Dinc Ibrahim. Refrigeration systems and applications 2th edition
Подождите немного. Документ загружается.

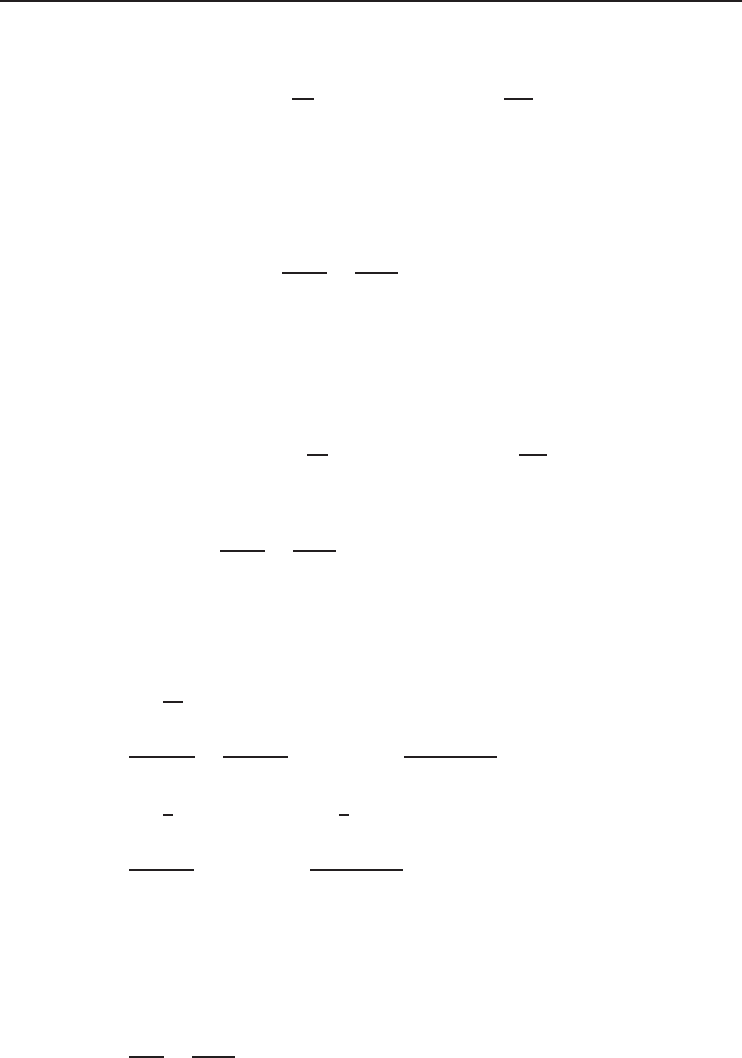
Refrigeration Cycles and Systems 181
(b) The exergy of the heat transferred from the low-temperature medium is
˙
Ex
˙
Q
L
=−
˙
Q
L
1 −
T
0
T
L
=−(13.91 kW)
1 −
280
233
= 2.81 kW
This is the minimum power input:
˙
W
min
=
˙
Ex
˙
Q
L
= 2.81 kW
The second-law efficiency of the cycle is
η
II
=
˙
Ex
˙
Q
L
˙
W
net
=
2.81
37.62
= 0.075 = 7.5%
The total exergy destruction in the cycle can be determined from
˙
Ex
dest,total
=
˙
W
net
−
˙
Ex
˙
Q
L
= 37.62 − 2.81 = 34.8kW
(c) If the temperature of the cooled space is T
L
=−15
◦
C = 258 K
˙
Ex
˙
Q
L
=−
˙
Q
L
1 −
T
0
T
L
=−(13.91 kW)
1 −
280
258
= 1.19 kW
˙
W
min
=
˙
Ex
˙
Q
L
= 1.19 kW
η
II
=
˙
Ex
˙
Q
L
˙
W
net
=
1.19
37.62
= 0.032 = 3.2%
˙
Ex
dest,total
=
˙
W
net
−
˙
Ex
˙
Q
L
= 37.62 − 1.19 = 36.4kW
(d) The simple gas refrigeration cycle analysis is as follows (Figure 4.18):
T
2s
= T
1
P
2
P
1
(k−1)/k
=
(
253 K
)(
4
)
0.4/1.4
= 376.0K
η
C
=
h
2s
− h
1
h
2
− h
1
=
T
2s
− T
1
T
2
− T
1
−−−→ 0.82 =
376.0 − 253
T
2
− 253
−−−→ T
2
= 402.9K
T
4s
= T
3
1
r
(k+1)/k
= (289 K)
1
4
0.4/1.4
= 194.5K
η
T
=
T
3
− T
4
T
3
− T
4s
−−−→ 0.84 =
289 − T
4
289 − 194.5
−−−→ T
4
= 209.6K
˙
Q
L
=˙mc
p
(T
1
− T
4
) = (0.45 kg/s)(1.005 kJ/kg · K)(253 − 209.6) kJ/kg = 19.63 kW
˙
W
net,in
=˙mc
p
(T
2
− T
1
) −˙mc
p
(T
3
− T
4
)
= (0.45 kg/s)(1.005 kJ/kg · K)
[
(402.9 − 253) − (289 − 209.6) K
]
= 31.91 kW
COP =
˙
Q
L
˙
W
net
=
19.63
31.91
= 0.615
182 Refrigeration Systems and Applications
s
T
1
2S
Q
H
16 °C
−20 °C
3
4s
·
Q
Refrig
·
2
4
Figure 4.18 Temperature–entropy diagram of simple gas refrigeration cycle considered in Example 4.5,
part (d).
