Gupta D. (Ed.). Diffusion Processes in Advanced Technological Materials
Подождите немного. Документ загружается.


222 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
TiAl and NiAl are the only phases that reveal a nonlinear Arrhenius
behavior of self-diffusion at high temperatures; that is, above 1470 K in
TiAl
[7]
and above 1500 K in NiAl.
[36]
The curvature in the Arrhenius plot
of TiAl is explained by an additional contribution of a specific ASB mech-
anism [Fig. 4.7(b)] and is related to the large concentration of thermal Ti
antistructure atoms at high temperatures, even in the Al-rich TiAl
alloys.
[7, 8]
The observed upward deviation of D
*
Ni
from the otherwise
straight Arrhenius line in NiAl at T 1500 K seems to have another ori-
gin. The concentration of thermal Ni antistructure atoms in NiAl is
notably lower than in TiAl [see Fig. 4.3(b)], which corresponds to a higher
degree of order in NiAl. Moreover, the upward deviation of D
*
Ni
is
observed in NiAl at about the same temperature, independent of the com-
position.
[36]
These features correlate with recent measurements of the
vacancy concentration in NiAl alloys.
[81]
The regular change of Fe diffusion in the B2 FeAl alloys of different
compositions (Fe
3
Al, Fe
2
Al, and FeAl) was related to the change in the
long-range order parameter S.
[92]
Using the long-range order parameter for
the B2 order, which is related to the Al content by the relation S
max
2X
Al
(X
Al
is the atomic fraction of aluminium), the activation enthalpy of Fe self-
diffusion in the B2 region can be described by the following relation:
[92]
Q
Fe
(220 46 S
2
max
)kJmol. (13)
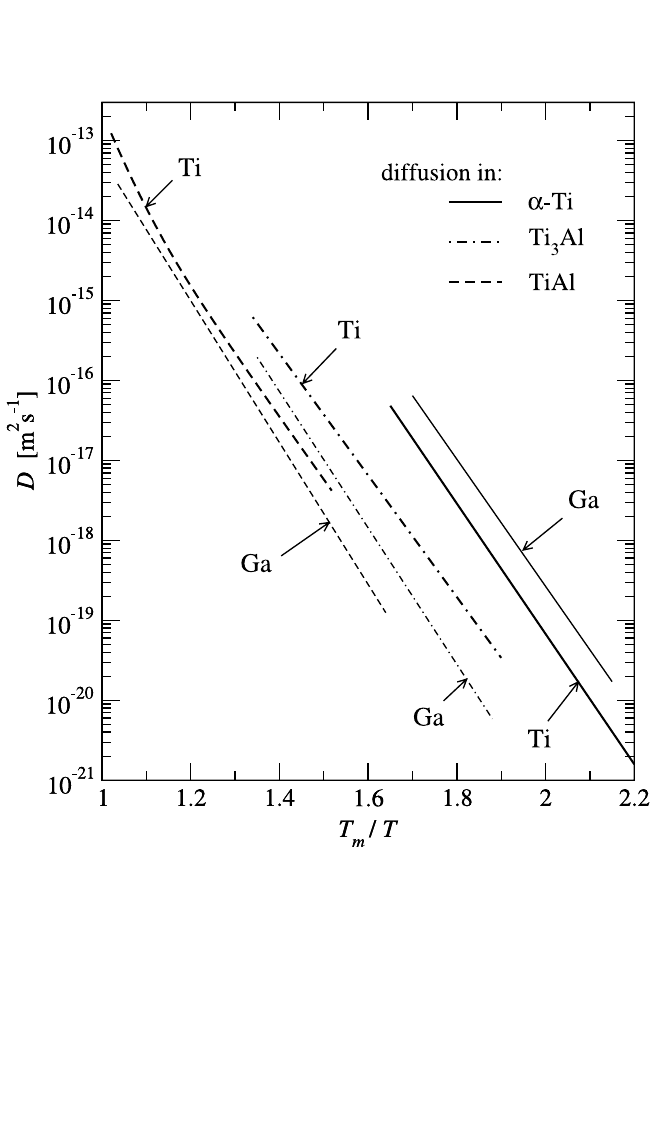
In Fig. 4.18, Ti self-diffusion and Ga solute diffusion in different tita-
nium aluminides are compared with respect to the homologous tempera-
ture, T
m
T. Here T
m
is the melting temperature of the corresponding stoi-
chiometric compound. Systematic changes in the diffusion behavior of the
Ti aluminides are clearly observed: Ti and Ga diffusion decreases gradu-
ally from pure a-Ti to Ti
3
Al and finally to TiAl.
