Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 279
TABLE 7.3 Relative Nucleophilicities of Some Common Species
Relative
Species Name Nucleophilicity
Excellent Nucleophiles
NC
Cyanide 126,000
HS
Mercaptide 126,000
I
Iodide 80,000
Good Nucleophiles
HO
Hydroxide 16,000
Br
Bromide 10,000
N
3
Azide 8,000
NH
3
Ammonia 8,000
NO
2
Nitrite 5,000
Fair Nucleophiles
Cl
Chloride 1,000
CH
3
COO
Acetate 630
F
Fluoride 80
CH
3
OH Methyl alcohol 1
H
2
O Water 1
So we can anticipate that energy match-
ing between the orbital on the nucleo-
phile and an empty carbon orbital
will be important in determining
nucleophilicity.
Table 7.3 and Figure 7.33 show
some nucleophiles segregated into gen-
eral categories. It is not possible to do
much better than this, because nucleo-
philicity is a property that depends on
the reaction partner. A good nucleophile
with respect to carbon may or may not
be a good nucleophile with respect to
displacement on some other atom.
Energy matching is critical in the reac-
tion. If we change the reaction partner,
and thus the energy of the orbital
involved, we change the stabilization
involved as well (Fig. 7.32).
NH
2
> NH
3
–
SH
> SH
2
–
OH
> OH
2
–
NH
2
> OR > OH
–
––
H
2
Se > H
2
S > H
2
O
R
3
P
> R
3
N
I
–
> Br
–
> Cl
–
> F
–
Based on charge Based on electronegativity
FIGURE 7.33 Some relative
nucleophilicities. Be careful!
Nucleophilicity (Lewis basicity) is a
hard-to-categorize quantity. Relative
nucleophilicity depends, for example,
on the identity of the reaction
partner, which is always a Lewis acid,
as well as the nature of the solvent.
Table 7.3 and Figure 7.33 reveal some spectacular exceptions to the “a good
Brønsted base is a good nucleophile” rule. Look at the halide ions, for example.
As we would expect from the pK
a
values of the conjugate acids, HX (where X is
a halide), the basicity order is F
Cl
Br
I
. The nucleophilicity order is
exactly opposite the basicity order (Fig. 7.34). Iodide is the weakest Brønsted base
but the strongest nucleophile. Fluoride is the strongest Brønsted base but the weakest
nucleophile. Why?
HI HBr HCl HF
–10 –9 –8 +3.2
I
–
Br
–
Cl
–
F
–
F
–
Cl
–
Br
–
I
–
Increasing pK
a
,
decreasing acidity
Increasing Brønsted
basicity in solution
Increasing nucleophilicity
in solution
FIGURE 7.34 For the halide ions in
solution, the orders of Brønsted
basicity and nucleophilicity are
opposite.
To answer this question, we need more data. It turns out that the effectiveness
of these (and other) nucleophiles depends on the so-often-neglected solvent. It is
easy to forget that most chemical reactions are run in “oceans” of solvent, and it is
280 CHAPTER 7 Substitution and Elimination Reactions
perhaps not too surprising that these oceans of other molecules are not always with-
out substantial effects on the reaction! Figure 7.35 examines rates for typical dis-
placement reactions by halides in solvents of different polarities and in the total
absence of solvent (the gas phase). Note that iodide is a particularly effective nucleo-
phile, relative to other halides, in water, a protic solvent. Recall our discussion in
Chapter 6 (p. 238): Protic solvents are polar solvents containing hydrogens (e.g., water
or alcohols). In polar solvents that do not contain hydroxylic hydrogen, such as ace-
tone, , the reactivity order is reversed. In the absence of solvent, in the
gas phase, the order is also reversed.
(CH
3
)
2
C
P
O
H
2
O
acetone
solvent
S
N
2
CH
3
CH
3
II
–
++
S
N
2
CH
3
CH
3
Br Br
–
++
gas phase
no solvent
S
N
2
CH
3
CH
3
Br Br
–
+
= halogens
+
–
–
–
X
X X
X X
X X
160 14 1 ---
---
---
15 11
<0.015 0.02 1
I Br
Relative Rates
Cl F
FIGURE 7.35 In the highly polar
and protic solvent water, iodide is
the best nucleophile (fastest
reacting). In the polar but aprotic
solvent (acetone), the
order of nucleophilicity is reversed.
In the gas phase, where there is no
solvent, fluoride emerges as the best
nucleophile! Read the table across,
not down.The dashed line means
“not measured.”
(CH
3
)
2
C
P
O
O
H
H
O
H
H
O
H
H
O
H
H
O
H
H
–
..
..
..
..
Cl
R
++
BrR
slower
––
..
..
..
..
Br
..
..
..
..
Cl R
..
..
..
Cl
..
..
R
+
R
H
H
O
O
H
H
O
H
H
O
H
H
–
..
..
..
..
I
–
..
..
..
..
I
+
Br
faster
–
..
..
..
..
Br
..
..
..
..
..
..
..
