Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

7.6 Substitution, Nucleophilic, Unimolecular: The S
N
1 Reaction 289
The S
N
2 reaction is by no means irrelevant to you—your life literally depends on
it happening properly, as in the example studied by Floss,and not in the wrong place—
as demonstrated by the sometimes lethal mutations induced by alkylating agents.
PROBLEM 7.15 Although tert-butyl bromide does not give tert-butyl alcohol on
reaction with hydroxide ion, an alkene product (C
4
H
8
) is produced. Suggest a
structure for the product and an arrow formalism for this bimolecular reaction.
7.6 Substitution, Nucleophilic, Unimolecular:
The S
N
1 Reaction
7.6a Rate Law tert-Butyl bromide does not undergo a substitution reaction when
treated with the good nucleophiles listed in Table 7.3. The rate of the second-order
S
N
2 displacement on tert-butyl bromide by hydroxide ion—or any nucleophile—is
effectively zero. Yet, tert-butyl bromide does react with the much less powerfully
nucleophilic molecule water to form the product of substitution, tert-butyl alcohol
(Fig. 7.52).
+
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
HO
–
H
3
C
CH
3
CH
3
Br
C
..
..
H
3
C
CH
3
CH
3
OHC
..
..
H
3
C
CH
3
CH
3
OHC
H
3
C
CH
3
CH
3
Br
HBr
C
+
Na
+
Br
H
2
O
..
Na
+
–
FIGURE 7.52 Despite the far greater
nucleophilicity of hydroxide ion, the
reaction of tert-butyl bromide with
water is successful in producing tert-
butyl alcohol, whereas the reaction
with hydroxide ion is not.
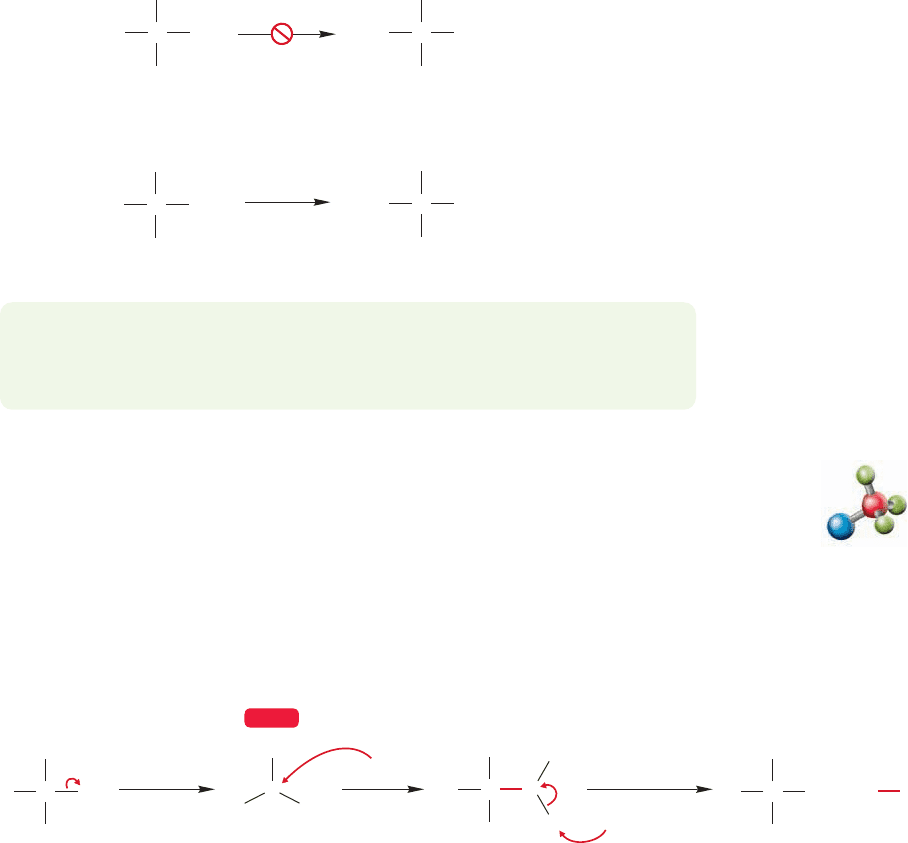
The first clue to what’s happening in Figure 7.52 comes from an examination of the
rate law for the reaction.It turns out that the formation of tert-butyl alcohol in this reac-
tion is a first-order process in which the concentration of added nucleophile is irrele-
vant to the rate.The rate law leads to the name Substitution,Nucleophilic,unimolecular,
or S
N
1 reaction, where k is the rate constant, a fundamental constant of the reaction.
