Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

19.2 Many Carbonyl Compounds Are Weak Brønsted Acids 939
C
H
O
..
..
CH
2
D
C
H
O
..
..
CD
3
C
H
O
CH
2
D
+
OD
2
..
..
..
D
2
O
..
..
D
3
O
..
deprotonate (D
+
)
on oxygen
protonate (D
+
)
on carbon
repeat
exchange
two times
Once-exchanged
acetaldehyde
Resonance-stabilized
intermediate
C
H
O
CH
2
D
..
..
+
+
C
H
O
CH
2
D
..
D
+
+
D
D
Tautomers
FIGURE 19.15 Reaction of the enol
with D
3
O
will generate deuterated
acetaldehyde.
WORKED PROBLEM 19.5 Write a mechanism for the equilibration of acetaldehyde
and its enol form in acid. This problem may seem mindless, and it certainly has
little intellectual content at this point, but it is an important drill problem. Doing
this problem is part of “reading organic chemistry with a pencil.”
ANSWER This process must become part of your chemical vocabulary from now
on. First the carbonyl oxygen is protonated and then the resulting cation is depro-
tonated at carbon to give the enol. This process is a keto–enol tautomerization
even though we are dealing with the aldehyde in this particular case.
+
H
3
O
H
3
C
C
H
..
..
O
..
H
2
C
C
H
H
..
O
H
+
..
..
H
2
O
H
2
C
C
H
..
O
H
..
..
+
OH
2
H
Notice that the steps in the enol acetaldehyde reaction are simply the reverse
of the acetaldehyde enol reaction (Fig. 19.15). Note also that in acid, as in base,
aldehydes and ketones that have α hydrogens are in equilibrium with their enol forms.
We will soon see that although enols are in equilibrium with the related keto forms,
it is usually the keto forms that are favored.This equilibrium is called the keto–enol
tautomerization.The carbonyl compound and its associated enol are called tautomers.
U
U
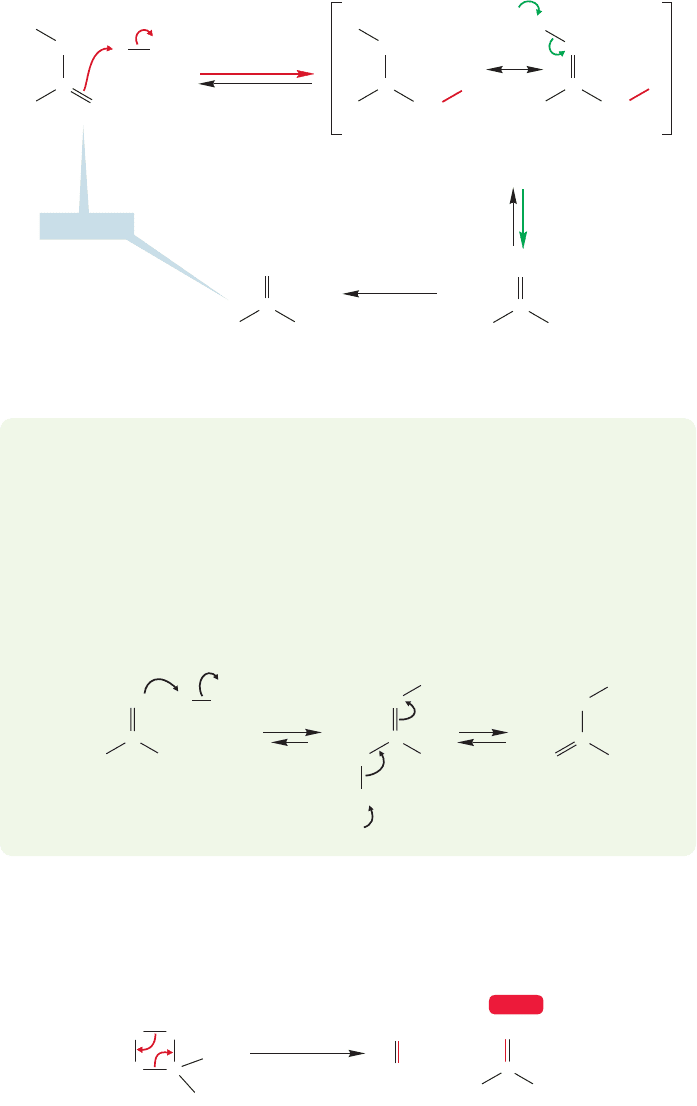
Recently, it has become possible to generate the simplest enol, vinyl alcohol
(hydroxyethene),in such a fashion that the acid or base catalysts of the previous reac-
tions are absent (Fig. 19.16). As long as it is protected from acids or bases, the enol
very low
pressure
Vinyl alcohol
(the simplest enol)
800–900 ⬚C
CH
2
CH
2
CH
2
H
OH
H
OH
H
2
C
H
2
C C
CH
2
+
C
WEB 3D
FIGURE 19.16 The parent of all
enols, vinyl alcohol, is stable as long
as it is protected from acid or base
catalysts.
940 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
CC
H
H
H
H
122.3⬚
119.1⬚
126.2⬚
109.7⬚
O
CC
H
H
H
H
1.08
1.34
1.08
0.96
O
121.8⬚
A
⬚
A
⬚
A
⬚
A
⬚
1.38
A
⬚
FIGURE 19.17 The structure of
vinyl alcohol.
C
R
R
O
..
..
CH
2
R
C
CHR
?
OH
..
..
FIGURE 19.18 Where does the
equilibrium between carbonyl
compound and enol lie? Which
tautomer is more stable?
C
HH
K ~ 6 x 10
–7
Acetaldehyde
O
..
..
CH
3
C
CH
2
OH
..
..
C
K ~ 5 x 10
–9
Acetone
O
..
..
CH
3
H
3
CH
3
C
C
CH
2
OH
..
..
FIGURE 19.19 For simple aldehydes
and ketones, it is the carbonyl form
that is favored at equilibrium.
