Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

18.16 Additional Problems 929
A
(C
8
H
10
O
4
)
COOH
COOH
COOCH
3
B
(C
5
H
5
N
3
O)
NaN
3
H
3
N
H
2
O
base
THF
C
(C
9
H
15
NO
2
)
D
(C
13
H
20
N
2
O
6
)
E
(C
13
H
19
NO
4
)
1. toluene
Δ
benzene, 25 ⬚C
2. (CH
3
)
3
COH
1. NaOH/H
2
O
55 ⬚C
2. H
2
O/H
3
O
+
F
(C
12
H
17
NO
4
)
G
HCl/H
2
O
55 ⬚C
O
2
N
EtN(i-Pr)
2
acetone
Cl
OEt
C
O
+
–
Cl
PROBLEM 18.56 Treatment of phthalimide (1) with bromine
in aqueous sodium hydroxide, followed by acidification with
acetic acid, affords compound 2. A summary of spectral data for
2 follows. Deduce the structure of 2, and then propose a mech-
anism for its formation.
1
2
1. Br
2
/NaOH/H
2
O
⌬
2. HOAc
NH
O
O
Compound 2
Mass spectrum: m/z 137 (M, 59%), 119 (100%), 93 (79%),
92 (59%)
IR (KBr): 3490 (m), 3380 (m), 3300–2400 (br), 1665 (s),
1245 (s), and 765 (m) cm
1
1
H NMR (DMSO-d
6
):
5
δ 6.52 (t, J = 8 Hz, 1H)
6.77 (d, J 8 Hz, 1H)
7.23 (t, J 8 Hz, 1H)
7.72 (d, J 8 Hz, 1H)
8.60 (br s, 3H, vanishes with D
2
O)
13
C NMR (DMSO-d
6
): δ 109.5 (s), 114.5 (d), 116.2 (d), 131.1 (d),
133.6 (d), 151.4 (s), 169.5 (s)
⌬
(CH
3
)
2
N
O
–
+
PROBLEM 18.57 Sketch the transition state for the Cope
elimination (p. 914) shown below.
O
..
..
NH
2
..
OH
..
..
HN
3
/H
2
SO
4
H
2
O
(72%)
PROBLEM 18.59 Write a mechanism for the Schmidt reaction
shown below. Hint: The first step is probably the formation of
an acylium ion, . Note also that the reagent is HN
3
,
not NH
3
.
R
O
C
+
P
O
5
Note that J
meta
and J
para
were not observed under these conditions.
PROBLEM 18.58
Design an experiment to test the stereo-
chemistry of the Cope elimination. How would one determine
that it is a cis hydrogen that is lost? Assume you can make any
labeled compounds you need.
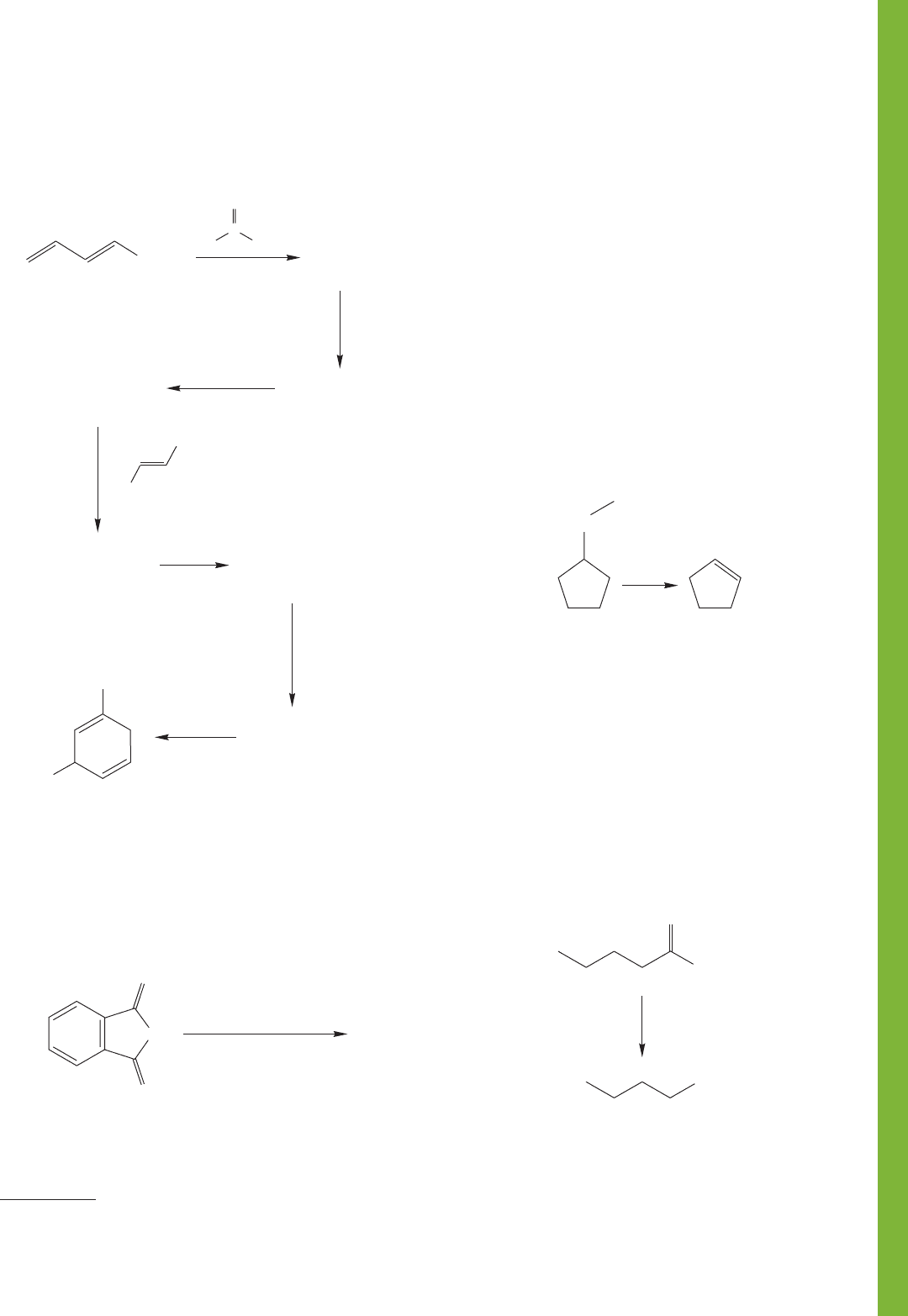
carboxylic acid (G), propose structures for intermediates A–F.