This phenomenon most likely originates from: (1) the sublattice dif-
fusion mechanism in these materials, (2) prevailing vacancy concentra-
tions on the transition metal sublattices, and (3) a systematic decrease of
the coordination number of the transition metal sublattice. Figure 4.18
reveals that the self-diffusivity follows the following relationship: D
*
Ti
D
*
Ti
3
Al
D
*
TiAl
, in a line with the coordination number of the transition
metal sublattices in the corresponding structures: z
Ti
Ti
12 z
Ti
Ti
3
Al
8
z
Ti
TiAl
4.
Figure 4.3(a) and (b) indicates that the vacancy concentrations on the
transition metal sublattices are similar in Ti
3
Al and TiAl. The ratio QT
m
also lies in the common limits of vacancy diffusion: 17.4k for Ti
3
Al and
17.5k for TiAl. These values are close to that in pure a-Ti, QT
m
18.8k.
The main difference in the absolute values of the diffusivities therefore
stems from the pre-exponential factors D
0
gfa
2
n
0
exp{S
f
/k}. Here, f is the
Chapter-04.qxd 11/29/04 6:36 PM Page 222
correlation factor; a is the lattice constant; n
0
is the attempt frequency; S
f
is the formation entropy; the numerical factor g reflects the smaller coor-
dination number of the sublattice with respect to the whole lattice: g 1,
23, and 13; f 0.781, 0.467,
[105]
and 0.71;
[80]
and a 2.95, 2.91, and
2.83 Å for the a-Ti, Ti
3
Al, and TiAl phases, respectively. Comparative
ratios of the products g gfa
2
for the three phases are g
Ti
:g
Ti
3
Al
:
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 223
Figure 4.18 Ti and Ga diffusion in a-Ti (solid lines
[68, 77]
), Ti
3
Al (dashed-dotted
lines
[65, 68]
), and TiAl (dashed lines
[7, 68]
) as functions of the reduced temperature
T
m
T.
Chapter-04.qxd 12/2/04 1:50 PM Page 223

g
TiAl
5.4:3.2:1. From the actual diffusion measurements shown in Fig. 4.18
and Tables 4.2 and 4.3, the corresponding ratios of 70:5:1 are obtained.
While the ratios g
Ti
3
Al
:g
TiAl
are reasonable, the one for Ti is rather large.
The large discrepancy in a-Ti may be attritubed to the entropy factor,
which should be taken into account, especially for pure Ti. Thus, the
observed systematics alone does not result from the structural limitations,
although the change in D follows the constraints imposed by the given
sublattice structures.
Note that the Ga diffusivity D
*
Ga
in these compounds follows a similar
tendency (Fig. 4.18). Such behavior was also established for the Al diffu-
sion data extracted from the interdiffusion measurements in the Ti alu-
minides.
[68]
This can be explained by the same arguments as for transition
metal self-diffusion and further indicates that the sublattice diffusion
mechanism operates for Ga and Al in the Ti aluminides.
4.6 Grain Boundary Diffusion
Grain boundary (GB) diffusion in intermetallic compounds was
investigated to a much smaller extent than bulk diffusion. Some exper-
imental information is available already for Ni,
[55, 106–108]
Ti,
[109]
and
Fe
[110]
aluminides. The primary problem, however, is to improve our
understanding of GB diffusion in intermetallic compounds on the atom-
istic level. For example, diffusion mechanisms in GBs, and the effects
of the order and of structural multiplicity, are still not well under-
stood.
[111]
It is not clear if the local disorder at GBs occurs by the same
mechanism as in the bulk lattice, that is, by means of antistructure
atoms or structural vacancy formation.
[111]
Therefore, in this overview,
experimental results will be described and unresolved problems will be
highlighted.
GB diffusion measurements in intermetallic compounds were per-
formed in the Harrison B-regime conditions.
