Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

19.4 Halogenation in the Position 949
␣
In NaOH/H
2
O,these trihalocarbonyl compounds react further because hydrox-
ide addition can lead to elimination of the stabilized trihalocarbanion. After these
addition–elimination steps, the carbanion can gain a proton from solvent or the
carboxylic acid to form a molecule of a haloform, which is a common name for a
trihalomethane (Fig. 19.38). Under these basic conditions the carboxylic acid will
remain deprotonated.
C
R
O
..
..
CI
3
C
R
O
–
..
..
–
HO
..
..
..
HO
..
..
..
–
–
OH
..
..
..
CI
3
CI
3
HCI
3
+
C
R
deprotonation
addition elimination
O
..
..
C
R
O
..
..
O
..
..
..
..
Tetrahedral
intermediate
Iodoform
(a yellow solid)
FIGURE 19.38 Addition of hydroxide to the carbonyl group leads to a tetrahedral intermediate that can lose
triiodomethide anion to generate the carboxylic acid.Transfer of a proton completes the reaction.
I
3
C I
3
C
HH H
pK
a
~ 14 pK
a
= 15.7
–
–
O
..
..
OH
..
..
..
..
+
FIGURE 19.39 The pK
a
of iodoform
is about 14. Iodoform is a relatively
strong acid, and the loss of
CI
3
as
a leaving group is a reasonable step.
Essentially all nucleophilic reagents add to carbonyl groups, often in a reversible
fashion. In this case, the addition reaction is strongly favored by the halogen substi-
tution (p. 780)! So, the addition of hydroxide to the carbonyl looks like a reaction
that is almost certain to occur. Once the addition reaction has taken place, there is
an opportunity to generate the acid and the haloform in an elimination step if the
triiodomethyl anion can be lost as the carbonyl group re-forms. Protonation of the
carbanion completes the reaction.
Is this mechanism reasonable? Is the triiodomethyl anion a good enough leav-
ing group to make this step a sensible one? The iodines are strongly electron with-
drawing, and that property will stabilize the anion. As a check we might look up
the pK
a
of iodoform to see how easily the molecule is deprotonated to form the anion
(Fig. 19.39). The value is about 14, and so iodoform is a relatively strong acid, at
least compared to water (pK
a
15.7).
PROBLEM 19.9 Write out the mechanism for the reaction between acetophenone
and excess chlorine in aqueous sodium hydroxide.
PROBLEM 19.10 If we measure the rates of three reactions of the ketone shown on the
next page, exchange of the α hydrogen for deuterium in D
2
O/DO
, racemization in
H
2
O/HO
, and α bromination using Br
2
/H
2
O/HO
, we find that they are identical.
(continued)
So the loss of
CI
3
in Figure 19.38 does look reasonable. In fact, the iodoform
reaction serves as a diagnostic test for methyl ketones. Formation of iodoform, a
yellow solid, is a positive test for a molecule containing a methyl group attached to
a carbonyl carbon.
950 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
19.4b Halogenation of Carboxylic Acids Halogenation can occur at the
α position of carboxylic acids. Treatment of carboxylic acids with Br
2
and PBr
3
,
or the equivalent, a mixture of phosphorus and bromine, leads ultimately to
formation of the α-bromo acid (Fig. 19.40). The process is known as the
Hell–Volhard–Zelinsky (HVZ) reaction after Carl M. Hell (1849–1926), Jacob
35 ⬚C
D
2
O/NaOD
Br
2
NaOH/H
2
O
H
2
O/NaOH
C
*
* Means optically active
O
H
3
C
CH
2
CH
3
H
3
C
CH
2
CH
3
H
3
C
CH
2
CH
3
D
H
3
C
H
C
Deuterium exchange
O
Br
C
α-Bromination
O
A
B
H
C
C
C
CH
2
CH
3
C
C
Racemization
O
C
dioxane
35 ⬚C
..
..
..
..
..
Br
O
COOH
P/Br
2
100 ⬚C
Br
(~80%)
..
..
..
A SPECIFIC EXAMPLE
..
..
..
..
..
..
H
2
O
CH
2
RC
O
P
Br
2
OH
..
..
OH
..
..
..
..
..
CH
Br
Br
..
..
..
RC
O
..
..
CH
Br
..
..
..
RC
O
THE GENERAL CASE
FIGURE 19.40 The
Hell–Volhard–Zelinsky reaction.
How can the rates of three such different reactions be the same? Explain, using
an Energy versus Reaction progress diagram.
19.4 Halogenation in the Position 951
␣
Volhard (1834–1910), and Nicolai D. Zelinsky (1861–1953). The first step is for-
mation of the acid bromide through reaction with PBr
3
. The acid bromide is in
equilibrium with its enol form (Fig. 19.41). Bromination of the enol form with
Br
2
gives an isolable α-bromo acid bromide.
..
..
..
..
CH
2
RC
O
PBr
3
Br
2
OH
..
..
..
..
..
CH
2
Br
RC
Acid bromide
O
..
..
..
..
..
CH
Br
..
..
..
Br
RC
O
..
..
..
..
..
CH
Br
RC
Enol form
α-Bromo acid bromide
OH
FIGURE 19.41 An intermediate in the Hell–Volhard–Zelinsky reaction is the α-bromo acid bromide.
