Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

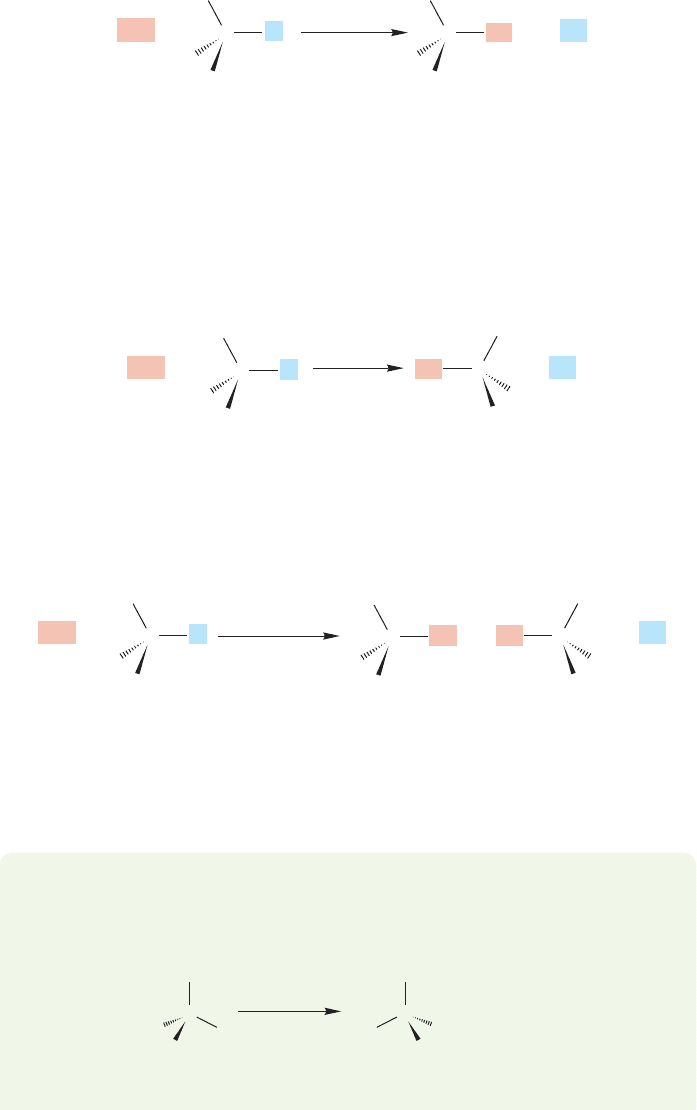
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 269
that will enable us to determine the stereochemical results. If we start with a single
enantiomer (Chapter 4, p. 151) (R) or (S), there are several possible outcomes of a
substitution reaction. We could get retention of configuration, in which the stereo-
chemistry of the starting molecule is preserved. The entering nucleophile occupies
the same stereochemical position as did the departing leaving group, and the prod-
uct has the same handedness as the starting material (Fig. 7.11).
A second possibility is inversion of configuration, in which the stereochemistry
of the starting material is reversed: (R) starting material going to (S) product and
(S) starting material going to (R) product. In this kind of reaction, the pyramid of
the starting material becomes inverted in the reaction like an umbrella in the wind
(Fig. 7.12). In this and Figure 7.11, notice how the numbers enable us to keep track
of inversions and retentions.
It is also possible that optical activity could be lost. In such a case, (R) or (S)
starting material goes to a racemic mixture—a 50:50 mixture of (R) and (S) product
(Fig. 7.13).
Finally, it is possible that some dreadful mixture of the (R) and (S) forms is pro-
duced. In such a case, it is very difficult to analyze the situation, as several reaction
mechanisms may be operating simultaneously.
retention
3
2
1
++
3
2
1
C C
Nu
L
–
..
L
Nu
..
–
FIGURE 7.11 One possible
stereochemical outcome for the S
N
2
reaction. In this scenario, the product
is formed with retention of configuration.
inversion
+
3
2
1
+
C
3
2
1
C
Nu
L
–
..
L
Nu
..
–
FIGURE 7.12 Another possible
stereochemical result for the S
N
2
reaction.The handedness of the
starting material could be reversed in
the product, in what is called
inversion of configuration.
(50%) (50%)
racemization
+
+
3
2
1
+
C
3
2
1
C
3
2
1
C
NuNu
L
Nu
..
–
–
..
L
FIGURE 7.13 A third possible
stereochemical result for the S
N
2
reaction.
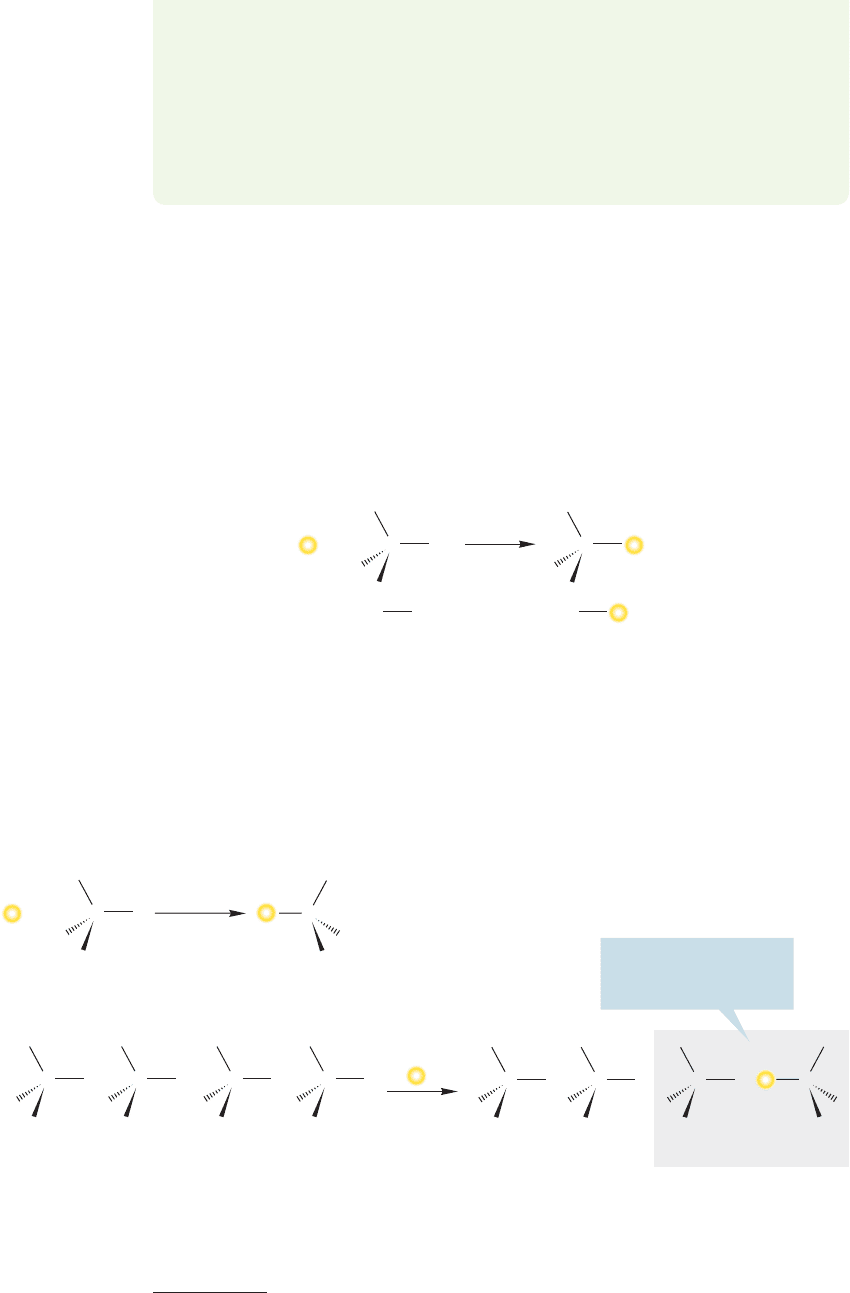
WORKED PROBLEM 7.5 Sometimes matters appear more complicated than they
really are. It is possible, for example, that an (R) starting material could produce an
(R) product by a process involving inversion.Here is a conceivable example.Explain.