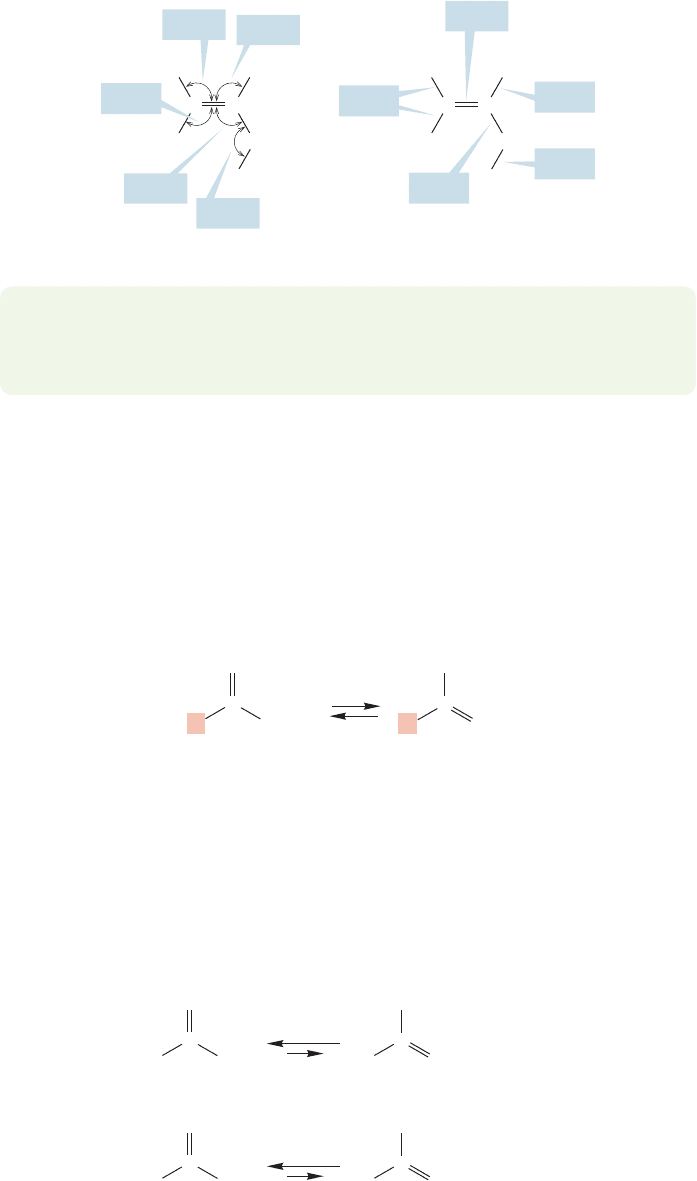
PROBLEM 19.6 It is impossible to protect vinyl alcohol from all acidic and basic
materials, and so it eventually reverts to the carbonyl form, acetaldehyde. Explain
what the unavoidable acid and base catalysts are.
So far we have seen that α hydrogens can be removed from carbonyl compounds,
and that carbonyl compounds are often in equilibrium with their enol forms. The
next question to address is the position of the equilibrium between a carbonyl com-
pound and its tautomeric form, the enol. Where does the equilibrium lie, and how
does it depend on structure (Fig.19.18)? Simple aldehydes and ketones are predom-
is stable and can be isolated and studied. The detailed structure of vinyl alcohol
provides no surprises. Bond lengths and angles are not unusual (Fig. 19.17).
inantly in their carbonyl forms.Figure 19.19 shows the equilibrium constants,K,for
acetone and acetaldehyde.These numbers are typical for other simple aldehydes and
ketones. Neither simple ketones nor simple aldehydes are very highly enolized,
although aldehydes generally exist somewhat more in their enol forms than ketones.
The second alkyl group in a ketone, which is a methyl group in the case of acetone,
19.2 Many Carbonyl Compounds Are Weak Brønsted Acids 941
stabilizes the carbon–oxygen double bond more than it does a carbon–carbon double
bond (Fig. 19.20).
Energy
Enol form
Enol form
Keto form
Aldehyde
form
C
ΔG' > ΔG
H
OH
CH
2
C
H
O
CH
3
C
H
3
C
O
CH
3
C
H
3
C
OH
CH
2
Stabilization of
C
O by CH
3
Stabilization of
C
C by CH
3
ΔG
ΔG'
FIGURE 19.20 Acetone is less
enolized than acetaldehyde because
of the greater stabilization provided
to the keto form by the second
methyl group.
PROBLEM 19.7 From a bond strength analysis (ΔH),decide whether the keto or enol
form of acetone is more stable. Use the data from Table 8.2 (p. 337).These mol-
ecules both contain sp
2
/sp
3
carbon–carbon bonds. Use a value of 101.4 kcal/mol
for these bonds.
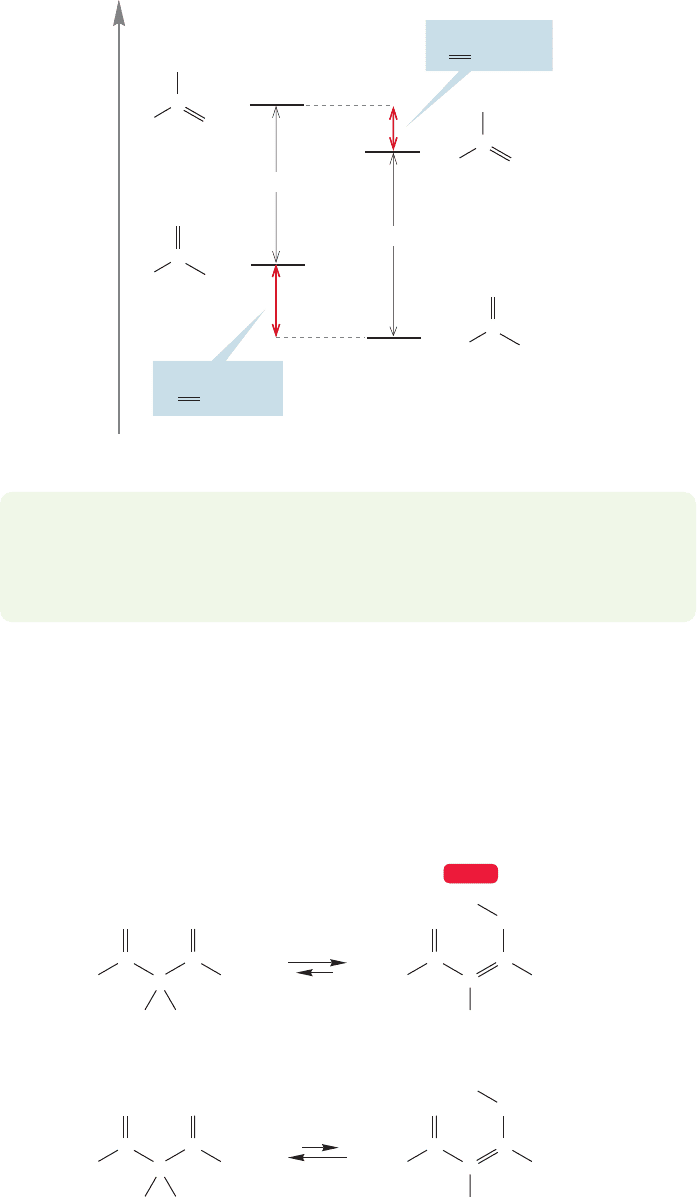
More complex carbonyl compounds can be much more strongly enolized.