I R
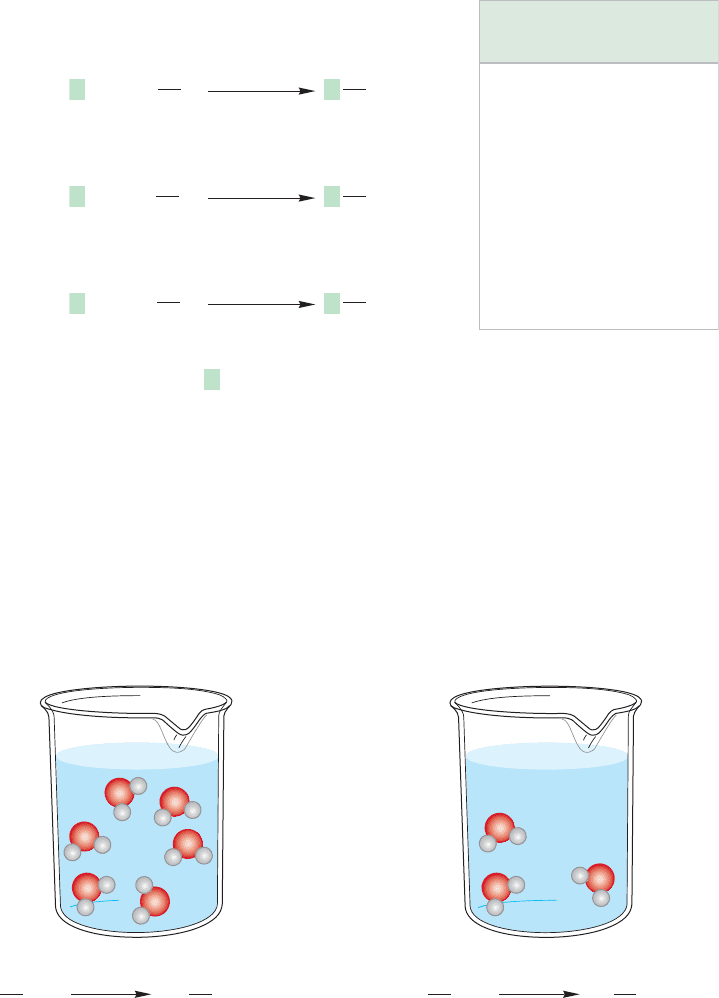
FIGURE 7.36 In a hydroxylated solvent, the highly solvated chloride ion reacts more slowly with than
the less encumbered iodide ion does.
R
O
Br
In a protic solvent, a halide can act as a nucleophile (Lewis base) and react with
the substrate through interaction with σ
*
of the bond. Alternatively, a halide
can behave as a Brønsted base by interacting with one of the protonic solvent mol-
ecules. Chloride and fluoride are by far the better bases and thus hydrogen bond to
protonic solvents much more strongly than iodide does. The halides become highly
solvated in protonic solvents and their relative sizes increase dramatically. They
become more encumbered, and therefore less effective nucleophiles. Iodide, the poorer
base, is less hydrogen bonded to protonic solvents and thus more free to do the S
N
2
reaction (Fig. 7.36).
C
O
X
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 281
As the polarity of the solvent decreases, fluoride becomes more competitive with
iodide. What’s the limit of this effect? A reaction with no solvent, of course. Pure
nucleophilicity, with no competing effects of solvent, can be examined only in the
absence of other bulk molecules. We can do that by running the reaction in the gas
phase.It is no trivial matter to examine reactions of ions in the gas phase, as the sta-
bility of ions depends heavily on solvation. Yet, since the 1990s such experiments
have become common. In the gas phase, fluoride, the better base, is also the better
nucleophile (Fig. 7.35).
7.4e Effect of the Leaving Group Many leaving groups depart as anions.
It seems obvious that the more stable is, the easier it will be to displace it.
The stability of the anion is related to how easily its conjugate acid, ,
dissociates and thus to the pK
a
of . Low-pK
a
acids (strong acids) are related
to good leaving groups, (weak bases). High pK
a
acids (weak acids) are related
to poor leaving groups (strong bases). Table 7.4 shows some good and poor leaving
groups and the pK
a
values of their conjugate acids, .
H
O
L + H
2
O
Z
U
H
3
O
+
+ L
:
-
H
O
L
L
:
-
H
O
L
H
O
LL
:
-
L
:
-
Nu
:
-
+ R
O
L
U
Nu
O
R + L
:
-
TABLE 7.4 Some Good and Poor Leaving Groups and Their Conjugate Acids
Acid pK
a
Leaving Group Name
Good Leaving Groups
HI 10
I Iodide
HBr 9
Br Bromide
HCl 8
Cl Chloride
HOSO
2
R 3
OSO
2
R Sulfonate
H
3
O
1.7 OH
2
Water
Poor Leaving Groups
HF 3.2
F Fluoride
H
2
S 7.0
SH Thiolate
HCN 9.4
CN Cyanide
H
2
O 15.7
OH Hydroxide
HOCH
2
CH
3
15.9
OCH
2
CH
3
Ethoxide
HOR 16–18
OR Alkoxide
7.4f How to Play “Change the Leaving Group” Many times it would be
convenient to convert an alcohol ( ), which is generally cheap and available
(in the chemical industry, those two terms are usually synonymous), into another
compound, . One naturally considers an S
N
2 substitution reaction of
(Fig. 7.37) with some nucleophile Nu .
:
-
R
O
OH
R
O
Nu
R
O
OH
OH
S
N
2
..
..
OH
..
..
..
+
?
–
Nu
..
Nu
RR
–
FIGURE 7.37 A hypothetical S
N
2
reaction converting an alcohol,
, into a new compound,
.Nu
O
R
R
O
OH
The problem is that
OH is an extraordinarily poor leaving group (Table 7.4).
The pK
a
of water is 15.7. Water is a rather weak acid, and its conjugate base, hydroxide
ion, is a strong base.There are several ways to circumvent the problem, the simplest
of which is to transfer a proton to the alcohol (protonate the alcohol) with a strong
282 CHAPTER 7 Substitution and Elimination Reactions
acid to give the conjugate acid of the alcohol, . Protonation converts the
leaving group from a very poor leaving group, hydroxide, into an excellent leaving
group, water (Fig. 7.38).
R
O
O
+
H
2
..
OH BRRB H
..
..
OH
2
..
+
++
–
FIGURE 7.38 The first step in the
reaction of an alcohol with the acid
is protonation of the Lewis
base oxygen.