[112]
Schematically, in such a
case, the tracer atoms diffuse fast along GBs. Then, at some depth, they
penetrate into the bulk of the grains and diffuse further, at a slower rate,
over a distance that is distinctly larger than the GB width d.
[112]
As a result,
the so-called triple product P sdD
gb
can be determined from the
detected diffusion profile. Here s is the segregation factor and D
gb
is the
GB diffusion coefficient. In GB self-diffusion experiments in pure metals,
s 1 and P represents a double product P dD
gb
. In intermetallic com-
pounds, especially in off-stoichiometric alloys, we can expect a certain
segregation of a constituent component to the GBs, resulting in s 1. The
total effect, however, is likely to be small and can be neglected in a first
approximation.
224 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-04.qxd 11/29/04 6:36 PM Page 224
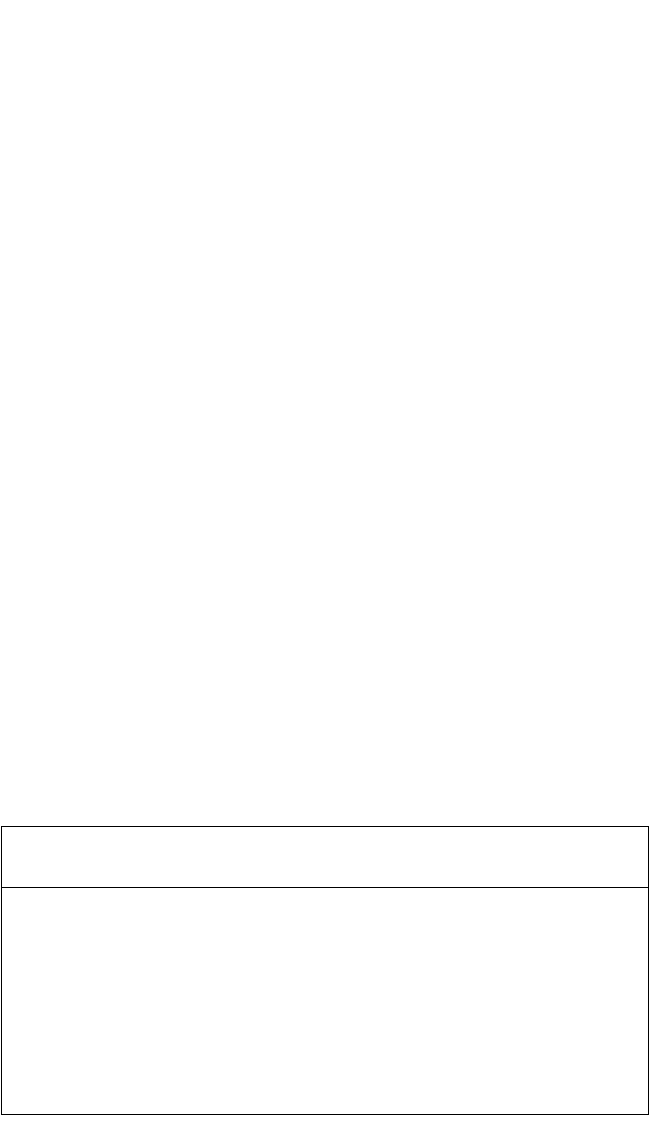
4.6.1 Ni
3
Al
Ni GB diffusion in the Ni
3
Al phase field was systematically investigated
in the temperature interval 968 to 1190 K.
[55]
Although only a marginal com-
positional dependence of Ni bulk diffusion is observed in this compound, a
distinct V-type dependence of the product P
Ni
on the composition is estab-
lished, with a minimum in P
Ni
about the stoichiometric composition
[55]
[see
Fig. 4.19(a)]. The resulting activation enthalpies reveal a maximum at about
the stoichiometric composition and decrease on both sides as the Ni content
deviates from stoichiometry [Fig. 4.19(b)]. The Arrhenius parameters of the
GB diffusivity P
Ni
in Ni
3
Al are summarized in Table 4.6.
Zulina et al. studied Ni GB diffusion in near-stoichiometric Ni
3
Al and
several Ni
3
Al-based alloys.
[108]
The resulting values of P
Ni
are smaller than
those measured by Frank and Herzig
[55]
at the given composition, and the
activation enthalpy of Ni GB diffusion in Ni
74.8
Al
25.2
is larger
[Fig. 4.19(b)]. However, note that Zulina et al. determined the activation
enthalpy Q
Ni
gb
by fitting only three experimental points in the Arrhenius
diagram. It thus includes a larger uncertainty.