This compound can be isolated if a full equivalent of PBr
3
is used in the reaction.
..
..
..
..
..
..
..
CH
..
..
..
Br
CH
2
C
O
..
..
O
Cl
..
..
..
CH
..
..
..
Br
CH
2
C
Cl
..
..
CH
..
..
..
Br
CH
2
C OCH
2
CH
3
..
..
OCH
2
CH
3
..
..
O
..
..
O
CH
..
..
..
Br
CH
2
C
(>90%)
CH
3
CH
2
OH
(cooling)
A SPECIFIC EXAMPLE
..
..
H
2
O
..
..
CH
..
..
..
Br
..
..
..
Br
R
C
O
..
..
O
..
..
..
CH
..
..
..
..
..
Br
HOR
OR
..
..
CH
..
..
..
Br
R
C
OH
CH
..
..
..
..
Br
NH
2
NH
3
..
..
O
R
C
..
..
O
R
C
THE GENERAL REACTIONS
FIGURE 19.42 Compounds available
from reactions of an α-bromo acid
halide.
PROBLEM 19.11 Write a general mechanism that accounts for the reactions of
Figure 19.42.
The α-bromo acid halide reacts like any other acid halide, and it can be used to
generate the α-bromo acid, ester, and amide, for example (Fig. 19.42).
..
..
..
..
CH
2
Br
2
/PBr
3
RC
O
OH
..
..
OH
..
..
CH
..
..
..
Br
C
O
R
..
..
..
..
CH
2
Br
2
catalytic PBr
3
(see Fig. 19.41)
RC
O
OH
..
..
..
Br
..
..
CH
..
..
..
Br
C
O
R
Anhydride
–
..
..
..
..
Br
addition
Br
2
R
..
..
O
..
..
O
..
..
O
O
C C
..
..
CH
2
R
CH
..
..
..
Br
+
CH
2
R
HO
..
..
..
..
O
C
R
elimination
..
–
..
..
..
Br
..
..
O
..
..
O
CH
2
R
CH
..
..
..
Br
C
..
..
O
..
..
..
Br
C
..
..
O
R
–
..
..
..
O
CH
..
..
..
Br
C
CH
2
R
(a)
(b)
FIGURE 19.43 (a) The overall
reaction of a carboxylic acid using a
catalytic amount of PBr
3
. (b) The
complex mechanism of the HVZ
reaction using a catalytic amount of
PBr
3
.
The HVZ reaction is limited to the formation of α-bromo compounds and is,
in truth, sometimes awkward to carry out. The reagents, bromine and phosphorus,
are noxious; reaction times are often long,and reaction conditions are harsh.For these
reasons, methods have evolved to extend and replace the classic HVZ process. For
example, David Harpp (b. 1937) and his colleagues at McGill University use the
reaction of acid chlorides and N-bromosuccinimide (NBS, p. 613), in the presence
of a catalytic amount of HBr, to form α-bromo acid chlorides conveniently and in
excellent yields (Fig. 19.44).
952 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
If a catalytic amount of PBr
3
is used, and not the full equivalent as shown in the
reaction in Figure 19.41, the product of the reaction is the α-bromo acid, not the
α-bromo acid bromide (Fig. 19.43a). The α-bromo acid bromide is still an inter-
mediate, but only a small amount of it can be made at any one time, because its for-
mation depends on PBr
3
. The small amount of α-bromo acid bromide produced is
attacked by a molecule of the acid in an anhydride-forming reaction (Fig. 19.43b).
The anhydride undergoes addition–elimination by bromide to give the carboxylate
anion and a new molecule of α-bromo acid bromide that recycles. A final hydrolysis
gives the α-bromo acid itself.This reaction surely involves a complicated mechanism.
OH
Br
1. SOCl
2
65 ⬚C
2. NBS, HBr
85 ⬚C
N BrNBS =
O
Not isolated (75%)
O
O
Cl
O
Cl
O
FIGURE 19.44 The Harpp modification of the HVZ reaction.