Mechanisms are not necessary, but may be helpful in
some cases.
930 CHAPTER 18 Derivatives of Carboxylic Acids: Acyl Compounds
PROBLEM 18.61 Reaction of 1,3-diphenylisobenzofuran (1)
and vinylene carbonate (2) gives compound 3. Acid hydrolysis
of 3 affords 4. Propose a structure for 3 and write a mechanism
for its formation and hydrolysis to 4.
Δ
xylene
acetic acid
Δ
O
O
O
O
+
HCl/H
2
O
Ph
Ph
Ph
Ph
HO
HO
(C
23
H
16
O
4
)
3
2
4
1
2. KCN/H
2
O
CN
–
1. Ph
Cl
C
O
N
O
N
+
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 18.63 Select the reaction “Ester hydrolysis” and
click on the play button. Note the products of the reaction. Is
this reaction catalyzed by base, or does this reaction require a
stoichiometric amount of base?
PROBLEM 18.64 Observe the LUMO animation of the “Ester
hydrolysis.” Describe the LUMO of the starting ester (methyl
methanoate). On what atom is the LUMO density concentrat-
ed? Where else is there LUMO density? Why?
PROBLEM 18.65 Select the reaction “Nitrile hydrolysis” and
watch the LUMO animation. Stop the animation at the first
intermediate. Notice that the first molecular orbital animated is
along the axis. Then the animation shifts to a molecular
orbital that is perpendicular to the axis. These orbitals
are very close in energy. Why does the animation shift to the
second LUMO? Does this mean that a nucleophile (a water
molecule in this case) has more than one option for reaction
with the first intermediate?
C
O
N
C
O
N
PROBLEM 18.62 Write an arrow formalism mechanism for the
following reaction:
PROBLEM 18.60 Provide structures for compounds A–E.
Mechanisms are not necessary.
COOH
A
(C
7
H
11
ClO)
B
(C
9
H
17
NO)
C
(C
9
H
19
N)
D
(C
9
H
19
NO)
E
(C
7
H
12
)
SOCl
2
150 ⬚C
1. LiAlH
4
ether, 35 ⬚C
2. H
2
O
(CH
3
)
2
NH
benzene 25 ⬚C
30% H
2
O
2
2 h
100 ⬚C

Carbonyl Chemistry 2:
Reactions at the α Position
931
19.1 Preview
19.2 Many Carbonyl Compounds Are
Weak Brønsted Acids
19.3 Racemization of Enols and
Enolates
19.4 Halogenation in the α Position
19.5 Alkylation in the α Position
19.6 Addition of Carbonyl
Compounds to the α Position:
The Aldol Condensation
19.7 Reactions Related to the Aldol
Condensation
19.8 Addition of Acid Derivatives to
the α Position: The Claisen
Condensation
19.9 Variations on the Claisen
Condensation
19.10 Special Topic: Forward and
Reverse Claisen Condensations
in Biology
19.11 Condensation Reactions in
Combination
19.12 Special Topic: Alkylation of
Dithianes
19.13 Special Topic: Amines in
Condensation Reactions, the
Mannich Reaction
19.14 Special Topic: Carbonyl
Compounds without
α Hydrogens
19.15 Special Topic: The Aldol
Condensation in the Real
World, an Introduction to
Modern Synthesis
19.16 Summary
19.17 Additional Problems
19
MAKING CARBON–CARBON BONDS All plants and animals such as these fish, the
coral, and the diver use enol chemistry in the citric acid cycle as a way to make
carbon–carbon bonds.
932 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
I want to beg you, as much as I can, dear sir, to be patient towards all that
is unsolved in your heart and try to love the
questions themselves
.
—RAINER MARIA RILKE,
1
LETTERS TO A YOUNG POET
19.1 Preview
In this chapter, we explore the chemistry of the α position in the carbonyl com-
pounds we met in Chapters 16–18. Our ability to understand complex reactions
and to construct complicated molecules will greatly increase. There really is lit-
tle fundamentally new chemistry—much of what we find from now on will be
applications of reactions we already know, placed in more complicated settings.
For example, in Chapter 16 we saw a vast array of additions of nucleophiles to
carbon–oxygen double bonds.Here that same reaction appears,when a new nucleo-
phile, an enolate, adds to carbonyl groups to yield products of increased com-
plexity. The reaction is the same old addition, but the context makes it look
complicated (Fig. 19.1).
1
Rilke (1875–1926) was a German lyric poet much influenced by the French sculptor, Rodin. The italics are
Rilke’s.
C
R
A nucleophile
R
..
O
..
..
..
NuNu
–
CH
3
CH
3
C
C
R
R
O
..
..
O
..
..
..
O
..
..
–
O
..
..
..
–
–
CH
3
C
R
An enolate
(a new nucleophile)
O
..
