Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

18.14 Special Topic: A Family of Concerted Rearrangements of Acyl Compounds 919
PROBLEM 18.30 Can you guess what nitrene chemistry will be like? What prod-
ucts do you anticipate from the reaction of a nitrene with the carbon–carbon dou-
ble bond? What product would you get from reaction with a carbon–hydrogen
bond? Remember that a nitrene is the nitrogen analogue of a carbene (p. 431).
Isocyanates, like ketenes, are very sensitive to nucleophiles. For example,
alcohols add to isocyanates to give carbamate esters (Fig. 18.71).
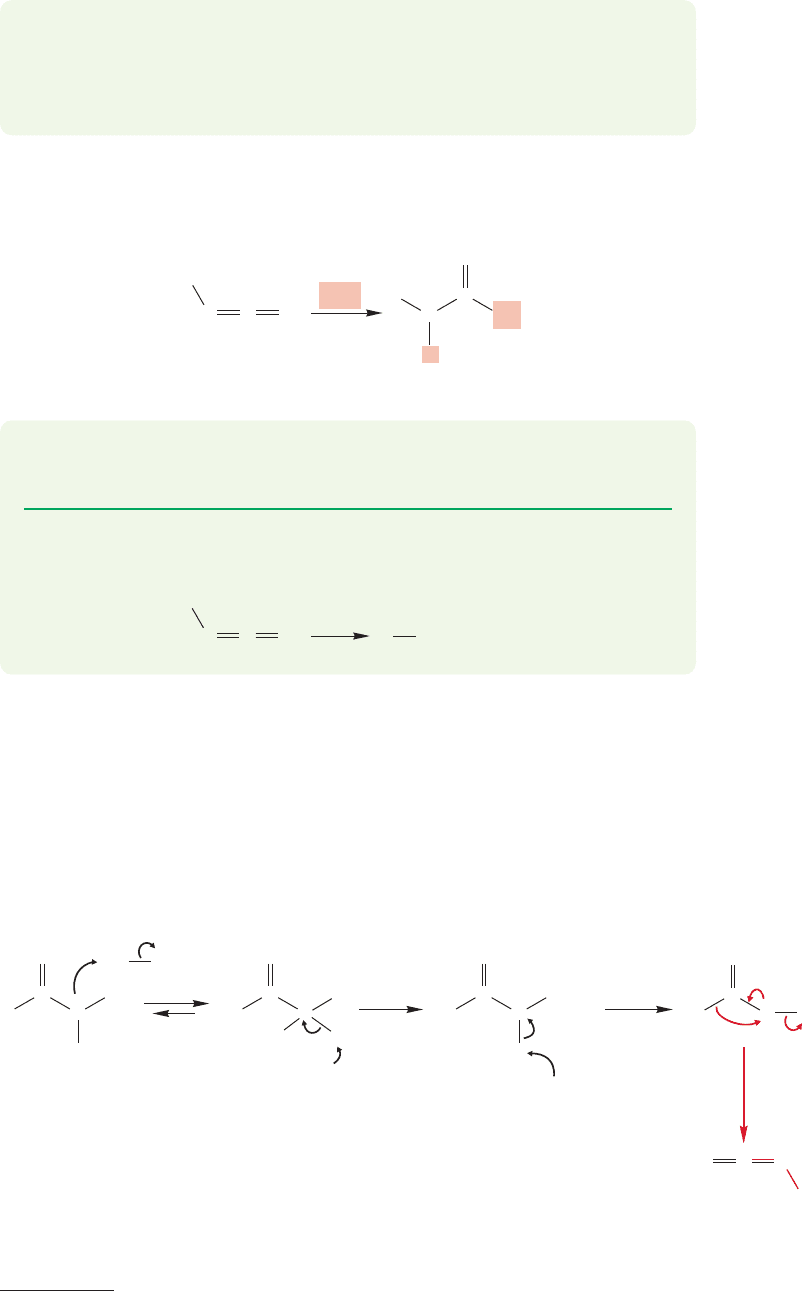
18.14c Hofmann Rearrangement of Amides Amides are able to take part
in a reaction similar to the Wolff and Curtius rearrangements.The reaction is called
the Hofmann rearrangement
3
and it allows you to convert an amide into an amine
with the loss of the carbonyl carbon.This process occurs in two steps.The amide is
first brominated to give the N-bromoamide (Fig. 18.72). Base then removes the
very acidic α hydrogen and rearrangement to the isocyanate ensues. The question
of whether a nitrene is involved in this rearrangement is not completely settled, but
we would bet on the direct rearrangement shown in Figure 18.72.
N
..
..
..
R
C
O
..
..
OR
A carbamate este
r
An isocyanate
N
H
CO
R
..
..
ROH
..
..
..
FIGURE 18.71 Isocyanates react with
alcohols to give carbamate esters.
PROBLEM 18.31 Write a mechanism for the addition of an alcohol to an isocyanate
to give a carbamate ester (Fig. 18.71).
PROBLEM 18.32 Write a mechanism for the formation of the amine in the
following reaction:
N
..
CO
R
R
..
H
2
O
..
..
NH
2
CO
2
..
..
+
N
..
CO
R
..
..
..
..
C
R
N
H
H
..
Br
..
..
..
Br
..
..
..
C
R
N
H
H
Br
..
..
..
OHNa
..
..
..
..
Br
..
..
..
–
–
Br
..
..
..
+
+
C
O
..
..
O
..
..
R
N
H
..
–
Br
..
..
..
C
R
N
migration of R
An isocyanate
(but not isolable under these conditions)
O
..
..
O
..
..
FIGURE 18.72 The first stages in the Hofmann
rearrangement. An isocyanate is an intermediate, but
cannot be isolated.
3
In earlier, more colorful times, this reaction was known as the “Hofmann degradation.”
920 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
The isocyanate is not isolable, as it is in the Curtius rearrangement. Instead,
in this reaction, it is born in the presence of base and is hydrolyzed to the carbam-
ic acid, which is unstable and decarboxylates (Fig. 18.73, recall Problem 18.32).
N
..
CO
R
..
..
HO
..
..
..
R
..
C
O
..
..
HO
N
..
–
–
–
R
C
O
..
..
HO
N
..
..
..
..
..
..
addition
decarboxylation
H
2
O
protonation
protonation
H
2
OCO
2
HO
..
..
..
..
–
–
++
R
CH
H
O
A carbamic
acid
An amine—
the end product
of the Hofmann
rearrangement
..
..
O
N
..
H
R
N
..
..
..