H
O
B
A variant of the “change the leaving group” game is very closely related to the
reactions of alcohols in halogenated acids to give halides. It is called ether cleavage,
and it follows essentially the same mechanism. In strong halogenated acids, such as
HI or HBr, ethers can be cleaved to the corresponding alcohol and halide. In the
presence of excess HBr, the alcohols formed in this reaction are often converted into
the bromides (Fig. 7.40).
..
..
..
..
..
..
..
..
..
..
..
..
CH
3
CH
2
OH CH
3
CH
2
OH
2
Hydroxide
(a very poor leaving group)
Ethyl alcohol
Ethyl bromide
Water
(an excellent leaving group)
C
C
H Br Br
+
..
+
–
Br
Br
–
OH
2
H
H
3
C
H
H
H
S
N
2
CH
3
H
2
O
+
+
+
..
..
..
..
..
..
..
FIGURE 7.39 Playing “change the
leaving group” with ethyl alcohol.
PROBLEM 7.10 What will happen when an alcohol is treated with a strong base?
Think “simple.”
Water is a weak base. Its conjugate acid, H
3
O
, is a strong acid (pK
a
−1.7).
Therefore, water is easily displaced by a good nucleophile. In the reaction of an alco-
hol with HBr, the strongest available nucleophile is Br
, the conjugate base of the
HBr used to protonate the alcohol. So, if we want to convert an alcohol into a bro-
mide we need only treat it with HBr. The reaction and its mechanism are shown for
the conversion of ethyl alcohol into ethyl bromide in Figure 7.39. Note especially
the technique of changing the leaving group from the very poor hydroxide to the
excellent water.
+
S
N
2
..
..
..
..
O
HBr
..
..
..
..
Br
excess 47% HBr
8 h
..
..
O
H
..
OH
..
..
Br
..
..
..
Br
..
..
..
..
..
+
–
HBr
FIGURE 7.40 The mechanism of the
cleavage of ethers by strong haloacids.
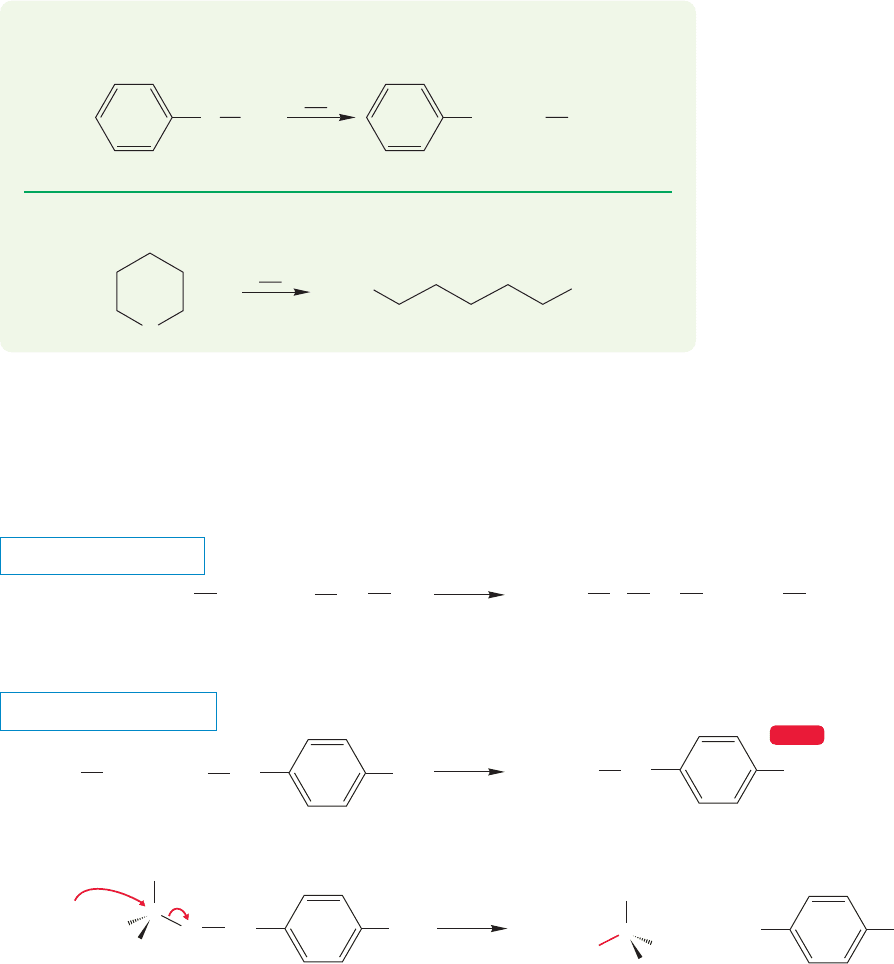
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 283
In this reaction, the ether oxygen is first protonated, turning the potential leav-
ing group in an S
N
2 reaction from alkoxide,
OR, into the much more easily dis-
placed alcohol, HOR. The displacement itself is accomplished by the halide ion
formed when the ether is protonated. This reaction is quite analogous to the reac-
tion of alcohols with haloacids.The halide must be a nucleophile strong enough to
do the displacement, and the reaction is subject to the limitations of any S
N
2 reac-
tion (Fig. 7.40).
PROBLEM 7.11 Clearly, an unsymmetrical ether can cleave in two ways. Yet often
only one path is followed. Explain the specificity shown in the following reaction.