H
3
C
S
N
2 with
inversion
+
H
H
I
HO
(
R
)
Still
(
R
)
!
Cl
Cl
CC
Na
+
Na
+
I
–
–
OH
CH
3
(continued)
270 CHAPTER 7 Substitution and Elimination Reactions
2
In this discussion, we indicate radioactive iodine with an asterisk in the text and with the asterisk and a
yellow circle in the figures.
ANSWER The designations (R) and (S) are determined for each molecule through the
Cahn–Ingold–Prelog priority system (Chapter 3, p. 111; Chapter 4, p. 152). If the
groups attached to carbon change, as in the example shown, there is no guarantee that
inversion will change an (R) carbon to an (S) carbon. In the example shown here, the
“umbrella” is inverted, but at the same time the substituents attached to the stereo-
genic carbon have also changed from (C, Cl, H, I) to (C, Cl, H, OH). Application of
the priority system shows that both molecules are (R) despite the inversion.
For most secondary and primary substrates, substitution occurs with complete
inversion of configuration. We now are faced with three questions: (1) How do we
know the reactions go with inversion? (2) What does this result tell us about the
reaction mechanism? (3) Why is inversion preferred to retention or racemization?
The answer to the first question is easy: We measure it. Suppose we start with the
optically active iodide in Figure 7.14 and monitor the reaction with radioactive
iodide ion (
I
*
).
2
We measure two things: the incorporation of radioactive iodide and
the optical activity of . If radioactive iodide reacts with with retention
of configuration, there will be no loss of rotation at all [I
*
and I are not significantly
different in their contributions to the rotation of plane-polarized light (Fig. 7.14)].
R
O
IR
O
I
*
R
O
I
I* I*
–
–
I
3
2
1
++
C
3
2
1
C
I
R I*R I
FIGURE 7.14 A mechanism involving
retention predicts that incorporation
of radioactive iodide will induce no
change in optical rotation.
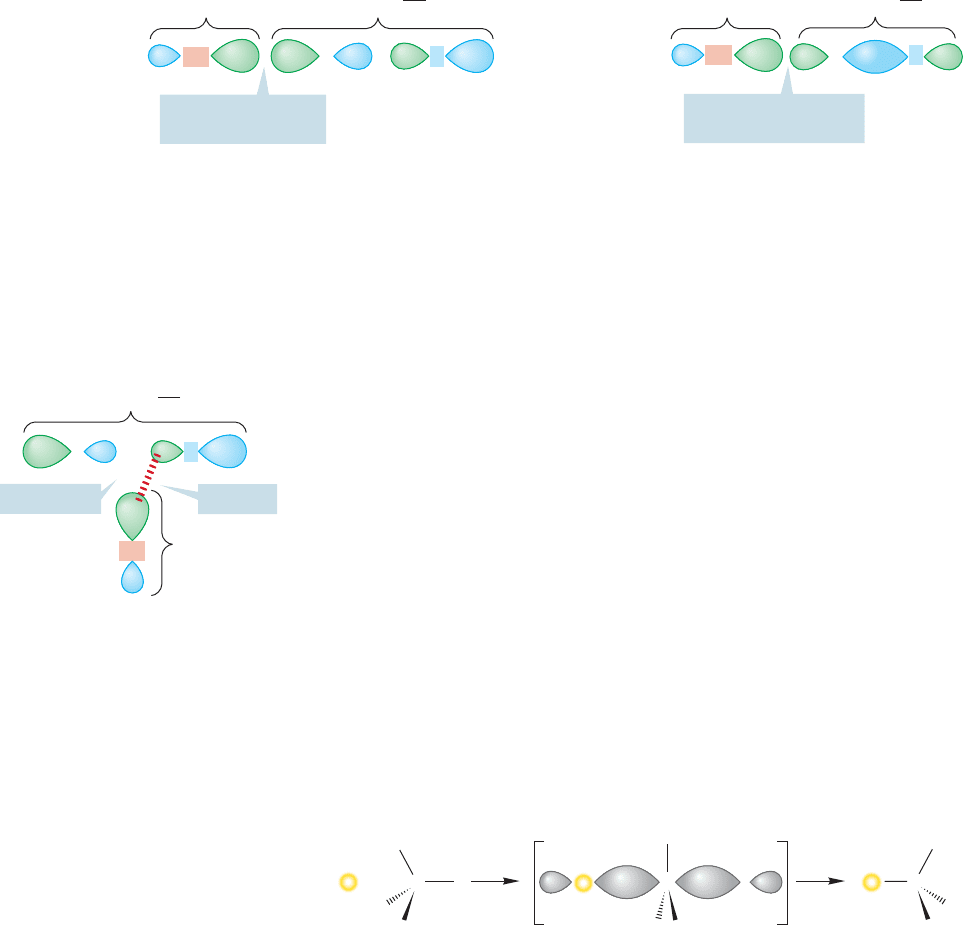
What happens if the reaction goes with inversion? The observed optical rotation
of is certainly going to decrease as, for example, (R)-iodide is converted into
(S)-iodide. But it is usually not obvious how the rate of loss of optical activity com-
pares with the rate of incorporation of I
*
. If every replacement occurs with inversion,
the rate of loss of optical activity must be twice the rate of incorporation of I
*
.Every
conversion of an (R)-iodide ( ) into an (S)-iodide ( ) generates a product
molecule ( ) whose rotation exactly cancels that of a molecule of starting
In effect, the rotation of two molecules goes to zero for every inversion (Fig. 7.15).
R
O
I.I
*
O
R
I
*
O
RR
O
I
R
O
I
inversion
Net rotation of this set of molecules = 4(R)
(R)
(R)(R)(R)(R)
(S)
3
2
1
(R)(R)
(R)(S)
…thus, the net rotation of this set of
four molecules = 2
(
R
)
The boxed set of two
molecules contributes
0⬚ to the rotation…
C
I*
–
I*
I*
I*
–
–
I
3
2
1
+
+
C
I
33
2
1
C
I
2
1
C
3
I
2
1
C
3
I
2
1
C
3
I
2
1
C
3
I
2
1
C
3
I
2
1
C
I
3
2
1
C
FIGURE 7.15 A mechanism involving inversion. For the incorporation of one atom of radioactive iodide, the
rotation of the set of molecules shown will be cut by a factor of 2.