As shown in Fig. 4.19(a), Cermak et al.
[106]
observed a maximum in
the P
Ni
versus composition plot instead of a minimum measured by Frank
and Herzig
[55]
at temperatures 950 K. On the other hand, at T 900 K,
a minimum of P
Ni
around stoichiometry was observed, in agreement with
Frank and Herzig.
[55]
The deduced activation enthalpies of Ni GB diffu-
sion
[106]
are remarkably larger than the values determined by Frank and
Herzig.
[55]
This behavior may be explained by GB segregation of residual
impurities in the alloys and/or by the applied experimental procedure.
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 225
Table 4.6. Arrhenius Parameters of Grain Boundary Self-Diffusion in Ni,
Ti, and Fe Aluminides
Composition P
0
Q
gb
Phase Tracer at.% Al (m
3
s
1
) (kJmol) Q
gb
Q
v
Ref.
Ni
3
Al Ni 26.6 7.3 10
14
153 0.51 55
24.8 4.4 10
14
154 0.51 55
22.4 2.2 10
15
115 0.38 55
NiAl Ni 50.0 4.6 10
15
152 0.53 80
46.5 9.0 10
14
182 0.65 80
Ti
3
Al Ti 25 4.8 10
13
195 0.68 109
33 3.2 10
11
252 0.88 109
TiAl Ti 56 4.6 10
15
123 0.47 109
Fe
3
Al Fe 25 4.0 10
9
227 0.8 110
Chapter-04.qxd 11/29/04 6:36 PM Page 225
73 74
75 76
77
78
Composition [at.% Ni]
10
-22
10
-21
10
-20
P [m
3
s
-1
]
22
23
24
252627
Composition [at.% Al]
1190 K
1066 K
968 K
1012 K
(a) Triple product P for Ni
3
Al alloys. Open circles
[55]
and solid
squares
[106]
at T = 1013 and 1073 K, respectivel
y
.
73 74
75 76
77
78
Composition [at.% Ni]
100
125
150
175
200
225
250
Q
gb
[kJ/mol]
22
23
24
252627
Composition [at.% Al]
Ni in Ni (Frank et al.)
( )
Ni in Ni (Mishin et al.)
(b) Activation enthalpy Q
gb
for Ni
3
Al alloys. Open circles
are from Frank and Herzig
[55]
; dashed lines represent
activation enthalpy of grain boundary diffusion of Ni in
pure Ni
[55,113]
; the full triangle is from Zulina et al.
[108]
Figure 4.19 The triple product P and the activation enthalpy Q
gb
of Ni GB diffusion in Ni
3
Al as functions of composition.
226 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MAT ERIALS
Chapter-04.qxd 11/29/04 6:36 PM Page 226

Frank and Herzig established
[55]
that by increasing the Ni content on
the Ni-rich side of Ni
3
Al, both P
Ni
and Q
Ni
gb
values approach the values
measured for pure Ni [see Fig. 4.19(b)]. This can be explained by the seg-
regation of Ni, which was experimentally observed
[114]
at GBs in Ni
3
Al on
the Ni-rich side. On the other hand, the increase of the P
Ni
values and the
decrease in Q
Ni
gb
on the Al-rich side can be explained by an excess of Al
segregating to GBs.
[114]
These Al atoms increase the free volume at GBs;
thus the formation enthalpy of the Ni vacancies at the GBs is
decreased.
[115]
These features most likely contribute to the enhancement of
Ni GB diffusion in these compositions.
[55]
Atomistic simulations are nec-
essary to explain the effect of GB segregation on diffusion behavior.
Polycrystalline Ni
3
Al is well known to reveal grain boundary brittle-
ness, which is successfully suppressed by microalloying with boron.
[116, 117]
The effect of boron addition on Ni GB diffusion in Ni
3
Al was carefully
studied.
[106, 118]
The doping of Ni
3
Al with 0.24 at.% B and the segregation
of B decrease Ni GB diffusion by a factor of 2 to 3 and increase the acti-
vation enthalpy Q
gb
slightly with respect to pure Ni
3
Al.