4.7 Absorption–Refrigeration Systems (ARSs)
Although the principle of the absorption–refrigeration cycle has been known since the early 1800s,
the first one was invented by French engineer Ferdinand P.E. Carre in 1860, an intermittent crude
ammonia absorption apparatus based on the chemical affinity of ammonia for water, and produced
ice on a limited scale. The first five Absorption–Refrigeration System (ARS) units Carre produced
were used to make ice, up to 100 kg/hour. In the 1890s, many large ARS units were manufactured
for chemical and petroleum industries. The development of ARSs slowed to a standstill by 1911 as
vapor-compression refrigeration systems came to the forefront. After 1950, large ARSs gained in
popularity. In 1970s, the market share of ARSs dropped rapidly because of the oil crisis and hence
the government regulations. Because of the increasing energy prices and environmental impact of
refrigerants, during the past decade ARSs have received increasing attention. So, many companies
have concentrated on ARSs and now do research and development on these while the market
demand increases dramatically.
ARSs have experienced many ups and downs. The system was the predecessor of the vapor-
compression refrigeration system in the nineteenth century, and water–ammonia systems enjoyed
a variety of applications in domestic refrigerators and large industrial installations in the chemical
and process industries. They were energized by steam or hot water generated from natural gas,
oil-fired boilers, and electrical heaters. In the 1970s, the shift from direct burning of oil and natural
gas struck a blow at the application of the ARSs but at the same time opened up other opportunities,
such as the use of heat derived from solar collectors to energize these systems.
The concept of absorption refrigeration developed well before the advent of electrically driven
refrigerators. In the last decades, the availability of cheap electricity has made absorption systems
less popular. Today, improvements in absorption technology, the rising cost, and the environmental
impact of generating electricity are contributing to the increasing popularity of absorption systems.
ARSs for industrial and domestic applications have been attracting increasing interest throughout
the world because of the following advantages over other refrigeration systems:
• quiet operation,
• high reliability,
• long service life,
• efficient and economic use of low-grade energy sources (e.g., solar energy, waste energy, geother-
mal energy),
• easy capacity control,
• no cycling losses during on-off operations,

Refrigeration Cycles and Systems 183
• simpler implementation, and
• meeting the variable load easily and efficiently.
Recently, there has been increasing interest in the industrial (Figure 4.19) and domestic use of
the ARSs for meeting cooling and air conditioning demands as alternatives, because of a trend
in the world for rational utilization of energy sources, protection of the natural environment, and
prevention of ozone depletion, as well as reduction of pollution. There are a number of applications
in various industries where ARSs are employed, including the following:
• food industry (meat, dairy, vegetables, and food freezing and storage, fish industry, freeze
drying),
• chemical and petrochemical industry (liquefying if gases, separation processes),
• cogeneration units in combination with production of heat and cold (trigeneration plants),
• leisure sector (skating rinks),
• HVAC,
• refrigeration, and
• cold storage.
