Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

6.13 Additional Problems 259
(a)
OH
(e)
NH
2
(c)
O
Cl
N
H
(b)
OH
N
(d)
O
Cl
OH
OH
OH
Cl
Cl
(a)
2
NH
2
F
(d)
NH
2
(b)
NH
4
(e)
N
+
I
–
(f)
3
(c)
N
NH
2
NH
2
(a) (b)
(c)
N
Ph
CH
3
NH
2
(d)
NH
2
CH
2
NH
2
CH
2
CH
3
OH
CH
3
N
H
3
C
H
3
C
PROBLEM 6.26 Indicate the hybridization of the carbons,
nitrogens, and oxygens in each of the following compounds:
PROBLEM 6.27 Which of the molecules in Problem 6.26 are
able to hydrogen bond with water? Which are able to hydrogen
bond with another molecule of the same structure (i.e., with
themselves)?
PROBLEM 6.28 In the following molecules, there exists the
possibility of forming more than one alkoxide or thiolate. In
each case, explain which will be formed preferentially, and why.
(a)
(solution) (gas phase)
(
solution
)
(b)
(c)
OH
OH
OH
OH
SH
OH
PROBLEM 6.29 Predict the relative acidities of the following
three alcohols:
PROBLEM 6.30 Write all the isomers of the alcohols of the
formula C
6
H
14
O.
PROBLEM 6.31 Name the following compounds (in more than
one way, if possible). Identify each as a primary, secondary, or
tertiary amine, or as a quaternary ammonium ion.
PROBLEM 6.32 Name the following compounds (in more
than one way, if possible). Identify each as a primary, secondary,
or tertiary amine.
PROBLEM 6.33 Explain why the compound below shows five
signals in the
13
C NMR spectrum at low temperature but only
three at higher temperature.

260 CHAPTER 6 Alkyl Halides, Alcohols, Amines, Ethers, and Their Sulfur-Containing Relatives
PROBLEM 6.34 Write the expressions for K
a
and K
b
and show
that pK
a
pK
b
14.
PROBLEM 6.35 The pK
b
values for a series of simple amines
are given below. Explain the relationship between pK
b
and base
strength, and then rationalize the nonlinear order.
Amine NH
3
NH
2
CH
3
NH(CH
3
)
2
N(CH
3
)
3
pK
b
4.76 3.37 3.22 4.20
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 6.36 Select the animation of the reaction titled
“Hofmann elimination.” Observe the reagents that are involved.
What type of amine is required for the reaction? Is it primary,
secondary, or tertiary?
PROBLEM 6.37 How would a polar solvent impact the
Hofmann elimination reaction? Would it slow the reaction
::::
down or make the reaction faster? Why? Use an energy diagram
to help explain your answer.
PROBLEM 6.38 There are three products formed in the
Hofmann elimination reaction. They can be seen moving off
the screen at the end at the reaction. Stop the animation while
all three are still on the screen. You should be able to see water,
an alkene, and an amine. Which do you suppose is the best
nucleophile? Select the HOMO representation for the
animation. Remember that the HOMO represents the most
available electrons, in other words, the most nucleophilic
electrons. Based on the calculated HOMO, which of the three
products is the most nucleophilic?
PROBLEM 6.39 Select the animation titled “Carbocation
rearrangement: E1.”There are several intermediates in this
reaction. Which one is the most stable? Which one is the least
stable? What process is occurring in the first step of the
reaction? Use Table 6.4 to explain why the first step occurs.

