Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

6.8 Ethers 249
PROBLEM 6.18 The stability of oxonium ions depends on the nature of the negatively
charged counterion. Fluoborate (
BF
4
) is an especially favorable counterion. Why?
Hint: Lewis bases, B
, can transfer methyl (and other) groups through a process
called the S
N
2 reaction. You will learn all about it in Chapter 7, but you might
want to use it here in this problem.
:
B
–
O
+
+
H
3
C
S
N
2
CH
3
CH
3
O
B
CH
3
CH
3
CH
3
Primary and secondary amines are Brønsted acids,but only very weak ones.Amines
are much weaker acids than alcohols. Removal of a proton from an alcohol gives an
alkoxide in which the negative charge is borne by oxygen. An amine forms an amide
3
in which the less electronegative nitrogen carries the negative charge (Fig. 6.49).
3
Be careful—there is another kind of amide that has the structure . There is no written or
verbal distinction made, so you need to know the context before you know which kind of amide is meant.
R
O
CO
O
NH
2
Amide ion
..
RB
HB
Primary amine
pK
a
~ 36
NH
2
..
..
R
NH
+
+
–
–
Amide ion
..
R
2
B
HB
Secondary amine
pK
a
~ 36
NH
..
..
R
2
N
+
+
–
–
..
..
R HB
HB
Alcohol
pK
a
~ 17
Alkoxide ion
O
+
+
–
..
..
R
O
..
–
FIGURE 6.49 Primary and secondary
amines are weak Brønsted acids.
It takes a very strong base indeed to remove a proton from an amine to give the
amide ion, which is itself a very strong base. Alkyllithium reagents (p. 228) are
typically used (Fig. 6.50).
LiBuH
BuLi
+
+
An amine (a good base
and a poor acid, pK
a
~ 36)
pK
a
> 50 An amide ion (an even stronger
base than the related amine)
..
R
NH
2
..
..
R
NH
–
An exceptionally
strong base
FIGURE 6.50 Very strong bases,
such as alkyllithium reagents,
can remove a proton from an
amine to give an amide ion
(Bu CH
2
CH
2
CH
2
CH
3
).
So, amines are good bases, but relatively poor acids. Amides, the conjugate bases
of amines, are even stronger bases than the amines themselves (Fig. 6.50).
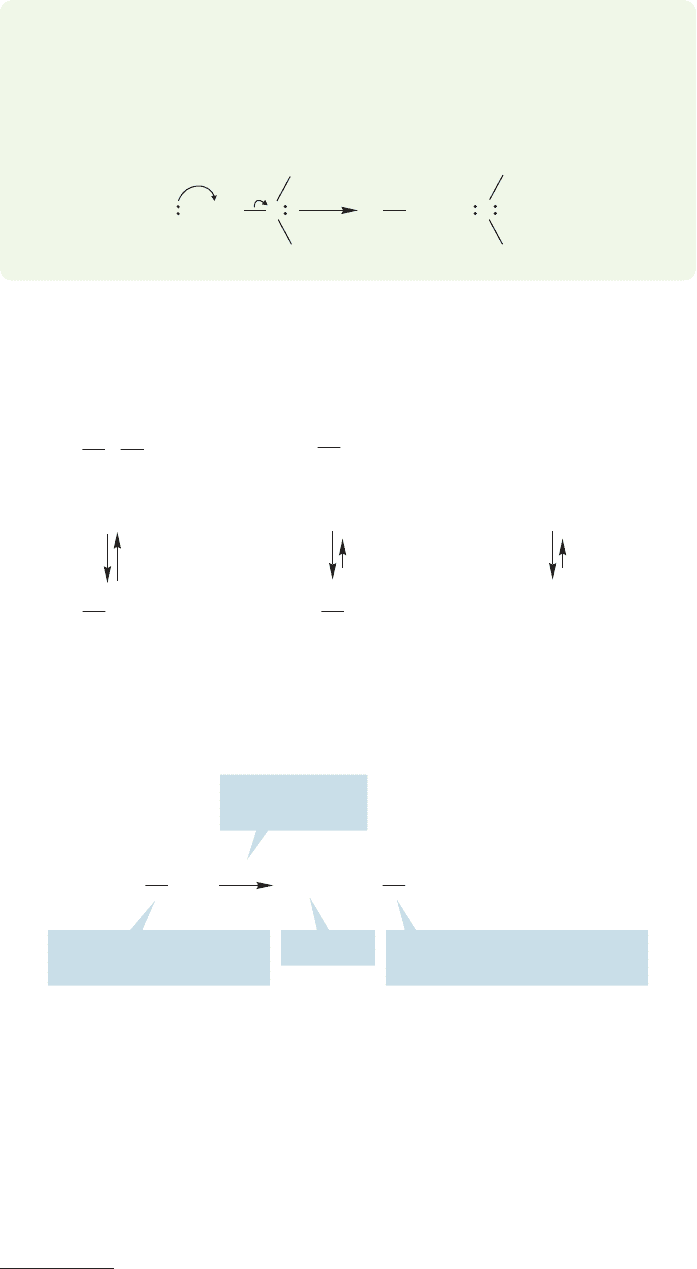
6.8 Ethers
Alcohol (ROH) and amine (RNH
2
) chemistry depends on both the OH or NH
and the OR or NR parts of the molecule. In this section, we look at molecules that
no longer have any OH groups,but retain the R group.These compounds are called
ethers, and have the structure or .R
O
O
O
R¿R
O
O
O
R
250 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
6.8a Nomenclature Naming ethers is simple. In the widely used common sys-
tem, the two groups joined by oxygen are named in alphabetical order, and the word
ether appended. In the IUPAC system, ethers are named as alkoxy (RO) alkanes.