Rate ν k [tert-butyl bromide]
At this point, a reasonable mechanistic hypothesis might simply view the S
N
1
reaction as beginning with the unimolecular ionization shown in Figure 7.53.
H
+
Br
..
..
..
..
..
..
..
..
..
..
ionization
(slow)
addition
(fast)
deprotonation
OHC
R
R
R
..
..
Br
R
R
R
RR
R
C
..
C
C
+
+
–
Br
..
..
..
..
..
–
BrH
..
..
..
OR
R
R
H
or
H
2
O
..
H
2
O
..
WEB 3D
FIGURE 7.53 A schematic mechanism for the S
N
1 reaction. A slow ionization is followed by a relatively
fast capture of the carbocation by a nucleophile, water in this case.
Unimolecular nucleophilic substitution: S
N
1
290 CHAPTER 7 Substitution and Elimination Reactions
The reaction continues as the carbocation is captured by the solvent, often water, as
in Figure 7.53, in what is called a solvolysis reaction. Solvolysis simply means that
the solvent, here water, plays the role of nucleophile in the reaction. Next, any base
present, here likely to be bromide or water, can remove a proton (deprotonate the
protonated alcohol) to give the alcohol itself (Fig. 7.53).
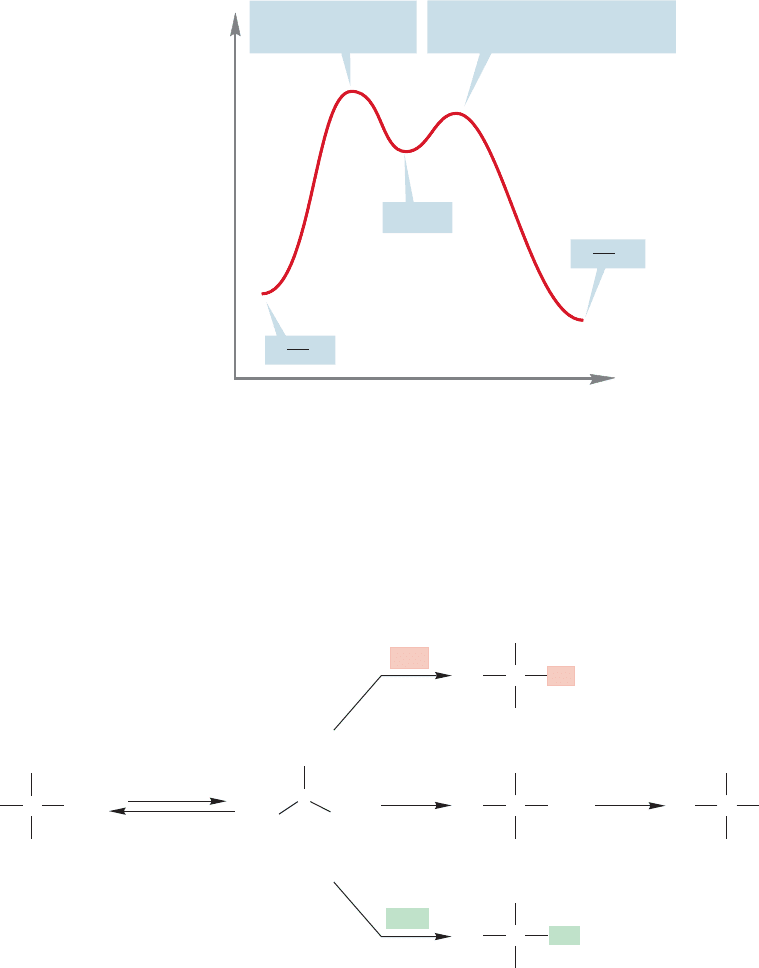
We can describe the S
N
1 reaction with an energy diagram, as we did for the S
N
2
reaction (pp. 273–274). The S
N
1 diagram is more complicated because of the for-
mation of the intermediate carbocation.The presence of the intermediate means that
there will be two transition states separating the starting material and the product.