H
H
R
N
..
WEB 3D
FIGURE 18.73 The isocyanate reacts
with hydroxide or water to make an
intermediate carbamic acid, which
decarboxylates to give the amine.
The end product is the amine. There is an essential difference between the Curtius
rearrangment and the Hofmann rearrangement. Both involve the formation of iso-
cyanates, but only in the Curtius rearrangement is this intermediate isolable. In the
Hofmann rearrangement,base is present and the isocyanate cannot survive (Fig.18.74).
This synthesis of amines is not easy to remember because it involves many steps, thus
making it a great favorite of problem writers (open-book, of course).
–
+
R
R
C
C
O
O
..
..
..
NNN
..
..
..
..
N
..
N
2
HO
–
H
2
O
+
H
2
O
H
2
O
+
CO
2
+
R
C
Br
Br
H
O
Isolable! No
nucleophiles
present
Curtius
But Hofmann
R
R
C
O
..
..
NNH
–
..
Not isolable—
born in the
presence of
–
OH
..
..
N
..
..
..
R
NH
2
..
–
OH
–
R
C
O
..
..
N
..
..
FIGURE 18.74 The Curtius and Hofmann rearrangements contrasted.
4
Do you remember the Hofmann elimination (p. 308)? It’s the same Hofmann.
Summary
In this section we have explored a number of rearrangement reactions that
involve ketenes or ketene-like intermediates.Notice that each of these rearrange-
ments is a name reaction (Wolff, Arndt-Eistert, Hofmann,
4
and Curtius).
Don’t panic about keeping the specifics of the reactions connected to the names.
Most instructors will not ask you to reproduce the reactions by name. However,
if your future includes organic chemistry, you will see them again and become
familiar with them. Of course if your future does not include organic chemistry,
at least you can appreciate the predictability and trends these reactions illustrate.
18.15 Summary 921
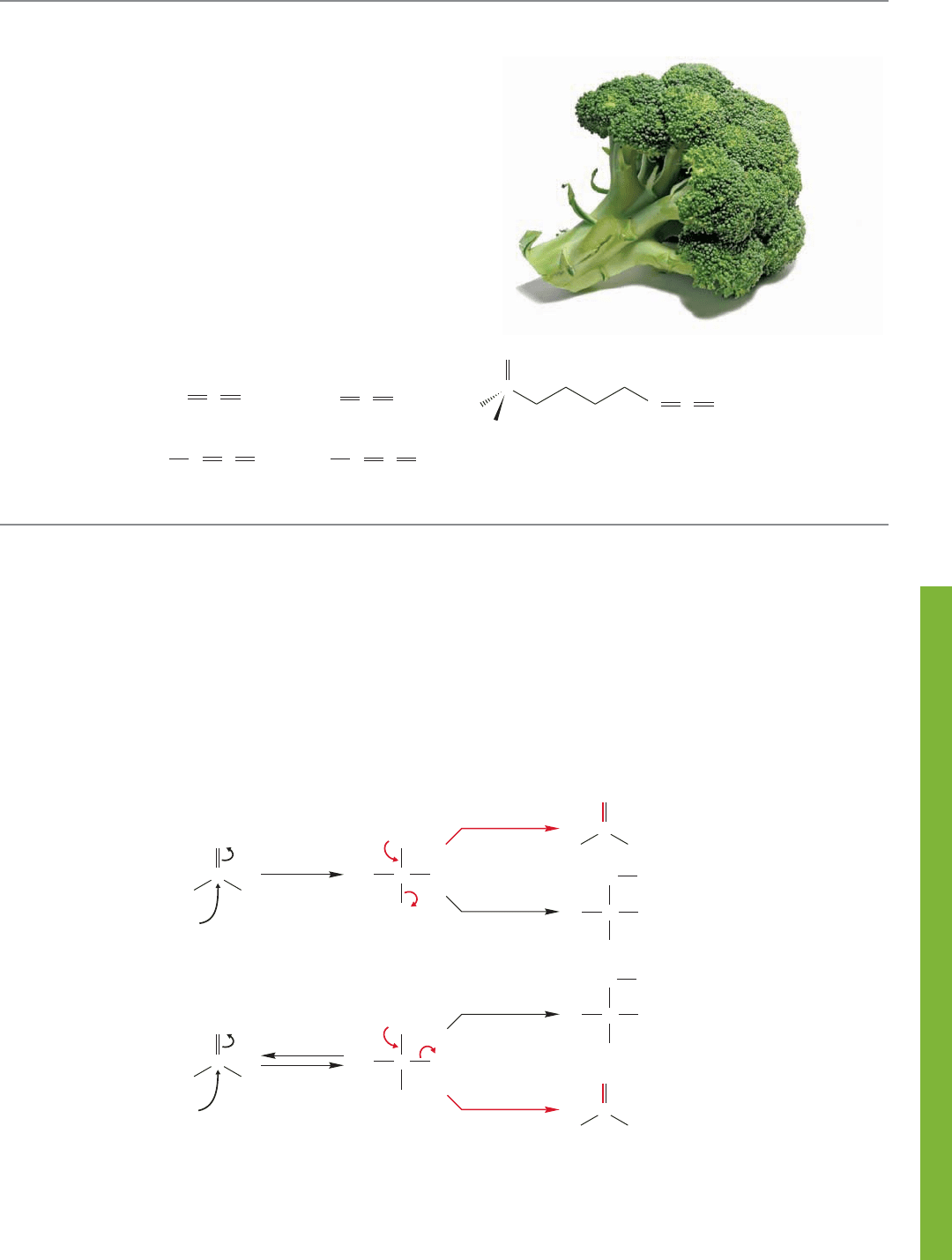
EAT YOUR BROCCOLI!
The last section showed a set of reactions unified by
formation of an intermediate containing two cumulated
(adjacent) double bonds. Their common ancestor is
allene, . Are these molecules mere
curiosities, chemical oddities of no real importance
outside a textbook or exam? Not at all. In 1992, broccoli
was shown to contain a sulfur-containing isocyanate, an
isothiocyanate, that induces formation of something called
a “phase II detoxication enzyme,” which is involved in the
metabolism of carcinogens. Eat your broccoli and study
your orgo.
H
2
C
P
C
P
CH
2
O
CH
3
..
..
..
CH
2
CCH
2
Allene Ketene
Isocyanate Isothiocyanate
C
OCH
2
..
..
CNOR
..
..
..
CNRS
..
..
..
C
1-Isothiocyanato-(4R)-
(methylsulfinyl)butane
(an anticancer agent found in broccoli)
NS
S
..