[55, 118]
This effect is
explained by an increase in GB cohesion and Ni–Ni atom bonding upon
boron alloying.
[118]
Moreover, B segregation is likely to block otherwise
energetically favorable diffusion paths along GBs and increases the
vacancy formation enthalpies in the boundary core.
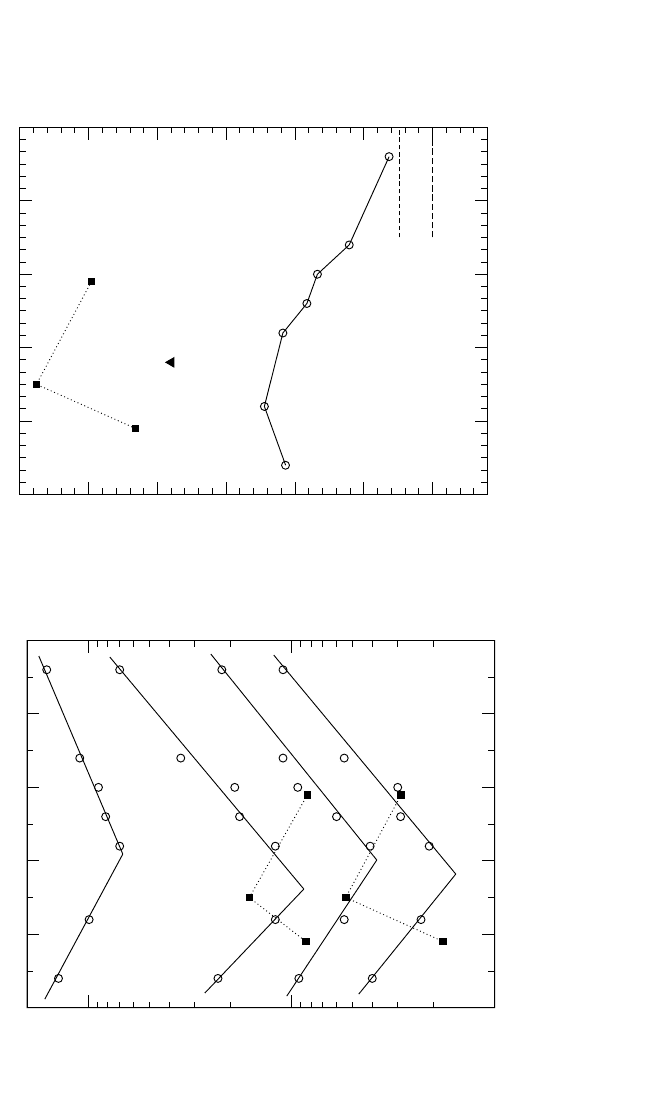
4.6.2 Ti
3
Al
Ti GB self-diffusion was studied as a function of composition in Ti
3
Al
within the temperature interval 940 to 1316 K.
[109]
As shown in Sec. 4.4.2.1,
Ti bulk self-diffusion is practically independent of composition in the
Ti
3
Al phase.
[65]
However, Ti GB self-diffusion reveals a distinct composi-
tional dependence: As the Al content on the Al-rich side of the compound
increases, the P
Ti
values systematically decrease [see Fig. 4.20(a)]. This
tendency is opposite to that observed in the other A
3
B compound investi-
gated, Ni
3
Al [Fig. 4.19(a)]. With increasing deviation from the stoichio-
metric composition, the GB diffusivity in Ti
3
Al is decreased and the acti-
vation enthalpy increases to an unusual large value in comparison with
that of bulk diffusion in this compound. In Fig. 4.20(b), the compositional
dependence of the activation enthalpy of Ti GB diffusion in Ti
3
Al is com-
pared with that of Ti GB diffusion in pure a-Ti.
[109]
Q
Ti
gb
in a-Ti is similar
within experimental accuracy to the value of Q
Ti
gb
in stoichiometric Ti
3
Al.
The absolute value of the product P
Ti
in a-Ti, however, is larger by one
order of magnitude than that in Ti
75
Al
25
.
[109]
This correlates with the geo-
metric limitations imposed by the particular structure of the Ti sublattice
in GBs of Ti
3
Al.