The transition state for the slow step, the initial endothermic ionization, is higher
than the transition state for the second, faster step, the capture of the ion by the
nucleophile (Fig. 7.54).
This second step determines the structure of the product but not the rate of the reac-
tion. If there are several nucleophiles present, they will compete with one another in
the capture of the carbocation to produce different products, but the rate of the reac-
tion will not be affected (Fig. 7.55). The nucleophile determines the structures of the
products, but not the rate of their formation. Note that the reverse of the ionization,
recapture of the carbocation by bromide, is nothing more than another example of a
reaction of this intermediate with a nucleophile.
Energy
Reaction progress
Transition state for
ionization—higher
Transition state for ion capture
by nucleophile—lower
+
Br
–
Br
R
R
R
Nu
FIGURE 7.54 A generic energy
diagram for the S
N
1 reaction.
Notice that the transition state for
the slow step in the reaction—the
ionization—is higher in energy than
the transition state for the capture of
the ion by a nucleophile.
FIGURE 7.55 Capture of the carbocation by four
nucleophiles (
Nu,
Nu′,
Br, and H
2
O).The
nucleophiles have no effect on the rate of formation of
the carbocation, which is the slow step in the reaction.
::
..
..
..
Br
..
..
..
..
..
Br
CR
R
R
C
C
C
C
H
2
O
..
..
..
slow
ionization
fast
fast
fast
+
–
OH
2
+
..
C
OH
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
Nu
Nu´
Nu
..
–
Nu´
..
–
7.6 Substitution, Nucleophilic, Unimolecular: The S
N
1 Reaction 291
We will now see if this simple hypothesis allows a reasonable prediction of the
stereochemical course of the reaction.
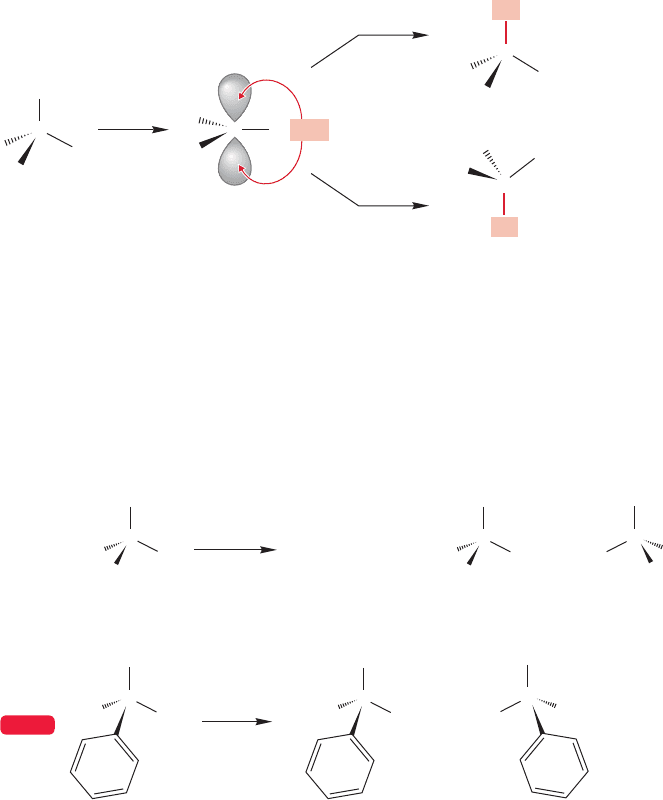
7.6b Stereochemistry of the S
N
1 Reaction If the S
N
1 reaction were as
straightforward as suggested by Figures 7.53–7.55, what would be expected if an
optically active starting material were used? The crucial intermediate in the reaction
is the planar carbocation, an achiral molecule! Once the free, planar carbocation is
formed, optical activity is inevitably lost. Addition of the nucleophile must take
place at both faces of the cation, forming equal amounts of (R) and (S) product.
This simple mechanism demands racemization, as Figure 7.56 shows.