Figure 6.51 gives some simple examples of both usages, IUPAC first.
WEB 3D
O
Methoxymethane
(dimethyl ether)
..
..
WEB 3D
WEB 3D
CH
3
H
3
C
Methoxyethane
(ethyl methyl ether)
CH
2
CH
3
H
3
C
O
Ethoxyethane
(diethyl ether or ether)
..
..
CH
2
CH
3
CH
3
CH
2
CH
3
O
2-Methoxy-2-methylpropane
(tert-butyl methyl ether)
..
..
O
..
..
CH
3
(CH
3
)
3
C
O
Ethoxyethene
(ethyl vinyl ether)
Furan
..
..
CH CH
2
CH
3
CH
2
O
..
..
Tetrahydrofuran (THF)
O
..
..
Anisole
(methyl phenyl ether)
O
..
..
FIGURE 6.51 Some ethers and their
common names.
6.8b Physical Properties Ethers are polar, but only for the smallest members
of the class does this polarity strongly affect physical properties. Other ethers are suf-
ficiently hydrocarbon-like so as to behave as their all-carbon relatives. For example,
diethyl ether has nearly the same boiling point as pentane and is only modestly
soluble in water.Table 6.10 gives some physical properties for a few common ethers.
TABLE 6.10 Some Physical Properties of Ethers
Compound bp (°C) mp (°C)
H
3
CCH
3
O
Dimethyl ether
–23 –138.5 0.668
34.6 –119.2
7.6 –139
Ethyl methyl ether
55.2 –109
Density (g/mL)
0.725
0.714
0.740
(CH
3
)
3
C
tert-Butyl methyl ether
Diethyl ether
–115.8
–85.6
35.5
31.4
Furan
67 –108
155.0 –37.5
0.759
0.951
0.889
0.996
Ethyl vinyl ether
THF
Anisole
O CH
3
O
O
CH
3
CH
2
CH
2
CH
3
O
H
3
CCH
2
CH
3
O
CH
3
O
CH
3
CH
2
CHO CH
2
6.9 Special Topic: Thiols (Mercaptans) and Thioethers (Sulfides) 251
6.8c Structure The carbon–oxygen bond length in ethers is similar to that in
alcohols, and the opening of the angle as we go from (104.5°) to
( 109°) to ( 112°) continues. As the angle in ethers is
approximately 112°, the oxygen is hybridized approximately sp
3
.
6.8d Acidity and Basicity Of course, ethers lack the OH group of their rela-
tives water and the alcohols and so are not Brønsted acids. The oxygen atom has
two pairs of nonbonding electrons, however, and they make ethers weak Brønsted
and Lewis bases. Ethers can be protonated, and the protonated form has a pK
a
in
the same range as those of the protonated alcohols, which means that ethers and
alcohols are strong bases of similar strength (Fig. 6.52).
'
R
O
O
O
R
'
R
O
O
O
H
H
O
O
O
H
THE GENERAL CASE
A SPECIFIC EXAMPLE
+
O
R
HA
R
..
..
+
O
H
R
pK
a
~ – 3.5
pK
a
= –1.74
A
R
..
..
+
–
+
O
CH
3
H
3
CCH
3
H
3
C
..
..
O
HH
..
..
+
O
H
pK
a
~ – 3.8
..
+
O
H
HH
..
+
FIGURE 6.52 Ethers are weak Brønsted bases and can be protonated. Protonated ethers (and protonated
alcohols) are strong acids.
We have already seen one instance in which the ability of ethers to donate
electrons, that is, to act as a Lewis base, was a critical property. Recall from p. 228
that an ether solvent is vital to success in the formation of the Grignard reagent
(Fig. 6.53). Ethers are stabilizing to Lewis acidic species. Boron trifluoride etherate
is a commercially available complex of BF
3
and ether that can even be distilled.
It is commonly used as a source of BF
3
. For similar reasons, the cyclic ether THF
(Fig. 6.51) is used to stabilize the highly reactive molecule BH
3
.
δ
–
δ
+
F
3
B
R
F
Cl
Boron trifluoride
etherate
RMg Cl
..
..
O
..
..
O
..
O
..
O
..
O
Mg
F
F
B
FIGURE 6.53 Ethers will react with
Lewis acids as well as Brønsted acids.
The BF
3
–etherate complex is a stable
source of boron trifluoride.