PROBLEM 7.12 Provide a mechanism for the following reaction:
I
I
O
excess
H I
CH
3
OH +OCH
3
I
H I
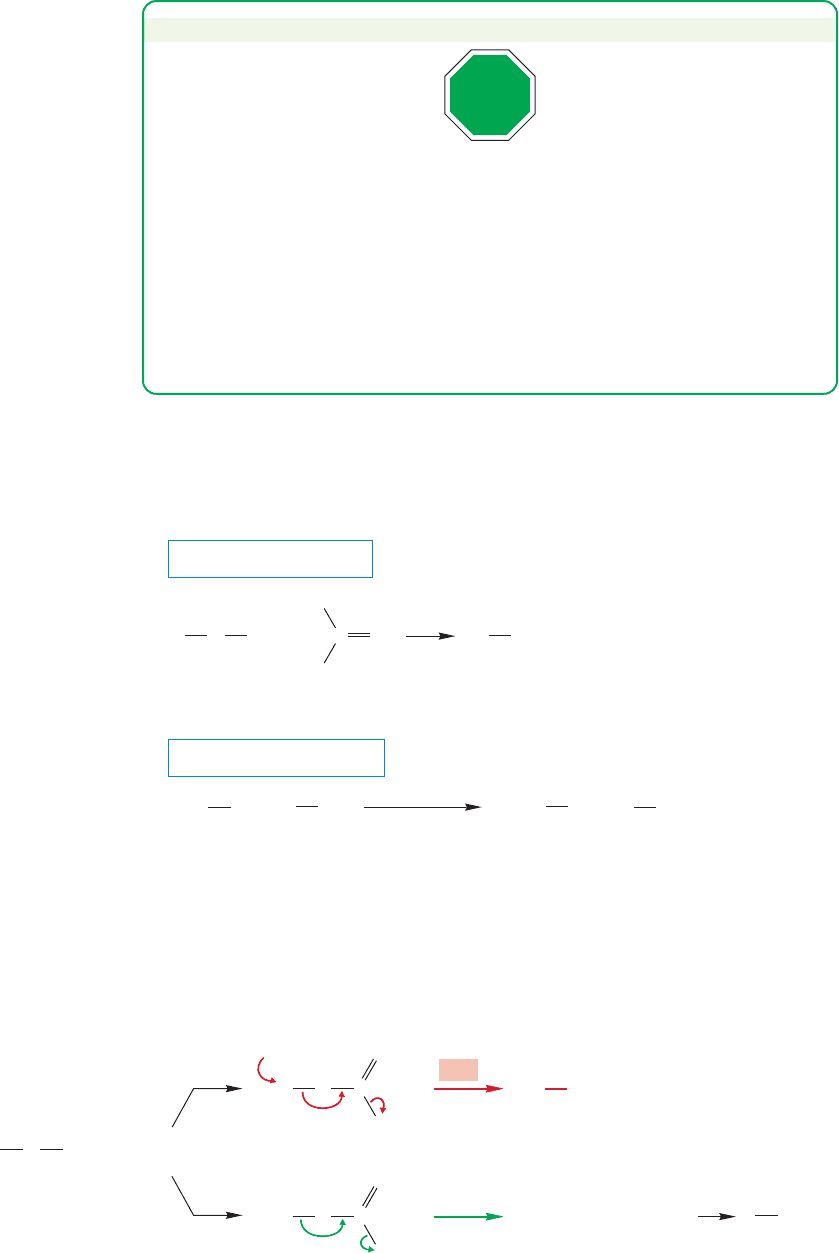
Another technique for changing the leaving group uses a two-step process in
which the alcohol is first converted into a sulfonate. Sulfonates are excellent leav-
ing groups (Table 7.4) and can be displaced by all manner of nucleophiles to give
desirable products (Fig. 7.41).
THE GENERAL CASE
A SPECIFIC EXAMPLE
+
+
+
+
An ethyl sulfonate
Ethyl alcohol
Ethyl tosylate
(an ethyl sulfonate)
Tosylate
(an excellent leavin
g group)
C
–
C
CH
3
CH
2
O
CH
3
CH
2
OH
Cl SO
2
SO
2
Cl SO
2
CH
3
O SO
2
SO
2
H
Cl
CH
3
CH
2
O
CH
3
CH
3
OSO
2
H
H
H
H
S
N
2
R
R
CH
3
CH
3
CH
3
–
CH
3
CH
2
O
CH
3
CH
2
O
..
..
..
..
..
CH
3
CH
2
OH
WEB 3D
FIGURE 7.41 The conversion of ethyl alcohol into another ethyl derivative. In this sequence of reactions the poor leaving
group OH is first converted into the good leaving group sulfonate.The leaving group is then displaced through an S
N
2
reaction with a nucleophile, here CH
3
CH
2
O
.
284 CHAPTER 7 Substitution and Elimination Reactions
PROBLEM SOLVING
The role of the tosylate group is essentially always to leave. It is a generic “good
leaving group.” Every time you see it in a problem, you are likely to have to break
the bond. There should be a little pull-down menu in your head that
says “leaving group!” when OTs appears.
There are other common “GO” signs, or clues, in many S
N
2 problems. Every
time you see “S
N
2” you must think “inversion”—all S
N
2 reactions go with
inversion. There are also good nucleophiles that appear often in S
N
2 problems.
Cyanide (NC
), mercaptan (HS
), and thiolate (RS
) are good examples of ions
that should make “good nucleophile” appear in your mental pull-down menu.
C
O
OTs
GO
THE GENERAL CASE
A SPECIFIC EXAMPLE
+
++
++
CH
3
S
H
Cl
HCl
SO
2
SO
2
CH
2
CH
2
OH
CH
3
S
CH
2
CH
2
100 ⬚C, 2 h
SOCl
2
CHCl
3
(80%)
HCl
R
R
S
Thionyl chloride
O
..
..
O
..
..
Cl
..
..
..
..
..
..
Cl
..
..
..
..
Cl
..
..
..
FIGURE 7.42 Treatment of an alcohol
with thionyl chloride results in the
formation of an alkyl chloride.
SOCl
2
Cl
SO
2
++
+
–
..
Cl
..
Cl
..