Substitution and Elimination
Reactions: The S
N
2, S
N
1, E1,
and E2 Reactions
261
7.1 Preview
7.2 Review of Lewis Acids and
Bases
7.3 Reactions of Alkyl Halides:
The Substitution Reaction
7.4 Substitution, Nucleophilic,
Bimolecular: The S
N
2
Reaction
7.5 The S
N
2 Reaction in
Biochemistry
7.6 Substitution, Nucleophilic,
Unimolecular: The S
N
1
Reaction
7.7 Summary and Overview of the
S
N
2 and S
N
1 Reactions
7.8 The Unimolecular Elimination
Reaction: E1
7.9 The Bimolecular Elimination
Reaction: E2
7.10 What Can We Do with These
Reactions? How to Do
Organic Synthesis
7.11 Summary
7.12 Additional Problems
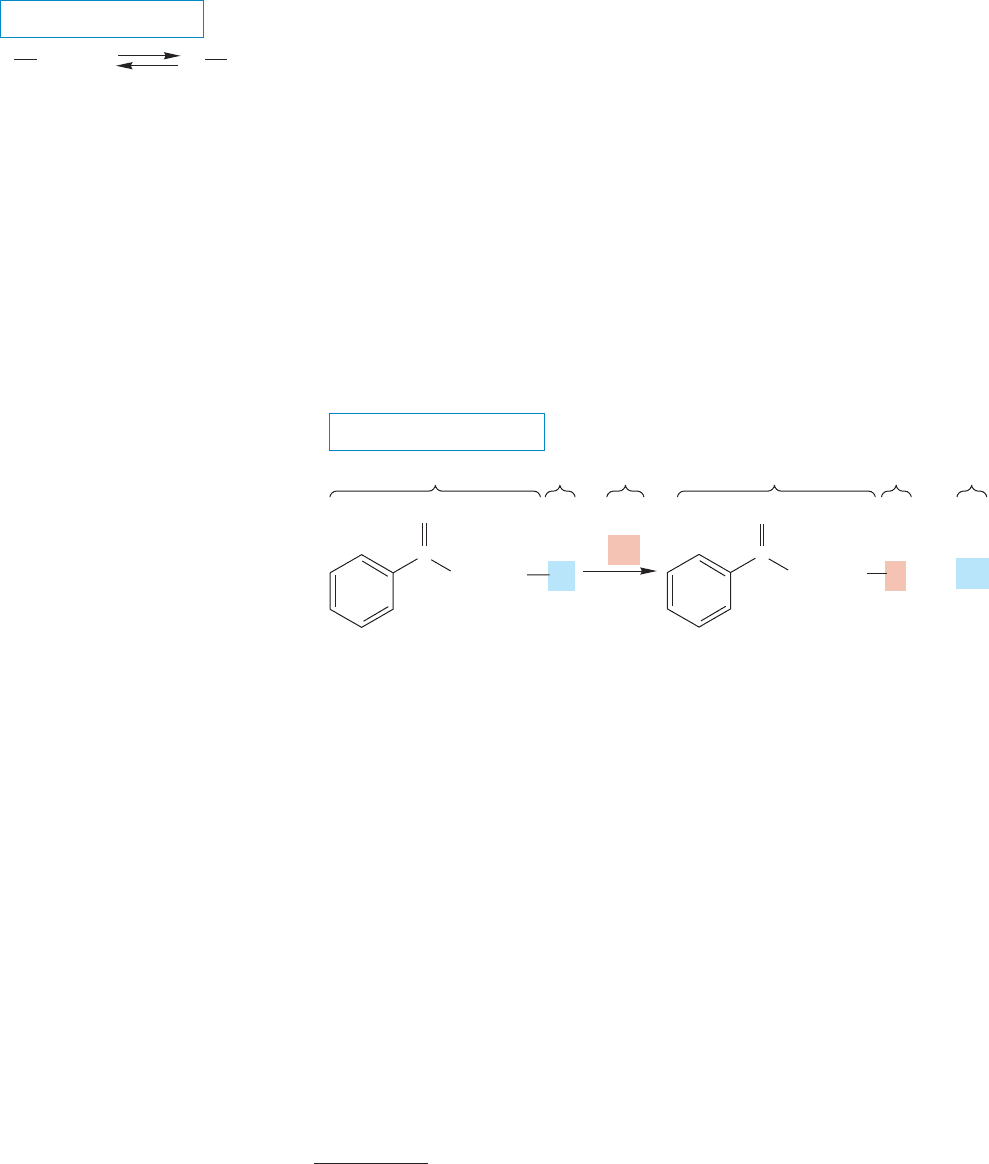
INVERSION In this chapter we will learn that some carbons are like umbrellas.
Both can undergo inversion.
7
THE GENERAL CASE
H
3
C
Methyl group Isopropyl group
tert-Butyl group
+
L
=
RL
R
R
(CH
3
)
2
CH
(CH
3
)
3
C
+
Ethyl group
CH
3
CH
2
Nu Nu
FIGURE 7.1 This generic reaction
presents an overall picture of the
substitution process. A leaving group,
L, is replaced by a new group, Nu, to
give . A standard set of alkyl
groups that will be used throughout
this chapter is presented.
R
O
Nu
A SPECIFIC EXAMPLE
–
..
..
..
..
RLN RNL
Cl
..
..
..
..
–
Na
Na
100
⬚C
24 h
(80%)
+
+
O
..
..
I
..
..
..
+
OCH
2
CH
2
..
..
I
Cl
OCH
2
CH
2
..
..
..
..
..
O
..
..
C
C
FIGURE 7.2 The replacement of one
halogen by another is an example of a
substitution reaction.
1
Satchel Paige (1906–1982) was a pitcher for the Cleveland Indians and later the St. Louis Browns. He was
brought to the major leagues by Bill Veeck after a long and spectacular career in the Negro Leagues.Some meas-
ure of his skill can be gained from realizing that Paige pitched in the major leagues after his 50th birthday.
Don’t look back, something might be gaining on you.