..
..
18.15 Summary
New Concepts
This chapter is filled with detail, but there are no new funda-
mental concepts. Most important, although not really new, is
the continuation of the exploration of the addition–elimination
reaction of carbonyl compounds begun in Chapter 17. When
nucleophiles add to carbonyl compounds that do not bear a
+
–
..
Nu
..
–
Nu
Nu
..
–
..
..
R
CCR
reversal
reversal
protonation
R
R
O
..
..
R
C
R
O
..
..
O
..
Nu
CR
H
R
..
O
+
–
..
L
..
–
Nu
Nu
..
–
..
..
L
CCL
protonation
elimination
R
R
O
..
..
Nu
C
R
O
..
..
O
..
Nu
CL
H
R
..
O
FIGURE 18.75 Carbonyl groups bearing good leaving groups can undergo the
addition–elimination reaction; others cannot.
leaving group (for example, aldehydes or ketones) reversal of the
addition or protonation are the only two possible reactions.
When there is a leaving group attached to the carbonyl, as there
is with most acid derivatives, another option is loss of the leav-
ing group (Fig. 18.75).
922 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
Key Terms
acid derivative (p. 877)
acid halide (p. 880)
acyl compound (p. 877)
Arndt–Eistert reaction (p. 917)
Baeyer–Villiger reaction (p. 907)
Beckmann rearrangement (p. 909)
Curtius rearrangement (p. 918)
diazo ketone (p. 915)
ester hydrolysis (p. 895)
Hofmann rearrangement (p. 919)
imide (p. 882)
isocyanate (p. 918)
nitrene (p. 918)
nitrile (p. 883)
Rosenmund reduction (p. 892)
transesterification (p. 896)
Wolff rearrangement (p. 916)
xanthate ester (p. 914)
Reactions, Mechanisms, and Tools
The addition–elimination reaction, introduced in Chapter 17,
dominates the reaction mechanisms discussed in this
chapter.
The chemistry of nitriles is similar to that of carbonyl com-
pounds. The carbon–nitrogen triple bond can act as an acceptor
of nucleophiles in addition reactions.
There is a class of intramolecular thermal elimination reac-
tions that provides a new route to alkenes. Esters, xanthates, and
amine oxides are commonly used in this reaction.The reactions
are concerted (one-step), and steric requirements dictate that a
syn elimination must occur in the reaction, as the carbonyl group
cannot reach a hydrogen in an anti position (Fig. 18.61).
Syntheses
Here are the many important synthetic reactions found in this
chapter. A few have been touched upon already in Chapter 17,
but most are new to you, at least in a formal sense. Many of the
following reactions share a common (addition–elimination)
mechanism.
A
cid- or base-induced hydrolysis of acid
halides, anhydrides, esters, and amides;
X = Cl, Br, I, O CO R, OR, NH
2
X
C
R
O
OH
C
R
O
H
2
O/H
3
O
or H
2
O/HO
–
Acid- or base-induced hydrolysis of
nitriles; the amide is an intermediate
CN R
OH
C
R
O
H
2
O/H
3
O
or H
2
O/HO
–
Hydration of ketenes
CO
OH
C
O
H
2
O
R
2
CH
C
R
R
+
+
2. Acyl Azides