6.9 Special Topic: Thiols (Mercaptans) and
Thioethers (Sulfides)
Thiols (RSH) and thioethers (RSR′) are the sulfur-containing counterparts of
alcohols and ethers, and their chemistries are generally similar to those of their
oxygen-containing relatives.These sulfur compounds have a poor reputation,because
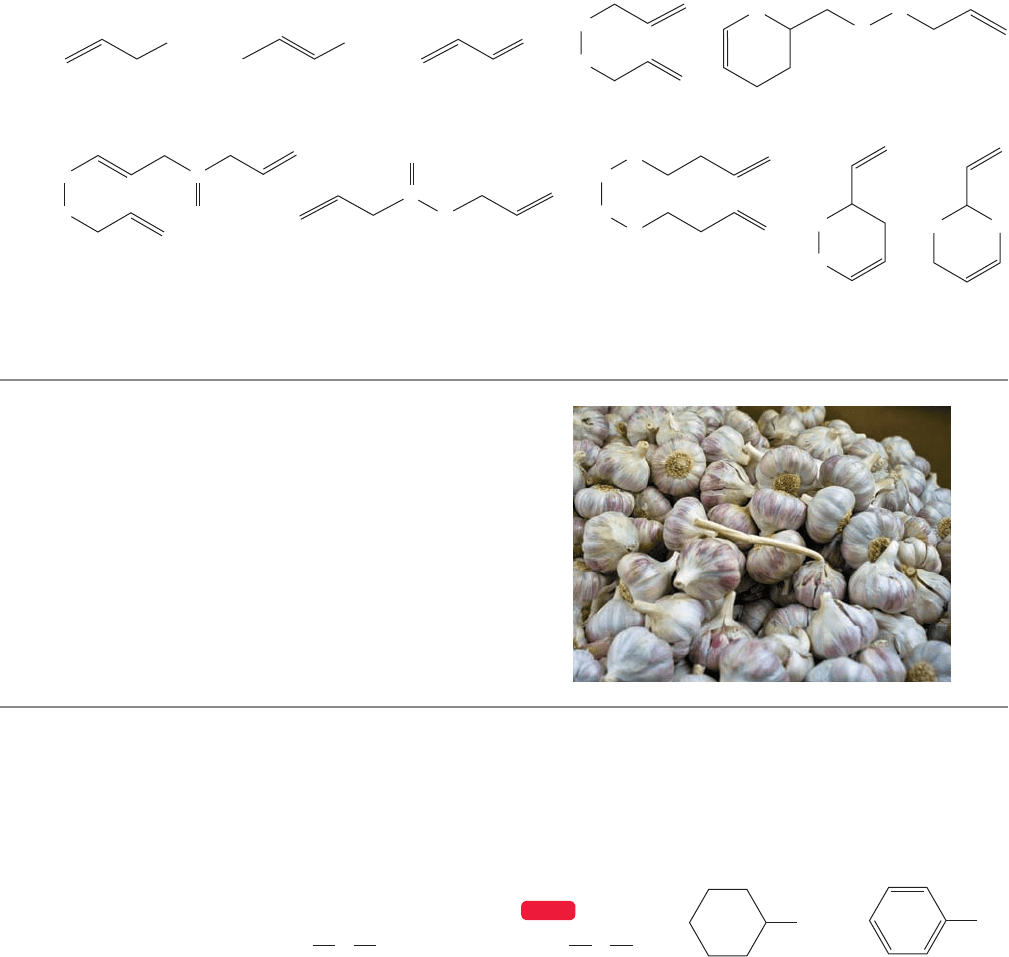
252 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
some are quite extraordinarily evil-smelling.The skunk uses a variety of four-carbon
thiols in its defense mechanism, for example. But this bad press is not entirely
deserved; not all sulfur compounds are malodorous. Garlic derives its odor from
small sulfur-containing compounds.You may never need to ward off a vampire with
it, but garlic contains many sulfur compounds that have quite remarkably positive
qualities. Figure 6.54 shows some of the sulfur-containing molecules that can be
found in garlic.
S
S
S
S
S
S
O
SOH SOH S
S S
S
S
S
S
S
S
S
O
S
S
S
Ajoene
FIGURE 6.54 Some of the sulfur-containing molecules in garlic.
AJOENE
Ajoene is only one of the beneficial compounds
present in garlic. Ajoene is lethal to certain tumor-prone
cells, promotes the antiaggregative action of some
molecules on human blood platelets, and seems to be
effective against viruses (including HIV) as well. It
appears to reduce repeat heart attacks among people who
have already suffered an initial attack. Garlic contains
many other compounds that also appear to have beneficial
medicinal properties. And, of course, if that vampire is hot
on your trail....
6.9a Nomenclature Thiols, also called mercaptans, are named by adding the
suffix “thiol”to the parent hydrocarbon name.Note that the final “e”is not dropped,
as it is, for example, in alcohol nomenclature (Fig. 6.55).
WEB 3D
Methanethiol
(methyl mercaptan)
H
3
CSH
..
..
1-Butanethiol
(butyl mercaptan)
CH
3
CH
2
CH
2
CH
2
S H
..
..
Cyclohexanethiol
SH
..
..
Benzenethiol
(phenyl mercaptan)
(thiophenol)
SH
..
..
FIGURE 6.55 Some thiols, or
mercaptans.