The absorption cycle is a process by which the refrigeration effect is produced through the use of
two fluids and some quantity of heat input, rather than electrical input as in the more familiar vapor-
compression cycle. In ARSs, a secondary fluid (i.e., absorbent) is used to circulate and absorb the
primary fluid (i.e., refrigerant), which is vaporized in the evaporator. The success of the absorption
process depends on the selection of an appropriate combination of refrigerant and absorbent. The
most widely used refrigerant and absorbent combinations in ARSs have been ammonia–water and
lithium bromide-water. The lithium bromide-water pair is available for air-conditioning and chilling
applications (over 4
◦
C, because of the crystallization of water). Ammonia-water is used for cooling
and low-temperature freezing applications (below 0
◦
C).
The absorption cycle uses a heat-driven concentration difference to move refrigerant vapors
(usually water) from the evaporator to the condenser. The high concentration side of the cycle
absorbs refrigerant vapors (which, of course, dilutes that material). Heat is then used to drive off
these refrigerant vapors thereby increasing the concentration again.
(a) (b) (c)
Figure 4.19 (a) An ARS of 2500 kW at −15
◦
C installed in a meat factory in Spain. (b) An ARS of 2700 kW
at −30
◦
C installed in a refinery in Germany. (c) An ARS of 1400 kW at −28
◦
C installed in a margarine
factory in The Netherlands (Courtesy of Colibri b.v.-Stork Thermeq b.v.).

184 Refrigeration Systems and Applications
Both vapor-compression and absorption-refrigeration cycles accomplish the removal of heat
through the evaporation of a refrigerant at a low pressure and the rejection of heat through the
condensation of the refrigerant at a higher pressure.
Extensive studies to find suitable chemicals for ARSs were conducted using solubility measure-
ments for given binary systems. Although this information is useful as a rough screening technique
for suitable binary systems, more elaborate investigations now seem necessary to learn more of the
fundamentals of the absorption phenomena.
During the last decade, numerous experimental and theoretical studies on ARSs have been
undertaken to develop alternative working fluids, such as R22-dimethyl ether tetraethylene gly-
col (DMETEG), R21-DMETEG, R22-dimethylformamide (DMF), R12-dimethylacetamide, R22-
dimethylacetamide, and R21-dimethyl ester. Previous studies indicated that ammonia, R21, R22,
and methylamine hold promise as refrigerants, whereas the organic glycols, some amides, esters,
and so on fulfill the conditions for good absorbents. Recently, environmental concerns have brought
some alternative working fluids to the forefront, for example, R123a-ethyl tetrahydrofurfuryl ether
(ETFE), R123a-DMETEG, R123a-DMF, and R123a-trifluoroethanol, because of the CFCs’ ozone
depletion effects.
The cycle efficiency and the operating characteristics of an ARS depend on the thermophysical
properties of the refrigerant, the absorbent, and their combinations. The most important properties
for the selection of the working fluids are vapor pressure, solubility, density, viscosity, and thermal
stability. Knowledge of these properties is required to determine the other physical and chemical
properties, as well as the parameters affecting performance, size, and cost.
Note that ammonia will quickly corrode copper, aluminum, zinc, and all alloys of these metals,
therefore these metals cannot be used where ammonia is present. From common materials only
steel, cast iron, and stainless steel can be used in ammonia ARSs. Most plastics are also resistant
to chemical attack by ammonia, hence plastics are suitable for valve seats, pump parts, and other
minor parts of the system.
4.7.1 Basic ARSs
It is considered that the ARS is similar to the vapor-compression refrigeration cycle (using the
evaporator, condenser, and throttling valve as in a basic vapor-compression refrigeration cycle),
except that the compressor of the vapor-compression system is replaced by three main elements – an
absorber, a solution pump, and a generator. Three steps, absorption, solution pumping, and vapor
release, take place in an ARS.