[119]
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 227
Chapter-04.qxd 11/29/04 6:36 PM Page 227
7.5
8
8.5
9
9.5
T
-1
[10
-4
K
-1
]
10
-23
10
-22
10
-21
10
-20
P [m
3
s
-1
]
1300 1200 1100
T [K]
Ti
3
Al
25
28
33
35
32
(a) Arrhenius dependence of triple product P in Ti
3
Al alloys.
Compositions in at.% are marked on each plot.
64 66 68
70 72 74
76
Composition [at.% Ti]
150
175
200
225
250
275
300
Q
gb
[kJ/mol]
24
262830323436
Composition [at. % Al]
Ti in α
2
-Ti
3
Al
Ti in α-Ti
(b) Activation enthalpy Q
gb
of Ti grain boundary diffusion
in α-Ti and α
2
-Ti
3
Al alloys.
[109]
Figure 4.20 Tr iple product P and the activation enthalpy Q
gb
of Ti GB diffusion in a
2
-Ti
3
Al.The dashed line is for grain bound-
ary diffusion in a-Ti.
228 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Chapter-04.qxd 11/29/04 6:36 PM Page 228

The Arrhenius parameters of Ti GB diffusion in several representative
Ti
3
Al alloys are given in Table 4.6. While in stoichiometric Ti
75
Al
25
the
ratio Q
Ti
gb
Q
v
Ti
adopts a typical value for GB diffusion, Q
Ti
gb
Q
v
Ti
0.68, this
value increases remarkably in Al-rich compositions. In Ti
67
Al
33
, Q
Ti
gb
hardly deviates from Q
v
Ti
, the activation enthalpy of Ti bulk self-diffusion
in this alloy. Note that such larger values of Q
gb
Q
v
were already observed
in other cases: Q
gb
Q
v
0.9 for Co diffusion in CoSi
2
[120]
and Q
gb
Q
v
0.8
for Fe diffusion in Fe
3
Al,
[110]
as discussed in Sec. 4.6.5.
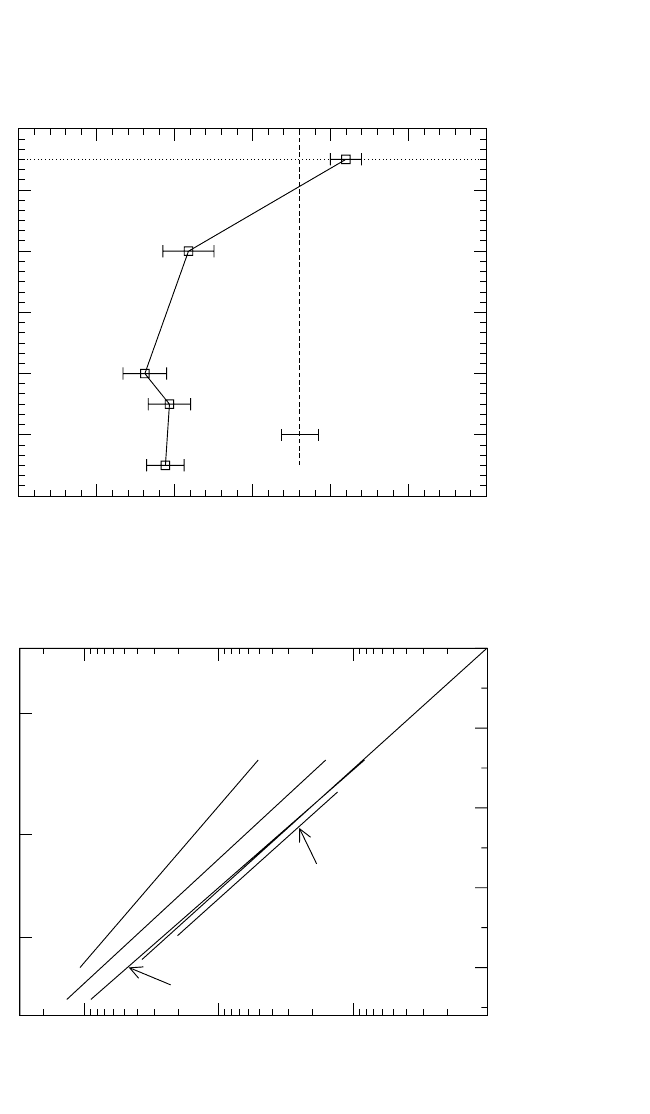
4.6.3 TiAl
Ti GB self-diffusion was recently measured in several TiAl alloys.