—LEROY ROBERT “SATCHEL” PAIGE
1
7.1 Preview
We are about to embark on an extensive study of chemical change.The last six chap-
ters mostly covered various aspects of structure, and still more analysis of structure
will appear as we go along. Without this structural underpinning no serious explo-
ration of chemical reactions is possible. The key to all studies of reactivity is an ini-
tial, careful analysis of the structures of the molecules involved. We have been
learning parts of the grammar of organic chemistry. Now we are about to use that
grammar to write some sentences and paragraphs. Let’s begin with a reaction cen-
tral to most of organic and biological chemistry, the replacement of one group by
another (Fig. 7.1).
When one group is replaced by another in a chemical reaction, the result is a
subsititution reaction. Conversion of one alkyl halide into another is one example
of the substitution reaction (Fig. 7.2). This reaction looks simple, and in some ways
it is. The only change is the replacement of one halogen by another.
Yet as we examine the details of this process, we find ourselves looking deeply into
chemical reactivity. This reaction is an excellent prototype that nicely illustrates
general techniques used to determine reaction mechanism.
One reason that we spend so much time on the substitution reaction is that it
is so general; what we learn about it and from it is widely applicable. Not only will
we apply what we learn here to many other chemical reactions, substitution reac-
tions have vast practical consequences as well. Substitution reactions, commonly
called displacement reactions, are at the heart of many industrial processes and are
central to the mechanisms of many biological reactions including some forms of
carcinogenesis, the cancer-causing activity of many molecules. Biomolecules are
bristling with substituting agents. It seems clear that when some groups on our DNA
(deoxyribonucleic acid,see Chapter 23) are modified in a substitution reaction, tumor
production is initiated or facilitated. Beware of substituting agents.
This chapter is long, important, and possibly challenging. In it, we cover four of
five building block reactions, two of them substitution reactions (S
N
1 and S
N
2) and
two of them elimination reactions (E1 and E2). We have encountered another fun-
damental reaction, additions, already in Chapter 3, and it will reappear shortly, in
Chapter 9. From now on, you really cannot memorize your way through the material;
262 CHAPTER 7 Substitution and Elimination Reactions

7.2 Review of Lewis Acids and Bases 263
you must try to generalize. Understanding concepts and applying them in new situa-
tions is the route to success. This chapter contains lots of problems, and it is impor-
tant to try to work through them as you go along.We know this warning is repetitious,
and we don’t want to insult your intelligence, but it would be even worse not to alert
you to the change in the course that takes place now or not to suggest ways of getting
through this new material successfully. You cannot simply read this material and hope
to get it all. You must work with the text and the various people in your course, the
professor and the teaching assistants, in an interactive way. The problems try to help
you do this. When you hit a snag or can’t do a problem, find someone who can and
get the answer.The answer is more than a series of equations or structures and arrows;
it also involves finding the right approach to the problem. There is much technique
to problem solving in organic chemistry; it can be learned,and it gets much easier with
practice. Remember: Organic chemistry must be read with a pencil in your hand.
ESSENTIAL SKILLS AND DETAILS
1. We have seen it before, as early as Chapter 1, but in this chapter the curved arrow
formalism becomes more important than ever. If you are at all uncertain of your ability to
“push”electrons with arrows,now is definitely the time to solidify this skill. In the arrow
formalism convention, arrows flow from electrons. But be careful about violating the rules
of valence; arrows either displace another pair of electrons or fill a “hole”—an empty orbital.
2. It is really important to be able to generalize the definition of Lewis bases and Lewis
acids. All electron-pair donors are Lewis bases, and nearly any pair of electrons can act
as a Lewis base under some conditions. Similarly, there are many very different
appearing Lewis acids—acceptors of electrons.
3. The four fundamental reactions we study in this chapter, S
N
2, S
N
1, E2, and E1, are
affected by the reaction conditions as well, of course, as by the structures of the
molecules themselves.These four reactions often compete with each other, and it is
important to be able to select conditions and structures that favor one reaction over the
others. You will rarely be able to find conditions that give specificity, but you will
usually be able to choose conditions that give selectivity.
4. At the end of this chapter, we will sum up what we know about making molecules—
synthesis. ALWAYS attack problems in synthesis by doing them backward. Do not
attempt to see forward from starting material to product. In a multistep synthesis, this
approach is often fatal. The way to do it is to look at the ultimate product and ask
yourself what molecule might lead to it in a single step.That technique will usually
produce one or more candidates.Then apply the same question to those candidates,
and before you know it, you will be at the indicated starting material. This technique
works, and can make what is often a tough, vexing task easy.
5. Alcohols are important starting materials for synthesis—they are inexpensive and easily
available. Alcohols can be made even more useful by learning how to manipulate the
OH group to make it more reactive—to make it a better “leaving group.”Several ways
to do that are outlined in Section 7.4f.
6. Keeping track of your growing catalog of useful synthetic reactions is not easy—it is
a subject that grows rapidly. A set of file cards on which you collect ways of making various
types of molecules is valuable. See page 320 for an outline of how to make this catalog.