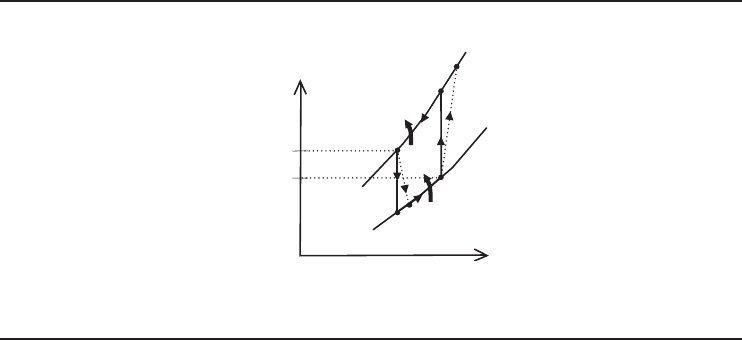
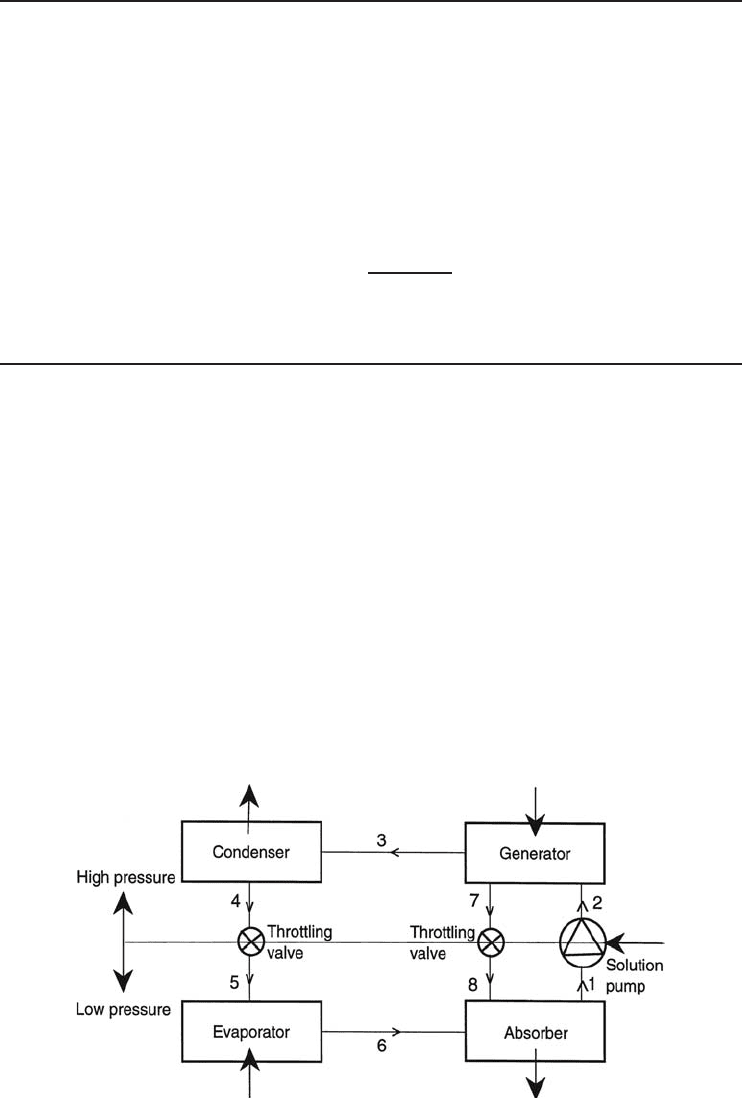
In Figure 4.20, a basic ARS, which consists of an evaporator, a condenser, a generator, an
absorber, a solution pump, and two throttling valves, is schematically shown. The strong solution
(a mixture strong in refrigerant), which consists of the refrigerant and absorbent, is heated in the
high-pressure portion of the system (the generator ). This drives refrigerant vapor off the solution.
The hot refrigerant vapor is cooled in the condenser until it condenses. Then the refrigerant liq-
uid passes through a throttling valve into the low-pressure portion of the system, the evaporator.
The reduction in pressure through this valve facilitates the vaporization of the refrigerant, which
ultimately effects the heat removal from the medium. The desired refrigeration effect is then pro-
vided accordingly. The weak solution (weak in refrigerant) flows down through a throttling valve
to the absorber. After the evaporator, the cold refrigerant comes to the absorber and is absorbed
by this weak solution (i.e., absorbent), because of the strong chemical affinity for each other. The
strong solution is then obtained and is pumped by a solution pump to the generator, where it is
again heated, and the cycle continues. It is significant to note that the system operates at high
vacuum at an evaporator pressure of about 1.0 kPa; the generator and the condenser operate at
about 10.0 kPa.
Refrigeration Cycles and Systems 185
Figure 4.20 AbasicARS.