..
..
..
..
..
..
..
..
–
S
R
S
R
A chlorosulfite
ester
R
S
N
2
+
H
R
R
O
..
..
R
++
SO
2
Cl
..
..
..
Cl
..
..
..
..
–
Cl
..
..
..
..
..
..
..
Cl
O
..
..
O
..
..
O
..
..
..
O
..
..
(a)
(b)
FIGURE 7.43 Mechanisms of chloride
formation from reaction of an alcohol
with thionyl chloride. (a) The S
N
2
pathway. (b) Direct decomposition.
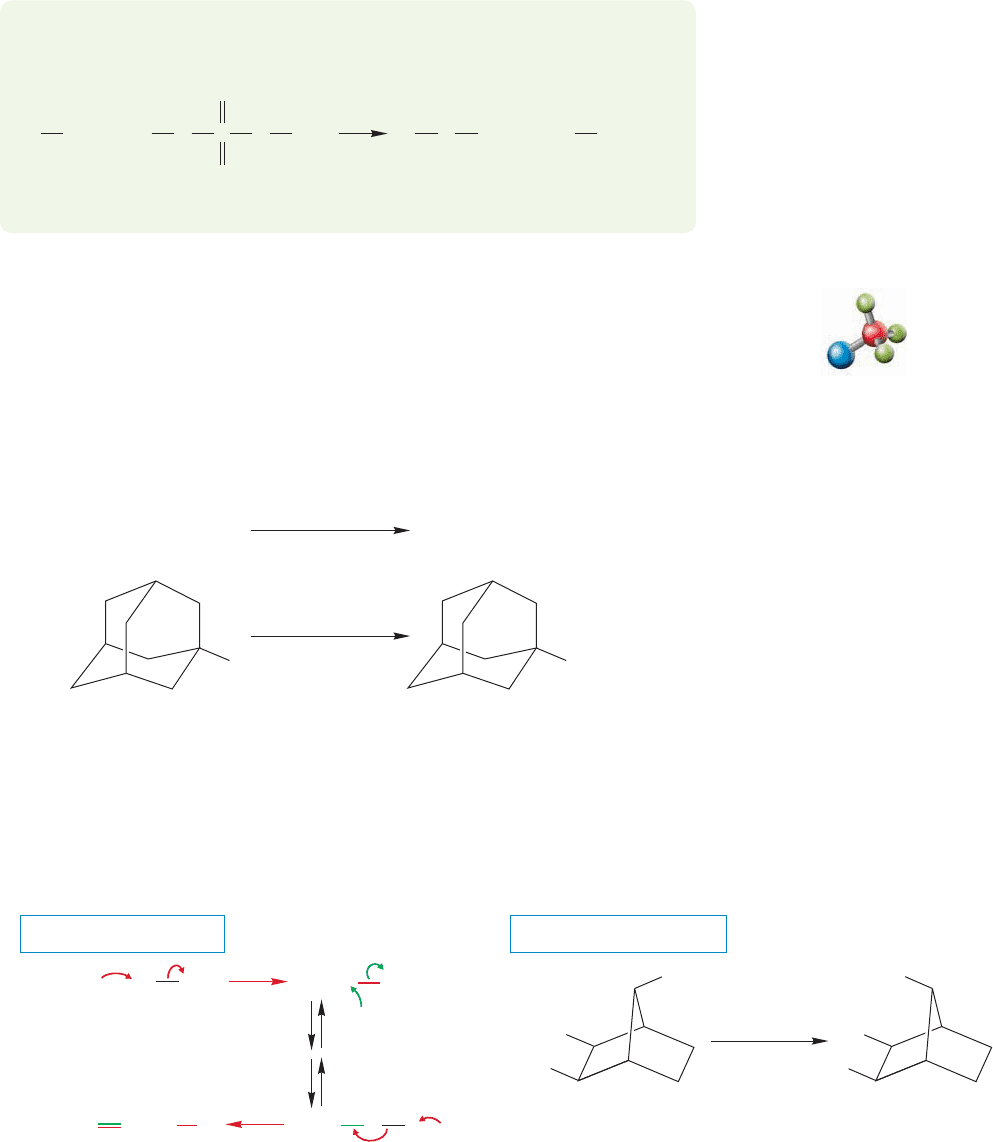
A variation on the sulfonate reaction of Figure 7.41 involves the treatment of an
alcohol with thionyl chloride (SOCl
2
).The end result is the conversion of an alco-
hol into a chloride (Fig. 7.42).
The initial product in this reaction is a chlorosulfite ester. Mechanisms of break-
down of the intermediate chlorosulfite ester involve both S
N
2 displacement by chlo-
ride ion to give the alkyl chloride, sulfur dioxide (SO
2
), and a chloride ion (Fig. 7.43a),
as well as direct decomposition to SO
2
and a pair of ions that recombine (Fig. 7.43b).
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 285
PROBLEM 7.13 Treatment of an alkoxide with dimethyl sulfate leads to methyl
ethers. Write a mechanism for this reaction.
+
+
–
R
CH
3
S
CH
3
RCH
3
SO
2
OCH
3
Dimethyl sulfate Meth
y
l etherAlkoxide
–
O
..
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
..