7.2 Review of Lewis Acids and Bases
7.2a The Curved Arrow Formalism Recall that there is a most important
bookkeeping technique called the curved arrow formalism (p. 23), which helps to
sketch the broad outlines of what happens during a chemical reaction.However,it does
not constitute a full reaction mechanism, a description of how the reaction occurs.
264 CHAPTER 7 Substitution and Elimination Reactions
–
..
..
Cl
..
..
..
..
HO H
..
..
–
Cl
..
..
..
HO H
..
..
..
..
Cl
..
..
..
..
H
3
NH
–
Cl
..
..
..
H
3
N
+
H
+
+
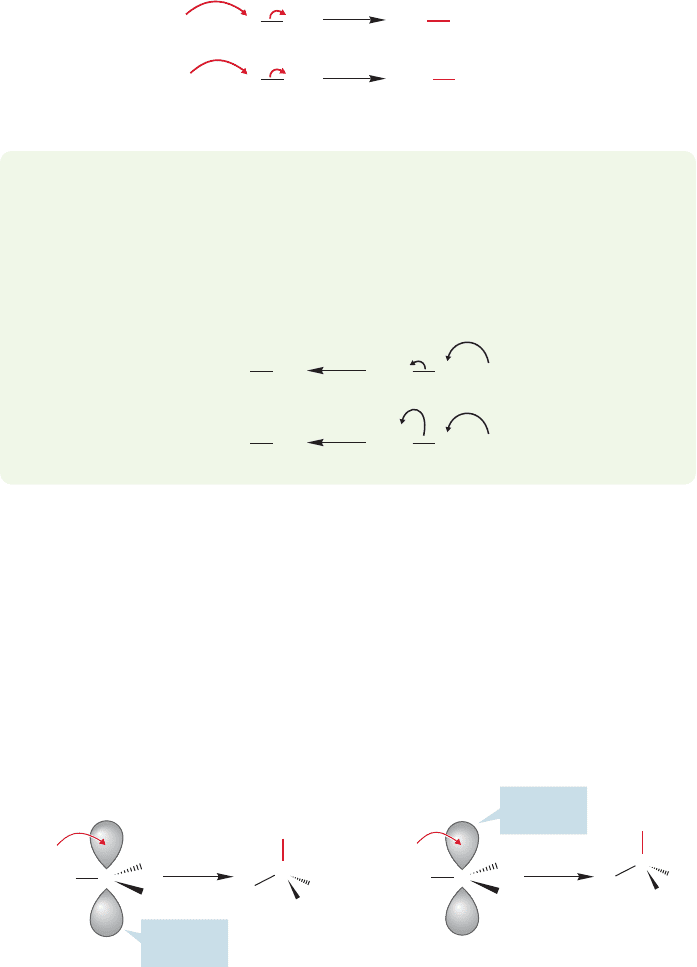
FIGURE 7.3 Two demonstrations of
the application of the curved arrow
formalism. We will see many other
examples.
+
Empty 2p
orbital
Empty 2p
orbital
F
..
..
..
F
..
F
..
..
..
..
..
..
..
..
..
..
..
..
F
F
–
BF
F
F
H
..
–
CH
H
H
B
–
C
H
H
H
H
..
..
..
..
..
..
..
..
..
FIGURE 7.4 The fluoride and hydride
ion are Lewis bases reacting with the
Lewis acids BF
3
and
CH
3
.
If a reaction mechanism is not an arrow formalism, what is it? A short answer
would be a description, in terms of structure and energy, of the intermediates and
transition states separating starting material and product. Implied in this descrip-
tion is a picture of how the participants in the reaction must approach each other.
The arrow formalism is a powerful device of enormous utility in helping to keep
track of bond makings and breakings. In this formalism, chemical bonds are shown
as being formed from pairs of electrons flowing from a donor toward an acceptor,
often, but not always, displacing other electron pairs. Figure 7.3 shows two exam-
ples. In the first, hydroxide ion, acting as a Brønsted base, displaces the pair of elec-
trons in the hydrogen–chlorine bond. The reaction of ammonia ( ) with
hydrogen chloride is another example in which the electrons on neutral nitrogen are
shown displacing the electrons in the hydrogen–chlorine bond.
:
NH
3
WORKED PROBLEM 7.1 Draw arrow formalisms for the reversals of the two reactions
in Figure 7.3.
ANSWER This problem may seem to be mindless, but it really is not. The key
point here is to begin to see all reactions as proceeding in two directions. Nature
doesn’t worry about “forward” or “backward.” Thermodynamics dictates the
direction in which a reaction will run.
Cl
..
..
..
HH
–
–
HO
..
..
..
HO
..
..
Cl
..
..
..
..
Cl
..
..
..
HH
–
H
3
N
..