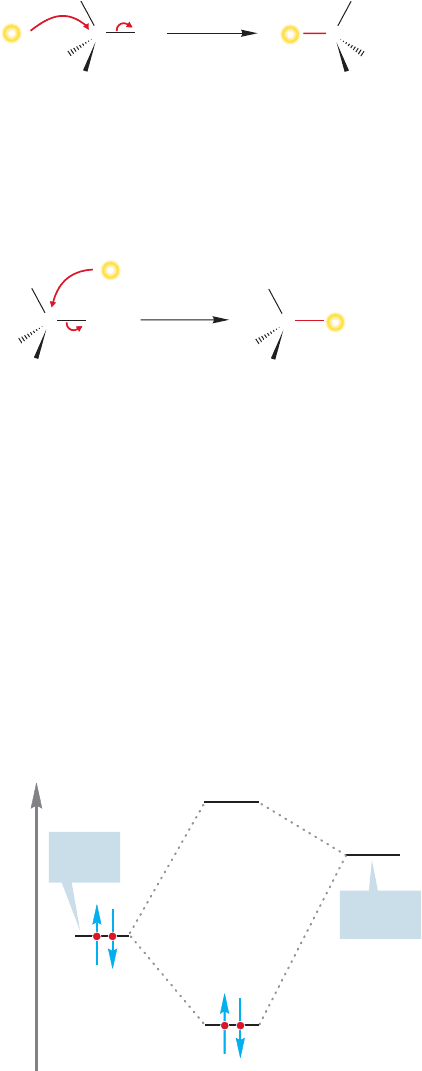
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 271
Had the reaction occurred with retention, a frontside approach would have been
demanded. The incoming nucleophile would have had to enter the molecule from
the same side as the departing leaving group.The tetrahedral “umbrella”would have
retained its configuration. This result is not observed (Fig. 7.17).
inversion
3
2
1
++
C
I*
I*
–
–
I
..
..
..
3
2
1
C
I
..
..
..
..
..
..
..
..
..
..
..
..
..
FIGURE 7.16 The observation of inversion
in the S
N
2 reaction means that the entering
nucleophile, radioactive (or labeled) iodide, must
enter the molecule from the side opposite the
departing leaving group, here unlabeled iodide.
This change is exactly what is observed experimentally. Every incorporation of I
*
is accompanied by the inversion of one (R)-iodide to an (S)-iodide.
Now, what does this result tell us about the mechanism? The observation of inver-
sion tells us that the nucleophile, the incoming atom or molecule, must be approaching
the substrate from the rear of the departing atom.Only this path can lead to the observed
inversion.Figure 7.16 shows the reaction,along with an arrow formalism description of
the bonds that are forming and breaking.The arrows show the path of approach of the
incoming nucleophile and the departing leaving group. The tetrahedral “umbrella” is
inverted during the reaction.
retention
I
..
..
..
3
2
1
C
I*
–
+
3
2
1
C
I
..
..
..
..
..
I*
–
..
..
..
..
..
..
..
..
FIGURE 7.17 If retention had been
the experimentally observed result,
the nucleophile would have added
from the same side that the leaving
group departed.
All this analysis is useful; we now know the path of approach for the nucleophile in
the S
N
2 reaction, and so we know more about the mechanism of this process. But our
newfound knowledge serves only to generate the inevitable next question.Why is inver-
sion preferred? Why is frontside displacement—retention—never observed? Here comes
part of the payoff for all the work we did in earlier chapters on molecular orbitals. One
of the key lessons of those early chapters was that interactions of filled and empty orbitals
are stabilizing, because the two electrons originally in the filled orbital can be accom-
modated in the new bonding orbital, and no electrons need occupy the antibonding
molecular orbital. Now let’s look at the S
N
2 reaction in orbital terms.A filled nonbond-
ing orbital “n” on the nucleophile interacts with an orbital on the substrate, ,
involved in the bond from R to the leaving group, L. Because the interaction of two
filled orbitals is not stabilizing, the filled orbital n must overlap with the empty, anti-
bonding σ
*
orbital of .This overlapping is nothing more than the interaction of
the nucleophile’s HOMO,n,with the sigma bond’s LUMO,σ
*
, shown schematically in
Figure 7.18.
R
O
L
R
O
L
Filled n
(HOMO)
Empty
σ*
(LUMO)
Energy
FIGURE 7.18 An orbital interaction
scheme for the stabilizing interaction
of a filled nonbonding orbital (n) on
the nucleophile overlapping with the
empty, antibonding (σ
*
) orbital on
the substrate.
What does σ
*
look like? Figure 7.19 shows us. Note that σ
*
has one of its large
lobes pointing to the rear of . That is where overlap with the filled n orbital
will be most efficient.
Now look what happens if the orbitals overlap in a way that would lead to reten-
tion through a frontside substitution (Fig. 7.20).Not only is the magnitude of inter-
action reduced by the relatively poor overlap with the smaller lobe of σ
*
, but there
is both a bonding and an antibonding interaction,and these will tend to cancel each
other. Frontside displacement is a very unlikely process indeed.
We can now map out the progress of the bond making and breaking in the S
N
2
reaction.The reaction starts as the nucleophile , approaches the rear of the sub-
strate. Because the HOMO, the nonbonding orbital n on , overlaps with the
LUMO, the antibonding σ
*
orbital of , the bond from C to L is weakened as
the antibonding orbital begins to be occupied. The bond length of increases
as the new bond between Nu and C begins to form. As lengthens, the other
groups attached to C bend back, in umbrella fashion, ultimately inverting (just like
the windblown umbrella) to form the product.For a symmetrical displacement such
as the reaction of radioactive
I
*
with discussed before, the midpoint of the
reaction will be the point at which the groups attached to C are exactly halfway
inverted (Fig. 7.21).
R
O
I
C
O
L
C
O
L
C
O
L
Nu
:
-
Nu
:
-
R
O
L
272 CHAPTER 7 Substitution and Elimination Reactions
What is the hybridization of the central carbon at this midpoint? It must be sp
2
,
because this carbon is surrounded by three coplanar groups.There are partial bonds
to the incoming nucleophile, , and the departing leaving group, . No picture
shows the competition between the two nucleophiles ( and ) as clearly as this
one.What Lewis acid are they competing for? A carbon 2p orbital,as the figure shows.
What is this structure? At least in the symmetrical displacement of by , it
is the halfway point between the equi-energetic starting material and product.
-
:
I*
-
:
I
-
:
I
-
:
I*
-
:
I
-
:
I*
L
L
(a) Stabilizing interaction of filled and empty orbitals (strong)
(b) Destabilizing interaction of two filled orbitals (weak
)
..
..