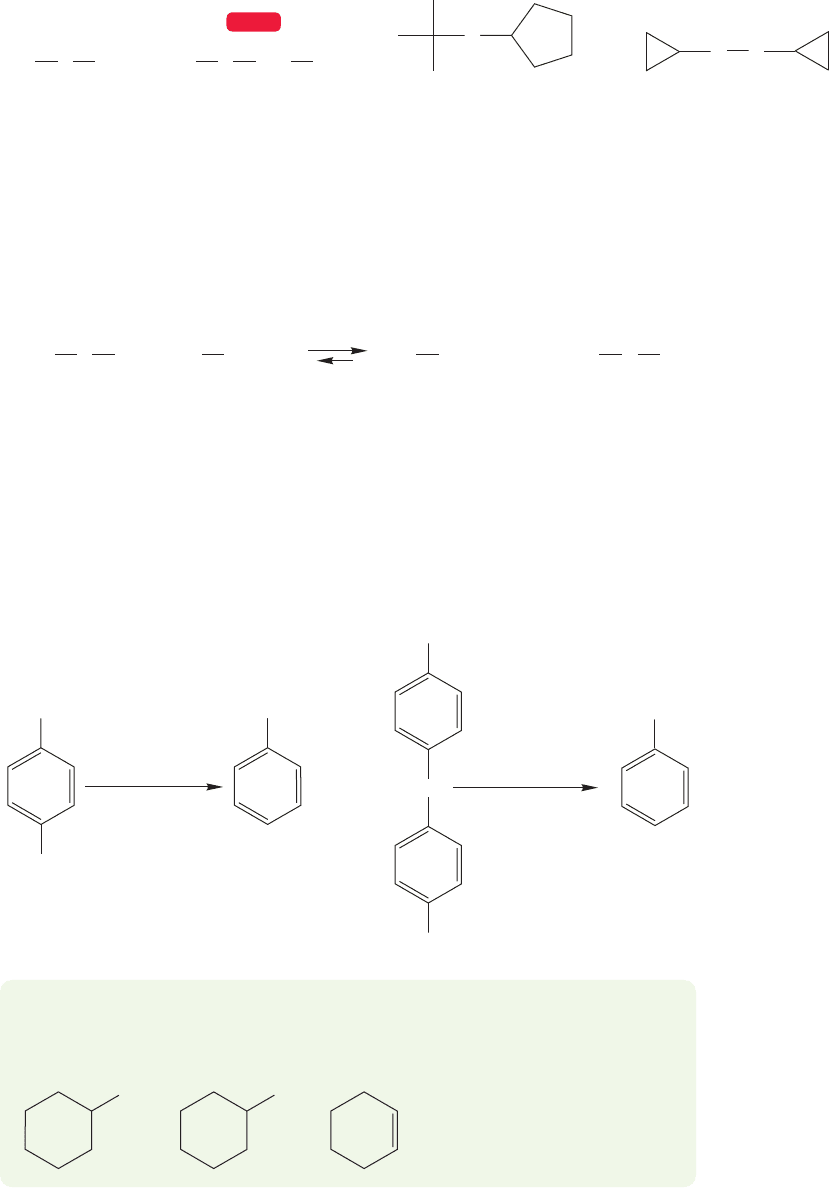
Thioethers, the sulfur counterparts of ethers, are also called sulfides, and are
named in the same way as ethers. The two groups attached to the sulfur atom are
6.9 Special Topic: Thiols (Mercaptans) and Thioethers (Sulfides) 253
followed by the word sulfide. Disulfides ( ) are the counterparts of
peroxides, , and have a significant biological role, which we will
discuss later. Peroxides are nastily unstable and tend to explode, but disulfides are
relatively benign.Disulfides are named in a similar fashion as are sulfides (Fig. 6.56).
R
O
O
O
O
O
R
R
O
S
O
S
O
R
WEB 3D
S
Dimethyl sulfide
..
..
H
3
CCH
3
S
Ethyl methyl sulfide
..
..
H
3
CCH
2
CH
3
tert-Butyl cyclopentyl sulfide
..
Dicyclopropyl disulfide
..
..
..
..
S
..
SS
FIGURE 6.56 Some thioethers (sulfides) and disulfides.
6.9b Acidity Thiols (pK
a
9–12) are stronger acids than alcohols and form
mercaptides, the sulfur counterparts of alkoxides, when treated with base.The rel-
atively high acidity of thiols makes formation of the conjugate base more favorable
than formation of alkoxides from alcohols (Fig. 6.57).
H ++
..
S
pK
a
= 10 Alkoxide
(conjugate base
of CH
3
OH)
Mercaptide
(conjugate base
of CH
3
SH)
pK
a
= 15.5
..
..
H
3
C HO
..
..
H
3
CONa
+
..
..
H
3
C
–
..
S
..
..
H
3
C
–
Na
+
FIGURE 6.57 Mercaptides are easier
to form than alkoxides because thiols
are much more acidic than alcohols.
RaNi
(80%)
(85%)
2
H
2
O/NaOH
40 ⬚C
SH
RaNi
Et = CH
3
CH
2
EtOH, 78 ⬚C, 2 h
S
CH
3
CH
3
CH
3
CH
3
CH
3
FIGURE 6.58 Desulfuration with
Raney nickel (RaNi).