..
R
C
CH
2
CH
3
CH
2
C
FIGURE 19.1 Additions of
nucleophiles to carbonyl compounds.
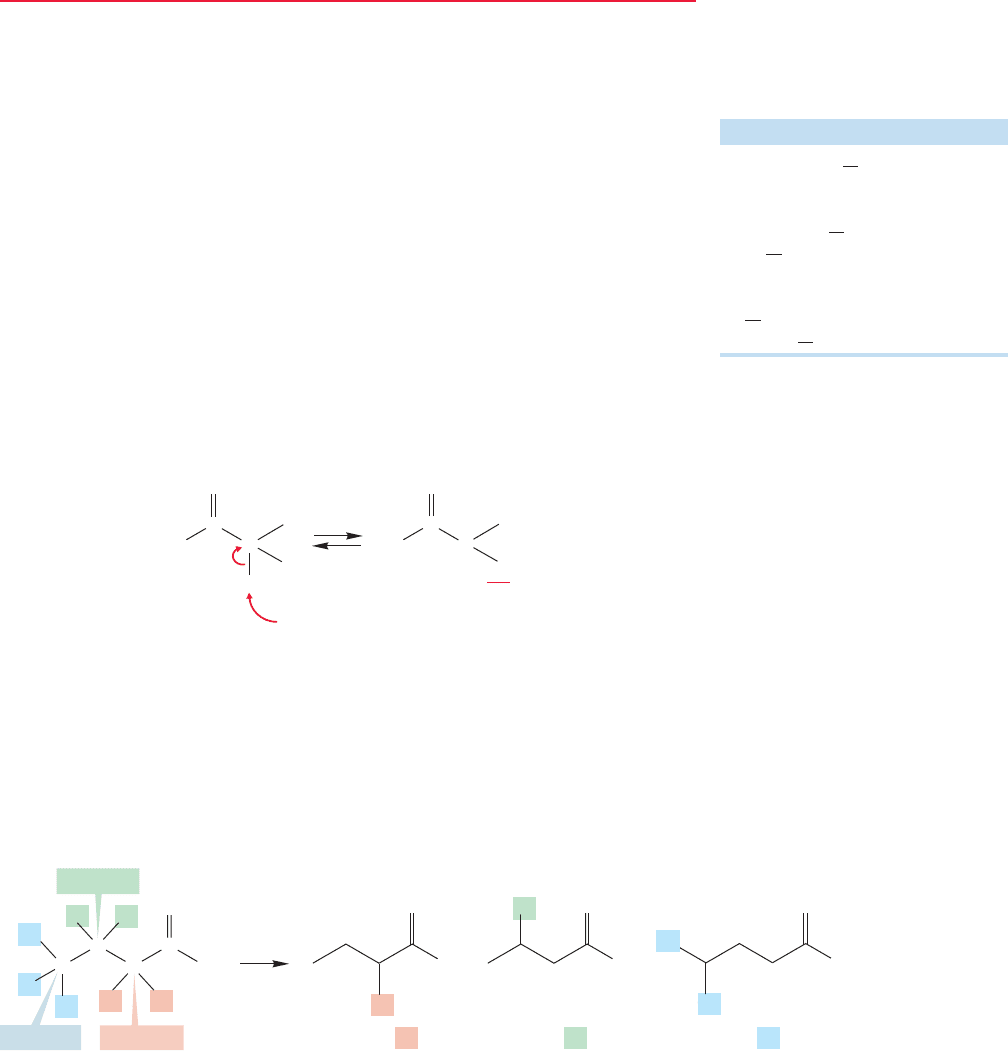
ESSENTIAL SKILLS AND DETAILS
1. Two reactions critical to understanding the material in this chapter are enol formation
in acid and enolate formation in base. Be sure you understand these two basic reactions
completely.
2. The central reactions of this chapter are the aldol and Claisen condensations. Both
acid- and base-catalyzed aldol reactions exist.The Claisen condensation only succeeds
under basic conditions.
3. Although both the acid- and base-catalyzed aldol condensations lead to the same
initial product, a β-hydroxy carbonyl compound, the reaction usually goes further
in acid (sometimes in base) to give an α,β-unsaturated carbonyl compound. All
β-hydroxy carbonyl compounds and all α,β-unsaturated carbonyl compounds can,
in principle, be made through aldol or closely related condensations.This observation
is an important problem-solving tool. Always look for the α,β-unsaturated carbonyl
compound.
19.2 Many Carbonyl Compounds Are Weak Brønsted Acids 933
4.
Watch out for the addition of nucleophiles to α,β-unsaturated carbonyl compounds to
give enolates.This process, called the Michael reaction, is very common in Nature, in
organic synthesis, and in organic chemistry problems.
5. The synthetic procedures known as the acetoacetate synthesis and the malonic ester
synthesis are very useful. Acetoacetate can be used to make ketones and malonic esters
can be used to make carboxylic acids.
6. Alkylation of enolates provides an excellent route for making carbon–carbon bonds.
TABLE 19.1 Some pK
a
Values
for Simple Ketones and
Aldehydes
Compound
a
pK
a
CH
3
CH
2
COCH
2
CH
3
19.9
CH
3
COCH
3
19.3
PhCOCH
3
18.3
PhCH
2
COCH
3
18.3
PhCH
2
COCH
3
15.9
Cyclohexanone
(α hydrogen) 18.1
CH
3
CHO 16.7
(CH
3
)
2
CHCHO 15.5
a
The proton to be lost is underlined when
there is a choice.
C
R
O
..
..
C
H
C
R
O
..
..
C
H
B
..
..