β-Dicarbonyl compounds (1,3-dicarbonyl compounds) exist largely in their enol
forms, for example (Fig. 19.21). In the enol forms of these compounds, it is possi-
ble to form an intramolecular hydrogen bond, thus forcing the equilibrium position
away from the diketo forms. There is also conjugation between the carbon–carbon
..
C
K = 3.2
O
..
..
CH
3
C
O
..
..
C
HH
H
3
C
C
O
..
CH
3
C
O
..
..
C
H
H
H
3
C
..
C
K = 0.1
O
..
..
CH
3
C
O
..
..
CH
3
OCH
3
O
C
O
..
CH
3
C
O
..
..
C
H
H
C
HH
..
..
..
..
WEB 3D
FIGURE 19.21 β-Dicarbonyl
compounds are more enolized than
are monoketones.
942 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
double bond and the remaining carbonyl group in these enols (Fig. 19.22). We will
revisit the β-dicarbonyl compounds in Section 19.5c.
..
C
O
..
..
CH
3
C
O
..
..
H
3
C
C
O
..
CH
3
C
O
..
..
C
H
H
H
3
C
Hydrogen bond
Conjugated
double bond
Conjugated
double bond
C
HH
FIGURE 19.22 The formation of an
intramolecular hydrogen bond, as
well as conjugation between the
carbon–carbon double bond and the
carbonyl group, contributes to the
increased stability of enols in
β-dicarbonyl compounds.
K > 10
13
2,4-Cyclohexadienone Phenol
O
..
..
H
H
OH
..
..
FIGURE 19.23 The equilibrium
constant for enolization of
cyclohexadienone is estimated
to be greater than 10
13
.
The ultimate stabilized enol is phenol. It is estimated that the equilibrium con-
stant for formation of the aromatic enol form from the nonaromatic ketone is greater
than 10
13
(Fig. 19.23).
19.2b Enolates of Acid Derivatives Hydrogens in the α position of acid
derivatives are acidic for the same reasons that α hydrogens are acidic in aldehydes
and ketones. The conjugate bases are resonance stabilized by the adjacent carbonyl
or nitrile group (Fig. 19.24). The ease of deprotonation at the α position varies,
..
–
B
..
–
–
–
B
H
H
O
..
..
C
Aldehyde
Enolate
H
2
C
H
O
..
..
..
..
C
H
2
C
H
O
..
..
C + BH
H
2
C
α
..
–
–
–
B
OR
O
..
..
C
Ester
Enolate
O
..
..
..
..
OR
..
..
OR
..
..
..
..
C
H
2
C
O
..
..
C + BH
H
2
C
..
–
–
B
Nitrile
..
H
2
C
–
..
..
+ BH
..
CN
..
CN
H
2
CCN
..
–
B
H
H
2
C
α
..
–
B
H
H
2
C
α
FIGURE 19.24 A hydrogen at the α
position of an acyl compound or
nitrile is acidic and can be removed
by base to give an enolate, or, in the
case of nitriles, a resonance-stabilized
species much like an enolate.
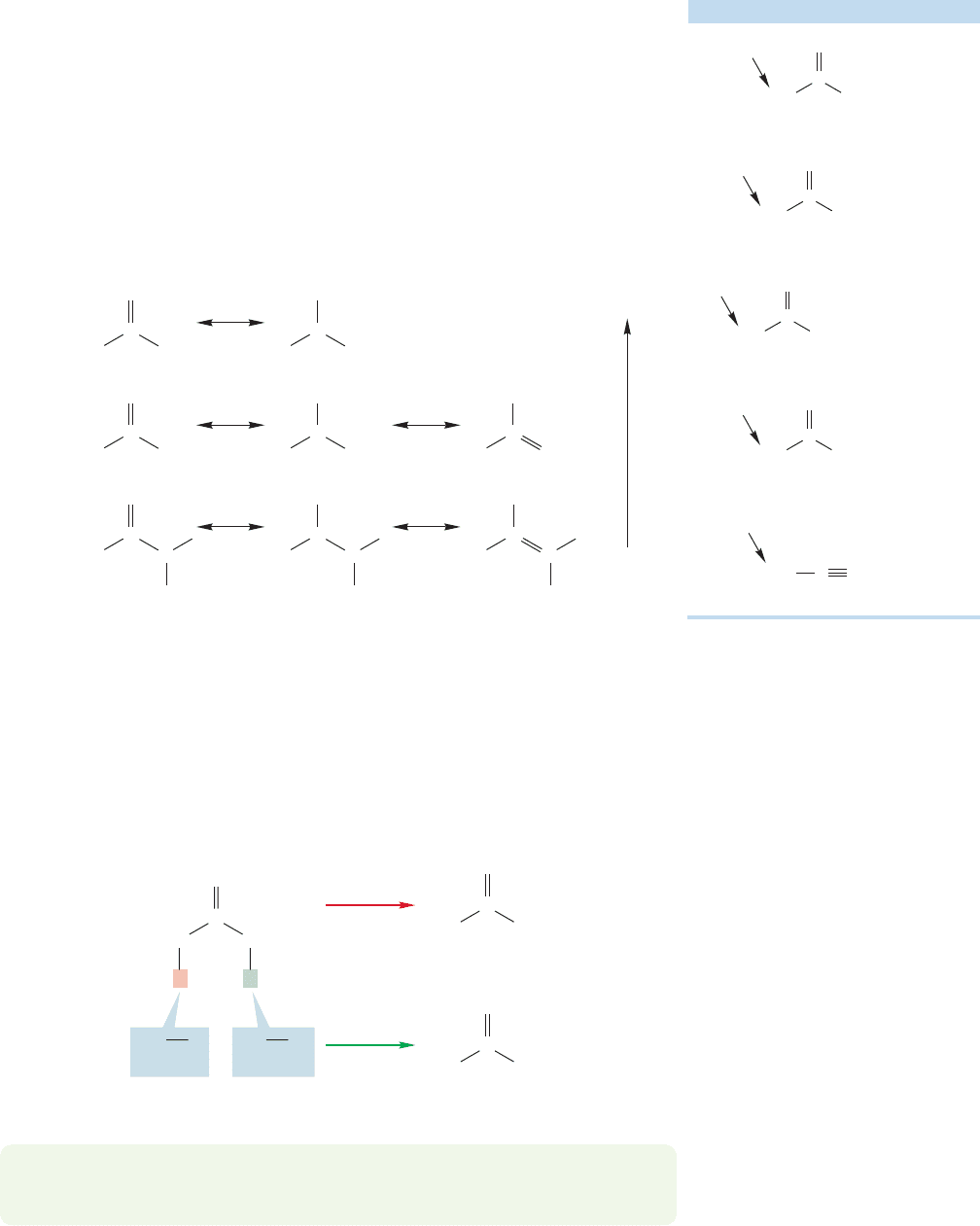
19.2 Many Carbonyl Compounds Are Weak Brønsted Acids 943
however, depending on the ability of the adjacent carbonyl group to provide stabi-
lization (Table 19.2).