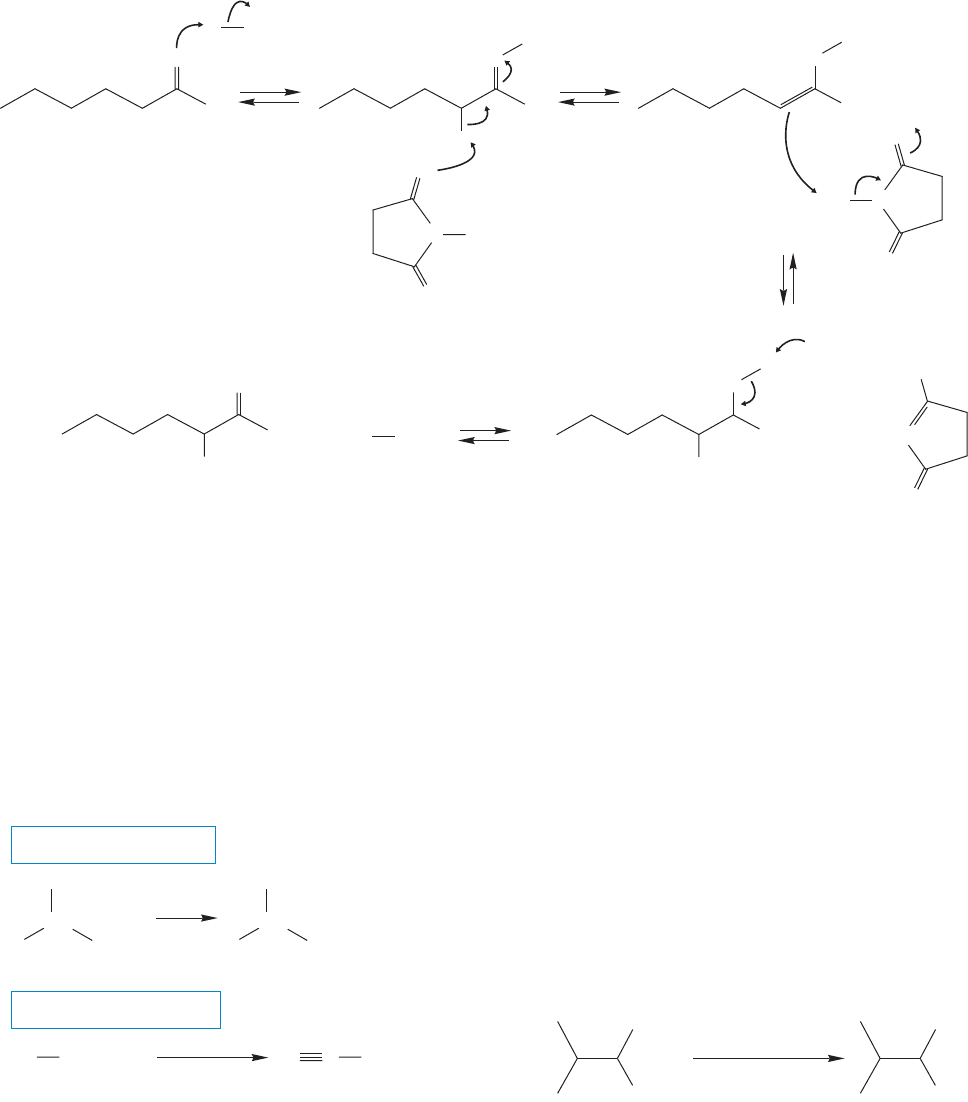
19.4 Halogenation in the Position 953
␣
Cl
O
H
Br N
O
O
HO
+
+
O
Cl
O
Cl
+
+
+
O
Br
Br
Cl
H
O
H
Cl
N Br
H Br
H Br
N
–
Br
–
Br
O
OH
O
H
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
FIGURE 19.45 The mechanism of the Harpp modification of the HVZ reaction.
The mechanism of Harpp’s modification is an ionic one, and uses many of the
steps we have seen already. The HBr catalyst protonates the carbonyl oxygen of
the acid halide (Fig. 19.45). Then NBS deprotonates the α carbon to form an enol.
Cl
..
..
..
..
..
..
Nu
KCN
K
2
CO
3
/H
2
O
95–100 ⬚C
1. NH
3
25 ⬚C, 1 week
2. H
2
O/H
3
O
+
S
N
2
..
–
..
..
..
–
Br
COOH
..
NH
2
COOH
CH
X
= Br or Cl
R
X
CH
2
COOH CH
2
COOH
(~90%)
An
α-amino
acid, valine
(48%)
CN
COOH
CH
R
Nu
COOH
+ X
THE GENERAL CASE
SPECIFIC EXAMPLES
FIGURE 19.46 Some reactions of α-halo acids.
The enol now functions as a base to remove a Br from NBS. Finally deproto-
nation of the protonated carbonyl gives the product and a new molecule of acid
catalyst.
Like the α-bromo aldehydes and ketones, the α-bromo acids are very reactive
in displacement reactions and therefore serve as sources of many α-substituted
carboxylic acids (Fig. 19.46). A particularly important example is the formation of
α-amino acids through reaction with ammonia.