..
n The filled
nonbonding orbital on
the nucleophile, Nu
n The filled
nonbonding orbital
on the nucleophile, Nu
Note good bonding
overlap here
Note weaker bonding
overlap here
σ* The empty,
antibonding
orbital of R L
σ The filled,
bonding
orbital of R L
Nu
Nu
R
R
FIGURE 7.19 (a) The overlap of the filled nonbonding orbital (n) with the “outside” lobe of the empty σ
*
orbital of
takes advantage of the good overlap available with this lobe of σ
*
. (b) Contrast (a) with the destabilizing interaction of n and
σ, the two filled orbitals.The interaction of two filled orbitals is destabilizing, but the overlap of the lobes involved,one fat,
the other thin, is poor.Therefore, this interaction is not important, and the stabilizing interaction shown in (a) dominates.
R
O
L
L
Nu
Bonding
σ* The empty antibonding
orbital of R
L
n The filled
nonbonding
orbital of the
nucleophile, Nu
..
Antibonding
R
FIGURE 7.20 Frontside substitution
(retention) requires overlap with the
inside part of σ
*
.There is poor
overlap with the small lobes of σ
*
as well as offsetting bonding and
antibonding interactions.
sp
2
I
In the transition state for this symmetrical S
N
2 reaction, the
umbrella is halfway inverted and the carbon is sp
2
hybridized
–
C
I*
++
C
I*
I*
–
I
C
I
–
FIGURE 7.21 In a symmetrical
inversion process, the midpoint,
called the transition state, is the point
at which the umbrella is exactly half-
inverted.
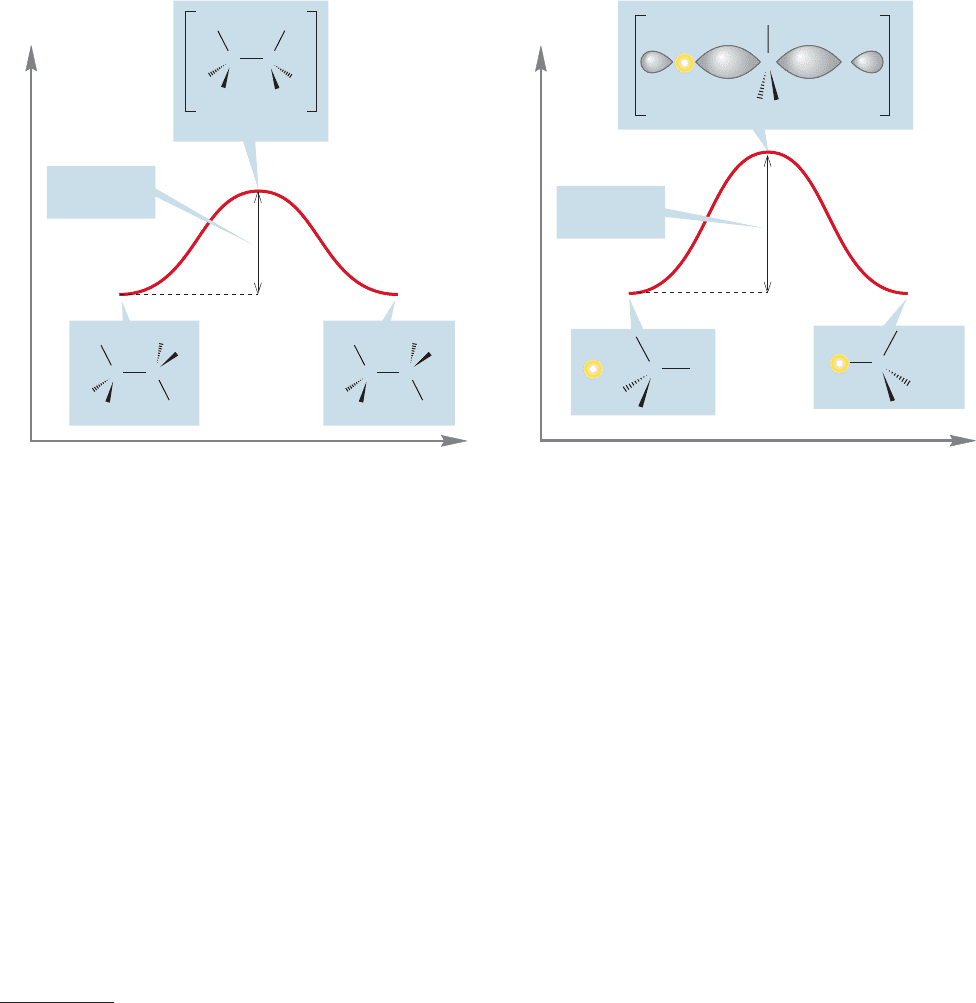
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 273
This structure is the transition state for this reaction. Is this transition state
something one could potentially catch and isolate? No! It occupies not an energy
minimum, as stable compounds do, but it sits on an energy maximum along the
path from starting material to product.We have seen such structures before: Recall,
for example, the eclipsed form of ethane or the transition state for the interconver-
sion of two pyramidal methyl radicals (Fig. 7.22).
3
Actually, even our picture of the displacement of by is ever so slightly wrong. Normal iodine (I) is
not exactly the same as radioactive iodine (I*), and even this reaction is not truly symmetrical. We can ignore
the tiny differences in isotopes, however, as long as we keep the principle straight. If you want to be more cor-
rect, insert the words “except for a tiny isotope effect” wherever appropriate.
-
:
I*
-
:
I
–
C
+
Transition state
Activation
energy
Energy
Reaction progress
+
–
I
C
I
C
I*
I*
I*
I
–
Transition state
Activation
energy
Energy
Reaction progress
H
H
H
H
H
H
CC
H
H
H
H
H
H
CC
HH
H
(a) (b)
H
CC
HH
FIGURE 7.22 Two analogous diagrams. (a) The rotation about the carbon–carbon bond in ethane; (b) the displacement
of
I by
I*. In each case, two equi-energetic molecules are separated by a single energy barrier, the top of which is called
the transition state, shown in brackets.
Now, let’s consider the change in energy as the S
N
2 reaction proceeds (Fig. 7.22b).
The starting material and products are separated by the high-energy point of
the reaction, the transition state. The energy difference between starting material
and transition state is related to the rate of the reaction. The larger this difference,
the higher is the barrier, and the slower is the reaction.So, a quantity of vital impor-
tance to any study of a chemical reaction is the energy difference between starting
material and the transition state, called the activation energy.The reaction progress
diagram is symmetrical only if the substitution reaction is symmetrical, that is, if
starting material and product are of equal energy.
3
This specific picture is transferable to a general second-order substitution reac-
tion, but some differences will appear. First of all, usually the starting material and
product will not be at the same energy, and the transition state cannot be exactly
halfway between starting material and product.It will lie closer to one than the other.