–
pK
a
= 15–20
B
–
FIGURE 19.2 Carbonyl compounds
bearing hydrogen at the α position
are weak acids, with pK
a
values in
the high teens.
C
C
CH
C
O
O
..
..
H
β
H
β
H
α
H
α
H
γ
H
γ
H
γ
..
B
–
–
H
H
α
H
α
Lose
H
β
Lose
H
γ
Lose
..
O
–
H
H
β
..
O
–
H
H
γ
..
γ Carbon
β Carbon
Butanal
α Carbon
H
γ
..
..
..
..
..
..
FIGURE 19.3 There are three possible anions that can be formed from butanal by breaking an sp
3
/1s
carbon–hydrogen bond.
We’ll start with butanal (pK
a
16.7) and look at the three possible anions we
might form from it by breaking an sp
3
/1s carbon–hydrogen bond (Fig. 19.3). First
of all, the dipole in the carbon–oxygen bond will stabilize an adjacent anion more
than a more remote anion. Loss of the α hydrogen will lead to a more stable anion
19.2 Many Carbonyl Compounds Are
Weak Brønsted Acids
One of the remarkable things about carbonyl compounds is that they are not only
electrophiles (see the addition reactions throughout Chapters 16–18 and Fig. 19.1),
but are proton donors as well.
19.2a Enolates of Ketones and Aldehydes The pK
a
values of typical alde-
hydes and ketones are generally in the high teens (Table 19.1). It is the hydrogen at
the ␣ position, the position adjacent to the carbon–oxygen double bond,that is lost
to a base as a proton (Fig. 19.2). Our first task is to see why these compounds are
weak acids at their α positions, and then to see what new chemistry results from
this acidity.
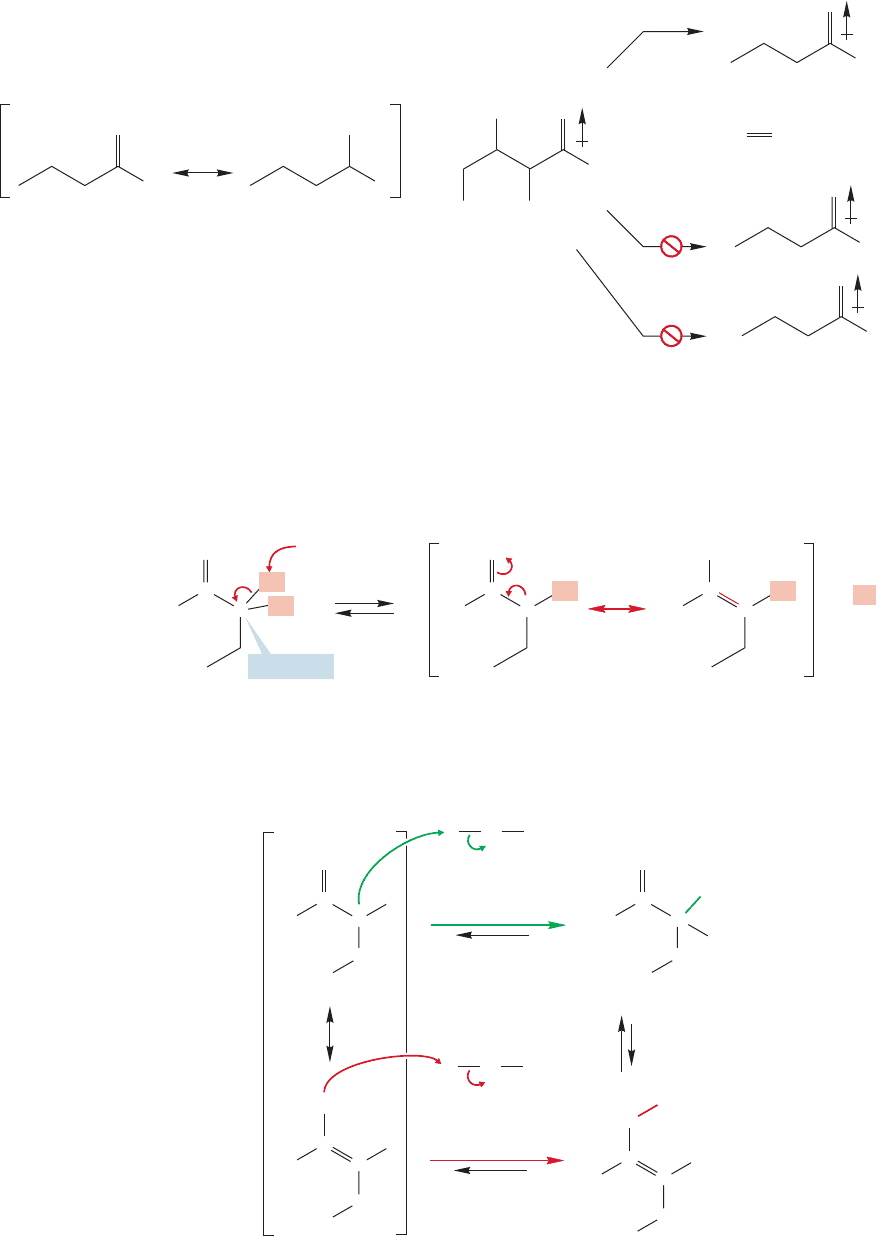
934 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
O
..
..
H
=
H
H
H
γ
H
β
H
α
–
O
..
..
..
O
..
..
+
This α anion is most
stabilized by the
C
O dipole
H
–
O
..
..
..
H
–
O
..
..
..
H
–
O
..
..
..