Halide formation
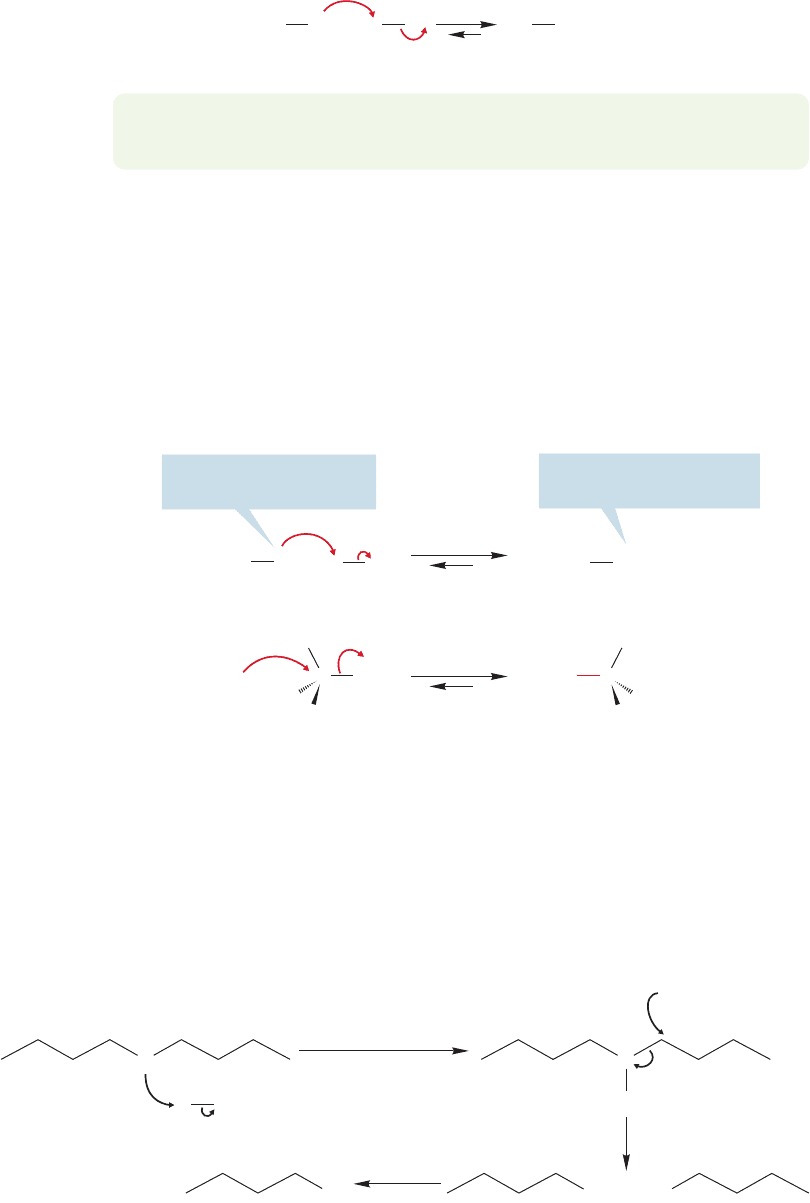
Several related reactions involve the use of phosphorus reagents. Alkyl halides
can be made from the treatment of an alcohol with phosphorus halides,PX
3
or PX
5
,
as shown in Figure 7.44. Many of these phosphorus-containing reagents cause
rearrangement, especially in secondary systems, and the stereochemical outcome,
retention or inversion,depends on solvent and other reaction conditions.Accordingly,
modifications have been worked out in recent years in order to avoid such stereo-
chemical problems.
OH Cl
(CH
3
)
2
CHCH
2
OH (CH
3
)
2
CHCH
2
Br + P(OH)
3
(58%)
PBr
3
–10 ⬚C, 4 h
PCl
5
CaCO
3
ether
0 ⬚C, 3 min
(100%)
FIGURE 7.44 Formations of alkyl
halides with phosphorus reagents.
For example, alcohols can be converted into the related bromides and chlorides
through treatment with triphenylphosphine and a carbon tetrahalide. The alkyl
halide is generally formed with inversion, and a rough mechanism involving a series
of S
N
2 reactions is sketched out in Figure 7.45.
THE GENERAL CASE
A SPECIFIC EXAMPLE
+
Cl CCl
3
..
..
CCl
3
..
..
(Ph)
3
P
Triphenylphosphine
(Ph)
3
P
(Ph)
3
P
CCl
4
25 ⬚C, 24 h
..
+
–
D
D
Cl
..
..
ROH
..
..
..
+
OR
..
H
..
(Ph)
3
P (Ph)
3
P
+
–
+
O RCl
..
..
..
..
Cl
..
..
..
OH
D
D
(~85%)
Cl
FIGURE 7.45 Halides are formed from the reaction of alcohols with the intermediate
produced from triphenylphosphine and a carbon tetrahalide.
In either case, the end product is the chloride. Once again, the strategy in this
reaction has been to convert a poor leaving group (
OH) into a good one (SO
2
and Cl
).