Esters and amides are more stabilized by resonance than are aldehydes and
ketones.Therefore their carbonyl groups have less double-bond character, and they
are weaker acids at the α position. Notice the connection between resonance stabi-
lization and acidity. The more resonance stabilized the starting material, the more
difficult it is to remove a proton from it.
Amides are the most resonance stabilized of acyl compounds and therefore the
weakest acids. In an amide’s polar resonance form it is the relatively electropositive
nitrogen atom that bears the positive charge (Table 19.2; Fig. 19.25).Thus the polar
form is relatively stable. There is no substantial chemistry of amide enolates.
H
C
O
HH
3
C
H
C
O
CH
3
H
3
C
H
C
O
OCH
2
CH
3
H
3
C
H
C
O
NH
2
H
3
C
H
CNH
3
C
TABLE 19.2 e Acidity of Some
Acyl Compounds
a
Compound pK
a
16.7
19.3
24
25
24
Acetone
Ethyl acetate
Acetamide
Acetonitrile
Acetaldehyde
a
e arrow shows the proton lost.
C
O
R
R
C
O
–
+
R
R
C
O
OR
R
C
O
–
+
OR
R
C
O
N
R
C
O
–
+
N
R
C
O
–
+
OR
R
C
O
–
+
N
R
Aldehyde/
ketone
Strongest
acid
Weakest
acid
Ester
Amide
FIGURE 19.25 Esters and amides are more stabilized by resonance than aldehydes and
ketones. It is harder to remove an α hydrogen from the R group of these more stable
species.They are weaker acids as shown by their higher pK
a
values.
α C
H
pK
a
~
25
α N
H
pK
a
~ 18
–
–
(–)
(–)
..
H
2
CNH
(harder)
base
(easier)
base
HH
O
..
..
C
..
H
2
CNH
2
O
..
..
C
Enolate
Amidate
..
..
..
H
3
CNH
O
..
..
C
FIGURE 19.26 There are two kinds
of α hydrogen in most amides. It is
much easier to remove the proton
attached to nitrogen than the
proton attached to carbon.
PROBLEM 19.8 Explain why α hydrogens on the nitrogen of amides are more
acidic than α hydrogens on carbon (Fig. 19.26).
The problem is that the α hydrogens on the amide nitrogen are more acidic than
the α hydrogens on carbon, and therefore are more easily removed by base to give
anions (Fig. 19.26).
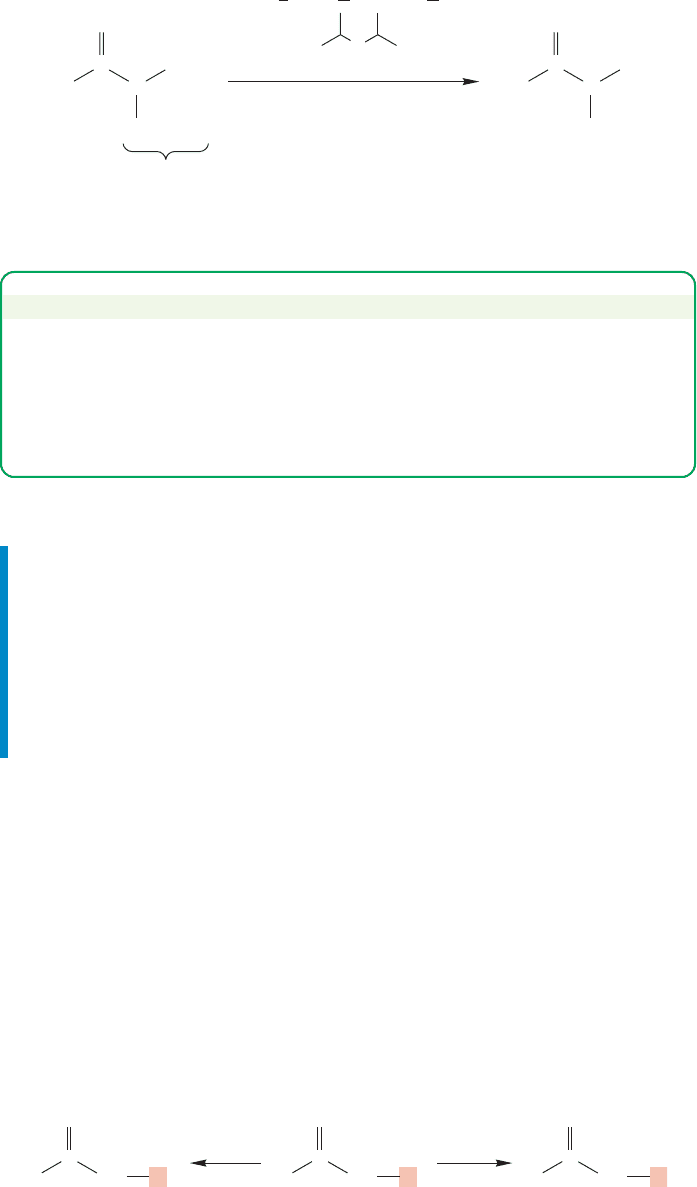
944 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
PROBLEM SOLVING
In many problems there are clues in the very question itself! Here’s one:
Whenever you see LDA on a problem—every time—think “deprotonation.”
LDA, a strong, very hindered base, is going to remove the most sterically
available hydrogen to give an anion. That anion must be stabilized by resonance,
however.
–
(–)
..
H
3
CN
No
α hydrogens
on nitrogen
CH
3
CH
3
O
..
..
C
..
..
H
2
CN
Enolate anion
CH
3
CH
3
O
..
..
C
LDA = Lithium Diisopropyl Amide
–
..
..