6.9c Reduction of Sulfur Compounds with Raney Nickel: A New
Alkane Synthesis
Thiols and thioethers are reduced by a catalyst called Raney
nickel (essentially just hydrogen adsorbed on finely divided nickel) to give hydro-
carbons (Fig. 6.58). This synthetic method, called desulfuration, gives you a second
way to make alkanes.
PROBLEM 6.19 What reagents would you use to convert the indicated starting
materials into cyclohexane?
(a) (b) (c)
SH
Br
254 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
6.10 Special Topic: Crown Ethers
Cyclic ethers are common solvents and, as we will see in Section 7.10, can often be
prepared rather easily (Williamson ether synthesis,p. 315).It is possible to make cyclic
polyethers, compounds in which there is more than one ether linkage. Figure 6.59
gives some examples of cyclic ethers and cyclic polyethers.
Ethylene oxide
(oxirane)
Trimethylene oxide
(oxacyclobutane
or oxetane)
Furan
1,4-Dioxan
(1,4-dioxacyclohexane)
1,3-Dioxolane
(1,3-dioxacyclopentane)
1,3,5-Trioxane
(1,3,5-trioxacyclohexane
or paraldehyde)
Tetrahydropyran
(pentamethylene oxide
or oxacyclohexane)
Tetrahydrofuran
(THF)
O
O
O
O
O
O
O
O
O
O O
O
FIGURE 6.59 Some cyclic ethers and
polyethers.
We have mentioned more than once the remarkable solvating powers of ethers.
Ethers are Lewis bases and can donate electrons to Lewis acids, thus stabilizing
them. One example of this stabilization is the requirement for an ether, usually
diethyl ether or THF, in the formation of a Grignard reagent. Ethers are also polar
compounds and therefore are able to stabilize other polar molecules through non-
covalent dipole–dipole interactions.
This stabilization was carried to extremes in work that ultimately led to the
1987 Nobel Prize in Chemistry for Charles J. Pedersen (1904–1989) of du Pont,
Donald J. Cram (1919–2001) of UCLA, and Jean-Marie Lehn (b. 1939) of
Université Louis Pasteur in Strasbourg for opening the field of “host–guest”chem-
istry. Pedersen discovered that certain cyclic polyethers (the hosts) had a remark-
able affinity for metal cations (the guests). Molecules were constructed whose
molecular shapes created different-sized cavities into which different metal ions
fit well. Because of their vaguely crown-shaped structures, these molecules came
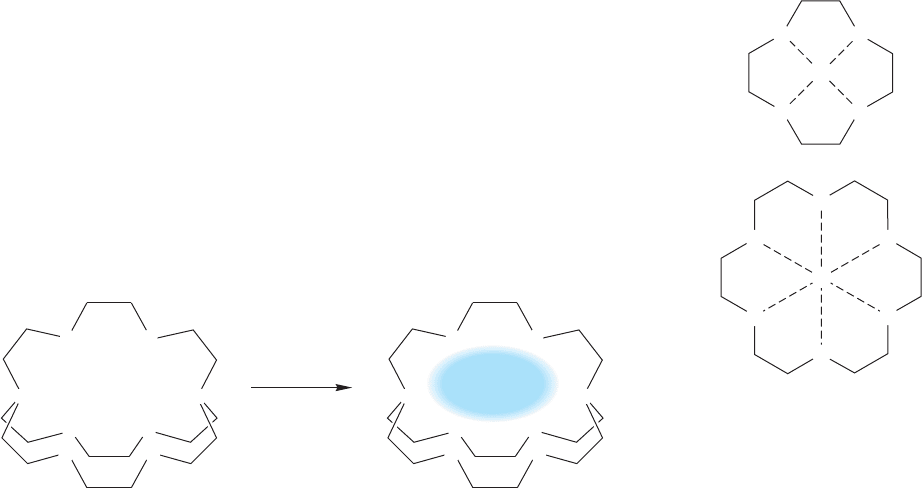
to be called crown ethers. Figure 6.60 shows two of them.
WEB 3D
12-Crown-4 18-Crown-6
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
FIGURE 6.60 Two crown ethers.The
first number in the name shows the
number of atoms in the ring, and the
final number shows the number of
heteroatoms in the ring. In these
molecules the heteroatoms are all
oxygen, but others are possible.
6.11 Special Topic: Complex Nitrogen-Containing Biomolecules—Alkaloids 255
The molecule 12-crown-4 is the proper size to capture lithium ions, and
18-crown-6 has a remarkable affinity for potassium ions. These crown ethers
can stabilize positive ions of different sizes, depending on the size of the cavity
(Fig. 6.61).
Three-dimensional versions of these essentially two-dimensional molecules
have now also been made, using both oxygen and other heteroatoms, such as sul-
fur and nitrogen, as the Lewis bases. These host molecules are called cryptands
and can incorporate positive ions into the roughly spherical cavity within the cage
(Fig. 6.62).
+
O
..
O
..
O
..
O
..
O
..
O
..
O
..
O
..
O
..
O
..
Li
K
+
FIGURE 6.61 Two crown ethers
stabilizing cations.