4.7.2 Ammonia–Water (NH
3
–H
2
O) ARSs
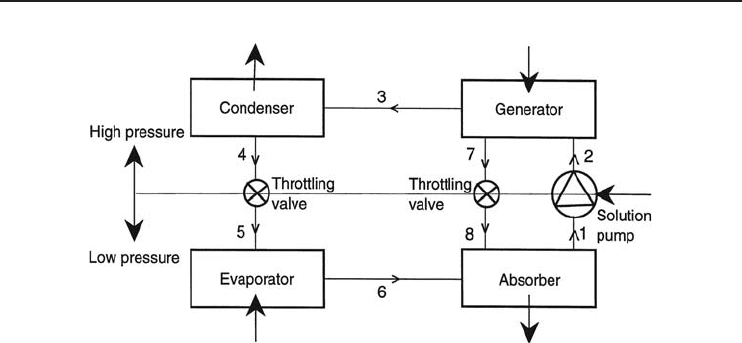
In practical ARSs, the utilization of one or two heat exchangers is very common. Figure 4.21
represents a practical ARS using a working fluid of ammonia as the refrigerant and water as the
absorbent, with two exchangers. As can be seen from the figure, in addition to two heat exchangers,
this system employs an analyzer and a rectifier. These devices are used to remove the water vapor
that may have formed in the generator, so that only ammonia vapor goes to the condenser.
The system shown in Figure 4.21 utilizes the inherent ability of water to absorb and release
ammonia as the refrigerant. The amount of ammonia vapor which can be absorbed and held in a
water solution increases with rising pressure and decreases with rising temperature. Its operation is
same as the system given in Figure 4.20, except for the analyzer, rectifier, and heat exchangers. In
the absorber, the water absorbs the ammonia at the condenser temperature supplied by circulating
water or air, and hence a strong solution (about 38% ammonia concentration) occurs.
Because of physical limitations, sometimes complete equilibrium saturation may not be reached in
the absorber, and the strong solution leaving the absorber may not be as fully saturated with water as
its pressure and temperature would require. This strong solution from the absorber enters the solution
pump (the only moving part of the system), which raises its pressure and delivers the solution into
the generator through the heat exchanger. Pumped strong solution passes into generator via heat
exchanger where strong solution is preheated before being discharged into ammonia generator.
Note that the pumping energy required is only a few percent of the entire refrigeration energy
requirement. The generator, which is heated by an energy source (saturated steam or other heat
source via heating coils or tube bundles), raises the temperature of the strong solution causing the
ammonia to separate from it. The remaining weak solution (about 24% ammonia concentration)
absorbs some of the water vapor coming from the analyzer/rectifier combination and flows down
to the expansion valve through the heat exchanger. It is then throttled into the absorber for further
cooling as it picks up a new charge of the ammonia vapor, thus becoming a strong solution. The
hot ammonia in the vapor phase from the generator is driven out of solution and rises through
the rectifier for possible separation of the remaining water vapor. Then it enters the condenser
and is released to the liquid phase. Liquid ammonia enters the second heat exchanger and loses
some heat to the cool ammonia vapor. The pressure of liquid ammonia significantly drops in the
throttling valve before it enters the evaporator. The cycle is completed when the desired cooling
load is achieved in the evaporator. Cool ammonia vapor obtained from the evaporator passes into
186 Refrigeration Systems and Applications
Analyzer
Rectifier
Generator
First heat
exchanger
Throttling valve
Throttling valve
Evaporator
Q
E
Absorber
7
8
9
10
12
11
Condenser
Second hea
t
exchanger
Solution pump
4
5
6
2
1
3
Figure 4.21 A practical ammonia–water ARS.
the absorber and is absorbed there. This absorption activity lowers the pressure in the absorber and
causes the vapor to be taken off from the evaporator. When the vapor goes into liquid solution
it releases both its latent heat and a heat of dilution. This energy release has to be continuously
dissipated by the cooling water or air.
The heat introduced into the absorption system in the generator (from steam heat) and the evapo-
rator (from actual refrigeration operation) has to be rejected to the outside. One heat ejection occurs
in the ammonia condenser and other heat ejection occurs in the ammonia absorber. Reabsorption of
ammonia into weak solution generates heat and unfortunately this heat has to be rejected so that the
absorption process can function. Aqua ammonia consists of water and ammonia. Water can easily
absorb ammonia and stay in solution under normal temperature; hence the absorber has to be cooled
with cooling water or air. Evaporated ammonia in the generator is passed through the distilling
column where the ammonia is concentrated into nearly pure ammonia vapor before going into the
condenser. Once ammonia is turned into liquid it is let down into the evaporator, low-pressure side,
where ammonia is again turned into vapor, by evaporation, while picking up heat from the confined
refrigerated space. Ammonia vapor is then absorbed in the absorber to complete the cycle.