Addition–elimination reaction
Cl
C
R
O
N
3
C
R
O
Na
+
N
3
–
3. Alcohols
Cl
C
R
O
R'
OH
R'
CR
R
OH
R
R
R
CR
1. 2 equiv. RLi
2. H
2
O
1. 2 equiv. RLi
2. H
2
O
OR
C
R
O
The ketone is an intermediate but cannot be isolated;
all three R groups may be the same, but all three
R’s cannot be different, RMgX also works
H
OH
H
CR
H
OH
H
CR
OH
R
RRC
Other metal hydride donors also work; the
aldehyde is an intermediate, but cannot be
isolated
Cl
C
R
O
1. LiAlH
4
2. H
2
O
Other metal hydride donors also work; the aldehyde
is an intermediate, but cannot be isolated
OR
C
R
O
1. LiAlH
4
2. H
2
O
The ester and ketone are intermediates,
but cannot be isolated; all three R groups
must be the same, RMgX also works
OR
C
RO
O
1. RLi
2. H
2
O
1. Acids
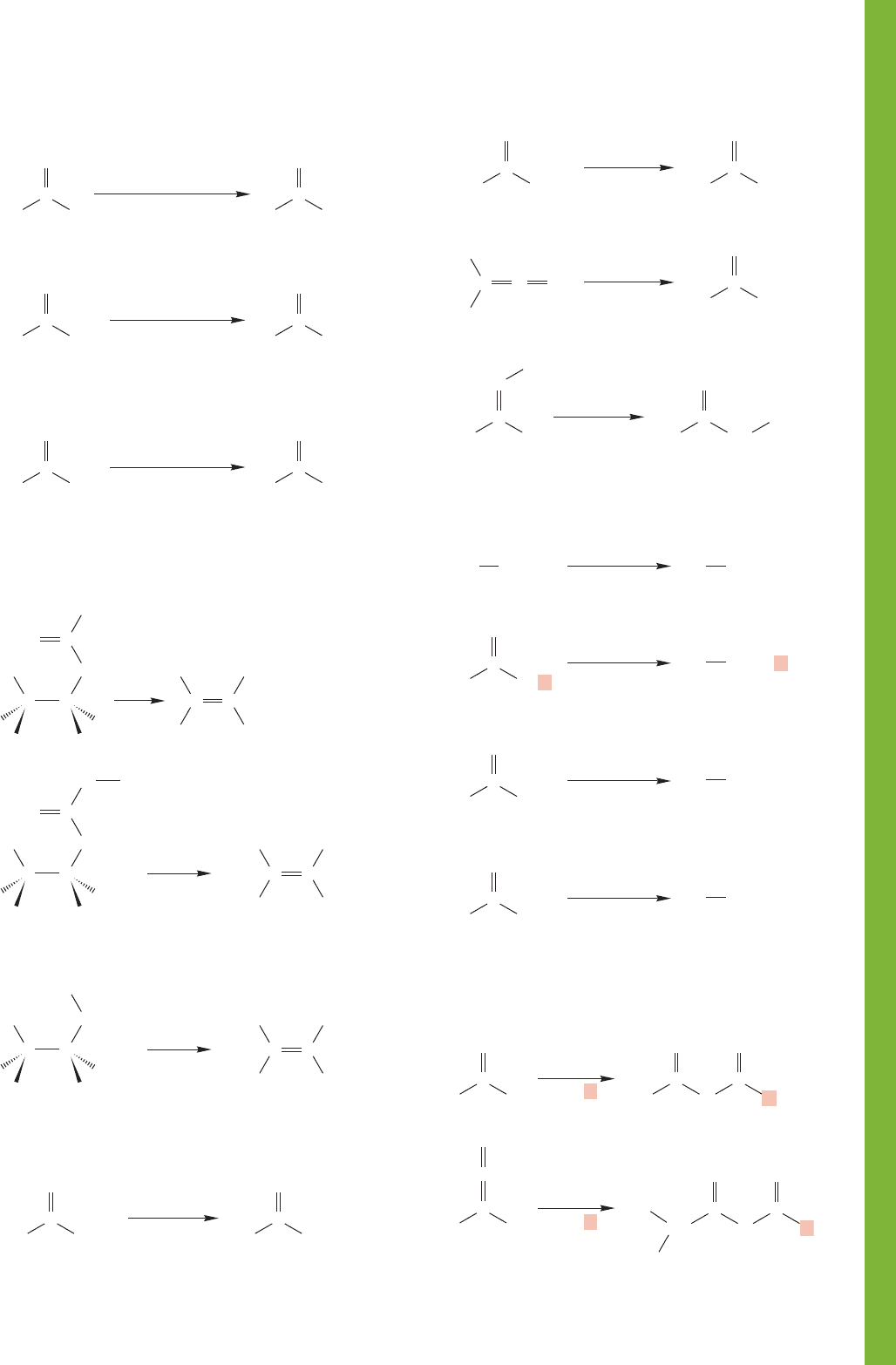
18.15 Summary 923
4. Aldehydes
Other encumbered metal hydrides also work
Cl
C
R
O
1. LiAlH[OC(CH
3
)
3
]
3
2. H
2
O
Diisobutylaluminum hydride (DIBAL-H) will not
reduce the aldehyde if the reaction is carefully run
OR
C
R
O
H
C
R
O
H
C
R
O
1. DIBAL-H
2. H
2
O
Rosenmund reduction
Cl
C
R
O
H
2
poisoned catalyst
H
C
R
O
5. Alkenes
–
+
C
Note that only a cis hydrogen can be removed
C
CO
OH
⌬
R
C RCOOH+C
C
Xanthate ester pyrolysis requires lower
temperature than ester pyrolysis
C
CH
3
CS
OH
⌬
S
CC
C
A Cope elimination
C
O
NR
2
H
⌬
CC
6. Amides
Substituted amines give substituted amides
Cl
C
R
O
NH
3
NH
2
C
R
O
Acid catalysis also works
OR
C
R
O
NH
2
C
R
O
NH
3
Na
+
–
NH
2
Substituted amines give substituted amides
NH
2
C
R
2
CH
O
NH
3
C CO
R
R
The Beckmann rearrangement
NH
CR
R
H
3
O
O
OH
R
C
R
N
+
7. Amines
Catalytic hydrogenation
H
2
/catalyst
Loss of a metal oxide from the initially
formed intermediate is the key to this reaction
NHR
C
R
O
CN R CH
2
NH
2
R
CH
2
NHRR
1. LiAlH
4
2. H
2
O
1. LiAlH
4
2. H
2
O
Loss of a metal oxide from the initially
formed intermediate is the key to this reaction, too
NH
2
C
R
O
CH
2
NH
2
R
The Hofmann rearrangement;
an isocyanate is an intermediate
NH
2
C
R
O
NH
2
R
1. Br
2
2. H
2
O/KOH
8. Anhydrides
Cl
C
R
O
R
C
R
C
O
O
C
R
R
R
O
R
C
O
O
C
CH
O
R
C
O
Na
+
–
OCOR
Na
+
–
OCOR
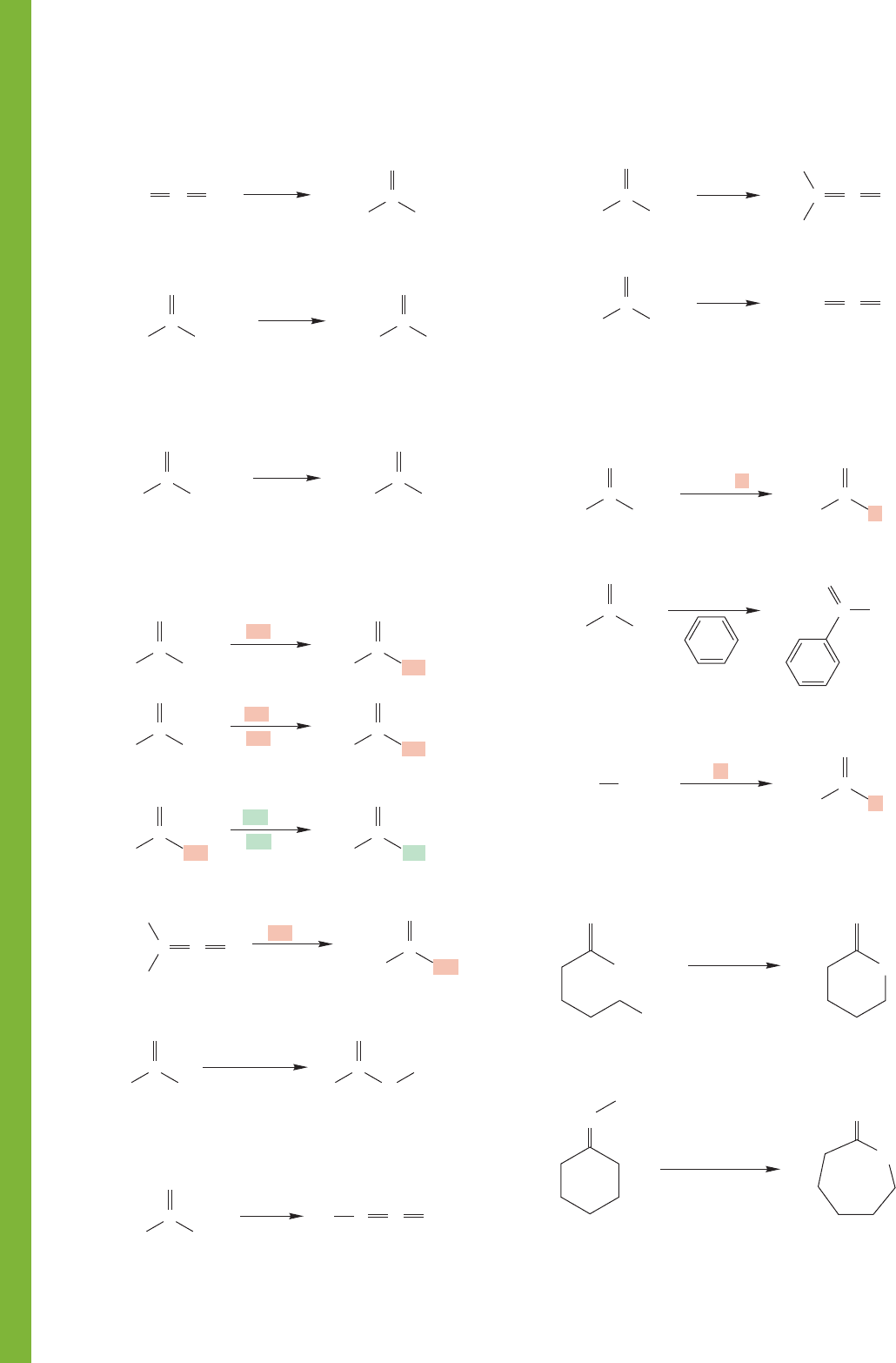
924 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
9. Carbamates
C ORN
ROH
OR
C
RNH
O
10. Carbonates
The mixed carbonate cannot be made this way
Cl
C
Cl
O
ROH
OR
C
RO
O
12. Esters
11. Diazo Ketones
Addition–elimination mechanism followed
by removal of an acidic hydrogen
Cl
C
R
O
CH
2
N
2
CHN
2
C
R
O
+
+
Cl
C
R
O
ROH
Fischer esterification
OH
C
R
O
OR
C
R
O
OR
C
R
O
ROH
2
ROH
ROH
2
ROH
Acid-catalyzed transesterification;
this transformation also works with base catalysis
OR
C
R
O
OR
C
R
O
OR
C
R
2
CH
O
ROH
CO
C
R
R
CF
3
COOOH
R
C
R
O
O
CR
R
O
The Baeyer–Villiger reaction
15. Ketones
The cuprate will react with the acid