[109]
The data suggest negligible compositional dependence of Ti GB diffusion
in TiAl. The Arrhenius dependence of Ti GB diffusion in two representive
compositions of a
2
-Ti
3
Al and a-Ti is compared in Fig. 4.21. Ti diffuses
very fast along GBs in TiAl, even in comparison with a-Ti. The ratio
Q
Ti
gb
Q
v
Ti
0.45 for TiAl is typical for pure close-packed metals.
The comparison of the absolute values of Ti GB self-diffusion in
Ti
3
Al and TiAl shows that the GB self-diffusivity in TiAl is larger by
about one order of magnitude than in Ti
3
Al with stoichiometric composi-
tion, and even larger by two orders of magnitude than P
Ti
in Ti
68
Al
32
at
similar temperatures.
Ti diffusion was measured along a
2
g phase boundaries between a
2
-
Ti
3
Al and g-TiAl phases.
[121]
Material, so-called polysynthetically twinned
(PST) single crystals, with a uniformly oriented lamellar structure, was
investigated.
[121]
In Fig. 4.21, these results are compared with Ti GB self-
diffusion in both a
2
and g phases. Diffusion in a
2
g phase boundaries is
very slow and is in line with the GB diffusion data in the Al-rich Ti
3
Al
phase. These low values of Ti GB diffusivity in the a
2
g interphase
boundaries reflect their compact structure and were explained by vacancy-
mediated nearest neighbor jumps in the Ti sublattice of the phase bound-
ary in correspondence with its special atomic arrangement.
[121]
4.6.4 NiAl
Ni GB self-diffusion in NiAl was measured within the temperature
interval 958 to 1194 K.
[80]
No distinct compositional dependence of Ni GB
self-diffusion has been established in the limited range of compositions
investigated. The results are given in Fig. 4.22. A slight tendency for an
increase of the activation enthalpy for Ni GB diffusion in Ni-rich NiAl
alloys is shown in Fig. 4.22(b). The Arrhenius parameters of Ni GB self-
diffusion in representative NiAl alloys are listed in Table 4.6.
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 229
Chapter-04.qxd 11/29/04 6:36 PM Page 229
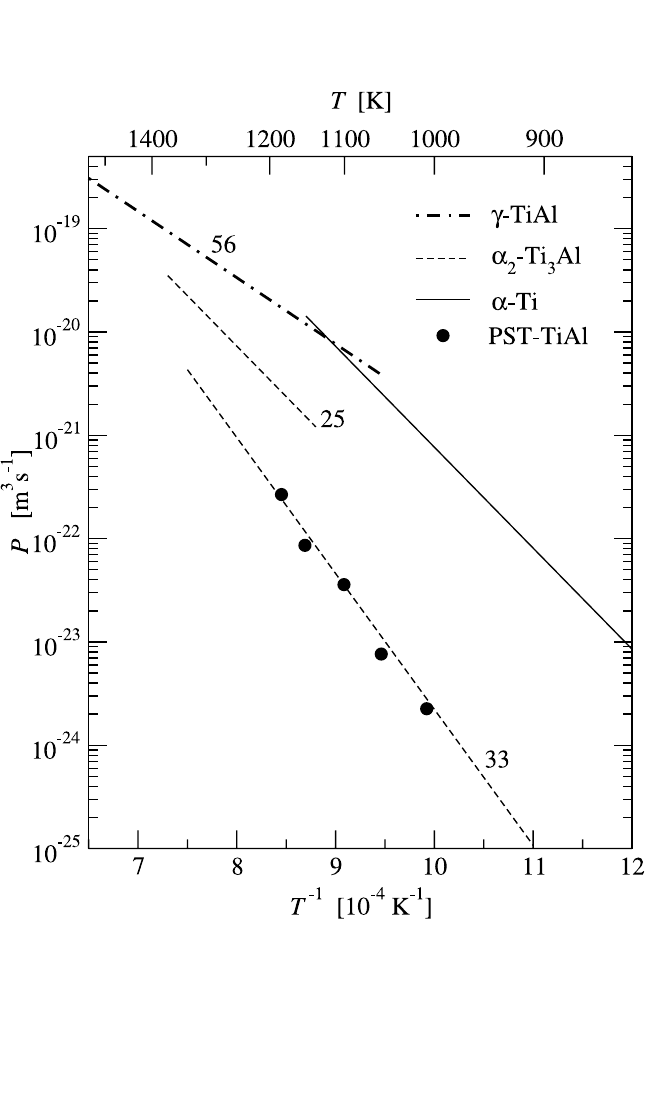
230 DIFFUSION PROCESSES IN ADVANCED TECHNOLOGICAL MATERIALS
Figure 4.21 Comparison of grain boundary diffusion in pure a-Ti (solid line),
a
2
-Ti
3
Al (dashed lines), g-TiAl (dotted-dashed line), and TiAl polysynthetically
twinned (PST) interphase boundaries (circles).