Sometimes racemization is the actual result, and there is 50% inversion and 50%
retention in the product.Usually, however the stereochemical results are not so clean,
and an excess of inversion is found. For example, the two optically active molecules
of Figure 7.57 give 21 and 18% excess inversion in the S
N
1 reaction with water.
Br
..
..
..
Chiral
50% Retention
50% Inversion
Achiral, planar carbocation
Racemic mixture
C*
C
1
1
2
2
3
3
C
1
2
3
C
1
2
3
+
Nu
Nu
Nu
..
–
FIGURE 7.56 Loss of optical activity
occurs as soon as the free, planar
carbocation is formed. Addition of
any nucleophile will occur equally at
the two available lobes of the 2p
orbital to give a racemic mixture of
products.
CH
3
CH
3
CH
2
(CH
3
)
2
CHCH
2
CH
2
CH
2
Cl
H
2
O
(39.5% retention) (60.5% inversion)
(41% retention) (59% inversion)
C
CH
3
H
Cl
C
CH
3
OH
HO
C
CH
3
C
CH
3
CH
3
CH
2
CH
2
CH
3
(CH
3
)
2
CHCH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH(CH
3
)
2
OH
C
CH
3
C
H
2
O
HO
+
+
acetone
H
H
WEB 3D
FIGURE 7.57 Incomplete
racemization is often found in S
N
1
reactions. An excess of inversion is
generally found.
Why is the product not racemic, and why is excess inversion (as opposed to reten-
tion) observed? These two questions are vitally important, as satisfactory answers
must be found if we are not to be forced to abandon the proposed mechanism for
the S
N
1 reaction.The answers turn out to be simple. In the mechanism outlined in
Figures 7.53–7.55, we assumed that the leaving group had diffused away from the
carbocation, leaving behind a symmetrical ion. What if reaction with solvent occurs
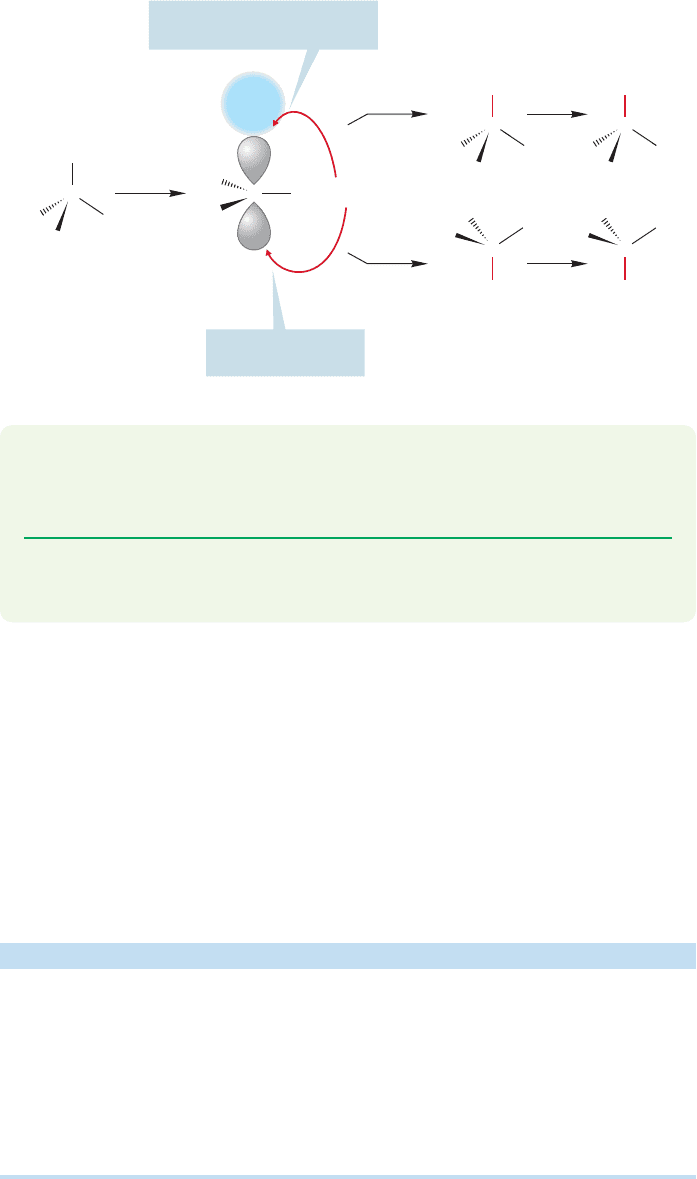
292 CHAPTER 7 Substitution and Elimination Reactions
before the leaving group diffuses completely away? What if reaction occurs with the
pair of ions shown in Figure 7.58? The leaving group guards the side of the carbo-
cation from which it departed, and makes addition by solvent water more difficult
at this “retention” side. The result is usually an excess of inversion.