For ammonia–water ARSs, the most suitable absorber is the film-type absorber for the following
reasons (Keizer, 1982):
• high heat and mass transfer rates,
• good overall performance, and
• large concentration rates.

Refrigeration Cycles and Systems 187
Further information and detailed discussion of energy and mass balances and limiting conditions
in the analyzer and rectifier, along with some examples, can be found in Gosney (1982).
4.7.3 Energy Analysis of an ARS
As mentioned earlier, energy analysis of an ARS refers to the first law of thermodynamic analysis
of an open (control volume) system. Therefore, each component in the ARS is considered a steady-
state steady-flow process, and we will write energy balance equations, equating that input energies
(including work) to output energies. Note that in vapor-compression refrigeration systems, the mass
flow rate of the refrigerant was constant throughout the cycle. However, here in ARS we have two
fluids (making a working fluid) as refrigerant and absorbent and their composition at different points
is different, particularly in the absorber and generator. Therefore, we also include mass balance
equations for those two components in addition to energy balance equations. We refer to Figure 4.21
for the state points in the following equations.
• Absorber:
Energy balance: ˙m
6
h
6
+˙m
12
h
12
=˙m
1
h
1
+
˙
Q
A
(4.42)
Mass balance equation: ˙m
ws
X
ws
+˙m
r
=˙m
ss
X
ss
(4.43)
where
˙
Q
A
is the absorber head load in kW; X is the concentration; ˙m
ws
=˙m
6
is the mass flow
rate of the weak solution in kg/s; ˙m
ss
=˙m
1
is the mass flow rate of the strong solution in kg/s;
and ˙m
r
is the mass flow rate of the refrigerant in kg/s. Here, state 1 is a saturated liquid at the
lowest temperature in the absorber and is determined by the temperature of the available cooling
water flow or air flow.
• Solution pump:
˙m
1
h
1
+
˙
W
P
=˙m
2
h
2
(4.44)
The compression is almost isothermal.
• First heat exchanger:
˙m
2
h
2
+˙m
4
h
4
=˙m
3
h
3
+˙m
5
h
5
(4.45)
• Generator:
Energy balance: ˙m
3
h
3
+
˙
Q
gen
=˙m
4
h
4
+˙m
7
h
7
(4.46)
Mass balance: ˙m
ws
X
ws
+˙m
r
=˙m
ss
X
ss
(4.47)
where
˙
Q
G
is the heat input to generator in kW; ˙m
ws
=˙m
4
and ˙m
ss
=˙m
3
.
• Condenser:
˙m
7
h
7
=˙m
8
h
8
+
˙
Q
H
(4.48)
• Second heat exchanger:
˙m
8
h
8
+˙m
11
h
11
=˙m
9
h
9
+˙m
12
h
12
(4.49)
• Expansion (throttling) valves:
˙m
5
h
5
=˙m
6
h
6
⇒ h
5
= h
6
(4.50)
˙m
9
h
9
=˙m
10
h
10
⇒ h
9
= h
10
(4.51)
The process is isenthalpic pressure reduction.
188 Refrigeration Systems and Applications
• Evaporator:
˙m
10
h
10
+
˙
Q
L
=˙m
11
h
11
(4.52)
For the entire system, the overall energy balance of the complete system can be written as
follows, by considering that there is negligible heat loss to the environment:
˙
W +
˙
Q
L
+
˙
Q
gen
=
˙
Q
A
+
˙
Q
H
(4.53)
The COP of the system then becomes
COP =
˙
Q
L
˙
W
P
+
˙
Q
gen
(4.54)
where
˙
W
P
is the pumping power requirement, and it is usually neglected in the COP calculation.
Example 4.6
Consider a basic ARS using ammonia–water solution as shown in Figure 4.22. Pure ammonia enters
the condenser at 2.5 MPa and 60
◦
C at a rate of 0.022 kg/s. Ammonia leaves the condenser as a
saturated liquid and is throttled to a pressure of 0.15 MPa. Ammonia leaves the evaporator as a
saturated vapor. Heat is supplied to the generator by geothermal liquid water that enters at 135
◦
C
at a rate of 0.35 kg/s and leaves at 120
◦
C. Determine (a) the rate of cooling provided by the system
and (b) the COP of the system. (c) Also, determine the second-law efficiency of the system if the
ambient temperature is 25
◦
C and the temperature of the refrigerated space is 2
◦
C. The enthalpies
of ammonia at various states of the system are given as h
3
= 1497.4 kJ/kg, h
4
= 482.5 kJ/kg,
h
6
= 1430.0 kJ/kg. Also, take the specific heat of water to be 4.2 kJ/kg·
◦
C.