[109]
The Al content of the corre-
sponding alloys is indicated in at.%.
Chapter-04.qxd 12/2/04 1:50 PM Page 230
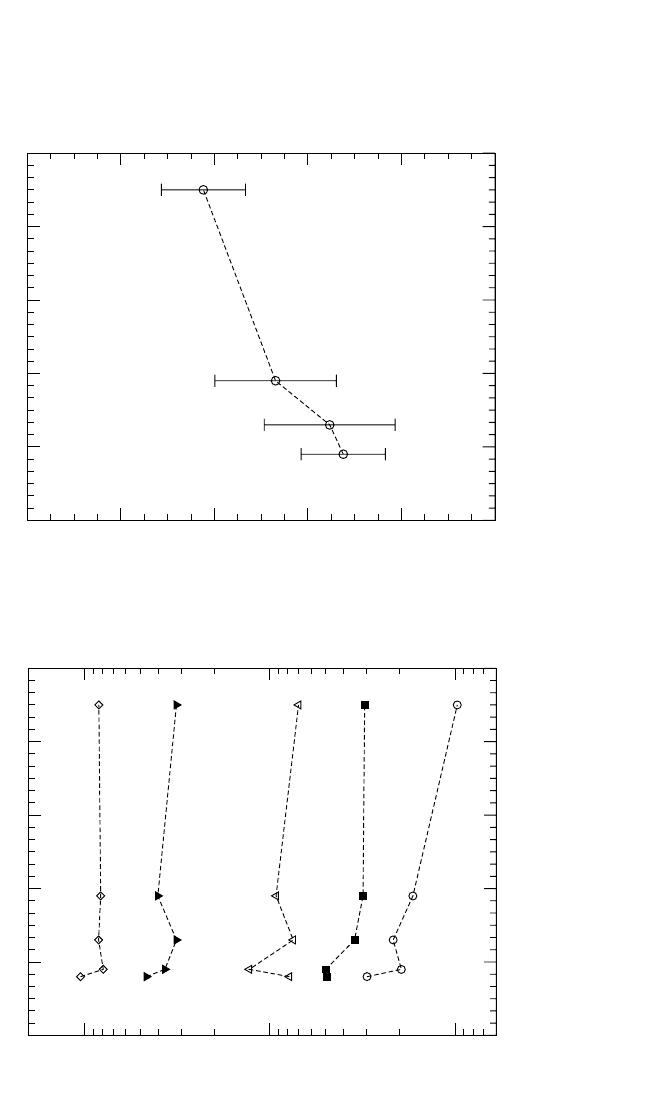
DIFFUSION IN INTERMETALLIC COMPOUNDS, HERZIG, DIVINSKI 231
49
50 51 52 53 54
Composition [at.% Ni]
10
-23
10
-22
10
-21
P [m
3
s
-1
]
464748
49
5051
Composition [at.% Al]
1194 K
958 K
1004 K
1052 K
1115 K
(a) Triple product P for Ni grain boundary diffusion in NiAl
[80]
49
50 51 52 53 54
Composition [at.% Ni]
120
140
160
180
200
220
Q
gb
[kJ/mol]
464748
49
5051
Composition [at.% Al]
(b) Activation enthalpy Q
gb
for Ni grain boundary diffusion in NiAl
[80]
Figure 4.22 Tr iple product P and the activation enthalpy Q
gb
of Ni grain boundary diffusion in NiAl alloys as a function of com-
position.
[80]
Chapter-04.qxd 11/29/04 6:36 PM Page 231