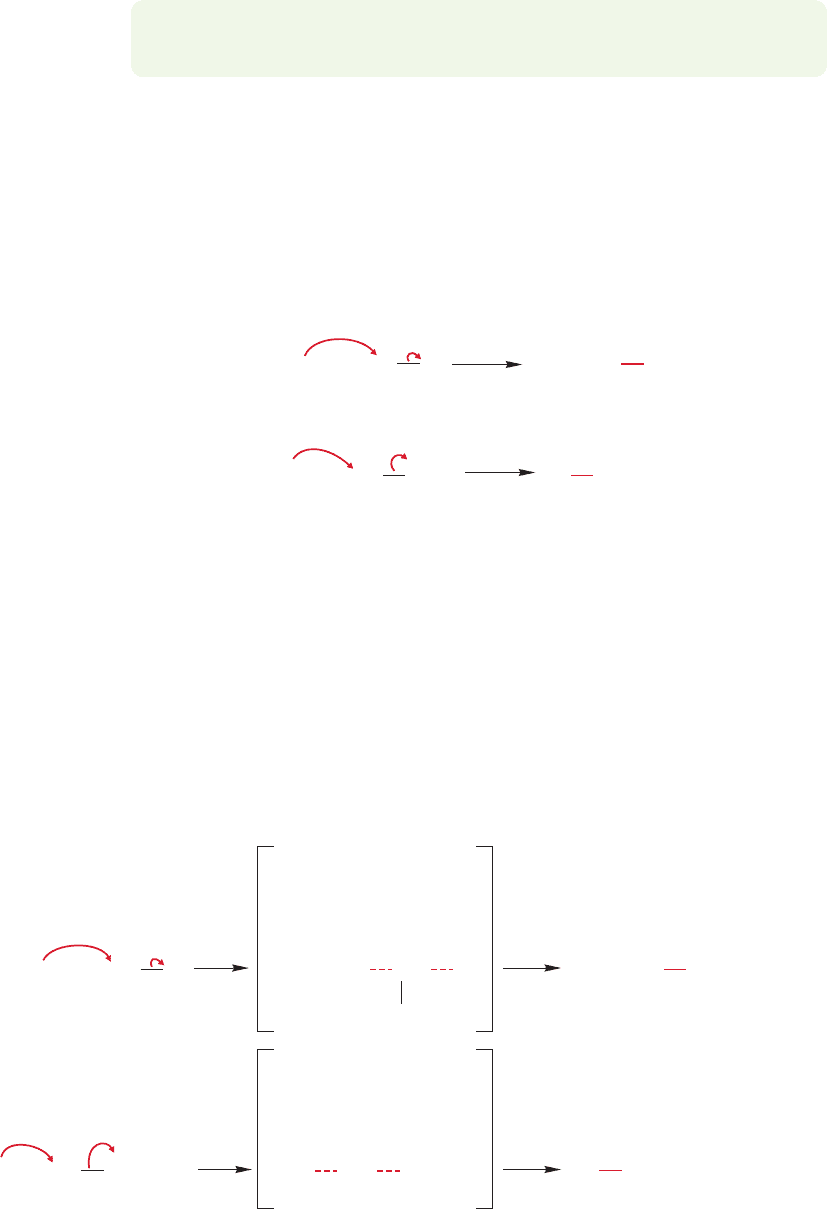
286 CHAPTER 7 Substitution and Elimination Reactions
+
(CH
3
CH
2
)
3
NCH
3
CH
2
CH
2
CH
3
..
(CH
3
CH
2
)
3
N
–
(a)
Rate increases as solvent polarity increases
+
I
..
..
..
I
..
..
..
..
+
..
..
..
..
..
..
..
..
CH
3
CH
3
OH
HO
–
S(CH
3
)
2
S(CH
3
)
2
(b)
+
Rate decreases as solvent polarity increases
FIGURE 7.46 Opposite effects of a
change in solvent polarity on the
rates of two S
N
2 reactions. Reaction a
goes faster when the solvent is made
more polar, whereas reaction b goes
more slowly.
7.4g Effect of Solvent At first, the behavior of the S
N
2 reaction as the
solvent polarity is changed is perplexing. As the solvent polarity is increased,
some S
N
2 reactions go faster, some slower. Two examples are shown in Figure
7.46. In the first reaction (a), a change to a more polar solvent results in a faster
reaction, but in the second reaction (b), the rate decreases when the solvent is
made more polar.
(a)
(CH
3
CH
2
)
3
N (CH
3
CH
2
)
3
N ICH
2
CH
2
CH
3
CH
3
CH
3
CH
2
I (CH
3
CH
2
)
3
N
–
..
..
+
–
+
+
δ
–
δ
+
In this transition state, a
partial positive charge is
developed on nitrogen and
a partial negative charge
on iodine
(b)
HO HO S(CH
3
)
2
S(CH
3
)
2
CH
3
HO CH
3
CH
3
S(CH
3
)
2
..
..
..
..
.. ..
..
..
..
+
δ
–
δ
+
In this transition state,
charge is dispersed. The
transition state is less polar
than the starting material
In the starting material
two full charges exist
Uncharged
starting materials
Charged products
Uncharged products
I
..
..
..
..
..
..
..
FIGURE 7.47 The transition state for reaction a is more polar than the starting material.The transition state for
reaction b is less polar than the starting material.
There is a way to attack almost every problem that poses a question having to
do with rates. The rate of a reaction is determined by the energy of the transition
state—the high point in energy as the reaction proceeds from starting material to
products. In order to solve a question involving rates, we must know the structure
of the transition state of the reaction.
Figure 7.47 shows the structures of the transition states for the two S
N
2
reactions of Figure 7.46. In reaction a, a more polar solvent will act to stabilize the
charged products and the polar, charge-separated transition state.There will be very
PROBLEM 7.14 Explain why sulfonates should give rather stable anions (weak
nucleophiles) and are therefore good leaving groups.
7.4 Substitution, Nucleophilic, Bimolecular: The S
N
2 Reaction 287
Reaction b is different. Here the starting materials bear full charges.This charge
is dispersed as the reaction goes on. A change to a more polar solvent will stabilize
the fully charged starting materials more than the transition state or products.