δ
+
δ
–
FIGURE 19.4 The dipole in the
carbon–oxygen bond will stabilize an
adjacent anion more than a more
distant anion.
sharing the negative charge (Fig. 19.5). It is this stabilization that makes the α posi-
tion, but only the α position, a proton source. The intermediate is called an enolate
anion,because it is part alkene (ene),part alcohol (ol ), and an anion (ate). Please note
again that the enolate anion is not “part of the time an alkoxide” and “part of the
time a carbanion,” but all of the time a single species, the resonance-stabilized enolate.
O
..
..
C
C
H
B
..
–
O
..
..
C
C
H
–
..
O
C
H
α
B
C
H
Resonance-stabilized enolate
–
..
..
..
+
α Carbon
H
α
H
α
H
α
H
α
FIGURE 19.5 Loss of the α hydrogen
leads to a resonance-stabilized
enolate anion.
O
..
..
C
H
H
C
H
–
–
O
..
..
C
H
HH
C
H
–
..
O
C
H
C
H
H
..
..
+
protonation
at carbon
(a)
Carbonyl compound
Enol
O
..
..
..
..
..
OH
..
..
..
–
+
OH
..
..
..
..
..
H
2
O
CH
2
H
3
C
CH
2
H
3
C
O
C
H
HH
C
H
–
protonation
at oxygen
(b)
O
..
..
..
..
H
2
O
H
2
O/HO
..
..
..
CH
2
H
3
C
CH
2
H
3
C
FIGURE 19.6 Reprotonation of the
enolate can lead either to (a) the
original carbonyl compound or to
(b) the related enol.
than loss of either the β or γ hydrogen (Fig. 19.4). Moreover, one of these anions,
but only one of them, is stabilized by the resonance resulting from the oxygen atom
The enolate anion can reprotonate in two ways. If it reacts with a proton source at
carbon, the original carbonyl compound is regenerated (Fig. 19.6a). If it protonates at
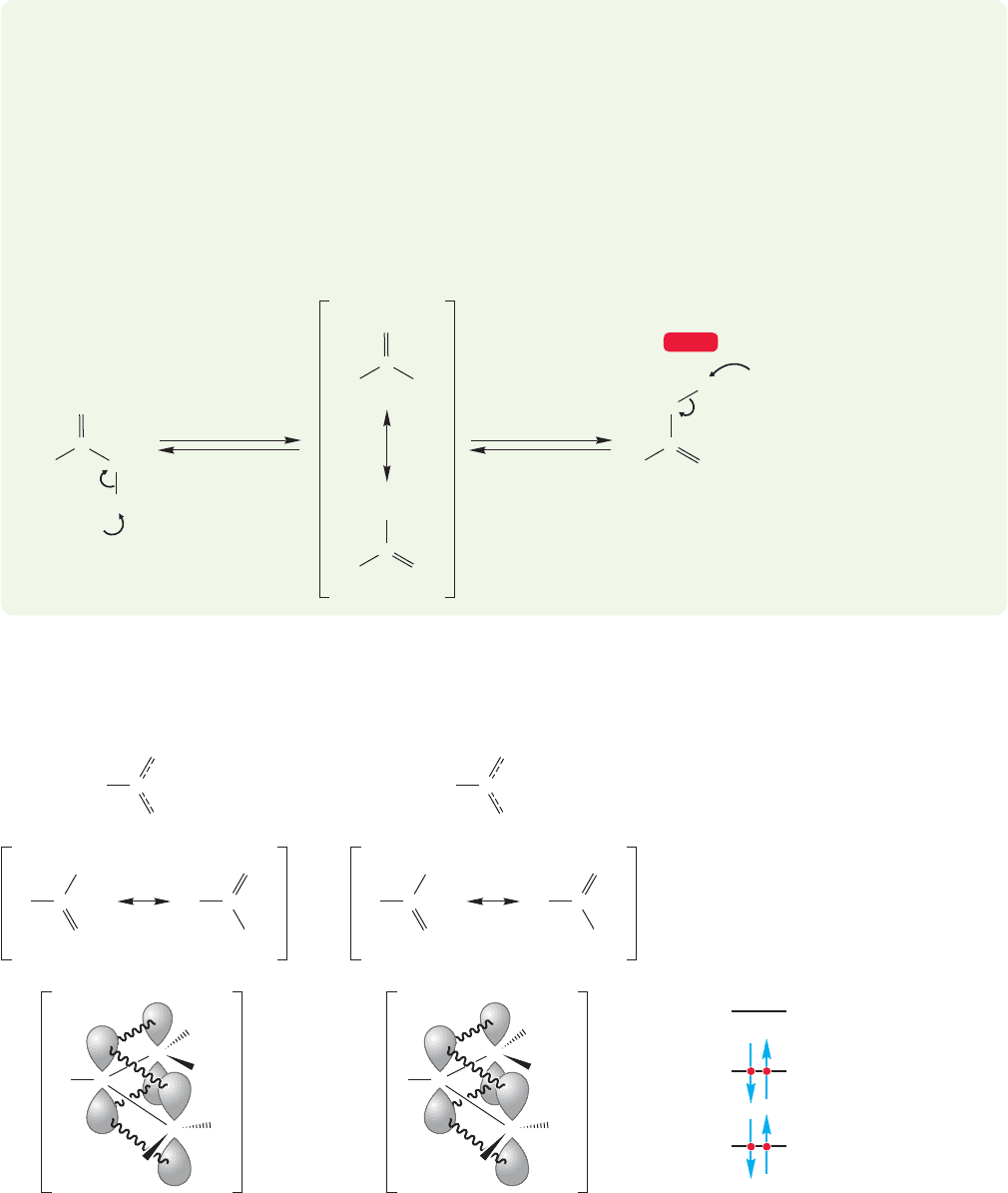
19.2 Many Carbonyl Compounds Are Weak Brønsted Acids 935
oxygen, the neutral enol (p. 448) is formed (Fig. 19.6b). In aqueous base, carbonyl
compounds are in equilibrium with their enol forms.