7.6c Effects of Substrate Structure: The R Group The rate of the S
N
1
reaction is much greater for tertiary substrates than for secondary substrates, and
simple primary alkyl and methyl halides do not react at all by the S
N
1 process. Our
mechanistic hypothesis shows us why. If carbocation formation is the key step, only
those compounds that can form relatively stable ions can react in S
N
1 fashion.
Modern measurements show that the stability of carbocations increases with
substitution (p. 137). Table 7.5 shows the heats of formation of carbocations in
the gas phase. Remember: The more positive its heat of formation, the less stable
a species is (p. 115). We can’t make too much of the quantitative data in Table 7.5,
Br
..
..
..
+
+
C* C
3
2
1
2
H
2
O
OH
2
3
1
..
..
+
OH
2
..
C
C
3
3
2
1
3
2
1
<50% Retention
>50% Inversion
Chiral,
optically
active
OH
..
C
3
2
1
OH
..
..
C
2
1
..
..
Inversion pathway
is unhindered
Retention pathway is
hindered by the bromide ion
Br
..
..
..
..
–
FIGURE 7.58 Until the leaving group,
here bromide ion, completely diffuses
away, it shields the carbocation along
the retention pathway, and this results
in an excess of inversion in most S
N
1
reactions.
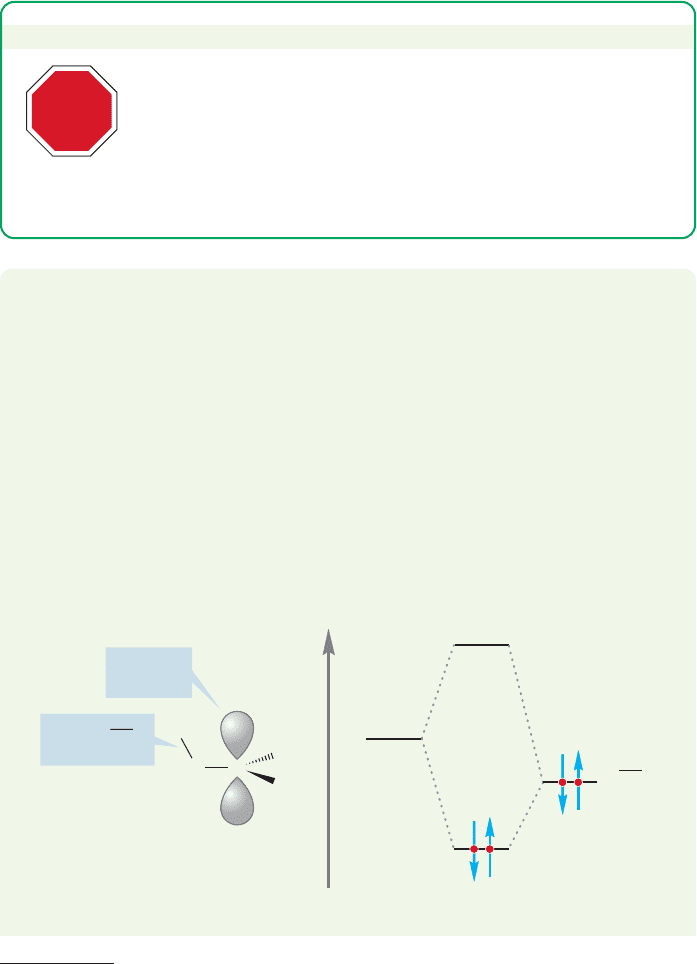
TABLE 7.5 Heats of Formation for Some Carbocations
Entry Cation Substitution (kcal/mol)
1
CH
3
Methyl 261.3 Least stable
2
CH
2
CH
3
Primary 215.6
3
CH
2
CH
2
CH
3
Primary 211
4 Secondary 190.9
5
CH
2
CH
2
CH
2
CH
3
Primary 203
6 Secondary 183
7(CH
3
)
3
C
Tertiary 165.8 Most stable
H
3
CC
+
HCH
2
CH
3
(CH
3
)
2
C
+
H
≤H °
f
PROBLEM 7.16 The second reaction of Figure 7.57 shows an S
N
1 reaction of a sec-
ondary halide. Explain why the S
N
1 reaction should be especially favorable for
this secondary halide.