Li
+
N
FIGURE 19.27 Amides in which there
are no hydrogens attached to
nitrogen can form enolates.
Summary
Ketones and aldehydes bearing α hydrogens are in equilibrium with their enol
forms, although for simple ketones and aldehydes the carbonyl forms are great-
ly favored.This equilibrium is the keto–enol tautomerization.Equilibration with
the enol form can be either acid- or base-catalyzed.The enol form can be favored
in special cases. Esters and other acid derivatives also have acidic α hydrogens.
LDA is a strong base that can be used to drive ketones, aldehydes, or esters com-
pletely to their corresponding enolates.
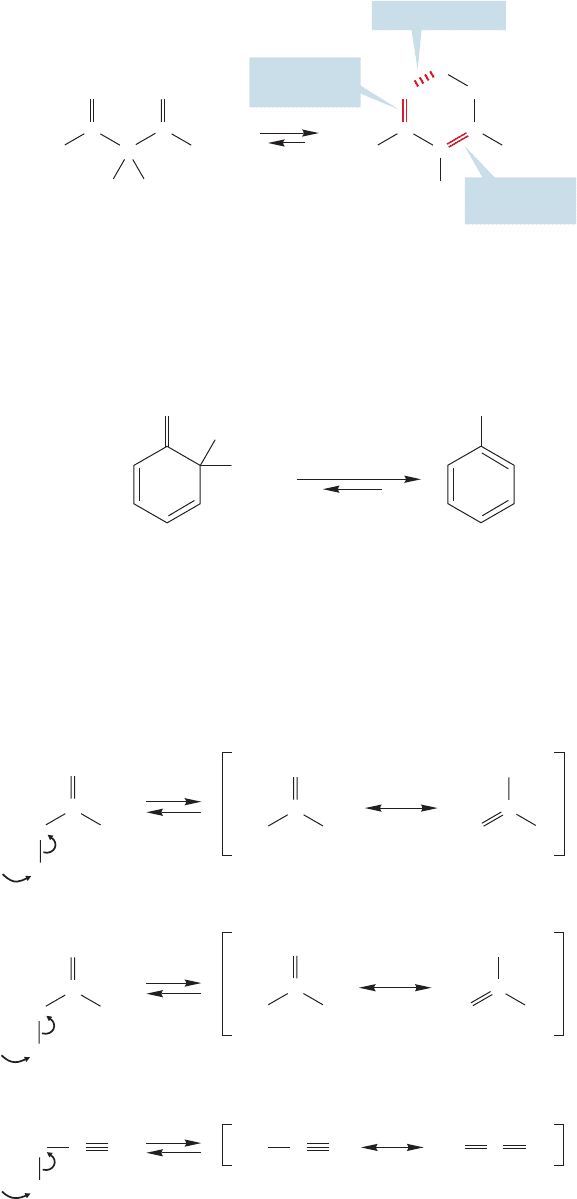
19.3 Racemization of Enols and Enolates
Now that we know something of the origins of enols and enolates, and we have seen
that enols are in equilibrium with carbonyl compounds, it is time to move on to a
look at reactions of these species.
We saw in the previous sections that as long as an enol or enolate can be formed,
hydrogens in the α position can be exchanged for deuterium through treatment with
deuterated acid or base (Fig. 19.28).Treatment of a simple optically active aldehyde
or ketone with acid or base results in loss of optical activity, racemization, as long as
C
RR
O
CD
2
D
2
O
C
RR
O
CH
2
C
RR
O
CD
2
DO
–
D
2
O
D
3
O
+
FIGURE 19.28 Exchange reactions
of carbonyl compounds bearing α
hydrogens can be either acid or base
catalyzed.
If there are no hydrogens on the nitrogen, it becomes possible to form enolates.
Strong,hindered bases such as lithium diisopropylamide (LDA, Fig. 19.27) are gen-
erally used because they are sufficiently basic and nonnucleophilic.
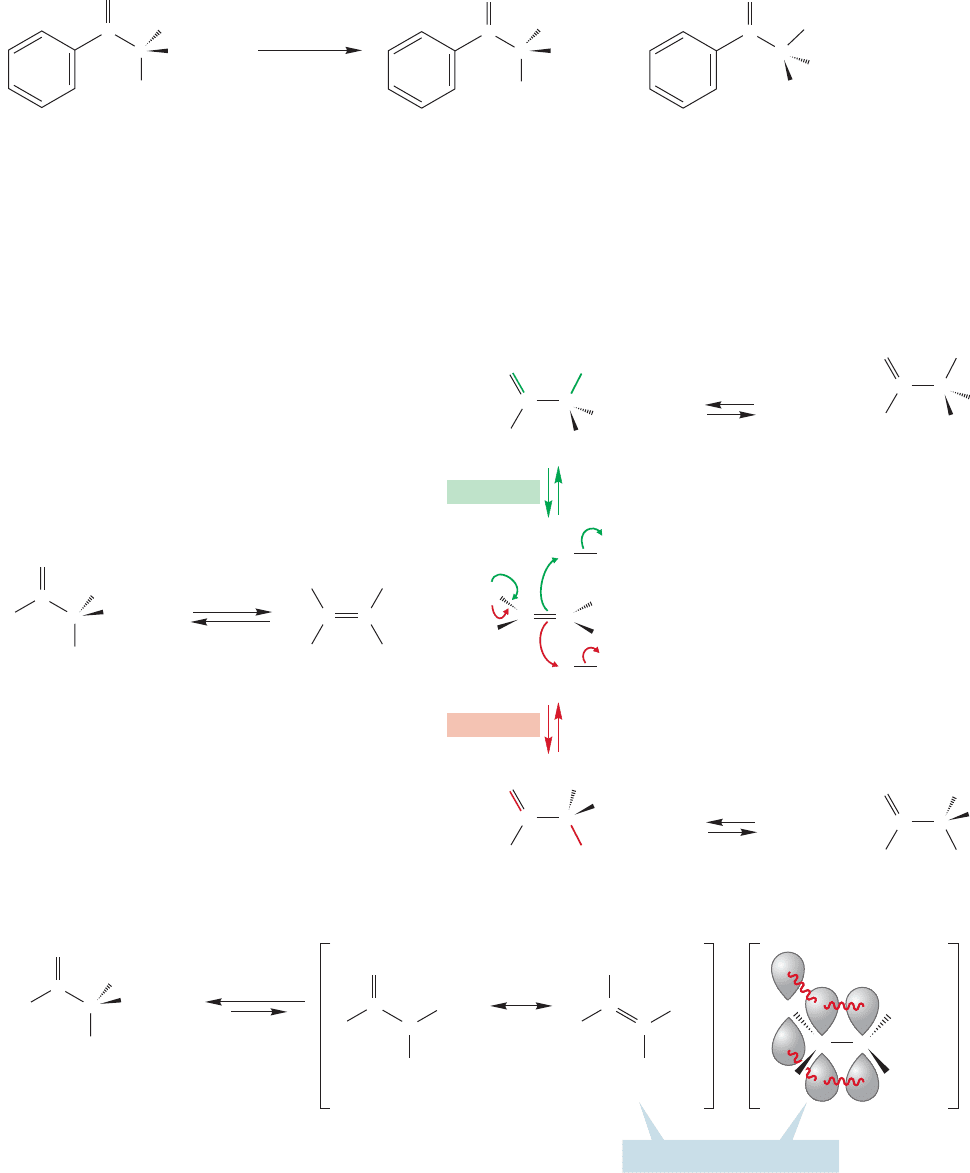
19.3 Racemization of Enols and Enolates 945
(S)
O
..