chloride but not with the product ketone
Cl
C
R
O
Li
+
–
CuR
2
Friedel–Crafts acylation; this reaction
works with the anhydride as well
Cl
C
R
O
R
C
R
O
CR
O
AlCl
3
The imine is an intermediate
1. RLi
2. H
2
O
R
C
R
O
CN R
13. Isocyanates
Δ
or hν
N
3
C
R
O
CO
Curtius rearrangement
NR
14. Ketenes
R
3
N
Cl
C
RCH
2
O
CO
Elimination using a tertiary amine as base
C
H
R
CHN
2
C
R
O
CO
Wolff rearrangement
RCH
Δ
or hν
16. Lactams
Amide-forming reactions applied in an
intramolecular way will work
Cl
OH
NH
2
O
NH
NH
O
Lactams are formed by the cyclic version
of the Beckmann rearrangement
N
polyphosphoric
acid
O

18.16 Additional Problems 925
17. Lactones
Ester-forming reactions applied in an
intramolecular way will work
Cl
OH
O
O
O
Lactones are formed by the cyclic version
of the Baeyer–Villiger reaction
O
O
O
O H
O
Ph
O
18. Nitriles
CN
S
N
2 displacement
NC
–
+
Dehydration of amides
NH
2
C
R
O
P
2
O
5
⌬
X R R NC
R
19. Xanthate Esters
–
RO
–
+ CS
2
S
C
RO
S
SCH
3
C
RO
S
CH
3
—I
S
N
2
PROBLEM 18.33 Write mechanisms for the following conversions:
(a)
(b)
C
O
(CH
3
)
2
CH
H
2
NCH
3
Cl
C
O
(CH
3
)
2
CH NHCH
3
C
O
(CH
3
)
2
CH
NaN
3
Cl
C
O
(CH
3
)
2
CH
N
3
PROBLEM 18.34 Write mechanisms for the following conver-
sions in acid:
(a)
(b)
C
O
(CH
3
)
2
CH
H
2
O
OCH
3
C
O
(CH
3
)
2
CH
HOCH
3
OH
C
O
(CH
3
)
2
CH
CD
3
OH
CD
3
OH
2
OCH
3
C
O
(CH
3
)
2
CH
OCD
3
+
HOCH
3
+
+
H
3
O
+
Analyze problems before starting. Do not be too proud to do the
obvious. Use the molecular formula to see what molecules must
be combined. In acid (H
3
O
, HCl, etc.), carbocations are the
likely intermediates. In base (
OH,
OR, etc.), carbanions are
probably involved. Try to identify what must be accomplished in
a problem. Although sometimes it will be hard to plan, and you
may have to try all possible intermediates, usually a problem will
tell you much of what you must do.
Ask yourself questions and set goals. What rings must be
opened? What rings must be closed? What atoms become
incorporated in the product? Pay attention to “stop signs.”
Primary carbocations are stop signs. Unstabilized carbanions are
stop signs. If you are forced to invoke such a species in an
answer, it is almost certainly wrong, and you must backtrack.
Hard problems become easier if you keep such questions in
mind. All of this advice sounds obvious, but it’s not. Thinking a
bit about what must be done in a problem saves much time in
the long run and avoids the generally hopeless random arrow
pushing that can trap you into a wrong answer.
Common Errors
Let’s warm up with some simple problems, just to be certain
that the basic (and acidic) mechanisms of this chapter are under
control. Then we can go on to other things. Please be sure you
can do the “easy” problems before you try the more difficult
ones at the end of this section.