H
3
N
Cl
..
..
..
..
+
+
+
+
+
7.2b Lewis Acids and Bases In Sections 1.7 and 2.15, we began a discus-
sion of acids and bases that will last throughout this book. Then we spent some
time in Chapter 3 reviewing Brønsted acids and bases in the context of the addi-
tion reaction and extended the subject of acidity and basicity to Lewis acids and
Lewis bases. These concepts will hold together much of the material in the
remaining chapters.
Consider two conceptually related processes: the reaction of boron trifluoride
with fluoride ion, F
, and the reaction of the methyl cation with hydride,
(Fig. 7.4).
H
:
-
7.2 Review of Lewis Acids and Bases 265
In these two reactions there is no donating or accepting of a proton, and so
by a strict definition of Brønsted acids and bases we are not dealing with acid–base
chemistry. As we already know, however, there is a more general definition of acids
and bases that deals with this situation. A little review may be helpful: A Lewis
base is defined as any species with a reactive pair of electrons (an electron donor).
This definition may seem very general and indeed it is.It gathers under the “base”
category all manner of odd compounds that don’t really look like bases. The key
word that confers all this generality is reactive. Reactive under what circumstances?
Almost everything is reactive under some set of conditions. And that’s true;
compounds that are extraordinarily weak Brønsted bases can still behave as
Lewis bases.
If the definition of a Lewis base seems general and perhaps vague, the defini-
tion of a Lewis acid is even worse. A Lewis acid is anything that will react with a
Lewis base (thus, an electron acceptor).Therein lies the power of these definitions.