PROBLEM 7.17 Consider the role of solvent on the stereochemistry of the S
N
1
reaction shown in Figure 7.58. A more polar solvent favors racemization. Why?
7.6 Substitution, Nucleophilic, Unimolecular: The S
N
1 Reaction 293
because these gas-phase measurements cannot take into account the effects of sol-
vent, which are very large indeed. Nonetheless, the data do give a good qualita-
tive picture of carbocation stability: Entries 5, 6, and 7 show that tertiary
carbocations (three alkyl groups) are more stable than secondary ones (two alkyl
groups), and secondary carbocations are more stable than primary ones (one alkyl
group). The methyl cation (
CH
3
), with no alkyl groups, is the least stable of all.
Do not confuse these relative stabilities with absolute stabilities. Carbocations are
almost all very unstable species. A small atom such as carbon does not bear a pos-
itive charge with any grace, and a carbocation will react with available Lewis bases
(e.g., halide ion) very quickly. Do not say, “The tert-butyl cation is very stable,”
but rather, “For a carbocation, the tert-butyl cation is quite stable.”
PROBLEM SOLVING
WORKED PROBLEM 7.18 We have again avoided (p. 138) a detailed discussion of
why more substituted carbocations are more stable than less substituted ones.
4
Nevertheless you can now see the outlines of why this is so. Draw a three-
dimensional picture of the ethyl cation. (Remember: The positively charged
carbon and the three surrounding atoms are coplanar—this part of the ethyl
cation is flat.) Now consider the overlap of one of the carbon–hydrogen bonds
of the methyl group with the empty 2p
z
orbital. Is this interaction stabi-
lizing? Why?
ANSWER Interactions between filled and empty orbitals are stabilizing. This fun-
damental tenet of chemistry can be applied in this case. If the filled orbital of the
carbon–hydrogen bond overlaps with the empty 2p orbital of the carbocation,
the result is the stabilizing interaction called hyperconjugation.
H
2
C
Filled C
H
bond
Empty 2p
orbital
Energy
+
C
H
C H
σ bond
2p
4
If avoiding the question drives you crazy, and we think it should at least make you a little uncomfortable, be
sure to do this problem—the answer falls right out. See also Chapter 9.
(continued)
STOP
There are many reactions in which tertiary and secondary
carbocations are intermediates but no reactions for which a simple
primary or methyl cation is required. These intermediates are
simply too unstable. In practice, this instability means that when
you find yourself writing a mechanism using a primary carbocation, think again,
it’s almost certainly wrong!