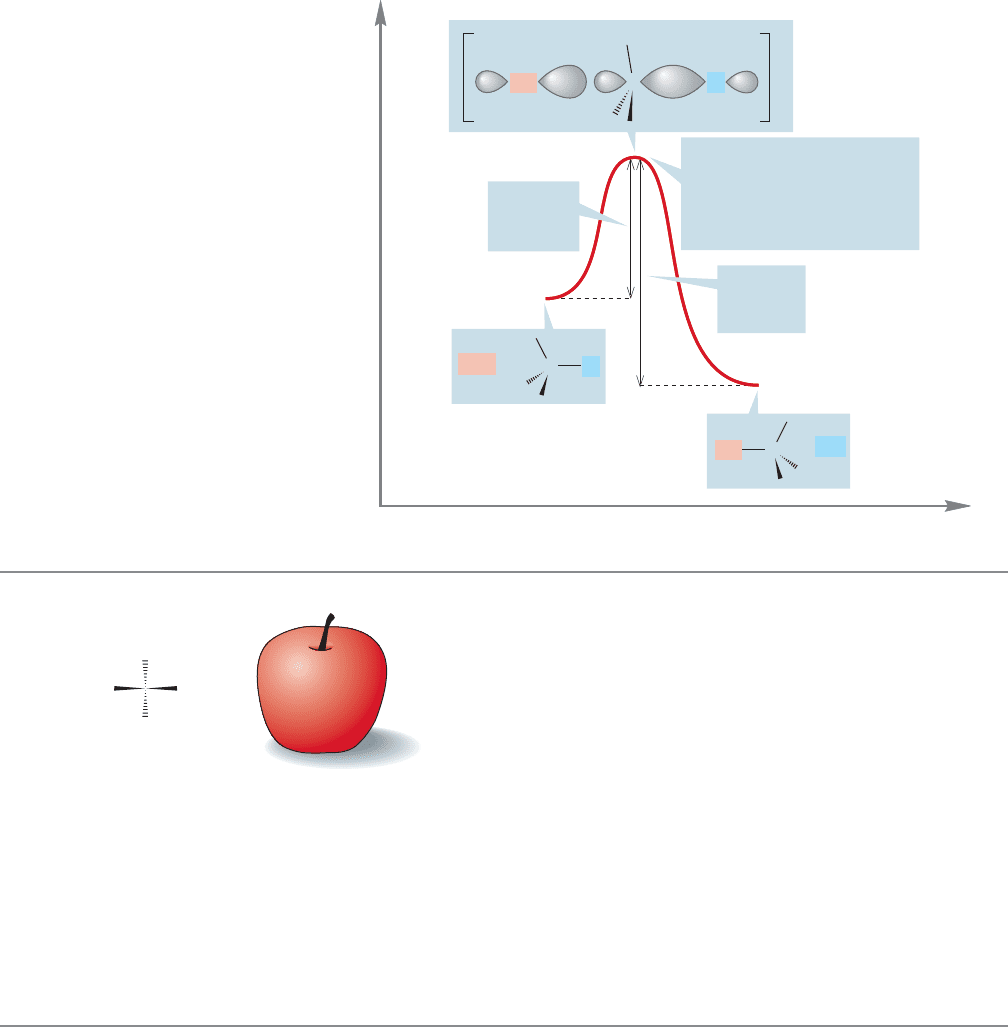
274 CHAPTER 7 Substitution and Elimination Reactions
The transition state cannot be exactly sp
2
, only nearly sp
2
(notice its slightly pyram-
idal shape) (Fig. 7.23).
Figure 7.23 shows both an exothermic and endothermic S
N
2 substitution reaction.
If we read the figure conventionally from left to right, we see the exothermic reaction,
but if we read it “backward,”from right to left,we see the endothermic reaction.Notice
that the activation energy for the forward process must be smaller than that for the
reverse reaction.Thermodynamics will determine where the equilibrium settles out,and
there may be practical consequences if one partner is substantially favored over the other,
but one diagram will suffice to examine both directions. Many of us are prisoners of
our language, which is read from left to right, and we tend to do the same for these
energy diagrams. Nature is more versatile, or perhaps less prejudiced, than we are.
Energy
Reaction progress
Slightly pyramidal transition
state—in the general case it
is not exactly at the midpoint
between starting material
and product
–
L
Nu
L
C
C
+
Nu
..
L
+
C
Nu
L
..
–
Activation
energy
forward
Activation
energy
reverse
–
FIGURE 7.23 A general S
N
2 reaction.
If the nucleophile (Nu) and the
leaving group (L) are different,
the transition state is not exactly
at the midpoint of the reaction, and
the hybridization of carbon is only
approximately sp
2
.
MALIC ACID
CH
2
COOH
OHH
COOH
now Latvia and who died in Tübingen, Germany, in 1957.
Indeed, the inversion now known to occur universally in the
S
N
2 reaction is sometimes called Walden inversion.
MJ’s teacher of organic chemistry, William Doering, told
a wonderful story about Paul Walden. Professor Doering was
visiting Tübingen in 1956 to talk about his work and overheard
a now-famous German chemist lamenting the fact that
Tübingen had not been bombed during the war. It seems that
most of his colleagues in other “more fortunate” cities had
newly built laboratories courtesy of Allied bombing.There was
an old gentleman sitting in the corner who had been wheeled
into the department from his nursing home to hear Doering’s
seminar. Paul Walden—for indeed it was he—was 92 at the
time. He overheard the remark and replied, scathingly, “Es ist
nicht der Käfig, sondern der kleine Vogel darin!” (It is not the
cage that matters, but the little bird inside!)
The chiral malic acid figures strongly in the original discovery
of inversion of configuration in the S
N
2 reaction. Malic acid is
sometimes called “apple acid” because of its high concentra-
tion in apples, nectarines, and some other fruits. In fact, it was
first isolated from apple juice as early as 1785. It functions as
a molecular carrier of the carbon dioxide absorbed by plants.
The CO
2
appears in the CH
2
COOH group of malic acid.
The principal actor in the early mechanistic work on the S
N
2
reaction was Paul Walden, a chemist born in 1863 in what is
PROBLEM 7.6 Explain the following change in rate for the S
N
2 reaction:
Rate for R CH
3
is much faster than for R (CH
3
)
3
C
R
O
O
-
+ CH
3
CH
2
O
I
U
R
O
O
O
CH
2
CH
3
+ I
-
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 275
The take-home lesson here is an important one: There is a strong preference for
inversion in the S
N
2 reaction. Indeed, there is no authenticated example of reten-
tion of configuration in this process, despite a great deal of searching by some very
clever people.
