physics, 67th ed.
SURFACTANTS 433
HO^
OH
SPAN
^CHOH
CH3(CH2)i6COOCH2
H0(CH2CH20)^
(OCH2CH2)xOH
^0^^
CH(OCH2CH2)yOH
CH3(CH2)i
QCOO(CH2CH20)2,CH2
TWEEN 80 w + x + y + z = 20
(0CH2CH2)n0H
R = CgH^gj n « 9 for NonoxynoI-9
Example: Triton N series
R = CH3C(CH3)2CH2C(CH3)2-;
n = 9 for Octoxynol-9; Ex-: Triton X~100
Fig. 21-8. One type of Span (top), Polysorbate
(Tween) 80 (middle), and the Triton family.
alkyl sulfates is: with 8 carbon atoms, 0.14 M;
with 12 carbon atoms, 0.0086 M (with about 40
molecules per micelle); and with 18 carbon atoms,
0.00023 M (with about 78 molecules per micelle).
Some cmc values are given in Table 21-3.
Addition of salts decreases the cmc of ionic
surfactants because it has a tendency to screen the
electrostatic repulsion at the surface of the micelle.
In the case of sodium alkyl sulfate with 12 carbon
atoms, the cmc decreases to 0.0056 M in 0.01 M
NaCl, 0.0015 M in 0.1 M NaCl, and 0.0007 M in
0.3 M NaCl. Increasing the temperature will
increase the cmc somewhat due to thermal motion.
Many surfactants that form micelles have a
solubility below their cmc. Thus at a certain
temperature, known as the Kraffi point, the solu-
bility increases dramatically since the micelles are
quite soluble. For the sodium alkyl sulfates the
Krafft temperature increases from 16°C for 12
carbon atoms, to 30°C for 14 carbon atoms, to
56°C for 18 carbon atoms.
Monomolecular films
Some surfactants that have low solubility in
water form monomolecular films on the surface of
water. Very small amounts of oil form iridescent
surfaces on water due to the thin films formed.
Usually these are alcohols or carboxylic acids of
long—chain fatty acids. These are routinely used
to combat mosquitoes in wet areas, decrease
evaporation from bodies of water, etc. These
concepts have application to internal sizing of
paper. The hydrophilic portion is oriented toward
the water and the hydrophobic tails form a layer
on top, where they stabilize each other.
Sulfated polyoxyethylated alcohols
Schwuger (Rosen, 1984) is a good reference
for the structure versus performance of sulfated
polyoxyethylated alcohols and was used for the
following paragraphs. The use of ethylene oxide
(EO) to form alkyl ether sulfates from fatty alco-
hols provides a surfactant with unique charac-
teristics, including favorable interfacial and appli-
cation properties especially with mixtures of other
surfactants. Although alkyl ether sulfates are
expensive anionic surfactants, they are widely used
in industry. Alkyl ether sulfates have characteris-
tics of both anionic and noncharged surfactants.
Ether groups in anionic surfactants lower the
Krafft temperature. While the Krafft point (in this
case defined as the temperature of solubility of a
1 %
solution of surfactant) of Ca dodecyl sulfate is
50°C,
one ethylene oxide group (the anion is
C14H29OCH2CH2OSO3) lowers it to 15X. A
second ethylene oxide unit lowers it to below 0°C.
Metal counter ions also play an important
role in the action of a surfactant. Tetradecyl (C14)
sulfate has a Krafft point of 21°C as the sodium
salt and 67°C as the calcium salt. For this reason,
in many applications (such as laundry detergents)
ionic surfactants are often used with complexing
agents or other methods to bind or remove higher
weight metal ions. Since calcium ions are often
unavoidable in pulp and paper processing slurries,
anionic surfactants often contain ethylene oxide
units when used in our industry.
Calcium or magnesium ions can improve the
washing effect of alkyl ether sulfates (relative to
sodium ions); this effect is attributed to compres-