Watch out! Arrows can emanate from either one of a pair of resonance forms.
One can always write an acceptable arrow formalism from any legitimate resonance
form. You should be able to write an arrow formalism using either resonance form.
WORKED PROBLEM 19.1 Write a mechanism for the base-catalyzed equilibration
of the carbonyl and enol forms of acetone.
ANSWER This problem is not hard, but it needs to be done right now. To be able
to do the more complicated material that will appear later, these simple intercon-
versions must become second nature to you. This problem is drill, but it is never-
theless very important.
Loss of either the α hydrogen from acetone or the hydroxyl hydrogen of the
enol form of acetone leads to the same enolate. Protonation at carbon gives the
ketone; protonation at oxygen gives the enol. It is formation of the enolate that
allows the equilibration to take place.
..
H
3
C
H
C
CH
2
..
..
O
–
B
..
H
C
CH
2
..
..
O
–
B
deprotonation
deprotonation
protonation
protonation
C
CH
2
..
..
O
–
..
C
CH
2
..
..
O
–
..
H
3
C
H
3
C
H
3
C
WEB 3D
The orbital picture of Figure 19.7 clearly shows the source of the stabilization
of the enolate. The three 2p orbitals overlap to form an allyl-like system (p. 373).
–
–
H
H
H
H
H
H
C
CH
2
CH
2
–
H
Allyl
C
CH
2
CH
2
–
–
HC
CH
2
CH
2
C
C
C
..
..
O
..
..
..
..
H
H
H
C
O
C
O
..
..
..
–
–
HC
CH
2
–
H
Enolate
C
O
CH
2
HC
CH
2
..
Φ
2
Φ
1
Φ
3
FIGURE 19.7 A comparison of the
enolate and allyl anions.
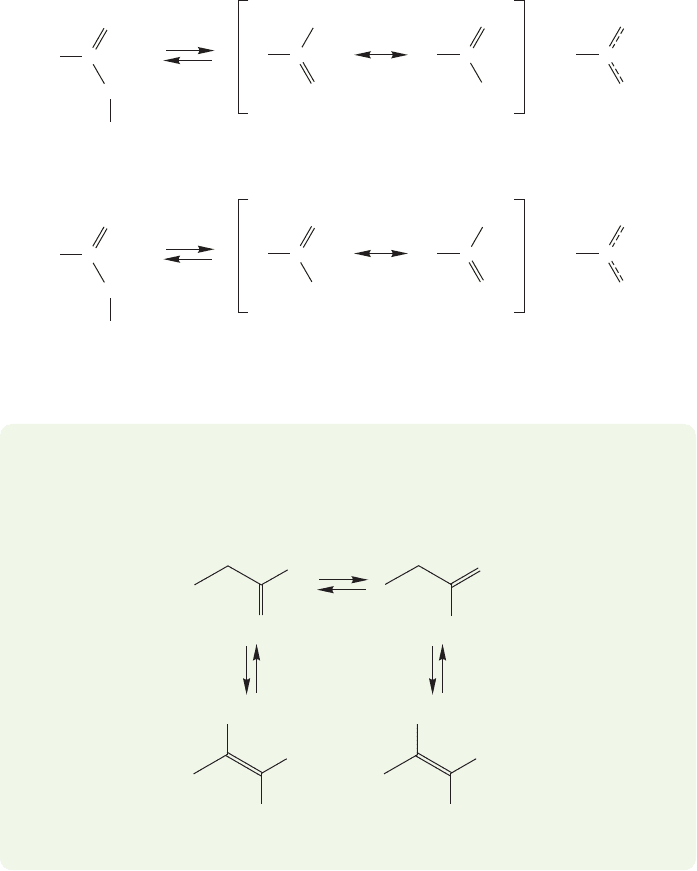
936 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
Two of the four π electrons are accommodated in the lowest bonding molecular
orbital, Φ
1
, and two in the nonbonding orbital, Φ
2
. The presence of the oxygen
atom makes the enolate less symmetrical than an allyl system formed from three
carbon 2p orbitals. The bonding orbital (Φ
1
) of the enolate has its highest
electron density on the oxygen atom. The electron density resides mostly on
the carbon of the enolate in the Φ
2
orbital, the HOMO. The carbon atom is
the usual nucleophilic site because it is the electrons in Φ
2
that react with an
electrophile.
How important is the electronegative oxygen atom to the stabilization of the eno-
late? The pK
a
of propene is 43 and that of acetaldehyde is 16.7. There is a 10
26
dif-
ference in acidity in the two molecules, which can be attributed to the influence of
the oxygen (Fig. 19.8).
Allyl anion
–
–
HC
CH
2
CH
2
–
B
..
–
HC
CH
2
CH
2
–
–
HC
CH
2
CH
2
..
..
H
pK
a
= 43
H
C
=
CH
2
CH
2
Enolate
HC
O
CH
2
–
B
..
HC HC
CH
2
–
..
H
pK
a
= 16.7
H
C
=
CH
2
O
..
..
..
O
..
..
CH
2
O
..
..
FIGURE 19.8 The oxygen atom of
the enolate plays a crucial role in
promoting the acidity at the α
position. Acetaldehyde is much
more acidic than propene.
WORKED PROBLEM 19.2 Propanal can form two enols. What are they?
ANSWER There are stereochemical differences between the two enols. Like any
other unsymmetrical alkene, this enol can exist in Z or E forms.
O
O
H
H
OH
H
..
–
B
OH
H
H
..