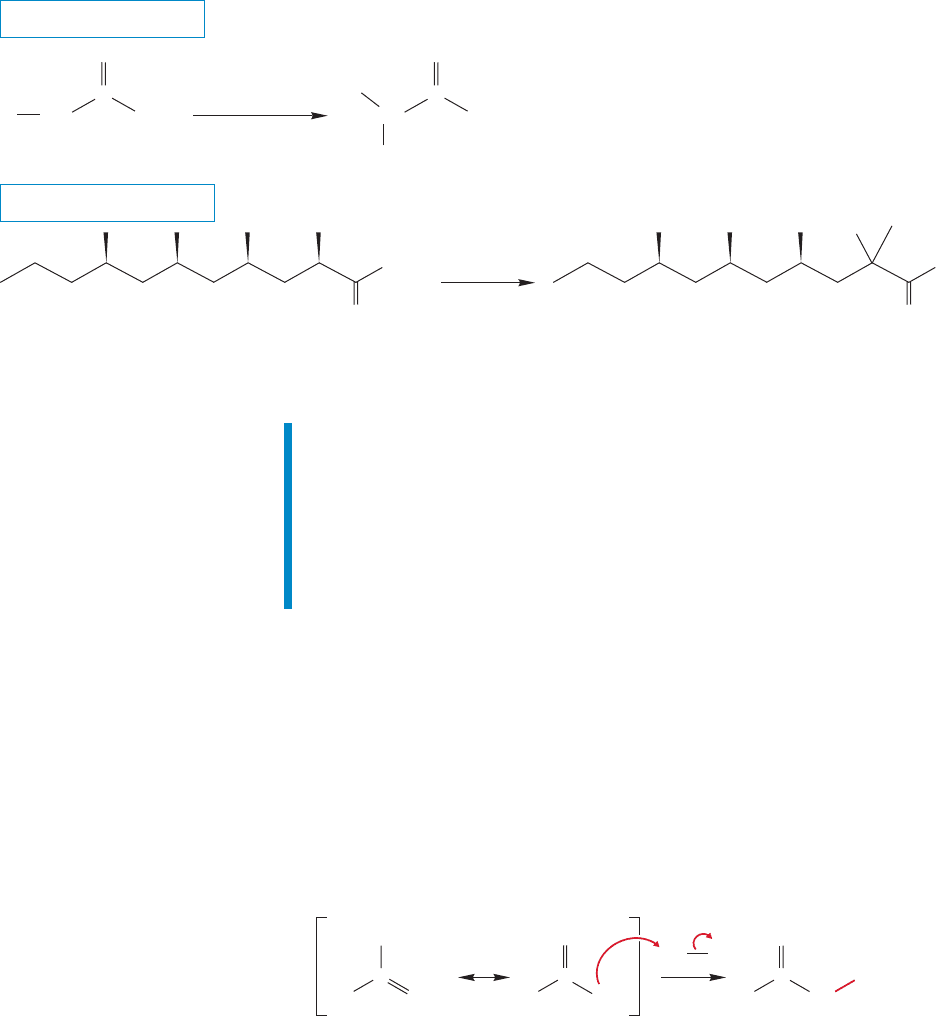
THE GENERAL CASE
A SPECIFIC EXAMPLE
1. LDA
THF
2. Br
2
OCH
3
O
OCH
3
Br
(87%)
O
OR
1. Strong base
2. Br
2
R
C
CH
2
O
OR
C
O
R
Br
CH
FIGURE 19.47 Formation of an α-bromo ester.
954 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
CH
2
–
..
C
R
O
..
..
CH
2
C
The methylated
enolate, the
product ketone
Enolate
R
O
..
..
CH
2
CH
3
CH
3
+
S
N
2
–
..
C
R
O
..
..
I
..
..
..
–
I
..
..
..
..
FIGURE 19.48 If the enolate could
act as a nucleophile in the S
N
2
reaction, we would have a way of
alkylating at the α position, thus
forming a new carbon–carbon bond.
Summary
We have now discussed two reactions that take place at the α position of car-
bonyl compounds.We will use these as prototypes on which to base further, more
complicated processes. The enolate can react with an electrophilic source of H
or the electrophile X
2
.The unifying theme is formation of an enolate ion (in base)
or an enol (in acid) that can act as a nucleophile. With what other electrophiles
might such a nucleophile react?
We can see some potential difficulties and limitations right away. Alkylation of
an enolate is an S
N
2 reaction,and that means we cannot use a tertiary halide, a species
too hindered for participation in the S
N
2 reaction.
19.4c Halogenation of Esters In theory, one should be able to halogenate an
ester in the α position.However, there are very few examples of this reaction. Here’s
one (Fig. 19.47).
19.5 Alkylation in the ␣ Position
In the previous sections,we saw several examples of enolate anions acting as nucleo-
philes. A logical extension would be to attempt to use enolates as nucleophiles in
S
N
2 displacements. If we could do this reaction,we would have a way to alkylate the
α position of carbonyl compounds,and a new and most useful carbon–carbon bond-
forming reaction would result (Fig. 19.48).
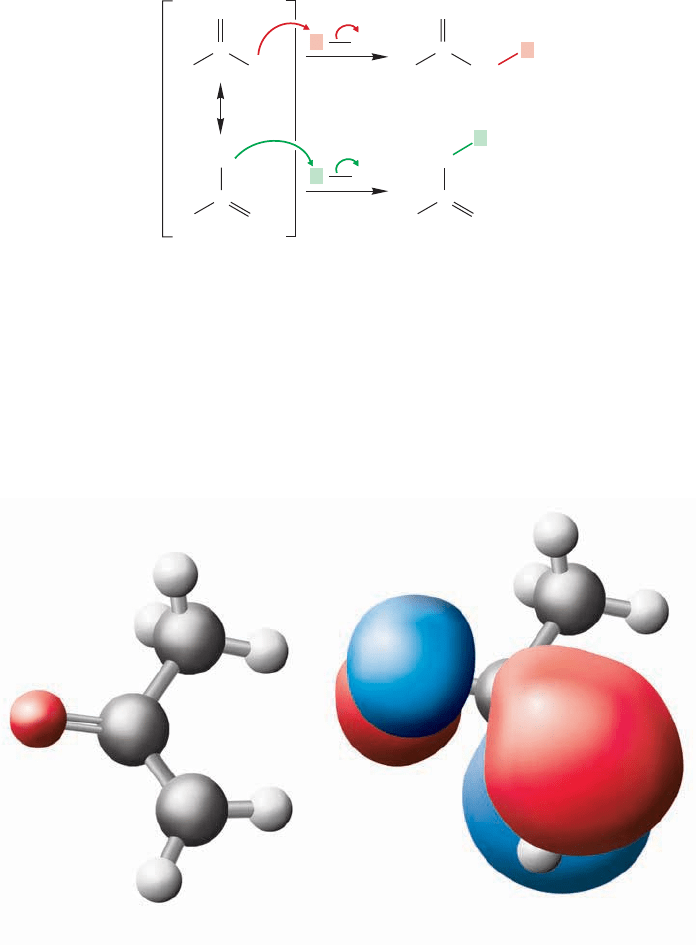
It turns out that most enolates are better nucleophiles at carbon. So, alkylation
takes place faster at carbon than at oxygen (Fig.19.50). One explanation for this fact
is that the highest occupied molecular orbital of the nucleophile has more electron
density at the carbon than at oxygen. Selectivity might also be a result of counter
ion (Li
, for example) coordination between the enolate and the electrophile. If the
counterion is complexed with the site of overall higher electron density, the oxygen
in this case, it sterically hinders alkylation at that position.