18.16 Additional Problems
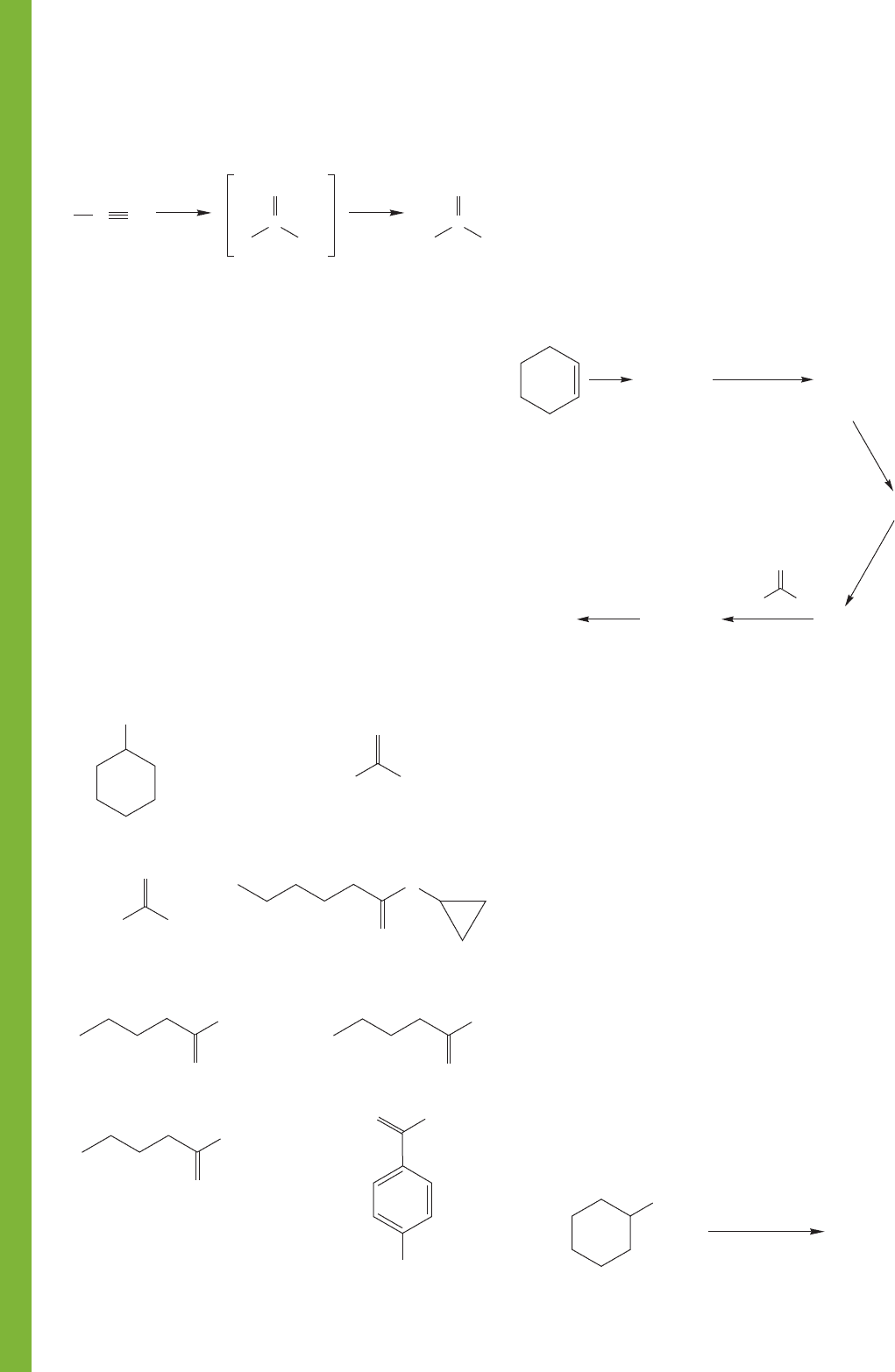
926 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
PROBLEM 18.35 Now write the mechanism for a slightly more
complicated acid-catalyzed hydrolysis.
C
C
N
O
P
h
Ph
HCl
H
2
O
HCl
H
2
O
NH
2
C
O
P
h
OH
PROBLEM 18.36 Write a mechanism for the acid-catalyzed
formation of ethyl hexanoate from ethanol and hexanoic acid.
PROBLEM 18.37 Show a synthetic route to methyl benzoate
starting with methanol and toluene. Assume you have access
to any other needed reagents.
PROBLEM 18.38 Write Lewis structures for the following
compounds:
(a) propanoic acid
(b) propanoyl chloride
(c) N,N-diethyl-2-phenylpropanamide
(d) methylketene
(e) propyl cyclopropanecarboxylate
(f) phenyl benzoate
PROBLEM 18.39 Give the IUPAC name for each of the
following molecules:
COOCH
3
OCH
2
CH
3
H
3
C
O
(a) (b)
OCH
3
CH
3
CH
2
O
O
Br
O
O
OH
O
Cl
O
O
(c) (d)
(e) (f)
(g) (h)
NH
2
NH
2
PROBLEM 18.43 Analysis of the IR stretching frequencies of
carbonyl compounds involves an assessment of resonance and
inductive effects. This assessment is a somewhat risky business,
and it must be admitted that near-circular reasoning is some-
times encountered. Nonetheless, see if you can make sense of
the following observations taken from Table 18.2. In each case,
it will be profitable to think of all the important resonance
forms of the acyl compound.
(a) Acetone absorbs at 1719 cm
1
, whereas acetaldehyde
absorbs at 1733 cm
1
.
(b) Explain why methyl acetate absorbs at higher frequency
(1750 cm
1
) than acetone (1719 cm
1
).
(c) N-Methylacetamide absorbs at much lower frequency
(1688 cm
1
) than methyl acetate (1750 cm
1
).
PROBLEM 18.44 Is your explanation in Problem 18.43 (b)
consistent with the observation that the carbonyl carbon of
methyl acetate appears at δ 169 ppm in the
13
C NMR spec-
trum, whereas the carbonyl carbon of acetone is far downfield
at δ 205 ppm? Explain.
PROBLEM 18.45 Give the major organic products expected in
each of the following reactions or reaction sequences.
PROBLEM 18.40 Show how you would synthesize N-phenyl-
1-pentanamine from 1-pentanol, aniline, tosylchloride, and any
necessary inorganic reagents.
PROBLEM 18.41 Amine oxides can be formed by the reaction
of a tertiary amine with a peroxy acid. Show the synthetic steps
you would use to make cyclohexene from cyclohexanamine
using any necessary reagents.