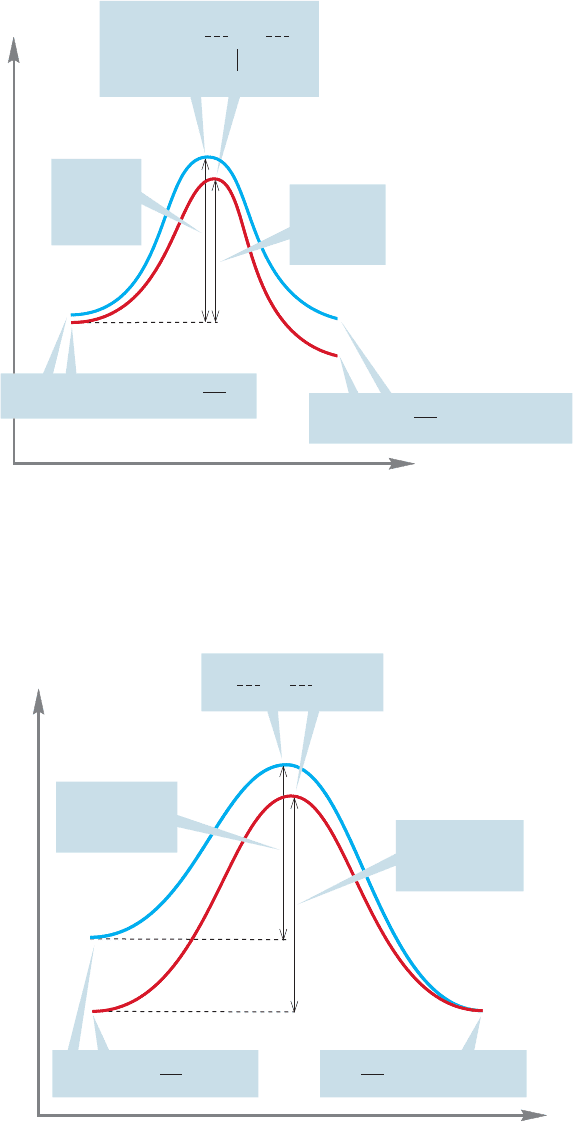
Therefore, the activation energy increases and the reaction slows (Fig. 7.49).
Energy
Reaction progress
(CH
3
CH
2
)
3
N ICH
2
CH
3
δ
–
δ
+
(CH
3
CH
2
)
3
NCH
3
CH
2
I
..
..
..
..
+
CH
2
CH
3
(CH
3
CH
2
)
3
N
–
I
..
..
..
..
+
+
Activation
energy in
more polar
solvent
Activation
energy in
less polar
solvent
FIGURE 7.48 The difference between
the energy of the starting materials
and the energy of the transition state
is smaller when the reaction is run in
a more polar solvent (red curve) and
larger when the reaction is run in a
less polar solvent (blue curve).The
result is a decreased activation energy
for the reaction and a faster rate.
little effect on the uncharged starting materials in reaction a. The energy diagram
in Figure 7.48 shows what this differential solvation does to the rate of this reac-
tion. The height of the transition state is decreased relative to the starting material
and the reaction goes faster. It takes less energy for the molecules to traverse the
barrier when the polarity of the solvent increases.
Energy
Reaction progress
HO S(CH
3
)
2
CH
3
δ
–
δ
+
–
+
HO CH
3
S(CH
3
)
2
..
....
..
+
S(CH
3
)
2
HO CH
3
..
..
..
..
+
Activation
energy in more
polar solvent
Activation
energy in less
polar solvent
FIGURE 7.49 The difference between
the energy of the starting materials
and the energy of the transition state
is larger when the reaction is run in a
more polar solvent (red curve) and
smaller when the reaction is run in a
less polar solvent (blue curve).The
result is an increased activation energy
for the reaction and a slower rate.
288 CHAPTER 7 Substitution and Elimination Reactions
Summary
The S
N
2 reaction—Substitution, Nucleophilic, bimolecular—is common for
methyl, primary, and secondary substrates. It is disfavored by steric hindrance in
either the substrate (the “R” group) or the entering nucleophile. It is favored by
a strong Lewis base (nucleophile) and a good leaving group.The response of the
S
N
2 reaction to changes in solvent polarity varies with the charge type of the reac-
tion. Each case must be examined individually. The S
N
2 reaction always involves
inversion of configuration at the center of substitution.
FIGURE 7.50 Replacement of the
hydrogens by an alkyl group
would hinder—or even eliminate—
hydrogen bonding. Thus, proper
association of the bases would no
longer be possible.
N
O
H
7.5 The S
N
2 Reaction in Biochemistry
As mentioned in Section 7.1, your vital biomolecules—DNA, for example—are
covered with nucleophiles, each of them ready to do the S
N
2 reaction if presented
with a suitable alkylating agent. Once that alkylation takes place, two
important changes are induced. First of all, the shape of the mol-
ecule is changed, and the critical fit, on which proper bioaction
depends, is altered. Second, alkylation often removes the possibility
for hydrogen bonding, and in turn that changes the ability of DNA
to associate properly with other molecules. As we will see in Chapter
23, DNA exists as pairs of associated “nitrogenous bases” and that
association is achieved through hydrogen bonding (Fig. 7.50).
Alkylation of one of the nucleophilic groups in DNA can disrupt base
pairing and lead to destructive mutations.
Remember the methyl transfer from S-adenosylmethionine (p. 142)? Heinz Floss
(b. 1934) and his co-workers have demonstrated that a similar transfer occurs through
an S
N
2 displacement. They did this experiment by showing that transfer takes place
with complete inversion of configuration—the hallmark of the S
N
2 reaction. How can
one show inversion in a simple methyl group, CH
3
? That’s not easy! Floss and his co-
workers used two isotopes of hydrogen, deuterium (D) and tritium (T), so that their
methyl was not CH
3
but CHDT.Use of a single enantiomer revealed the diagnostic inver-
sion in the transfer shown in Figure 7.51.The work involved in synthesizing that single
enantiomer and in analyzing the product was prodigious,but these prototypal techniques
have been borrowed several times in similar studies of biochemical transformations.
Note inversion
C
D
H
Modified
S-adenosylmethionine
C
D
H
T
S
R
_
+
NH
3
COO
_
O
“methyl”
transfer
S
N
2
T
O
N
H
N
H
+
FIGURE 7.51 Methyl transfer involves
an S
N
2 reaction with inversion.
Deoxyribose
C(1')
O
..
CH
3
O
..
..
..
N
N
..
N
Deoxyribose
C(1')
..
N
H
..
N
..
N
..
..
H
..
N
H