19.5 Alkylation in the Position 955
␣
CH
2
–
..
C
R
–
..
O
..
..
CH
2
C
R
O
..
..
R
R
R
X
CH
2
C
C-Alkylation
O-Alkylation
R
O
..
..
..
O
..
CH
2
C
R
RX
FIGURE 19.49 In principle, alkylation
of the enolate could take place at
either carbon or oxygen.
(a) (b)
FIGURE 19.50 (a) The enolate of acetone. (b) The highest occupied molecular orbital of the
enolate of acetone is represented. Note the greater contribution of electron density on the
carbon of the enolate. In practice, alkylation generally takes place at carbon because of the
greater electron density of the orbital of the nucleophile involved in the reaction.
19.5a Alkylation of Ketone and Aldehyde Enolates Even though
alkylation at oxygen is not common, there are other problems with the alkylation
reaction of simple ketones using hydroxide base. Consider the alkylation of
2-methylcyclohexanone in H
2
O/HO
. Both α positions are active, and two enolates
The resonance formulation of enolate anions clearly shows that the negative
charge is shared between an oxygen and a carbon atom (Fig. 19.49). At which atom
will alkylation be faster? If there is little or no selectivity in the alkylation reaction,
it will surely be of limited use.
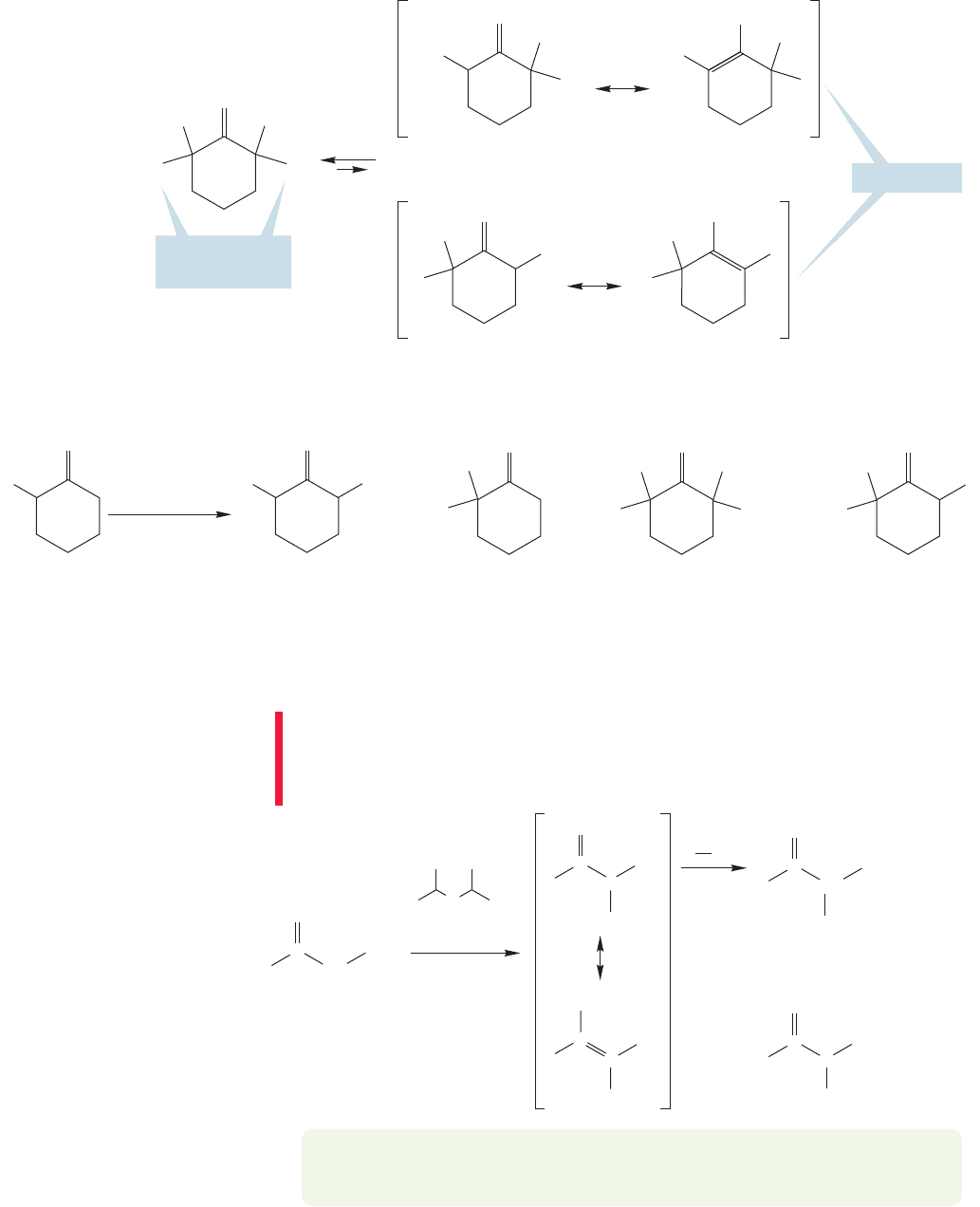
..