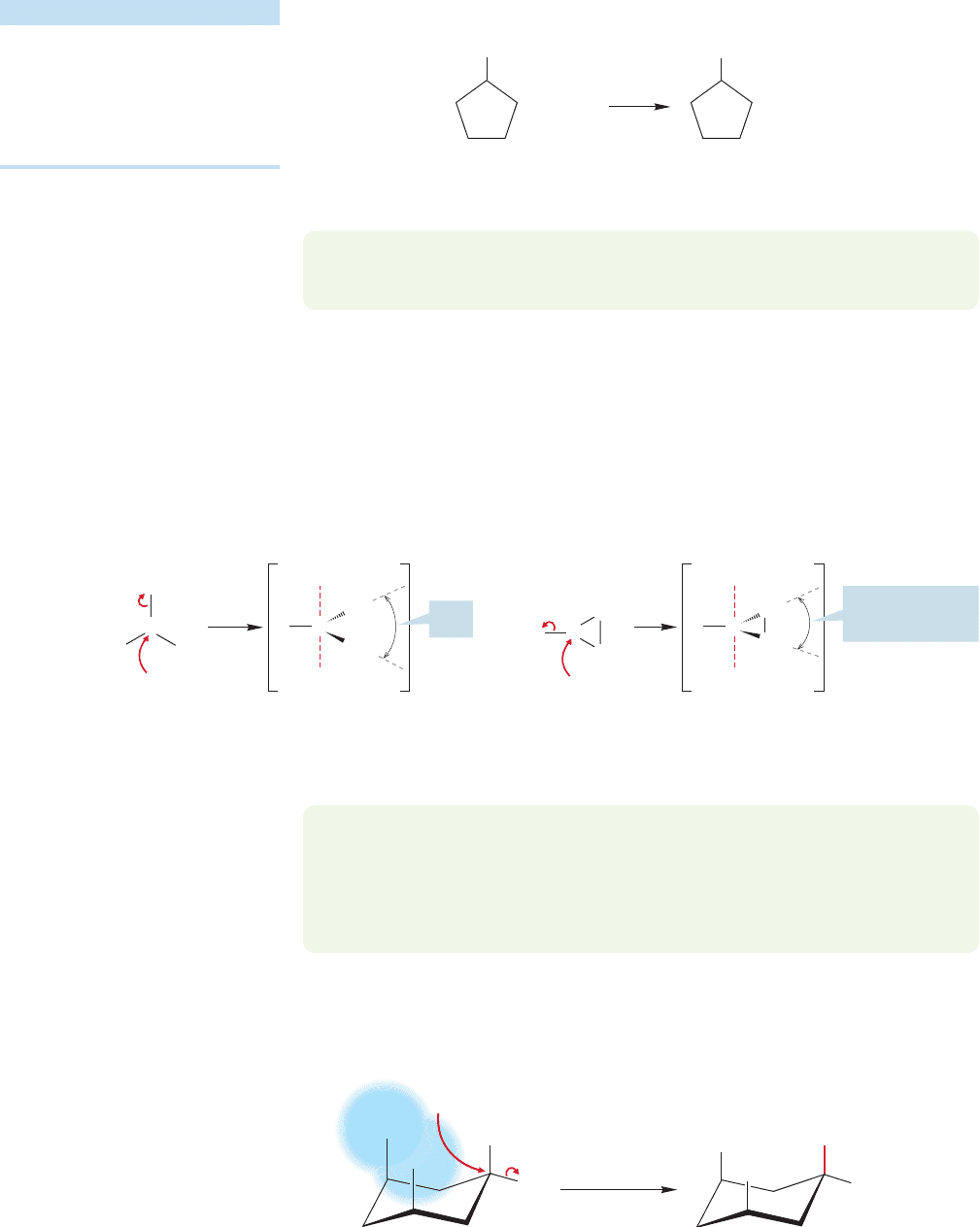
Now let’s examine the effects of structural change in the various participants in
the S
N
2 reaction, the R group, the nucleophile, the leaving group, and the solvent.
7.4c Effects of Substrate Structure: The R Group The structure of the
R group makes a huge difference in the rate of the S
N
2 reaction.We anticipated this
result when we mentioned earlier that the practically useful S
N
2 reaction was
restricted to methyl, primary, and secondary substrates.By implication, the rate of the
S
N
2 reaction with tertiary substrates is zero, or at least negligibly small (Table 7.1).
Why should this be? The simple answer seems to be that in a tertiary substrate the
rear of the bond is guarded by three alkyl groups, and the incoming nucleo-
phile can find no unhindered path along which to approach the fat lobe of σ
*
(Fig. 7.24). So the S
N
2 reaction is disfavored for tertiary substrates, for all of which
steric hindrance to the approaching nucleophile is prohibitively severe. Another
substitution mechanism, favorable for tertiary substrates, becomes possible. It is
called the S
N
1 reaction, and we will deal with its mechanism in Section 7.6.
If this steric argument is correct, secondary substrates should react more slowly
than primary substrates, and primary substrates should be slower than methyl com-
pounds. In general, this is the case (Table 7.1). In practice, the S
N
2 reaction is usu-
ally useful as long as there is at least one hydrogen attached to the same carbon as
the leaving group. Thus, the S
N
2 reaction works only for methyl, primary, and sec-
ondary substrates, all of which have at least one hydrogen attached to the carbon at
which the substitution is occurring.The small size of hydrogen opens a path at the
rear for the incoming nucleophile.
C
O
L
TABLE 7.1 Average Rates of
S
N
2 Substitution Reactions
for Different Groups
Average
R Relative Rate
1.3
CH
3
1
CH
3
CH
2
0.033
CH
3
CH
2
CH
2
0.013
(CH
3
)
2
CH 8.3 10
4
(CH
3
)
3
CCH
2
2 10
7
(CH
3
)
3
C~0
CH
2
P
CHCH
2
C
CH
3
H
3
C
H
3
C
L
Nu
..
–
No S
N
2 substitution
FIGURE 7.24 For tertiary substrates,
approach from the rear is hindered by
the alkyl groups, here all shown as
methyls.This steric effect makes the
S
N
2 reaction impossible.
This picture of the S
N
2 reaction, which emphasizes steric effects, allows us to
make a prediction. In principle, there must be some primary group so gigantic that
the S
N
2 reaction would be unsuccessful.
In practice, it is rather easy to find such groups. Even the neopentyl group,
(CH
3
)
3
CCH
2
, is large enough to slow the bimolecular displacement reaction severely,
because the tert-butyl group blocks the best pathway for rearside displacement of
the leaving group (Fig. 7.25; Table 7.1).
Very slow substitution
Neopentyl –
C
CH
3
CH
3
H
3
C
L
L
H
H
C
Nu
..
–
WEB 3D
FIGURE 7.25 Even neopentyl
compounds, in which a tert-butyl
group shields the rear of the
bond, are hindered enough so that
the rate of the S
N
2 reaction is
extremely slow.
C
O
L
276 CHAPTER 7 Substitution and Elimination Reactions
Br
..
..
..
–
+
Br
..
..
..
+
I
..
..
..
..
–
..
I
..
..
..
FIGURE 7.26 The S
N
2 reaction takes place in a
normal fashion with cyclic substrates.
–
..
..
..
..
..
Transition state
Br
..
..
..
H
3
C
CH
CH
3
–
H
CH
3
CH
3
C
Br
..
..
..
120⬚
I
..
..
..
Br
..
..
..
–
Transition state
Isopropyl bromide
Cyclopropyl bromide
Br
..
..
..
H
CH
2
CH
2
CH
2
CH
2
C
Wants 120⬚
but must be 60⬚!
CH
–
I
..
..
..
..
..
I
..
..
..
I
FIGURE 7.27 A comparison of S
N
2 transition states for isopropyl bromide and cyclopropyl bromide.
slow S
N
2
displacement
Br
..
..
..
..
..
..
..
–
+
..
..
–
H
Br
H
H
I
H
H
H
I
..
..
..
..
..
..
FIGURE 7.28 The slow S
N
2
displacement of bromide by iodide in
cyclohexyl bromide. Incoming iodide
is apparently blocked somewhat by
the axial carbon–hydrogen bonds.