Solution
(a) The rate of cooling provided by the system is
˙
Q
L
=˙m
R
(h
6
− h
5
) = (0.022 kg/s)(1430.0 − 482.5) kJ/kg = 20.9kW
Figure 4.22 The basic ARS considered in Example 4.6.
Refrigeration Cycles and Systems 189
(b) The rate of heat input to the generator is
˙
Q
gen
=˙m
geo
c
p
(T
geo,in
− T
geo,out
) = (0.35 kg/s)(4.2kJ/kg·
◦
C)(135 − 120)
◦
C = 22.1 kW
Then the COP becomes
COP =
˙
Q
L
˙
Q
gen
=
20.9kW
22.1kW
= 0.946
(c) In order to develop a relation for the maximum (reversible) COP of an ARS, we consider a
reversible heat engine and a reversible refrigerator as shown in Figure 4.23. Heat is absorbed
from a source at T
s
by a reversible heat engine and the waste heat is rejected to an environment
T
0
. Work output from the heat engine is used as the work input in the reversible refrigerator,
which keeps a refrigerated space at T
L
while rejecting heat to the environment at T
0
.Usingthe
definition of COP for an ARS, thermal efficiency of a reversible heat engine and the COP of
a reversible refrigerator, we obtain
COP
abs,rev
=
˙
Q
L
˙
Q
gen
=
˙
W
˙
Q
gen
˙
Q
L
˙
W
= η
th,rev
COP
R,rev
=
1 −
T
0
T
s
T
L
T
0
− T
L
Substituting,
COP
abs,rev
=
1 −
T
0
T
s
T
L
T
0
− T
L
=
1 −
(25 + 273) K
(127.5 + 273) K
(2 + 273) K
(25 − 2) K
= 3.06
Reversible
heat engine
T
0
T
s
Reversible
refrigerator
T
L
T
0
Q
gen
Q
L
W
·
·
·
Figure 4.23 The system used to develop reversible COP of an absorption-refrigeration system.
The temperature of the heat source is taken as the average temperature of geothermal water.
Then the second-law efficiency of this absorption system is determined to be
η
II
=
COP
COP
abs,rev
=
0.946
3.06
= 0.309 = 30.9%
190 Refrigeration Systems and Applications
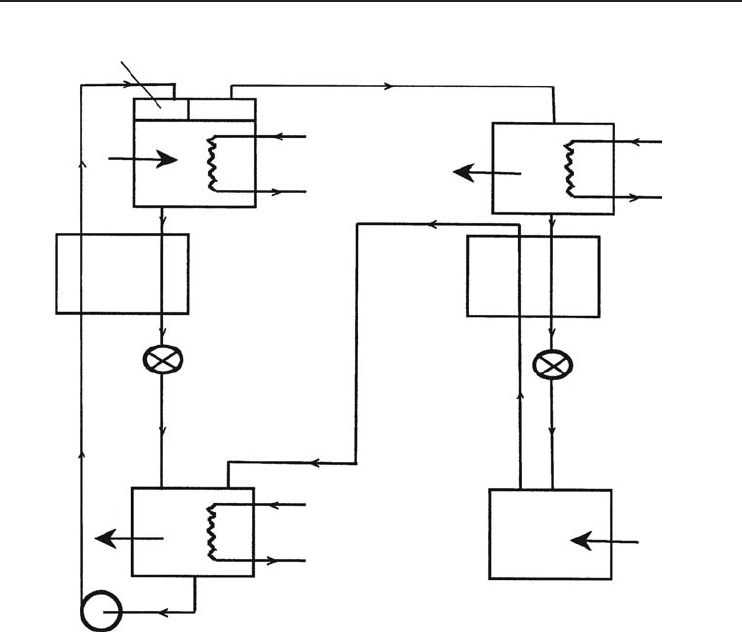
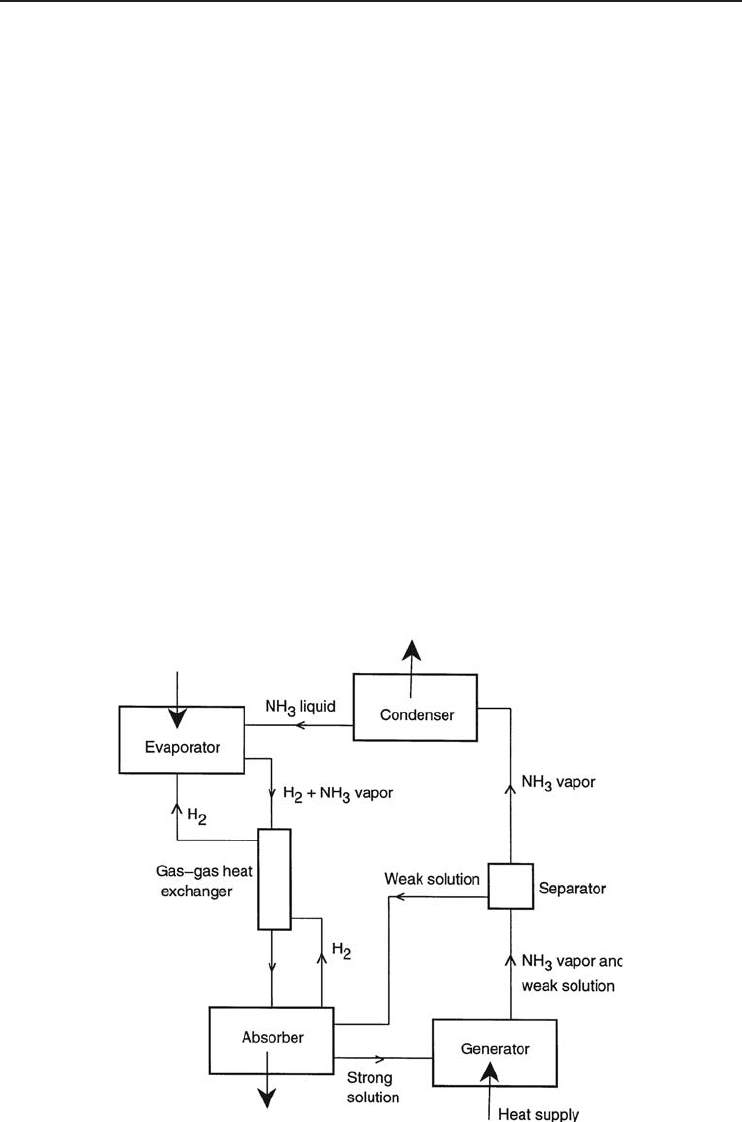
4.7.4 Three-Fluid (Gas Diffusion) ARSs
The two-fluid ARS succeeded in replacing a compressor which requires a large amount of shaft