–
..
–
O
..
..
O
..
..
H
Two enolates
H
3
C
O
..
..
H
H
H
H
3
C
H
H
3
C
H
H
3
C
H
..
–
O
..
..
..
–
O
..
..
H
3
C
Both α positions
are active
+
H
H
H
H
FIGURE 19.51 For many ketones there
are at least two possible enolates, and
therefore mixtures are obtained in the
alkylation reaction.
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
..
O
1. (Ph)
3
C K
+
2. CH
3
I
H
3
C
O
(9%) (41%) (21%)(6%)
O
H
3
C
CH
3
CH
3
OO
+
++
–
CH
3
CH
3
FIGURE 19.52 For ketones, there can
often be more than one alkylation.
Mixtures of products can be formed.
LDA
–78 °C
+
O
..
..
CH
2
CH
3
S
N
2
H
C
O
..
..
C
CH
3
RX
X
H
C
..
–
..
–
(–)
–
=
–
O
..
..
CH
CH
3
H
R
C
O
..
..
..
C
CH
3
H
H
H
C
..
O
..
..
C
CH
3
H
H
C
–
..
..
Li
+
N
FIGURE 19.53 Strong base (LDA)
and low temperatures are effective at
forming enolates.
PROBLEM 19.12 LDA is a nonnucleophilic base. It does not add to the carbonyl
carbon. Explain why LDA is a poor nucleophile.
956 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
CONVENTION ALERT
will be produced (Fig. 19.51). Moreover, once alkylated, the compounds can undergo
further alkylation. Reactions of the initial products compete with the desired single
alkylation reaction. Mixtures of products can be formed, and that is not a very useful
situation (Fig. 19.52). Most aldehydes encounter similar problems.
However, if a strong, poorly nucleophilic base such as LDA is used to form the
enolate at very low temperatures (Fig. 19.53), then one can usually control the reac-
tion.LDA is strong enough to convert all available carbonyl compound into an eno-
late.This technique has become a useful tool for the synthesis of organic molecules.
Note again the use in Figure 19.53 of the shorthand notation for resonance
forms. Instead of always drawing out each important resonance form, one summa-
ry form is drawn. The positions sharing the charge or electron are indicated with
parentheses, (), (), or ( ).
.
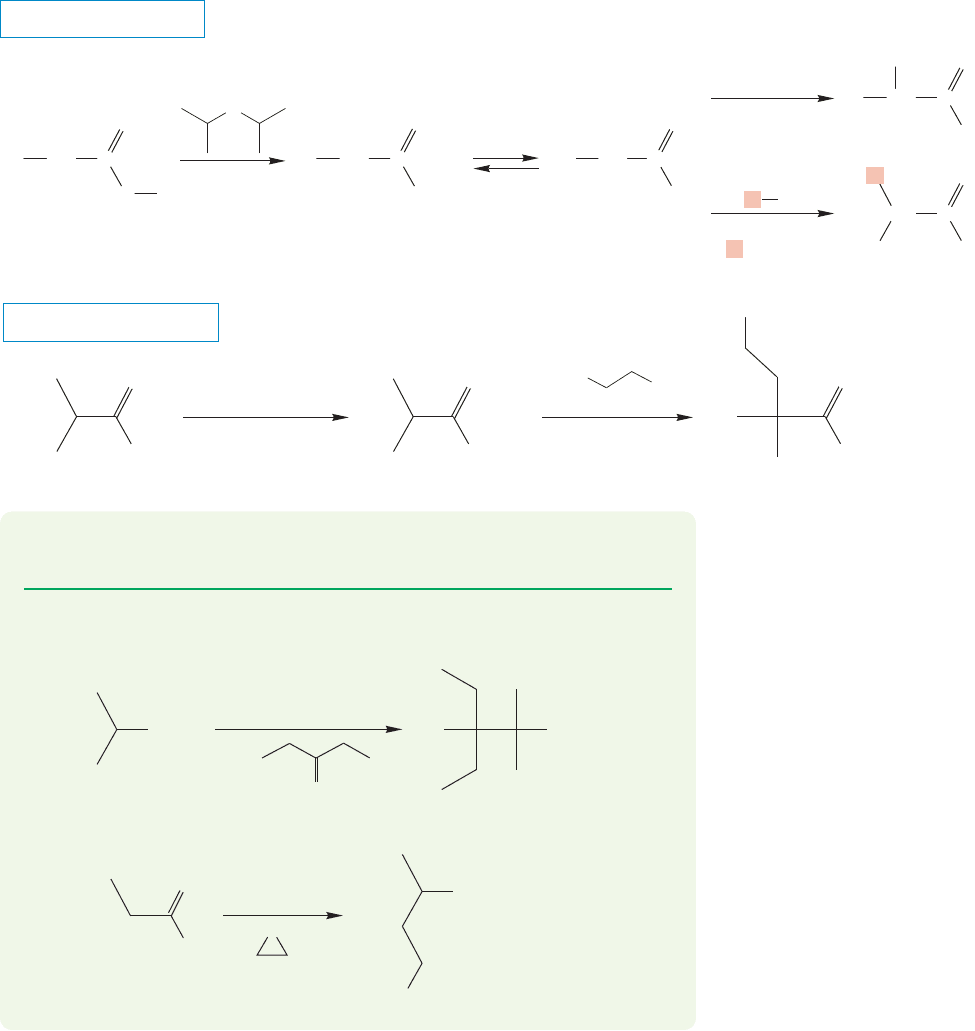
19.5 Alkylation in the Position 957
␣
C
O
O
..