TABLE 7.2 Relative
Reactivities of Cycloalkyl
Bromides in the S
N
2 Reaction
Compound Relative Rate
Cyclopropyl bromide 10
4
Cyclobutyl bromide 8 10
3
Cyclopentyl bromide 1.6
Cyclohexyl bromide 1 10
2
Isopropyl bromide 1.0
Why should small rings be so slow in the S
N
2 reaction? As with any question involv-
ing rates, we need to look at the structures of the transition states for the reaction to
find an answer.In the transition state,the substrate is hybridized approximately sp
2
,and
that requires bond angles close to 120°. The smaller the ring, the smaller are the
angles. For a three-membered ring, the optimal 120° must be squeezed
to 60°, and for a cyclobutane, squeezed to 90°.This contraction introduces severe angle
strain in the transition state, raises its energy, and slows the rate of reaction (Fig. 7.27).
C
O
C
O
C
PROBLEM 7.7 Use a ring compound to design a test of the stereochemistry of the
S
N
2 reaction.
PROBLEM 7.8 Wait! The argument just presented ignores angle strain in the start-
ing material. Cyclopropane itself is strained. Won’t that strain raise the energy
of the starting material and offset the energy raising of the transition state?
Comment. Hint: Consider angle strain in both starting material and transition
state—in which will it be more important?
In cyclohexane, the reaction rate is apparently slowed by steric interactions with
the axial hydrogens. There can be no great angle strain problem here (Fig. 7.28).
The S
N
2 reaction also takes place when ring compounds are used as the sub-
strates,although there are some interesting effects of ring size on the rate of the reac-
tion (Table 7.2; Fig. 7.26).
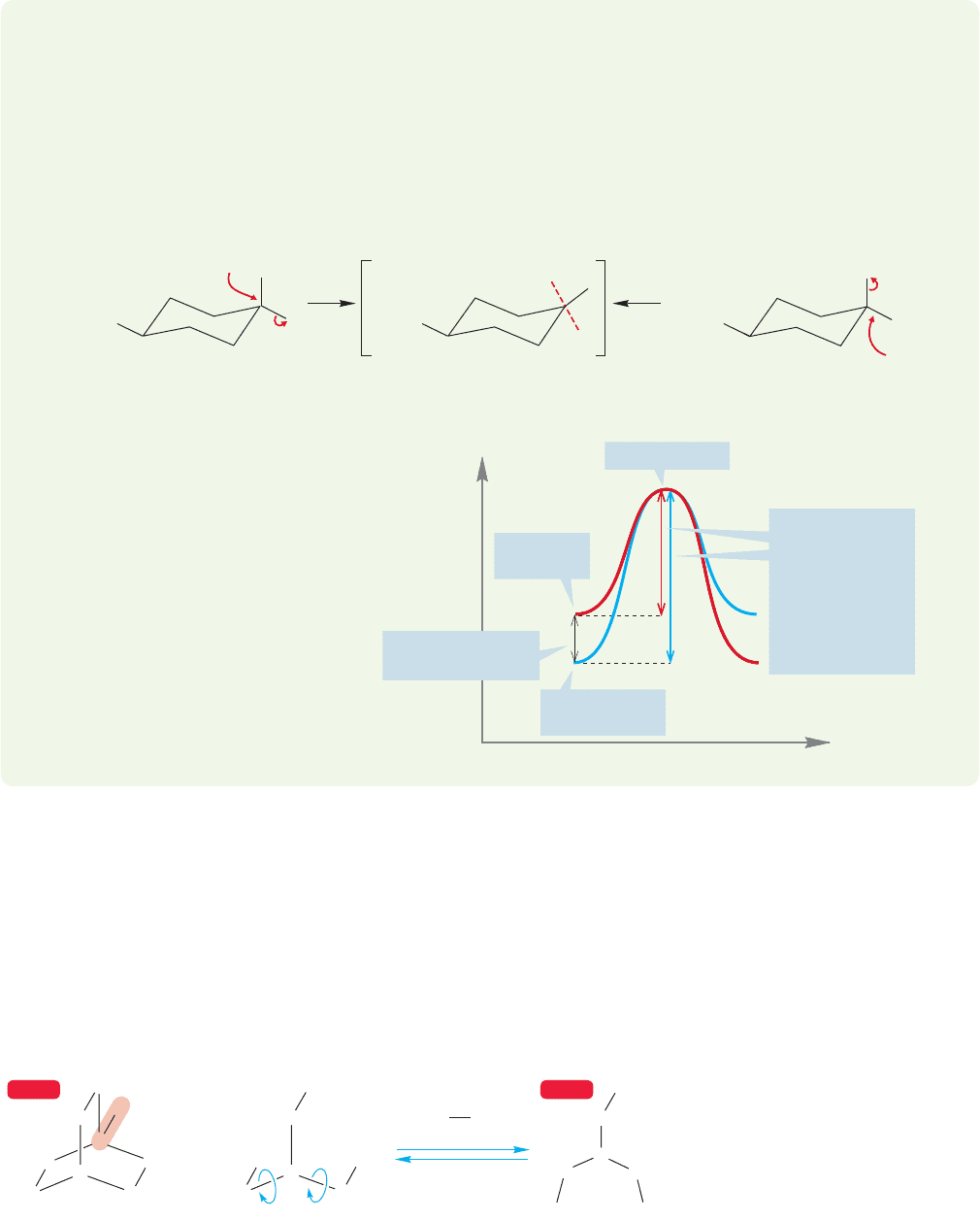
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 277
WORKED PROBLEM 7.9 The axial cyclohexyl iodide shown below reacts more
quickly in S
N
2 displacements than the equatorial cyclohexyl iodide. Draw the
transition states for displacement of iodide from axial cyclohexyl iodide by iodide
ion and for the analogous reaction of equatorial cyclohexyl iodide. What is the
relation between these transition states? Why does the axial iodo compound react
faster than the equatorial compound? Caution! Hard, tricky question.
ANSWER A tricky problem indeed. The transition states for the two reactions are
the same! There is no energy difference between the two transition states:
–
–
..
..
H
H
–
I
..
..
..
..
..
I
..
..
..
H
The transition state is the same for displacement of axial and equatorial cyclohexyl iodide
from 4-iodo-tert-butylcyclohexane
S
N
2
(CH
3
)
3
C
S
N
2
I
..
..
..
I
..
..
..
I
..
..
..
I
..
..
..
(CH
3
)
3
C (CH
3
)
3
C
Energy
Reaction progress
Energy increase
in starting material
Equatorial iodo
compound
Axial iodo
compound
Transition state
Activation energy
for the axial
compound (red
arrow) is lower
than that for the
equatorial
compound
(blue arrow)
Therefore, the reason that axial cyclo-
hexyl iodide reacts more quickly than
equatorial cyclohexyl iodide cannot lie
in the transition state energies.
However, the axial iodo group raises
the energy of the starting material,
thus lowering the activation energy.
7.4d Effect of the Nucleophile Some displacing agents are more effective
than others.They are better players in the competition for the carbon 2p orbital,the
Lewis acid. In this section, we examine what makes a good nucleophile, that is,
what makes a powerful displacing agent.
Remember: Size can be important. We have already seen how the S
N
2 reaction
can be slowed,or even stopped altogether,by large R groups (Fig.7.25). Presumably,
large nucleophiles will also have a difficult time in getting close enough to the sub-
strate to overlap effectively with σ
*
(Problem 7.6, p. 275). Figure 7.29 shows two
nucleophiles, carefully chosen to minimize all differences except size.