They allow us to generalize—to see as similar reactions that on the surface appear
very different.
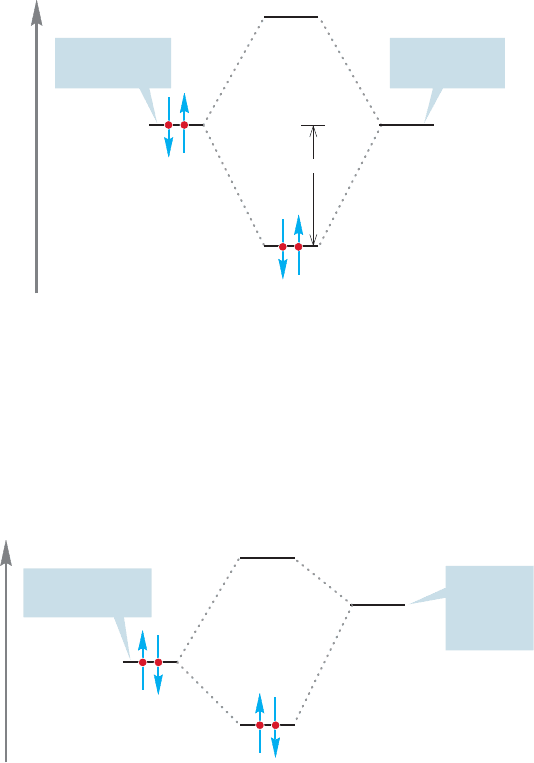
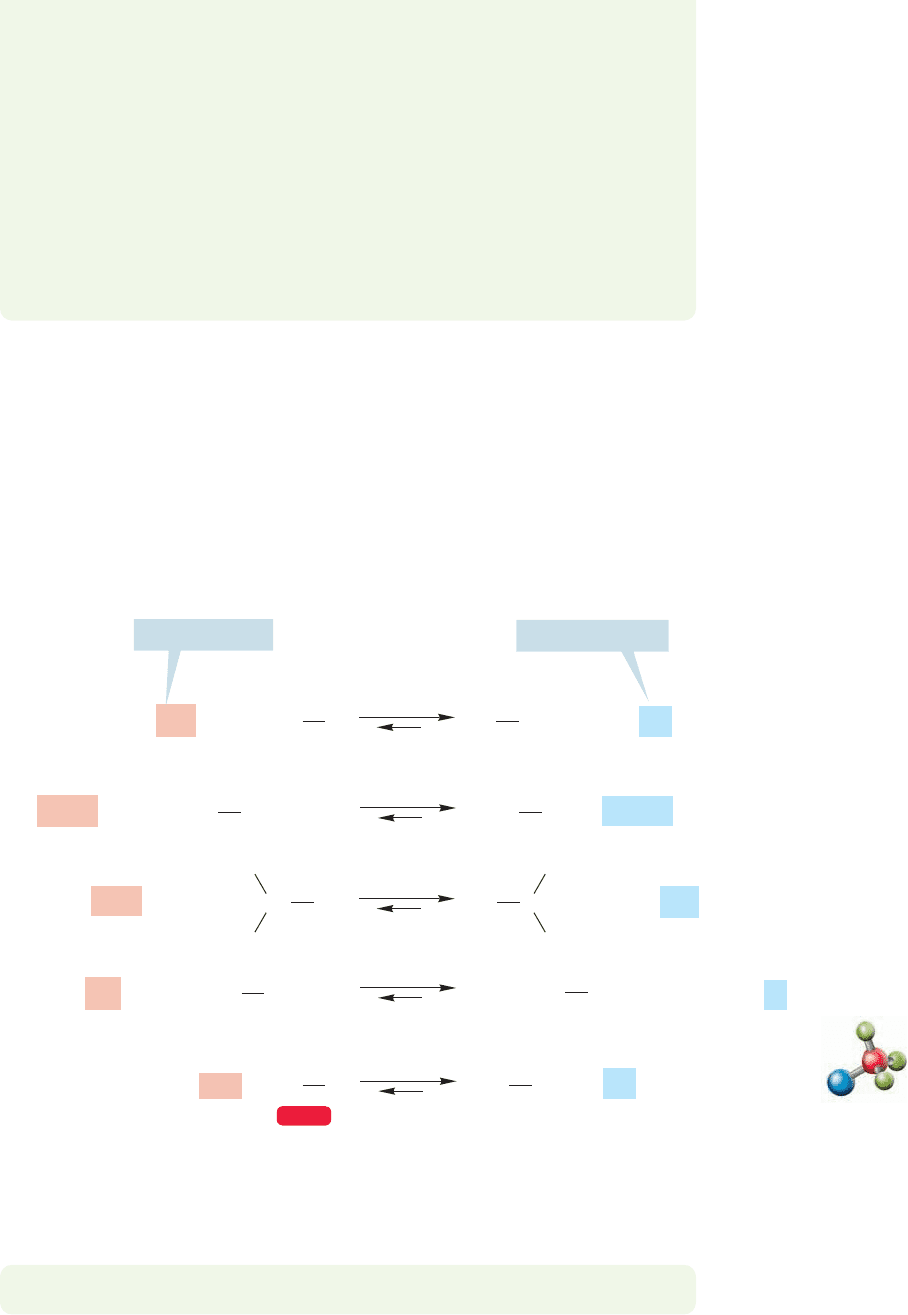
To describe Lewis acid–Lewis base reactions in orbital terms, we need only point
out that a stabilizing interaction of orbitals involves the overlap of a filled orbital
(Lewis base) with an empty orbital (Lewis acid) (Fig. 7.5).
Energy
Empty orbital
(Lewis acid)
Filled orbital
(Lewis base)
Stabilization
FIGURE 7.5 The interaction of a
filled orbital with an empty orbital
is stabilizing.
In the examples in Figure 7.4, the Lewis bases are the fluoride and hydride
ions. In fluoride, the electrons occupy a 2p orbital and in hydride, a 1s orbital.
The Lewis acids are boron trifluoride and the methyl cation, each of which
bears an empty 2p orbital. A schematic picture of these interactions is shown in
Figure 7.6.
Empty 2p
orbital on
carbon or
boron
Filled H (1s)
or F (2p) orbital
Energy
FIGURE 7.6 A schematic interaction
diagram for the reactions of
Figure 7.4.
266 CHAPTER 7 Substitution and Elimination Reactions
AB
LUMO
HOMO
Energy
FIGURE 7.7 In two typical molecules,
the strongest stabilizing interaction is
likely to be between the HOMO of
one molecule (A) and the LUMO of
the other molecule (B), because the
strongest interactions are between the
orbitals closest in energy.
HOMO
F
LUMO
σ∗
B
Energy
F
σ
B
F (2p)
B (2p)
FIGURE 7.8 The interaction of the
LUMO for BF
3
(an empty 2p orbital
on boron) and the HOMO of F
(a filled 2p orbital on fluorine)
produces two new molecular orbitals:
an occupied bonding σ orbital and an
unoccupied antibonding σ* orbital.