..
..
..
LDA
Anion
1. NaH, 55 ⬚C, THF
2. LDA, 10 –35 ⬚C
heptane
0–35 ⬚C
1.
2. H
2
O
(63%)
CH
2
R
H
O
OH
..
..
O
Ph
Br
O
..
..
..
..
..
..
..
..
C
O
O
..
..
..
..
..
CH
2
R
..
..
–
–
Li
+
Li
+
–
–
Li
+
Na
+
O
O
..
..
..
..
..
–
Na
+
C
O
O
..
..
..
..
..
..
CH
Br
2
R
–
–
C
O
O
..
..
..
..
..
..
..
..
CH
R
–
C
O
O
..
..
..
..
..
CH
R
–
N
LDA
Dianion
α-bromination
α-alkylation
(R primary)
R I
Ph
R
Br
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 19.54 Dianions of carboxylic
acids can function as enolates.They
can be alkylated in the α position
with primary or other reactive
halides.
PROBLEM 19.13 Draw the resonance forms for the dianion formed from butanoic
acid. Why do you suppose the electrophile adds to the α carbon?
PROBLEM 19.14 Provide mechanisms for the following reactions that show related
electrophiles that can react at the α position of carboxylic acids:
O
O
COOH
COOH
O
OH
COOHHO
1. 2 equiv. LDA
2.
3. H
2
O/H
3
O
+
1. 2 equiv. LDA
2.
3. H
2
O/H
3
O
+
..
..
HO
..
..
..
..
..
..
19.5b Alkylation of Carboxylic Acids Carboxylic acids can also be alkylated, but
two equivalents of strong base are necessary, because this reaction proceeds through a
dianion.The first equivalent of base removes the carboxyl hydrogen to give the carboxy-
late salt. If the base used is a strong base and a weak nucleophile, a second hydrogen can
be removed, this time from the α position.The typical base used is LDA. The dianion
can now be brominated or alkylated at the α position, as long as the alkylating agent is
reactive (Fig. 19.54). After the alkylation step the resulting carboxylate anion can be
reprotonated to obtain the neutral product. Primary halides work well in these S
N
2
alkylation reactions, but more substituted halides lead mostly to the products of an E2
reaction.Steric hindrance around the carbon–halogen antibond blocks the S
N
2 path.As
a result, the enolate reacts as a base to give the alkene product in the E2 elimination.