..
CH
3
CH
2
CH
3
HNO
3
37 ⬚C
CH
3
COOH
C
C
H
(S)
O
..
..
CH
3
CH
2
CH
3
C
C
H
(R )
O
..
..
CH
3
CH
2
CH
3
C
C
+
H
FIGURE 19.29 Optically active carbonyl compounds are racemized in acid or base, as long as an α hydrogen
is present on the stereogenic carbon.
the stereogenic carbon is in the position α to the carbon–oxygen double bond, and as long
as there is an α hydrogen (Fig. 19.29).
(S)
(S)
(R)
O
..
..
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
CH
3
C
Ph
C
H
..
..
..
..
H
2
O
Planar, achiral
enol
protonation
protonation
..
..
HO
HO
H
3
O
..
+
+
H
3
O
..
+
CC CC=
Ph
Ph
H
CH
3
CH
2
CH
3
..
HO
C
Ph
CH
3
CH
3
H
OH
2
..
OH
2
..
..
..
H
2
O
deprotonation
H
C
+
CH
2
CH
3
..
HO
C
Ph
CH
3
H
2
O
deprotonation
H
C
CH
2
CH
3
C
Ph
CH
3
H
C
CH
2
CH
3
C
Ph
CH
3
H
C
+
O
..
..
H
3
O
..
+
+
O
..
..
+
+
FIGURE 19.30 Protonation of the planar enol must
result in formation of equal amounts of two
enantiomers. An optically active carbonyl compound is
racemized on enol formation.
(S)
O
..
..
O
..
..
CH
3
CH
2
CH
3
CH
2
CH
3
CH
3
C
*
Ph
C
H
H
2
O / OH
The planar, achiral enolate
..
..
..
–
–
–
–
C
O
C
C
The chiral (S) carbonyl
compound
C
Ph
O
..
CH
2
CH
3
CH
3
C
C
=
Ph
CH
2
CH
3
CH
3
..
..
..
..
..
Ph
FIGURE 19.31 Resonance stabilization of the enolate depends on overlap of the 2p orbitals on carbon and oxygen. Maximum
overlap requires planarity, and the planar enolate is necessarily achiral. Racemization has been accomplished at the enolate stage.
Remember that the asterisk tells you that the structure is a single enantiomer.
Once again the mechanism depends on enol formation in acid, or enolate for-
mation in base. In acid, an achiral, planar enol is formed (Fig. 19.30). Re-formation of
the keto form occurs by equal protonation from either side of the enol.In base,removal
of the α hydrogen generates a planar enolate anion that is also achiral (Fig. 19.31).
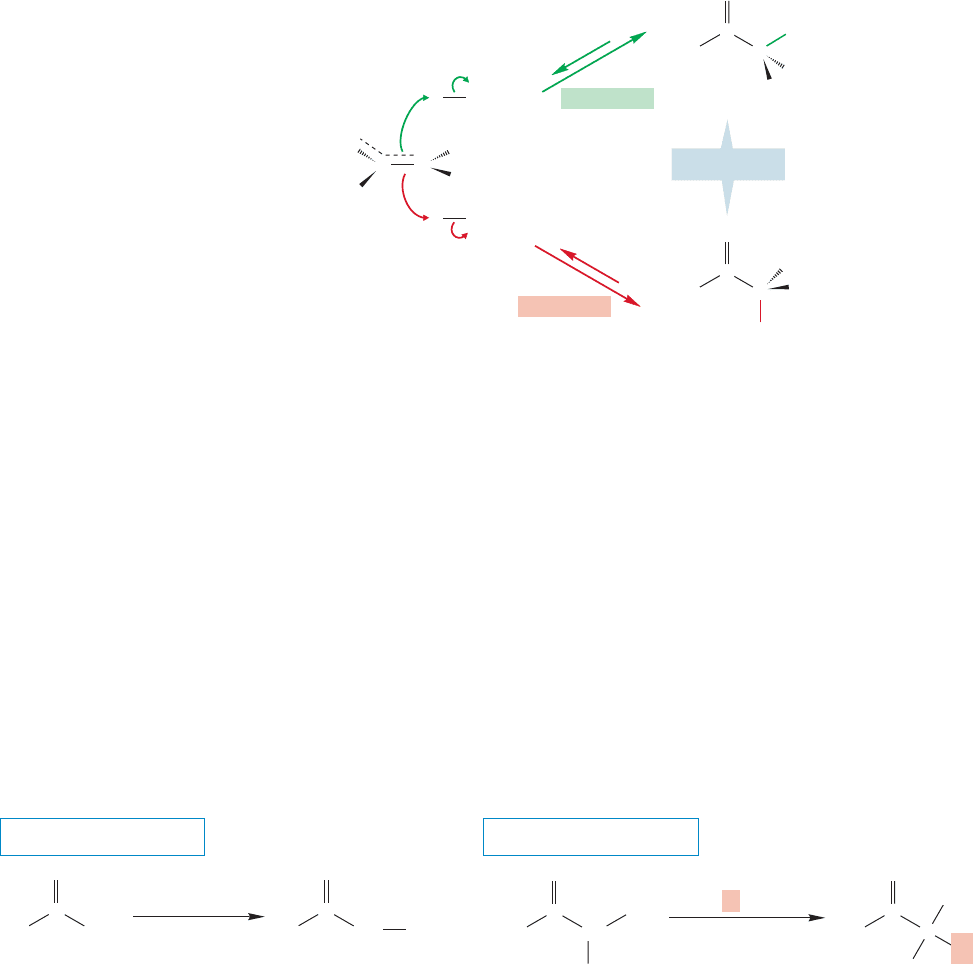
946 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
For overlap between the 2p orbitals on carbon and oxygen to be maximized, the eno-
late must be planar, and the planar structure has a nonstereogenic carbon at the α
position. A racemic mixture is formed when the enolate reprotonates (Fig. 19.32).