–
B
(Z )-Enol (E )-Enol
H
Enolate formation reveals itself most commonly through the exchange
of α hydrogens in deuterated base. For example, treatment of acetaldehyde
with D
2
O/DO
results in exchange of all three α hydrogens for deuterium
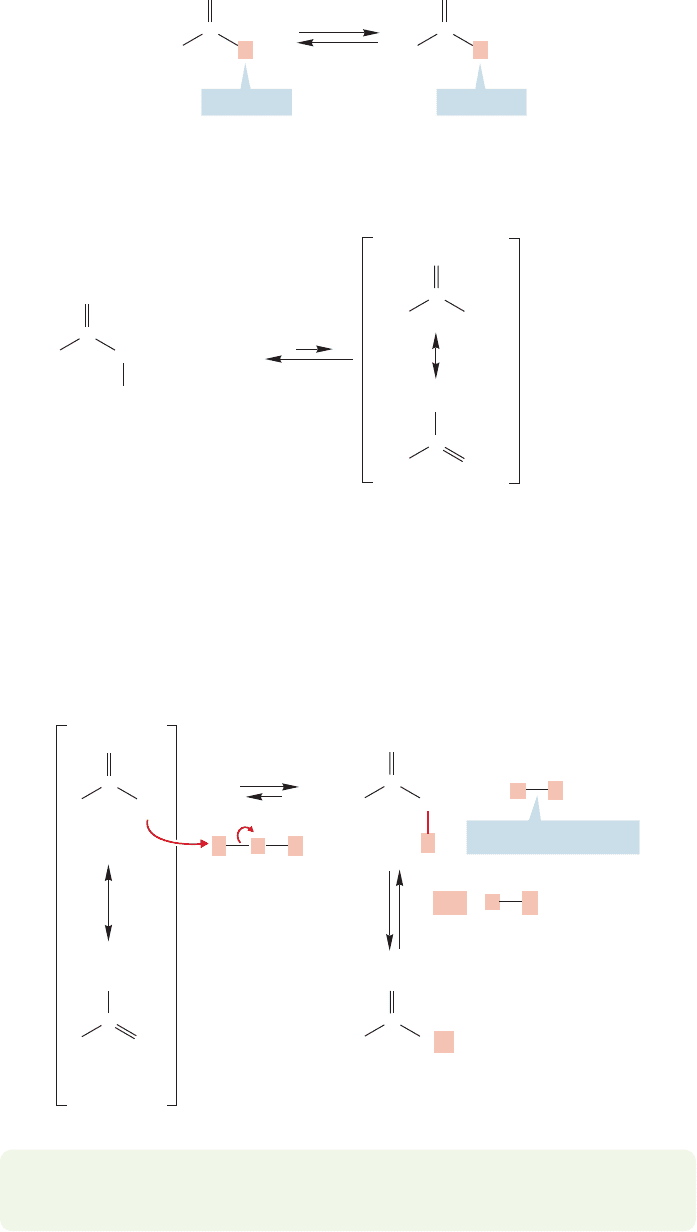
19.2 Many Carbonyl Compounds Are Weak Brønsted Acids 937
hyde is 16.7 and that of water is 15.7. So, hydroxide is a weaker base than the
enolate anion and at equilibrium only a small amount of the enolate anion will
be present. Enolate formation is endothermic in this case (Fig. 19.10).
C
H
O
..
..
–
CH
3
C
H
O
..
..
CD
3
..
..
D
2
O
OD
..
..
..
α Carbon α Carbon
FIGURE 19.9 In D
2
O/DO
, the three
α hydrogens of acetaldehyde are
exchanged for deuterium.
(Fig. 19.9). In order to understand this exchange process we need to consider the
reversibility of the deprotonation reaction. In hydroxide (here deuteroxide), not
all of the acetaldehyde will be transformed into the enolate.The pK
a
of acetalde-
C
H
H
Weaker
base
pK
a
= 16.7
Weaker
acid
Enolate is stronger base
pK
a
~ 15.7
Stronger
acid
O
..
..
–
–
CH
2
C
H
O
..
..
CH
2
OD
..
..
..
–
..
HOD
..
..
..
C
H
O
..
..
CH
2
+
+
FIGURE 19.10 Enolate formation
is an equilibrium reaction and is
endothermic in the case of
acetaldehyde.
Catalyst regenerated
D
D
Enolate
–
–
C
H
O
..
..
C
H
repeat two times
O
..
..
CH
2
C
Fully exchanged
acetaldehyde
H
O
..
..
CD
3
CH
2
O
..
..
..
..
O
..
..
–
..
..
C
H
O
..
..
CH
2
+
D
D
–
D
2
O/ O
..
..
..
D
FIGURE 19.11 The exchange
reaction is a catalytic process, with
deuteroxide ion (
OD) acting as
the catalyst.
These exchange reactions are typically run using catalytic amounts of base, DO
,
in a large excess of D
2
O. Under such conditions only a small amount of enolate can
be formed. However, any enolate present will react with the available water, in this
case D
2
O, to remove a deuteron and produce a molecule of exchanged acetaldehyde
(Fig. 19.11). A new molecule of DO
that can carry the reaction further is also
formed. Eventually all the available α hydrogens will be exchanged for deuterons.
PROBLEM 19.3 Explain why the aldehydic hydrogen in acetaldehyde (the one
bonded to the carbonyl carbon) does not exchange in D
2
O/DO
.