M
+
N
M
+
A cr
yp
tand
O
O
O
N
S S S S
O
N
O
O
O
N
O
FIGURE 6.62 Three-dimensional cryptands can incorporate metal ions into a
central cavity.
There are chemical and larger implications of this work. Potassium perman-
ganate, KMnO
4
, is an effective oxidizing agent. Its great insolubility in organic
reagents limits its use, however. For example, the deep purple KMnO
4
is
completely insoluble in benzene. It simply forms a solid suspension when
added to benzene, which remains a colorless liquid. The addition of a little
18-crown-6 results in the dissolution of the permanganate, and the benzene
takes on the deep purple color of the permanganate ion. The potassium ion
has been effectively solvated by the crown ether and now dissolves in the
organic solvent.The permanganate counterion, though not solvated by the crown
compound, accompanies the positive ion into solution, as it must to preserve
electrical neutrality. The purple benzene solution can be used as an effective
oxidizing agent.
Schemes have been envisioned in which specially designed crown compounds
are used to scavenge poisonous heavy metal ions from water supplies.There have
been less humanitarian, though admittedly clever, schemes for extracting the
minute amounts of gold ions present in seawater by passing vast amounts of water
over a crown compound.
6.11 Special Topic: Complex Nitrogen-Containing
Biomolecules—Alkaloids
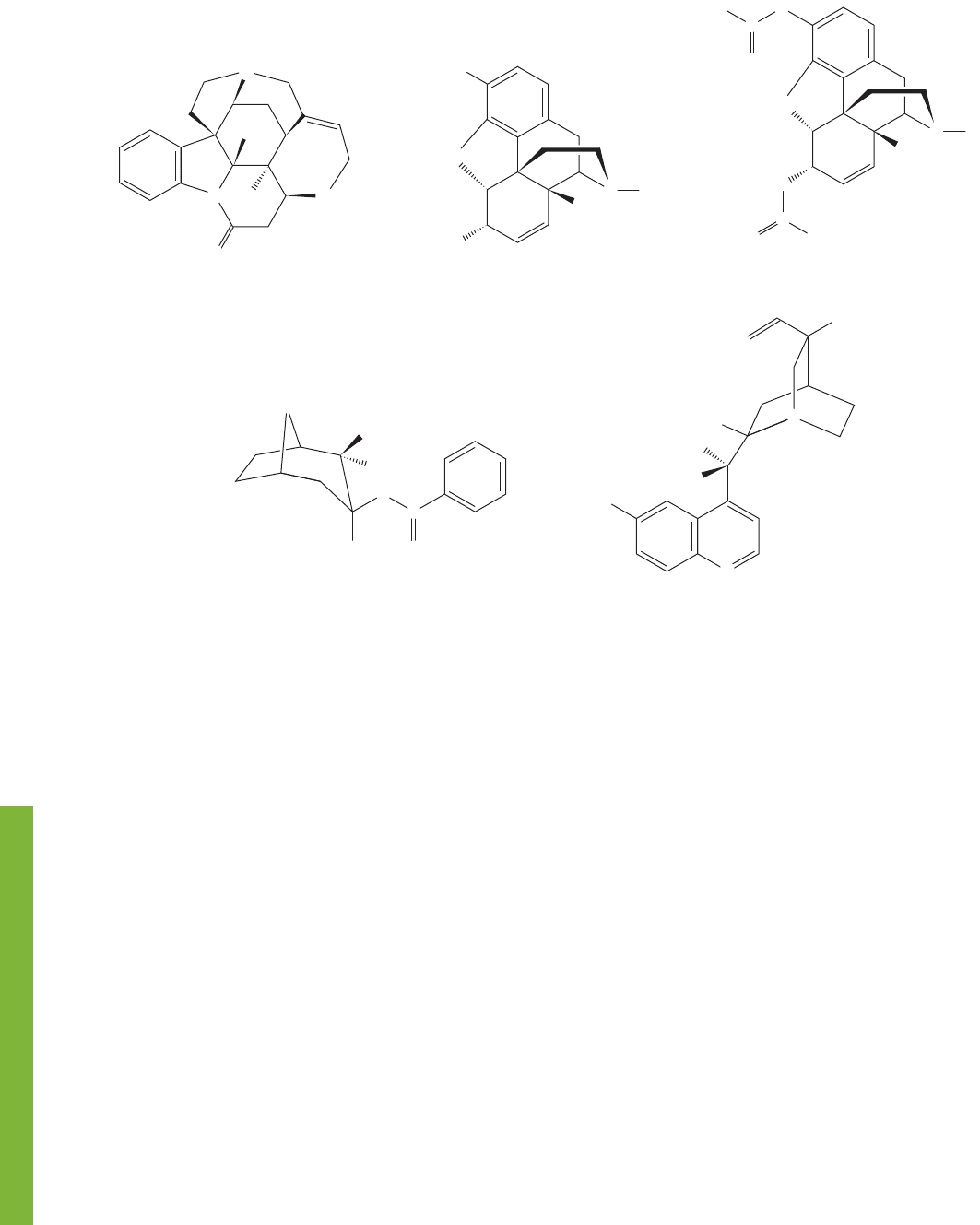
Alkaloids are naturally occurring amines that are powerfully active biologically.They
are among the most useful medicinal agents known. The painkiller morphine and
the antimalarial agent quinine are typical examples. At the same time, some of the
256 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
most destructively addictive substances known are also alkaloids. Heroin and cocaine
are examples from our own time, and anyone at all familiar with popular literature
knows of the notorious poison strychnine (Fig. 6.63).