Addition of a proton must take place with equal probability at the two equivalent
faces (“top” green; “bottom” red) of the planar molecule. Racemization will also
occur with esters and other acid derivatives that have a hydrogen on a stereogenic
α carbon.
(S)
(R)
O
..
..
C
Ph
C
O
..
..
C
Ph
C
CH
2
CH
3
O
CC
Ph
H
CH
3
OH
..
..
H
OH
–
–
–
..
..
protonation
protonation
OH
..
..
..
+
OH
..
..
..
+
Enantiomers!
H
CH
3
CH
2
CH
3
CH
3
CH
2
CH
3
H
FIGURE 19.32 Reprotonation of the
enolate from the two equivalent faces
must give a pair of enantiomers.
19.4 Halogenation in the ␣ Position
We have already learned that halogens (X
2
) are able to react with alkenes, alkynes,
and (with a little help) aromatic rings. Halogenation is also a useful tool for adding
functionality to ketones, aldehydes, carboxylic acids, and esters.
19.4a Halogenation of Ketones and Aldehydes Halogenation at the α
position of ketones and aldehydes is one of the relatively rare examples of a reaction
that takes a different course in its acid- and base-catalyzed versions. As you will see,
though, the two reactions are closely related.
Treatment of a ketone or an aldehyde containing an α hydrogen with halogen
(X
2
,X I, Cl, or Br) in acid results in the replacement of an α hydrogen with a
halogen atom (Fig. 19.33). For the specific example shown in Figure 19.33, the
C
R
O
..
..
O
..
..
CH
3
CH
2
CH
3
CH
3
CH
2
CH
3
acid
X
2
C
RX
CH
2
C
Ph
O
..
..
O
..
..
CH
HNO
3
/CH
3
COOH
I
2
C
Ph
C
37 ⬚C
X = I, Br, or Cl
CH
3
I
..
..
..
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 19.33 Treatment of a ketone containing an α hydrogen with iodine, bromine, or chlorine in acid leads to α
halogenation.
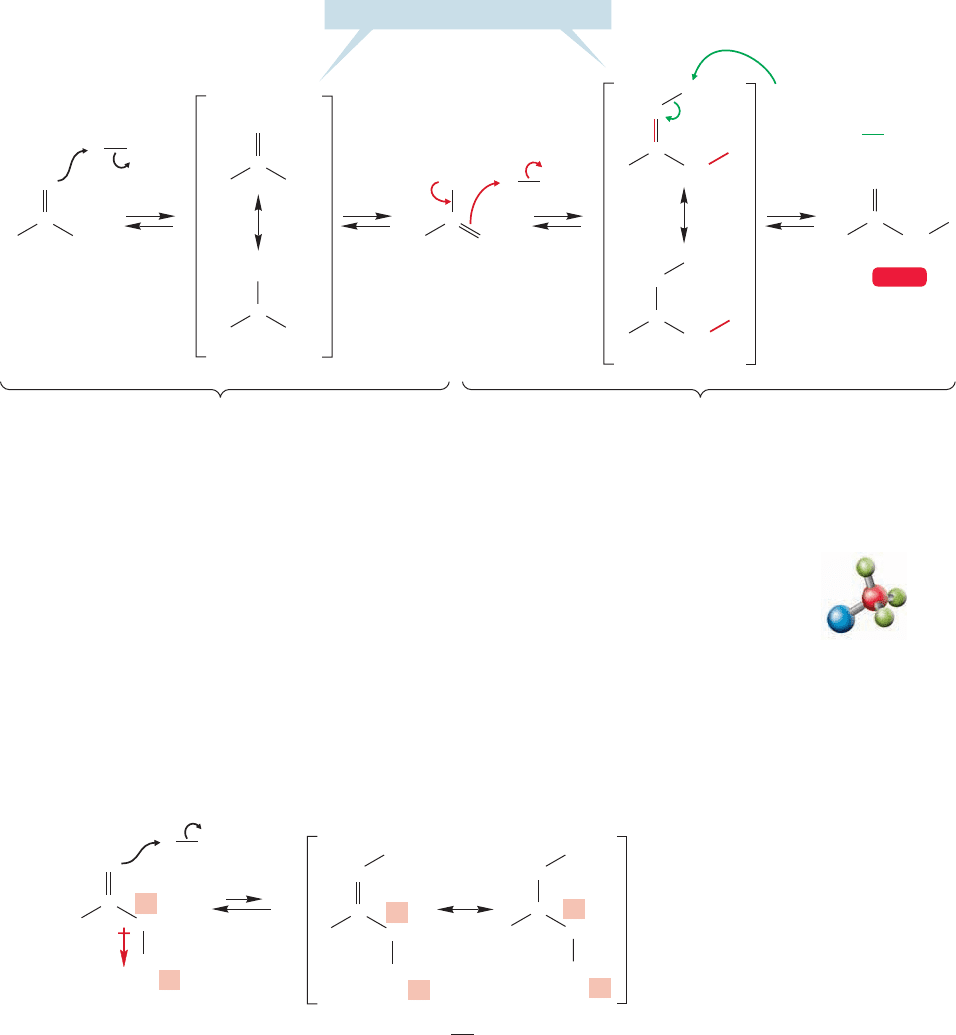
19.4 Halogenation in the Position 947
␣
enol is formed first and, like most alkenes, reacts with iodine to displace iodide
and form a resonance-stabilized cation (Fig. 19.34). The iodide ion deprotonates
the intermediate cation generating the α-iodo carbonyl compound, and ending
the reaction.
C
R
OH
OH
..
CH
3
C
R
Enol formation
Iodination
..
CH
3
C
R
O
..
..
CH
3
C
R
O
..
..
CH
2
H
OH
2
+
+
+
..
..
OH
..
..
C
Enol
R
CH
2
C
R
O
H
H
O
..
H
CH
2
C
R
..
CH
2
+
+
..
I
..
..
..
I
..
..
..
I
..
..
..
I
..
..
..
I
..
..
..
I
..
..
..
I
..
..
..
..
–
Resonance-stabilized intermediates
WEB 3D
FIGURE 19.34 Under acidic conditions the enol is formed and then reacts with iodine to give the resonance-stabilized cation.