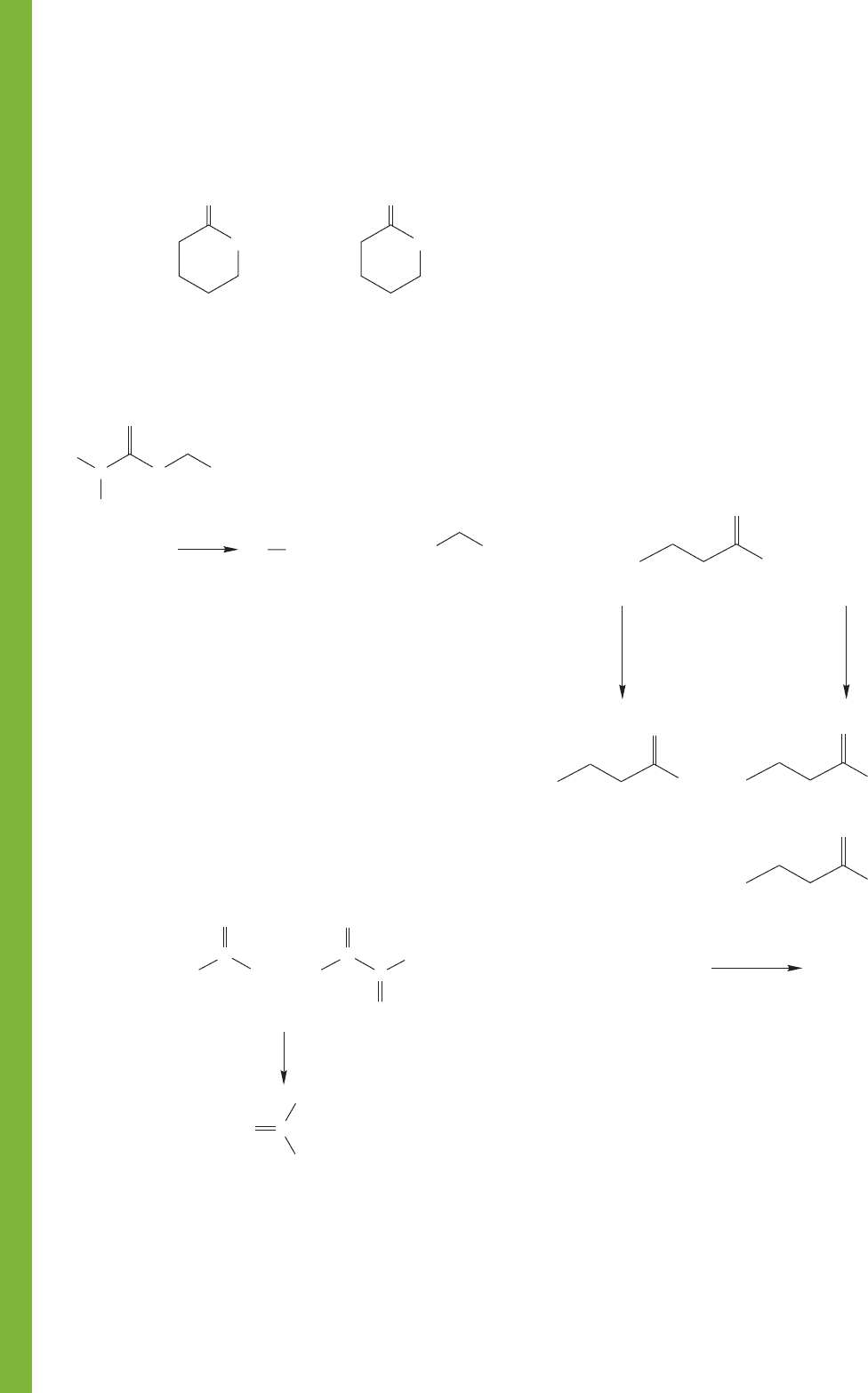
PROBLEM 18.42 Provide structures for compounds A–F in
this series of reactions.
A
(C
6
H
11
Br)
B
(C
7
H
12
O
2
)
C
(C
8
H
14
O
2
)
F
(C
7
H
12
)
E
(C
9
H
16
O
2
)
D
(C
7
H
12
O)
H
3
CCl
HBr
500 °C
1. Mg
2. CO
2
3. H
2
O/H
3
O
+
1. LiAlH
4
2. H
2
O
O
CH
3
OH
H
3
O
+
COOH
(a)
1. SOCl
2
2. HN(CH
3
)
2
3. LiAlH
4
4. H
2
O
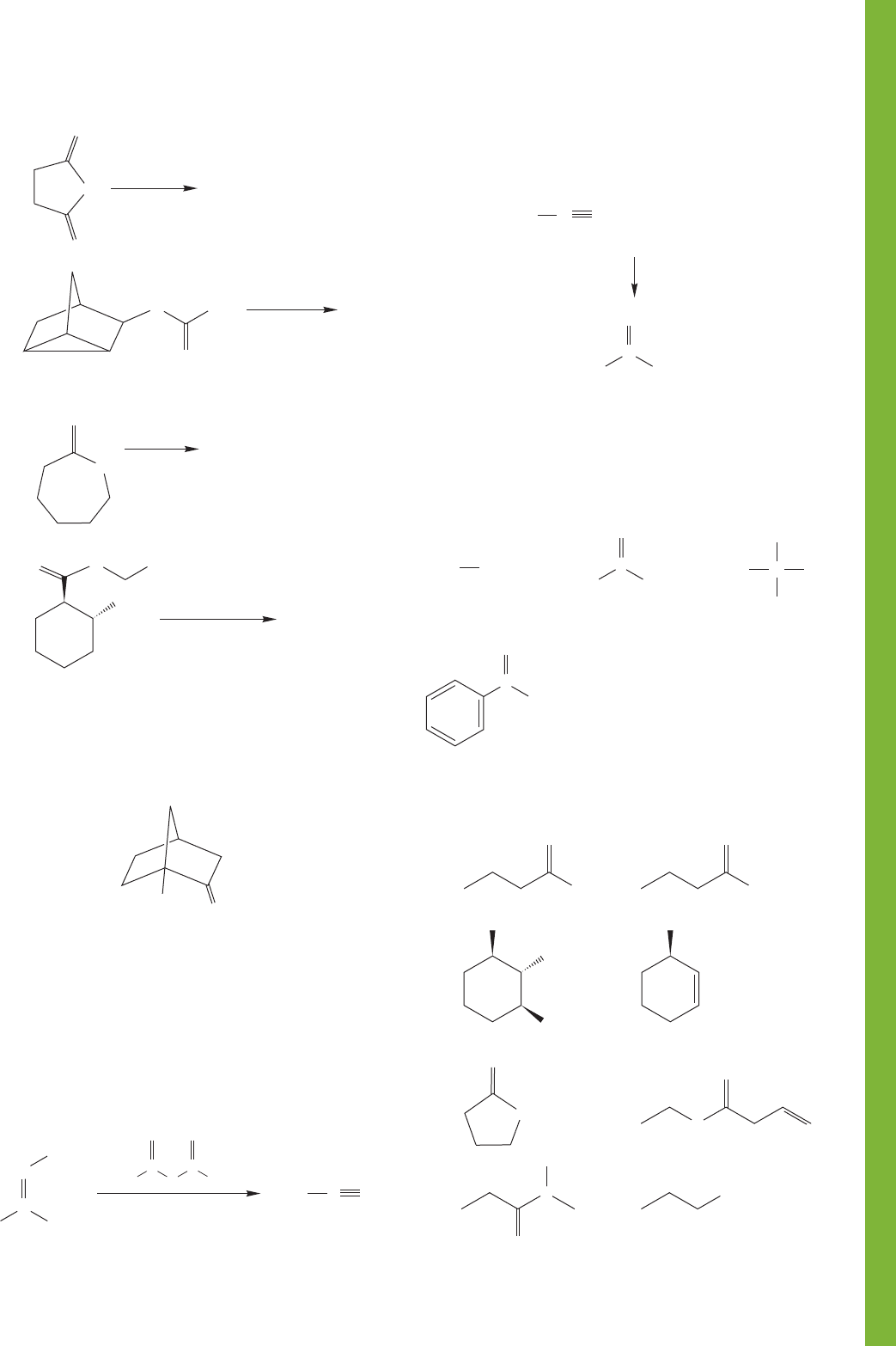
18.16 Additional Problems 927
(b)
O
O
O
(c)
(d)
(e)
2. CS
2
3. CH
3
I
1. NaH
4. 210–235 C
H
2
O, Δ
CH
3
OH
CH
3
OH, Δ
OH
CH
3
ONa
O
Δ
HCl
NH
O
O
CH
3
OO
O
HOOC
PROBLEM 18.46 We learned in Chapter 17 that most β-keto
acids decarboxylate very easily (p. 858). The compound below is
an exception, as it survives heating to very high temperature
without losing carbon dioxide. Explain.