C
C
bond rotations
H
2
C
H
H
2
C
N
CH
2
CH
2
C
H
2
C
CH
2
H
2
C
Azabicyclooctane Triethylamine
CH
3
H
2
C
CH
3
..
H
3
C
H
2
C
N
CH
2
CH
3
..
H
2
C
H
3
C
N
CH
2
..
CH
3
WEB 3D WEB 3D
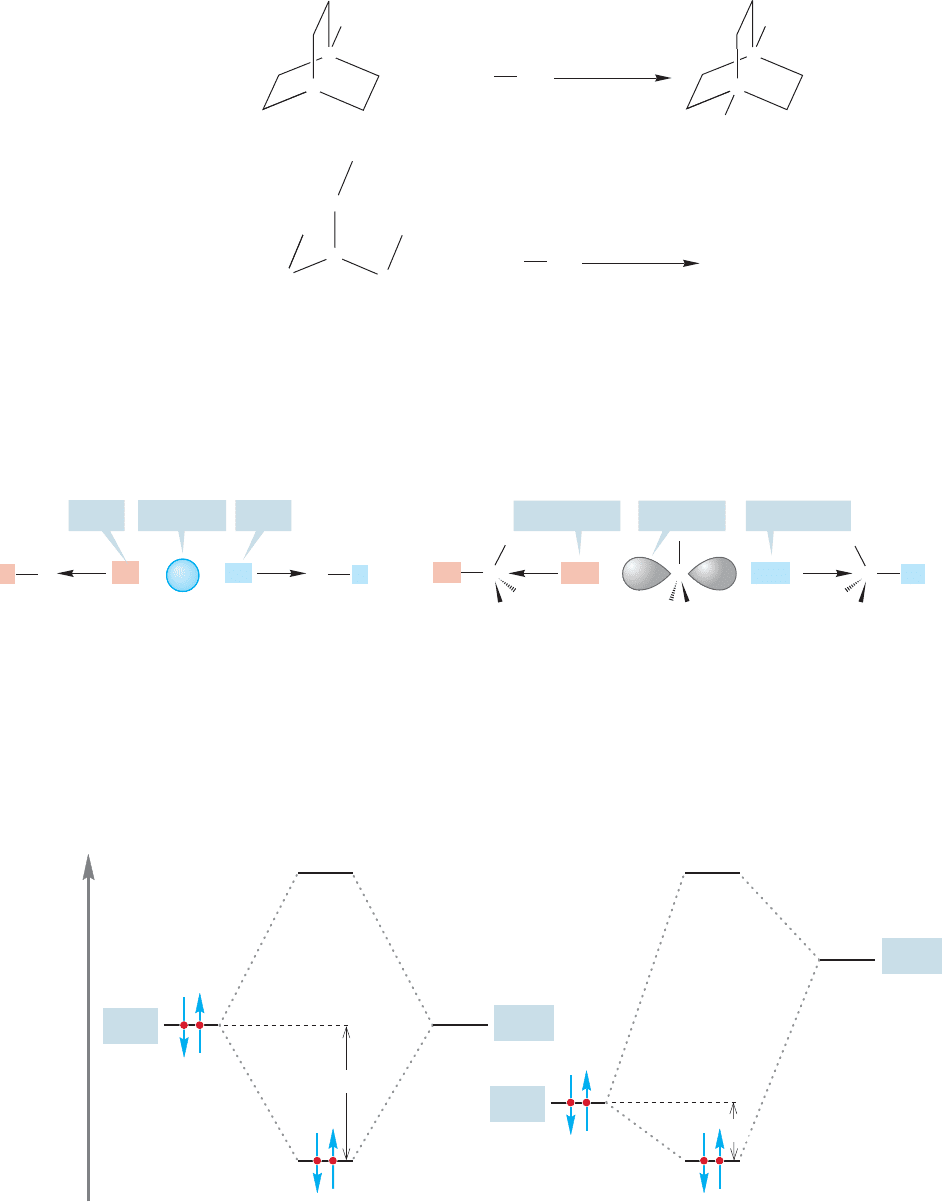
FIGURE 7.29 In both azabicyclooctane
and triethylamine, the nucleophilic
nitrogen atom is flanked by three two-
carbon chains. In the bicyclic cage
compound azabicyclooctane, they are
tied back by the CH group shown in
red and are not free to rotate. In
triethylamine, they are free to rotate
and effectively increase the bulk of the
nucleophile.
The bicyclic molecule, in which the three ethyl-like groups are tied back out of
the way, is a more effective displacing agent than is triethylamine,in which the three
278 CHAPTER 7 Substitution and Elimination Reactions
–
..
..
..
..
25 ⬚C
nitrobenzene
S
N
2
+
+
+
+
H
H
2
C
N
CH
2
CH
3
CH
3
H
3
C
CH
3
CH
2
CH
2
CH
3
..
I
25 ⬚C
nitrobenzene
S
N
2
Relative rate = 1
Relative
rate = 252
+
CH
3
CH
2
(CH
3
CH
2
)
4
N
+
I
..
..
..
..
..
..
–
I
..
..
..
..
H
2
C
I
H
C
N
..
N
C
FIGURE 7.30 The tied-back bicyclic
compound reacts faster with ethyl
iodide than does triethylamine,
which is effectively the larger
compound.The larger nucleophile
has a more difficult time in attacking
the rear of the carbon–iodine bond
in ethyl iodide.
Nucleophilicity: The competition between two nucleophiles for an
empty carbon 2p orbital
C
CC
H
H
H
+
+
Basicity: The competition between two bases for
an empty hydrogen 1s orbital
1s Orbital Base
2p OrbitalNucleophile
Nucleophile
Nu
..
Nu
Nu
–
Nu
..
–
–
..
BB
Base
B
–
..
B
FIGURE 7.31 Brønsted basicity and nucleophilicity are different, but related, phenomena.
ethyl groups are freely rotating.Triethylamine is effectively larger than the cage com-
pound in which the alkyl groups are tied back (Fig. 7.30).
Energy
Stabilization
Empty
Filled
Empty
Filled
Stabilization
FIGURE 7.32 Stabilization is greater when filled and empty orbitals of equal or nearly equal energy interact
than it is when orbitals that differ greatly in energy interact.The further apart two orbitals are in energy, the
smaller is the stabilization resulting from their overlap.
Actually, it is simple to refine our discussion of good nucleophiles.What do we mean
by “a good competitor for a carbon 2p orbital”? We are talking about the overlap of a
filled orbital and an empty orbital, and the resulting stabilization is a measure of how
strong the interaction is. Remember: The strongest orbital interactions, and hence the
greatest stabilizations, come from the overlap of orbitals close in energy (Fig. 7.32).
Nucleophilicity,Lewis basicity, is a measure of how well a nucleophile competes
for an empty carbon 2p orbital, and Brønsted basicity is a measure of how well a
base competes for an empty hydrogen 1s orbital. One would expect there to be a
general correlation of nucleophilicity with base strength although the two are not
exactly the same thing, because 1s and 2p orbitals are different in energy and shape.
Nevertheless, basicity and nucleophilicity are related phenomena (Fig. 7.31).