Deprotonation leads to the α-iodide.
Earlier, we saw how deuterium replaces all α hydrogens in acetaldehyde. Why
doesn’t the α-iodo compound react further to add more iodines? The way to answer
a question such as this one is to work through the mechanism of the hypotheti-
cal reaction and see if you can find a point at which it is disfavored. In this case,
that point appears right away. It is enol formation itself that is slowed by the intro-
duction of the first halogen. The introduced iodine inductively withdraws elec-
trons and disfavors the first step in enol formation, protonation of the carbonyl
group (Fig. 19.35).
C
R
H
H
CH
2
C
R
CH
2
C
R
O
..
..
O
..
CH
2
+
+
+
The dipole in the C
I bond destabilizes
the protonated carbonyl and makes it
difficult to form
O
..
..
δ
–
δ
+
δ
+
δ
+
H
OH
2
..
I
..
..
..
δ
–
I
..
..
..
δ
–
I
..
..
..
FIGURE 19.35 Protonation of the α-iodoketone is disfavored by the
electron-withdrawing character of the halogen.
Enol halogenation
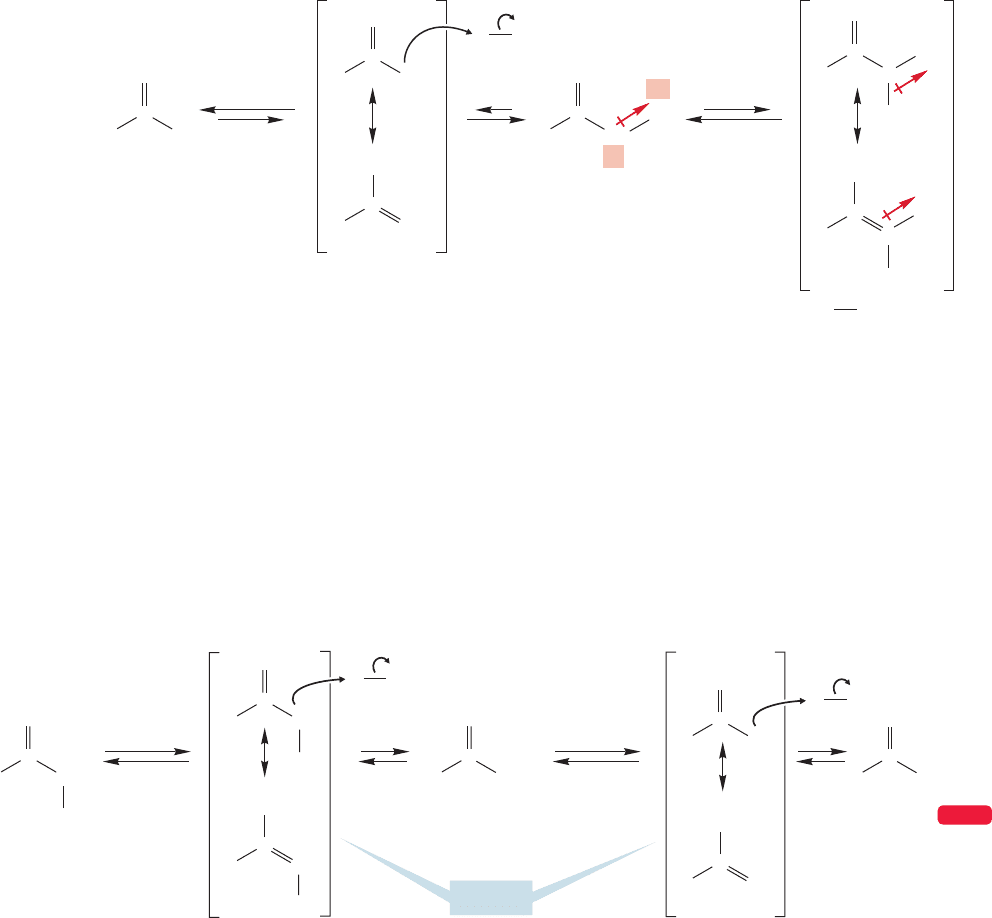
948 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
It is not easy to predict the extent of this sequence of enol formations and
halogenations. It is not reasonable to expect you to anticipate that further enol
formation would be sufficiently slowed so as to make the monoiodo compound
the end product. On the other hand, it is not so difficult to understand this effect.
Rationalization of results is much easier than predicting new reactions (or in this
case, no reaction).
Under basic conditions matters are quite different. In fact, it is very difficult
to stop the reaction at the monoiodo stage. The general result is that all α hydro-
gens are replaced with halogen. Note that ketones might have α hydrogens on
either side of the carbonyl. For molecules with three α hydrogens on one side,
methyl ketones, the reaction goes even further in what is called the haloform
reaction. In base, it is not the enol that is formed, but the enolate (Fig. 19.36).
C
R
CH
2
C
R
CH
2
C
R
O
..
..
O
..
..
CH
3
base base
C
R
O
..
..
O
..
..
C
–
–
–
–
C
R
CH
2
I
..
..
..
I
..
..
..
I
..
..
..
..
C
The C
I dipole stabilizes
the iodonated enolate
R
C
H
H
I
..
..
..
I
..
..
..
O
..
..
..
..
δ
+
δ
–
O
..
..
..
FIGURE 19.36 The initially formed α-iodo carbonyl compound is a stronger acid than the carbonyl compound
itself.The introduced iodine makes enolate formation easier.
This anion reacts in S
N
2 fashion with iodine to generate the α-iodo carbonyl
compound. Now, however, the electron-withdrawing inductive effect of iodine
makes enolate formation easier, not harder, as for enols. The negative charge of
the enolate is stabilized by the electron-withdrawing effect. A second and third
displacement reaction on iodine generates the α,α,α-triiodo carbonyl compound
(Fig. 19.37). Similar reactions are known for bromine and chlorine.
I
..
..
..
..
I
..
..
..
I
..
..
..
C
R
CH
C
R
CH
C
R
O
..
..
O
..
..
CH
2
base
C
R
O
..
..
O
..
..
–
–
–
C
R
CI
2
CHI
2
CI
2
C
R
I
..
..
..
I
..
..
..
O
..
..
..
..
O
..
..
..
base
+
–
–
I
..
..
..
..
O
..
..
C
R
CI
3
+
–
I
..
..
..
..
A triiodo
ketone
Enolates
I
..
..
..
I
..
..
..
WEB 3D
FIGURE 19.37 Sequential enolate formations and iodinations lead to the α,α,α-triiodo carbonyl compound.