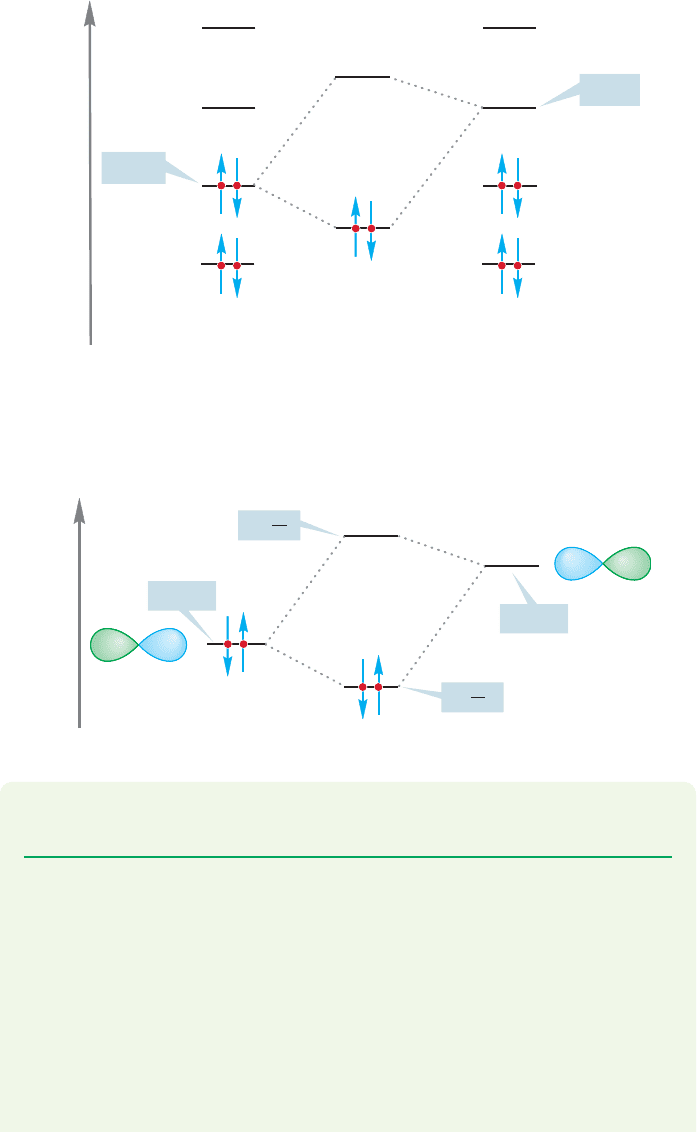
7.2c HOMO–LUMO Interactions It is possible to elaborate a bit on the gen-
eralization that “Lewis acids react with Lewis bases” by realizing that the strongest
interactions between orbitals are between the orbitals closest in energy. For stabiliz-
ing filled–empty overlaps, this will almost always mean that we must look at the
interaction of the highest occupied molecular orbital (HOMO) of one molecule (A)
with the lowest unoccupied molecular orbital (LUMO) of the other (B) (Fig. 7.7).
PROBLEM 7.2 Identify the HOMO and LUMO in the reaction of the methyl
cation with hydride in Figure 7.4.
WORKED PROBLEM 7.3 Point out the HOMO and LUMO in the following
reactions:
(a)
(b)
(c)
(d)
(e)
HO
.
.
.
.
:
-
+ H
3
O
+
:
U
2 H
2
O
.
.
:
H
3
N
:
+ H
O
Cl
.
.
.
.
:
U
+
NH
4
+
:
Cl
.
.
.
.
:
-
:
-
CH
3
+ H
O
Cl
.
.
.
.
:
U
CH
4
+
:
Cl
.
.
.
.
:
-
H
2
C
P
CH
2
+ BH
3
U
+
CH
2
O
CH
2
O
-
BH
3
HO
.
.
.
.
:
-
+ Li
+
U
HO
.
.
.
.
Li
In the reaction of BF
3
with fluoride, it is the filled fluorine 2p orbital that is the
HOMO and the empty 2p orbital on boron that is the LUMO (Fig. 7.8). All Lewis
acid–Lewis base reactions can be described in similar terms.
(continued)
7.3 Reactions of Alkyl Halides: The Substitution Reaction 267
7.3 Reactions of Alkyl Halides: The Substitution
Reaction
A great many varieties of substitution reactions are known, but all involve a com-
petition between a pair of Lewis bases for a Lewis acid. Figure 7.9 shows five exam-
ples with the substituting group, the nucleophile (from the Greek,“nucleus loving”),
and displaced group, the leaving group,identified. Both the nucleophile (usually Nu)
and leaving group (usually L) are Lewis bases.
ANSWER
(a) HOMO: Filled nonbonding orbital on HO
LUMO: Empty 2s orbital on Li
(b) HOMO: Filled π orbital of ethylene
LUMO: Empty 2p orbital on BH
3
(c) HOMO: Filled sp
3
nonbonding orbital of methyl anion
LUMO: Empty σ
*
orbital of
(d) HOMO: Filled sp
3
nonbonding orbital of ammonia,
LUMO: Empty σ
*
orbital of
(e) HOMO: Filled nonbonding orbital of hydroxide
LUMO: Empty σ
*
orbital of an bond of H
3
O
O
O
H
H
O
Cl
:
NH
3
H
O
Cl
PROBLEM 7.4 Identify the HOMOs and LUMOs in the reactions of Figure 7.9.
K
+
Na
+
(a)
Nucleophile (Nu )
I
..
..
HO
..
..
..
CH
3
CH
2
CH
2
H
3
C
N
2
N
2
H
3
CK
+
CH
3
–
..
HO
..
..
..
..
CH
3
CH
2
CH
2
I
..
..
..
H
3
C
H
3
C
CH Cl
–
–
(b)
I
..
..
..
..