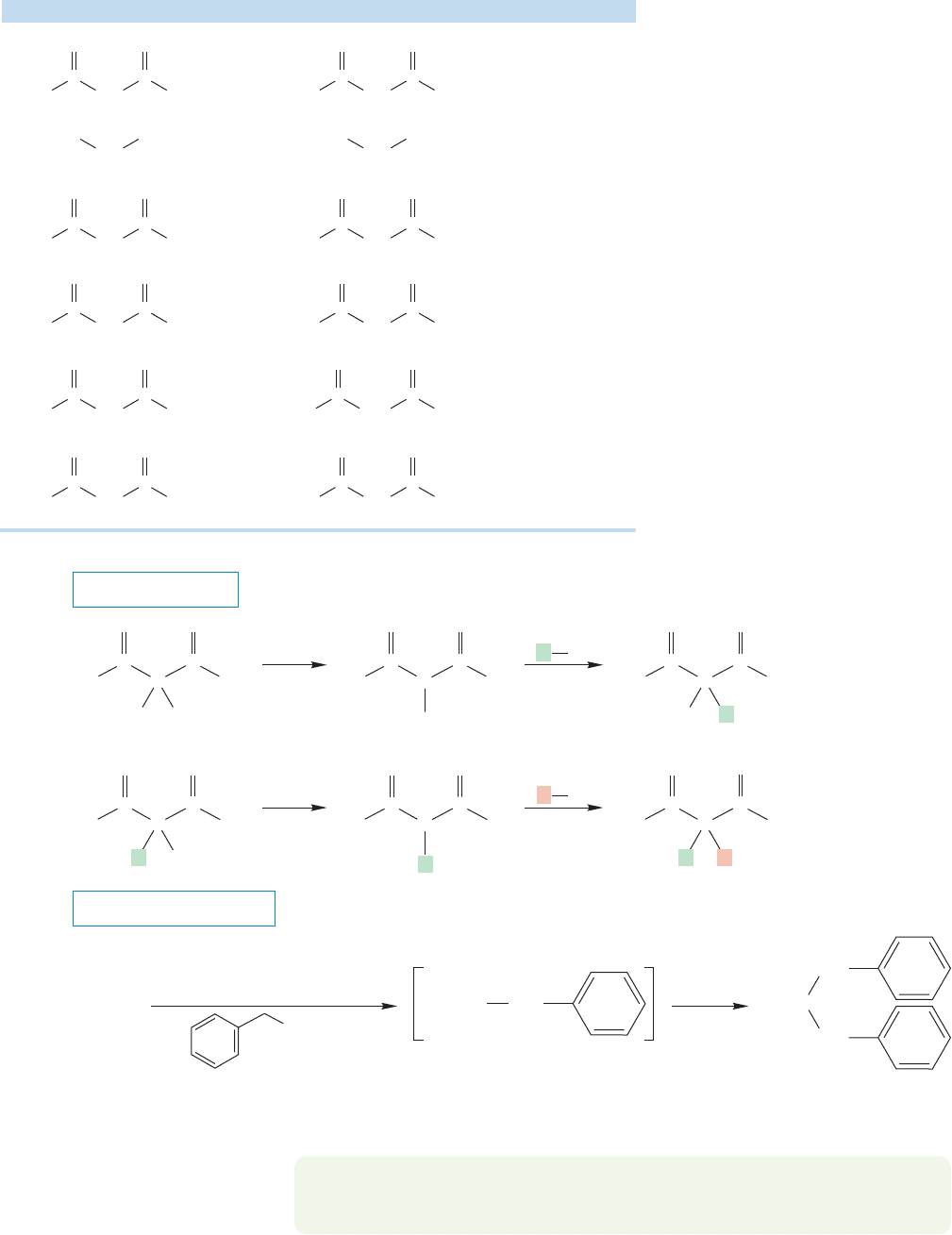
958 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
19.5c Alkylation of -Dicarbonyl
Compounds
β-Dicarbonyl com-
pounds are quite strong acids. The
pK
a
of diethyl malonate is about 13
and other β-dicarbonyl compounds,
β-cyano carbonyl compounds, and
dicyano compounds are similarly
acidic (Table 19.3). The anions
formed from these compounds can
be alkylated in S
N
2 reactions (Fig.
19.55). The once-alkylated com-
pounds still contain one doubly α
hydrogen, and therefore remain
acidic. A second alkylation reaction
is often possible, as long as the
requirements of the S
N
2 reaction are
kept in mind. These β-dicarbonyl
compounds are sufficiently acidic so
that alkylation through the enolate
using an alkoxide base is easy. Both
mono- and dialkylation reactions of
β-keto esters and related diesters are
common.
GENERAL CASES
A SPECIFIC EXAMPLE
(–) (–)
–
..
C
O
..
..
O
..
..
C
HH
CH
3
..
..
RO
C
O
..
..
O
..
..
C
Once alkylated
H
CH
3
..
..
RO
C
O
..
..
O
..
..
C
H
CH
3
..
..
RO
..
..
RO
..
..
HOR
RX
(S
N
2)
R
(–) (–)
–
..
C
O
..
..
O
..
..
C
H
CH
3
..
..
RO
C
O
..
..
O
..
..
C
Twice alkylated
CH
3
..
..
RO
C
O
..
..
O
..
..
C
CH
3
..
..
RO
..
..
RO
..
..
HOR
R
X
(S
N
2)
R
R
R
R
(75%)
Not isolated
CH
2
Cl, 25 ⬚C
(NC)
2
CH
2
(NC)
2
CH
1. NaH, dimethyl sulfoxide
repeat
2.
CH
2
CH
2
(NC)
2
C
C C C
CCC
–
..
–
..
FIGURE 19.55 Alkylations of some β-dicarbonyl compounds.
TABLE 19.3 pK
a
Values for Some Dicarbonyl Compounds
Compound Conjugate Base (Enolate) pK
a
13.3
11
10.7
8.9
8.5
5
EtO
C
O
CH
2
C
O
OEt
NC
CH
2
CN
C
O
CH
2
H
3
C
C
O
OEt
C
O
CH
2
H
3
CCH
3
C
O
C
O
CH
2
Ph CH
3
C
O
C
O
CH
2
HH
C
O
EtO
C
O
–
C
O
NC
CH
CN
OEt
H
3
C
C
O
C
O
OEt
H
3
C
CH
3
C
O
C
O
Ph CH
3
C
O
C
O
HH
C
O
C
O
..
–
CH
..
–
CH
..
–
CH
..
–
CH
..
–
CH
..
PROBLEM 19.15 Write a mechanism for the base-induced alkylation of
1, 1-dicyanomethane (malononitrile) with ethyl iodide.