CH
3
H
Strychnine
O
CH
3
O
Quinine
N
H
H
HO
H
C
Heroin
O
O
O
H
N
O
O
C
CH
3
H
3
C
COOCH
3
NCH
3
O
H
H
C
O
Cocaine
HO
HO
Morphine
O
H
N
H
CH
3
N
N
N
O
FIGURE 6.63 Some alkaloids.
We have already seen strychnine and its close relative brucine in Figure 4.44,
where they served as reagents for the resolution of optically active carboxylic acids
into separate (R) and (S) enantiomers (p. 171). As we work through the book, alka-
loids will reappear now and again. The important thing to “take home” from this
picture is not the detail but rather a broad view of the great structural diversity—
molecular architecture—of these bioactive molecules.
A measure of a compound’s acidity—its pK
a
value—is
introduced. The lower the pK
a
value, the more acidic is the
compound in question.
Solvents appear as important players in questions of
stability. For example, both the conjugate bases of alcohols
(alkoxides) and the conjugate acids of amines (ammonium
ions) depend greatly on solvation for stabilization. In
assessing basicity or acidity, one must take account of the
solvent.
A general discussion of solvents and solvation also appears.
Protic solvents that can participate in hydrogen bonding
dissolve other protic, polar molecules well. By contrast, polar
solvents do a poor job of dissolving “greasy” nonpolar organic
molecules such as hydrocarbons, which are dissolved by
nonpolar, similarly “greasy” solvents. Like dissolves like—polar
solvents dissolve polar molecules, and nonpolar solvents dissolve
nonpolar molecules.
Organic halogenated molecules can be used to form
organometallic reagents. Many of the important synthetic
reactions of organic chemistry use organometallic reagents.
In this chapter, we see them only as very strong bases and as
sources of hydrocarbons through their reaction with water.
6.12 Summary
New Concepts

6.12 Summary 257
Amines, like alkanes with sp
3
-hybridized carbons, are tetra-
hedral. Unlike alkanes, amines invert their “umbrellas” through
an sp
2
-hybridized transition state.
The hydrogen bond is introduced. This kind of bond is
nothing more than a partially completed Brønsted acid–base
reaction in which a partial bond is formed between a pair of
electrons on one atom and a hydrogen on another (Fig. 6.64).
Hydrogen bond
O
H
H
H
..
..
O
H
..
..
FIGURE 6.64 A hydrogen bond.
alkoxide ion (p. 236)
alkyl halide (p. 225)
amide (p. 249)
amine (p. 240)
amine inversion (p. 243)
ammonia (p. 240)
ammonium ion (p. 240)
aprotic solvent (p. 238)
aziridine (p. 242)
conjugate acid (p. 234)
conjugate base (p. 234)
crown ether (p. 254)
cryptand (p. 255)
diol (p. 240)
ether (p. 249)
free radical (p. 228)
glycol (p. 240)
Grignard reagent (p. 228)
hydrogen bonding (p. 232)
mercaptan (p. 252)
mercaptide (p. 253)
organolithium reagent (p. 228)
organometallic reagent (p. 227)
peptide (p. 224)
pK
a
(p. 235)
primary amine (p. 240)
protein (p. 224)
protic solvent (p. 238)
Raney nickel (p. 253)
secondary amine (p. 240)
solvation (p. 237)
sulfide (p. 252)
tertiary amine (p. 240)
thioether (p. 251)
thiol (p. 251)
Key Terms
People sometimes get the connection between pK
a
and acid
strength wrong. A strong acid has a low pK
a
; a weak acid has a
high pK
a
.
It is easy to get confused when using the pK
a
of an ammo-
nium ion as a measure of the acidity of the related amine.
Review the discussion (p. 246) or use the more direct (but less
common) pK
b
values.