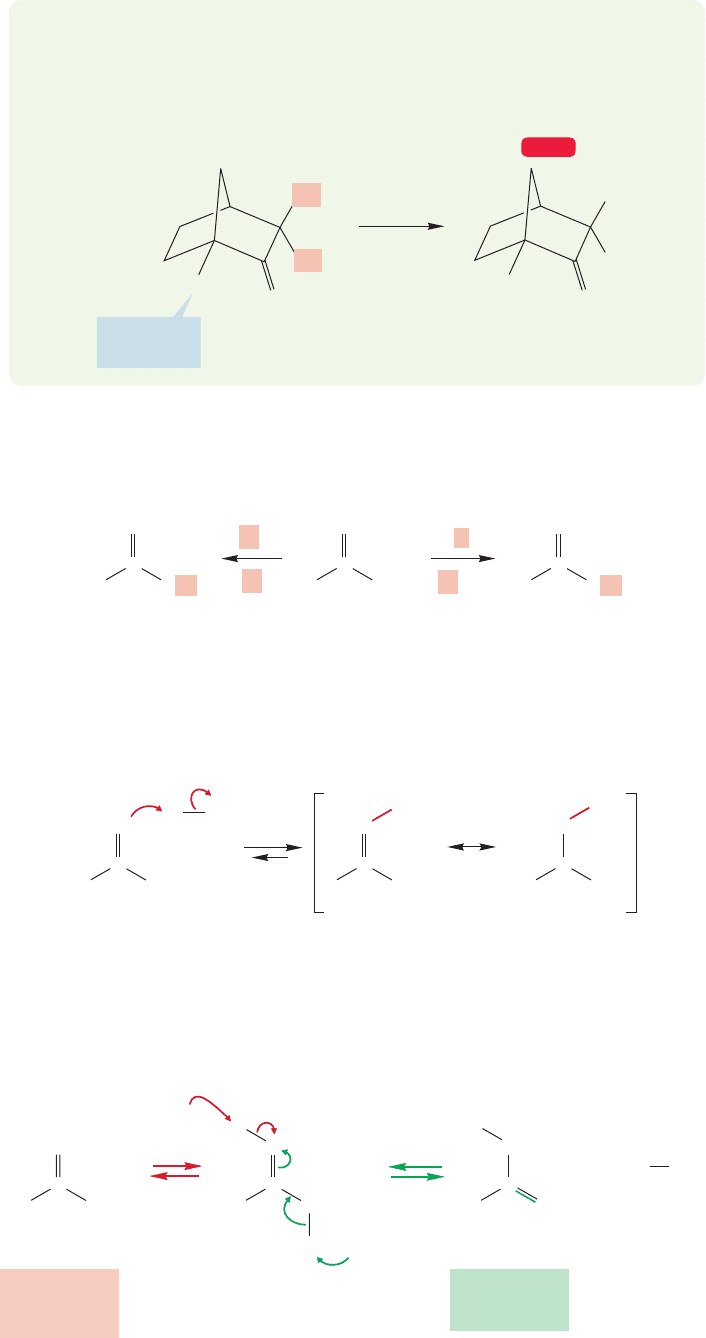
938 CHAPTER 19 Carbonyl Chemistry 2: Reactions at the Position␣
PROBLEM 19.4 Explain why the bicyclic ketone in the following reaction exchanges
only the two α hydrogens shown (H
α
) and not the bridgehead hydrogen, which is
also “α.” Hint: Use models and examine the relationship between the bridgehead
carbon–hydrogen bond and the π orbitals of the carbonyl group.
The α hydrogens of acetaldehyde can also be exchanged under acidic conditions
(Fig. 19.12). This result illustrates a general principle: In carbonyl chemistry, there will
usually (not quite always) be an acid-catalyzed version for every base-catalyzed reaction.
–
..
..
..
..
..
OD
D
2
O
H
Bridgehead
hydrogen
H
D
D
O
..
..
O
..
..
H
α
H
α
WEB 3D
C
H
O
..
..
CD
3
C
H
O
..
..
CD
3
C
H
O
..
..
CH
3
..
..
D
3
O
+
D
2
O
..
..
..
D
2
O
–
OD
..
..
..
FIGURE 19.12 Exchange of the α
hydrogens of acetaldehyde can also
be carried out in deuterated acid,
D
3
O
/D
2
O.
C
D
H
O
..
..
CH
3
C
H
O
..
..
CH
3
C
H
D
O
..
D
CH
3
..
+
+
+
OD
2
OD
2
Resonance-stabilized intermediate
..
..
FIGURE 19.13 The first step in the
acid-catalyzed exchange is addition
of a deuteron to the carbonyl oxygen.
A resonance-stabilized cation results.
C
H
O
..
..
..
CH
3
C
H
O
..
..
CH
2
C
H
H
D
O
..
D
CH
2
+
+
D
3
O
D
2
O
OD
2
Acetaldehyde
Enol
Product from
removal of H
+
from carbon
Protonated (D
+
)
acetaldehyde
..
..
..
..
+
Product from
removal of D
+
from oxygen
..
+
OD
2
H
FIGURE 19.14 Removal of a proton
from carbon (green) generates the
neutral enol form. Removal of a
deuteron from oxygen (red)
regenerates starting material.
Now we have created a powerfully acidic species,the protonated carbonyl compound.
Removal of a deuteron from the oxygen simply reverses the reaction to regenerate
acetaldehyde and D
3
O
, but deprotonation at the α carbon generates the enol
(Fig. 19.14). Note that the product is not an anionic enolate, but the neutral enol. If this
enol regenerates the carbonyl compound in D
3
O
, exchanged acetaldehyde will result.
The mechanisms of the two reactions will be different, however, even though the
end results are similar or even identical.For example, in this acid-catalyzed exchange
reaction there is no base strong enough to remove an α hydrogen from the starting
acetaldehyde. The only reasonable reaction is protonation of the Brønsted basic
oxygen by D
3
O
to give a resonance-stabilized cation (Fig. 19.13).