294 CHAPTER 7 Substitution and Elimination Reactions
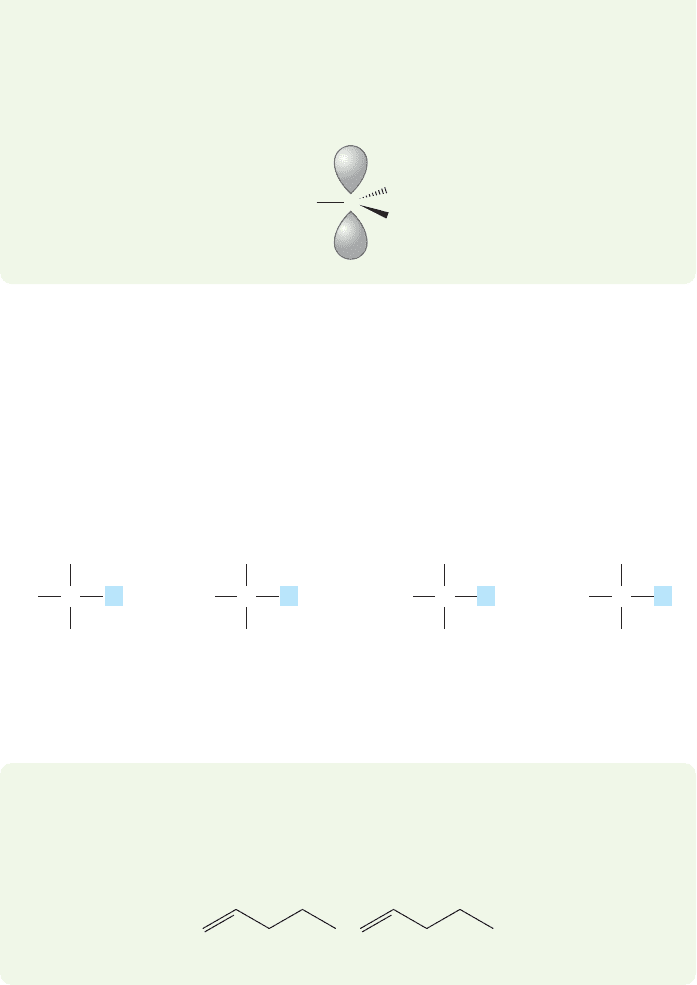
This interaction can occur only if the appropriate carbon–hydrogen bonds exist.
The more alkyl groups, the more stabilization there is. For example, in the methyl
cation no such stabilization is possible because there are no alkyl groups to sup-
ply the needed bonds.
+
C
H
H
H
C
O
H
MethylPrimarySecondaryTertiary
faster
than
much
faster
than
C
H
R
R
R
RR
R
C
H
H
orC H
H
H
C
L L L L
FIGURE 7.59 Relative reactivities in
the S
N
1 reaction.
PROBLEM 7.19 The heat of formation for cation A is 201 kcal/mol, and that of
cation B is 218.6 kcal/mol. Draw the two cations carefully and explain why A is
more stable than B.
A
+
B
+
So, tertiary compounds, which form the most stable, tertiary carbocations, com-
monly react through the S
N
1 mechanism, and methyl and primary compounds,
which would have to form extremely unstable carbocations, do not.Naturally enough,
secondary compounds react faster than primary ones in the S
N
1 reaction but more
slowly than tertiary compounds (Fig. 7.59).
7.6d Effects of the Nucleophile We have already seen that the nucleo-
phile does not affect the rate of the S
N
1 reaction—the reaction is first order in
substrate.Yet, the nucleophile does determine the product structure.Note the dis-
tinction between the rate-determining step (or rate-limiting step) and the
product-determining step. For example, reaction of tert-butyl bromide in water
containing a small amount of an added nucleophilic ion, such as cyanide, leads to
substantial amounts of tert-butyl cyanide. Even though water is present as solvent
and thus has an immense advantage over cyanide ion because of concentration,
the more powerfully nucleophilic cyanide still competes. Cyanide is many times
more reactive toward the carbocation than the solvent water is (Table 7.3, p. 279).
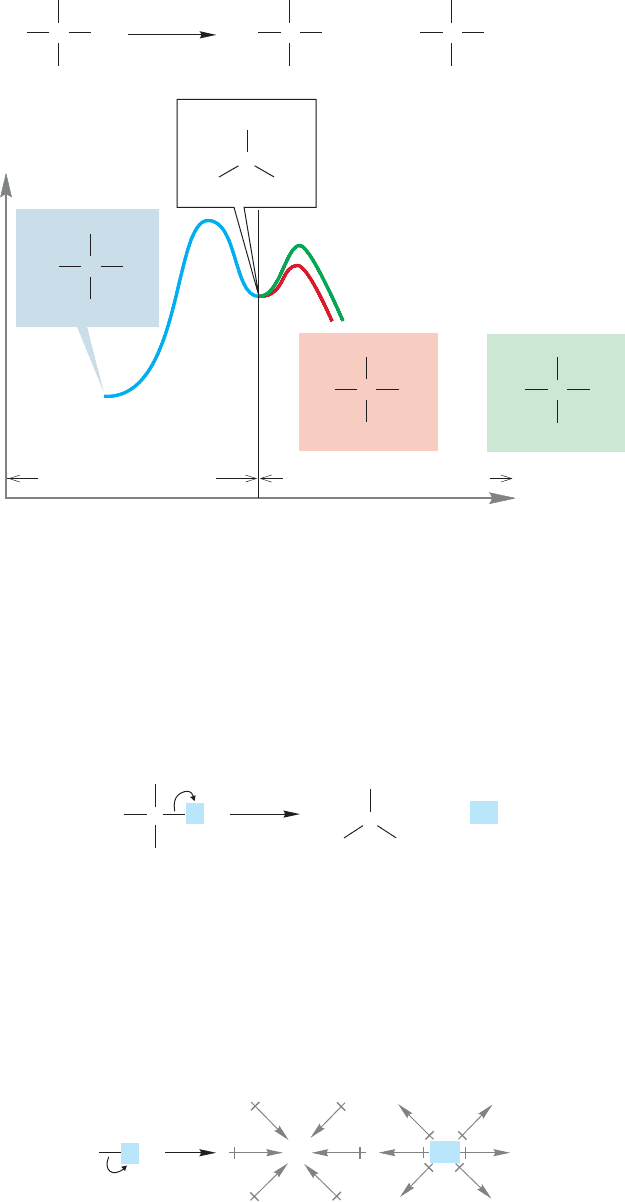
7.6 Substitution, Nucleophilic, Unimolecular: The S
N
1 Reaction 295
CH
3
H
3
C
CH
3
C
Energy
H
2
O
CH
3
H
3
C
CH
3
C
..
K
+
CN
..
..
..
–
CN
..
CH
3
H
3
C
CH
3
C
OH
..
..
+
Br
..
..
..
and
CH
3
H
3
C
CH
3
C
OH
..
..
Reaction progress
Product-determining steps
Br
..
..
..
..
CH
3
CH
3
H
3
C
+
–
CH
3
H
3
C
CH
3
C
Br
..
..
..
C
Rate-determining step
CH
3
H
3
C
CH
3
C
CN
..
FIGURE 7.60 The rate-determining