Common Errors
1. Grignard Reagents 3. Organolithium Reagents
Syntheses
RX
Mg
R MgX
R can be almost any organi
c
structure. X is I, Br, or Cl
R MgX
H
2
O
RH
If D
2
O is used instead
of H
2
O, deuterated
hydrocarbons can
be made
RLi
H
2
O
RH
RSH
Raney
nickel
RH
Raney
nickel
Reduction of thiols by
Raney nickel
Reduction of sulfides
by Raney nickel
RSR 2 R H
2. Hydrocarbons
RX
Li
RLi
R can be almost any organi
c
structure. X is I, Br, or Cl
258 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
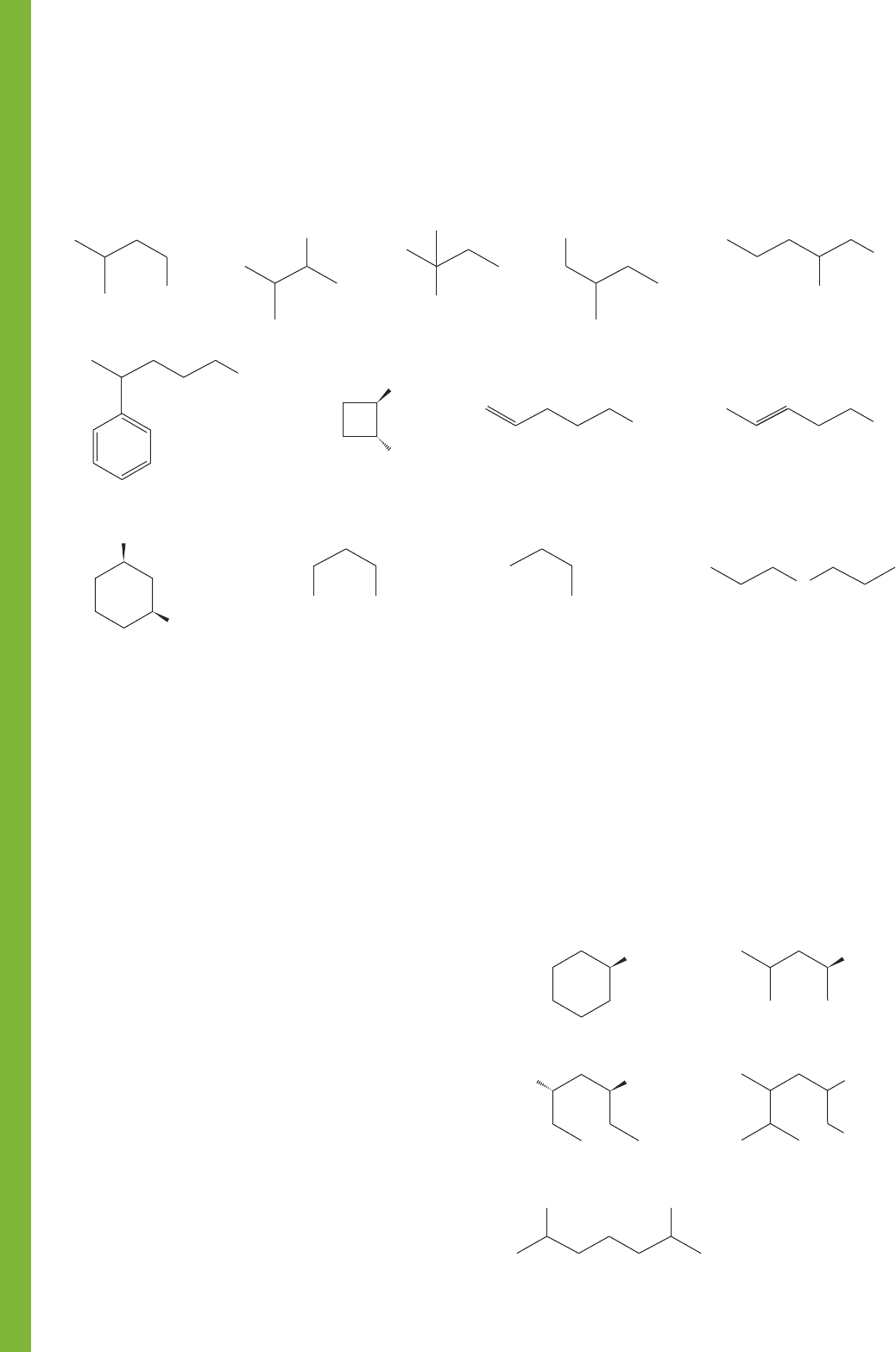
PROBLEM 6.20 Name the following compounds:
PROBLEM 6.21 Draw structures for the following compounds:
(a) 1,4-butanediol
(b) 3-amino-1-pentanol
(c) cis-4-tert-butoxycyclohexanol
(d) 2,2,4,4-tetramethyl-3-pentanol
(e) (R)-sec-butyl alcohol
(f) trans-3-chlorocyclohexanol
(g) tert-butyl cyclopentyl ether
PROBLEM 6.22 Draw structures for the following compounds:
(a) neopentyl alcohol
(b) 18-crown-6
(c) tetrahydropyran
(d) THF
(e) furan
PROBLEM 6.23 Draw structures for the following compounds:
(a) (R)-3-methyl-2-butanamine
(b) cis-2-ethylcyclopentanamine
(c) 1-hexanamine
(d) 3-methoxy-2-heptanamine
(e) N-methyl-3-hexanamine
6.13 Additional Problems
(a)
OH
(b)
OH
(d)
OH
(c)
OH
(e)
OH
F
(h)
OH
(i)
OH
(g)
OH
OH
(f)
OH
(m)
S
(l)
SH
(k)
OH
OH
(j)
OH
OCH
2
CH
3
OCH
3
NH
2
(a)
NH
2
(c)
NH
2
H
2
N
(b)
NH
2
(d)
(e)
NH
2
OH
PROBLEM 6.24 Draw structures for the following compounds:
(a) N-ethyl-1-ethanamine
(b) N,N-diethyl-1-ethanamine
(c) (E)-2-penten-1-amine
(d) (Z)-2-penten-2-amine
(e) 2,3-pentanediamine
PROBLEM 6.25 Name the following compounds according to
the official naming system (IUPAC):