work by a liquid pump with a negligible energy requirement compared to the refrigeration effect.
By addition of a third fluid, the pump is removed, completely eliminating all moving parts. This
system is also called the von Platen–Munters system after its Swedish inventors. This type of system
is shown in Figure 4.24. The most commonly used fluids are ammonia (as refrigerant), water (as
absorbent), and hydrogen, a neutral gas used to support a portion of the total pressure in part of
the system. Hydrogen is called the carrier gas. The unit consists of four main parts: the boiler,
condenser, evaporator, and absorber. In gas units, heat is supplied by a burner, and when the
unit operates on electricity the heat is supplied by a heating element. The unit charge consists of a
quantity of ammonia, water, and hydrogen at a sufficient pressure to condense ammonia at the room
temperature for which the unit is designed. This method of absorption refrigeration is presently
used in domestic systems where the COP is less important than quiet trouble-free operation. In
the system shown in Figure 4.24, the cold ammonia vapor with hydrogen is circulated by natural
convection through a gas–gas heat exchanger to the absorber, where the ammonia vapor comes
in contact with the weak solution from the separator. At the low temperature of the ammonia
and hydrogen, absorption of the ammonia occurs and hence hydrogen alone rises through the heat
exchanger to the evaporator, while the strong solution flows down by gravity to the generator.
4.7.5 Water–Lithium Bromide (H
2
O–LiBr) ARSs
These ARSs utilize a combination of water (as the refrigerant) and lithium bromide (as the
absorbent), as the working fluid. These systems are also called absorption chillers and have a wide
range of application in air conditioning and chilling or precooling operations and are manufactured
Figure 4.24 A three-fluid ARS.