PROBLEM 18.47 In Section 18.12c, we saw that primary amides
could be dehydrated to nitriles (cyanides) with P
2
O
5
. Nitriles
can also be prepared by treating aldoximes (1) with dehydrating
agents such as acetic anhydride. Propose a mechanism for the
formation of benzonitrile from the reaction of 1 with acetic
anhydride. How are oximes prepared?
H
1
Ph
Ph C N
N
OH
C
CH
3
Δ
C
O
C
H
3
C
OO
PROBLEM 18.48 Reaction of benzonitrile (1) and tert-butyl
alcohol in the presence of concentrated sulfuric acid, followed
by treatment with water, gives N-tert-butylbenzamide (2).
Provide an arrow formalism mechanism for this reaction.
NHC(CH
3
)
3
1
2
Ph
Ph C N
O
C
(CH
3
)
3
COH
1. conc. H
2
SO
4
2. H
2
O
+
PROBLEM 18.49 Devise syntheses for the following molecules.
You may start with benzene, methyl alcohol, sodium methoxide,
ethyl alcohol, sodium ethoxide, butyl alcohol (BuOH), phos-
gene, and propanoic acid as your sources of carbon. You may
also use any inorganic reagent and solvents as needed.
Bu
3
C
OH
C
C
Bu
C
CH
3
CH
2
CH
3
CH
2
CN
CH
3
CH
2
CH
2
CH
3
O
O
Bu
Bu
OH
(a)
(e)
(b) (c)
(d)
PROBLEM 18.50 Propose syntheses for the following target mol-
ecules starting from the indicated material. You may use any other
reagents that you need. Use a retrosynthetic analysis in each case.
(a) from
(b) from
(c) from
O
H
O
O
OH
CO
2
H
C(CH
3
)
3
C(CH
3
)
3
O
OH
O
O
(d) from
O
N OH
HBr
Br Ph
R NH
2
CO
2
++
928 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
PROBLEM 18.51 Propose syntheses of the following molecules
starting from cyclopentanone:
O
NH
(a) (b)
O
O
PROBLEM 18.52 Provide an arrow formalism mechanism for
the following reaction:
H
O
O Ph
R
N
PROBLEM 18.53 Here is the problem concerning the forma-
tion of the Vilsmeier reagent we promised you in Chapter 17.
As we saw in Section 17.7d, acid chlorides can be prepared
from carboxylic acids and reagents such as thionyl chloride,
phosphorus pentachloride, phosgene, and oxalyl chloride. The
conditions for these reactions are sometimes quite severe. It is
possible to prepare acid chlorides under appreciably milder
conditions by using the Vilsmeier reagent (Problem 17.42).
The Vilsmeier reagent is prepared from DMF and oxalyl
chloride. Write an arrow formalism mechanism for its forma-
tion. Hint: DMF acts as a nucleophile, but not via the nitro-
gen atom.
O
O
O
C
+
(CH
3
)
2
NCl
H
DMF Oxalyl chloride
C
C
Cl
+
(CH
3
)
2
N
Vilsmeier reagent
H
C
Cl
–
Cl
[CH
3
CH
2
CH
2
CH
2
OAlR
2
]CH
3
CH
2
CH
2
CH
2
OH
(major)
1. DIBAL-H
–70 ⬚C
2. H
2
O
1. DIBAL-H
–70 ⬚C
2. warm to
0 ⬚C
3. H
2
O
H
2
O
O
H
(90%)
O
H
(minor)
O
OCH
2
CH
3
(major)
O
OCH
2
CH
3
PROBLEM 18.55 γ-Aminobutyrate transaminase (GABA-T)
is the key enzyme controlling the metabolism of γ-aminobu-
tyric acid (GABA), a mammalian inhibitory neurotransmitter.
In the following synthesis of the hydrochloride salt of the
putative GABA-T inhibitor, 3-amino-1,4-cyclohexadiene-1-
PROBLEM 18.54 In Section 18.8, we saw that aldehydes
are available from the reduction of esters at low temperature
(70 °C) by diisobutylaluminum hydride (DIBAL-H; see
Fig. 18.38). If the reaction mixture is allowed to warm before
hydrolysis, the yield of aldehyde drops appreciably. For example,
reduction of ethyl butyrate with DIBAL-H at 70 °C followed
by hydrolysis at 70 °C, affords a 90% yield of butyraldehyde.
However, if the reaction mixture is allowed to warm to 0 °C
before hydrolysis, the yield of aldehyde is less than 20%.
Among the products under these conditions are ethyl
butyrate (recovered starting material) and butyl alcohol
(CH
3
CH
2
CH
2
CH
2
OH), formed from hydrolysis of
(R isobutyl). Rationalize these
observations with arrow formalism mechanisms.
CH
3
CH
2
CH
2
CH
2
O
O
AlR
2