..
..
F
..
..
..
..
F
..
..
..
Cl
..
..
..
..
Na
+
..
H
3
C N(CH
3
)
3
N(CH
3
)
3
CH
3
–
..
..
..
..
..
..
Cl
..
..
..
..
–
Cl
..
..
..
..
CH
3
CH
3
CH
Leaving group ( L)
CH
3
SH
3
CS
+
K
+
Li
+
Li
+
K
+
+
+
+
+
++
+
+
+
+
+
+
+
(c)
–
..
..
NC
(d)
H
3
N
CH
3
H
3
N
+
(e)
–
–
..
NC
..
–
–
I
..
..
..
..
–
I
..
..
..
..
I
..
..
..
..
WEB 3D
FIGURE 7.9 Five examples of the substitution reaction. The nucleophiles for the reactions
reading left to right are (a) hydroxide (HO
), (b) methyl mercaptide (CH
3
S
), (c) cyanide
(NC
), (d) fluoride (F
), and (e) ammonia ( NH
3
).The leaving groups are iodide (I
),
trimethylamine [N(CH
3
)
3
], chloride (Cl
), nitrogen (N
2
), and iodide (I
) again.
:
Bimolecular nucleophilic substitution: S
N
2
268 CHAPTER 7 Substitution and Elimination Reactions
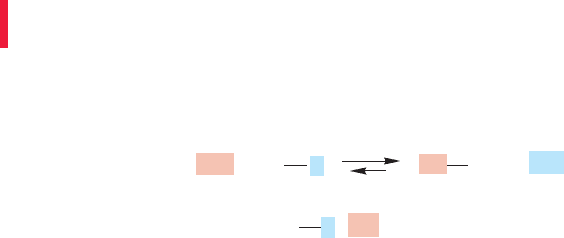
–
..
+
+
R
L
LR
Nu
..
Nu
–
..
–
Rate (ν) = k [R L] [ ] Nu
FIGURE 7.10 A bimolecular reaction.
Notice that the reactions in Figure 7.9 are all equilibrium reactions.We will con-
sider equilibrium in detail in Chapter 8, but we need to see here that these compe-
tition reactions are all, in principle at least, reversible. A given reaction may not be
reversible in a practical sense, but that simply means that one of the competitors has
been overwhelmingly successful. There are many possible reasons why this might
be so. One of the equilibrating molecules might be much more stable than the other.
If one of the nucleophiles were much more powerful than the other, the reaction
would be extremely exothermic in one direction or the other.Reactions a–c in Figure 7.9
are examples of this.We are used to thinking of reactions running from left to right,
but this prejudice is arbitrary and even misleading. Reactions run both ways. There
are other reasons one of these reactions might be irreversible. Perhaps the equilib-
rium is disturbed by one of the products being a solid that crystallizes out of the
solution, or a gas that bubbles away. If so, the reaction can be driven all the way to
one side, even against an unfavorable equilibrium constant. Under such circum-
stances, an endothermic reaction can go to completion. In reaction d of Figure 7.9
both propyl fluoride (bp 2.5 °C) and nitrogen are gases, and their removal from the
solution will drive the equilibrium to the right.
The second point to notice is that there is no obligatory charge type in these
reactions. The displacing nucleophile is not always negatively charged (reaction e),
and the group displaced—the leaving group—does not always appear as an anion
(reactions b and d).
If we examine a large number of substitution reactions, two categories emerge.
The boundaries between them are not absolutely fixed, as we will see, but there are
two fairly clear-cut limiting classes of reaction. One is a kinetically second-order
process, common for primary and secondary substrates ( , Section 7.4). The
other is a kinetically first-order reaction,common for tertiary substrates (Section 7.6).
We take up these two reactions in turn, examining each in detail.
7.4 Substitution, Nucleophilic, Bimolecular:
The S
N
2 Reaction
7.4a Rate Law The rate law tells us the number and kinds of molecules involved
in the transition state—it tells you how the reaction rate depends on the concentra-
tions of reactants and products. For many substitution reactions, the rate ν is propor-
tional to the concentrations of both the nucleophile, , and the substrate,
This bimolecular reaction is first order in and first order in substrate,
. The rate constant, k, is a fundamental constant of the reaction (Fig. 7.10).
Here, R stands for a generic alkyl group. Don’t confuse this R with the (R) used to
specify one enantiomer of a chiral molecule. This bimolecular reaction is second
order overall, which leads to the name Substitution, Nucleophilic, bimolecular, or
S
N
2 reaction.
R
O
L
Nu
:
-
[R
O
L].
[Nu
:
-
]
R
O
L
7.4b Stereochemistry of the S
N
2 Reaction One of the most powerful of
all tools for investigating the mechanism of a reaction is a stereochemical analysis.
In this case, we need first to see what’s possible and then to design an experiment
CONVENTION ALERT