step and product-determining step
are sharply distinguished in the S
N
1
reaction.
In this reaction, the rate-determining step (the slow ionization) and the product-
determining step (the faster capture of the intermediate ion by nucleophiles) are
sharply separated (Fig. 7.60).
7.6e Effect of the Leaving Group The identity of the leaving group has a
large influence on the rate of the S
N
1 reaction because the structure of the depart-
ing group affects the ease of ionization. We have already discussed how the
structure—and hence stability—of the carbocation affects the rate of ionization, but
obviously the stability of the negatively charged counterion ( ) will be important
as well. The more stable the anion, the more easily it will be lost (Fig. 7.61). Some
good and poor leaving groups are shown in Table 7.4 (p. 281).
L
:
-
+
H
3
C
CH
3
CH
3
CH
3
H
3
C
S
N
1
CH
3
–
C
C
+
LL
..
FIGURE 7.61 The rate of ionization is
affected by the stability of the leaving
group, . The more stable the
leaving group, the faster is the
ionization.
L
:
-
7.6f Effect of Solvent For the S
N
2 reaction, the effect of solvent is rather compli-
cated and depends on the charge type of the reaction. Some S
N
2 reactions are accelerat-
ed by a change to a more polar solvent;others are slowed down by such a change (p.286).
For most S
N
1 reactions the situation is simpler.Ions are usually formed in the slow step
of the reaction. In a polar solvent, represented by the dipole arrows in Figure 7.62, the
charged species formed in the S
N
1 reaction are stabilized. A nonpolar solvent cannot
stabilize as well. One can expect that the S
N
1 reaction will usually be favored by a
polar solvent.
+
R
R
L
L
..
–
FIGURE 7.62 A polar solvent
stabilizes the ions formed in
an S
N
1 reaction.
296 CHAPTER 7 Substitution and Elimination Reactions
7.7 Summary and Overview of the S
N
2
and S
N
1 Reactions
These two substitution reactions are interconnected by more than the ultimate for-
mation of a substitution product.They complement each other—when one is inef-
fective, the other is likely to work well. For example, the S
N
2 reaction works best for
methyl and primary substrates, which is exactly the point at which the S
N
1 reaction
is least effective. When the S
N
2 reaction fails, as with tertiary substrates, the S
N
1
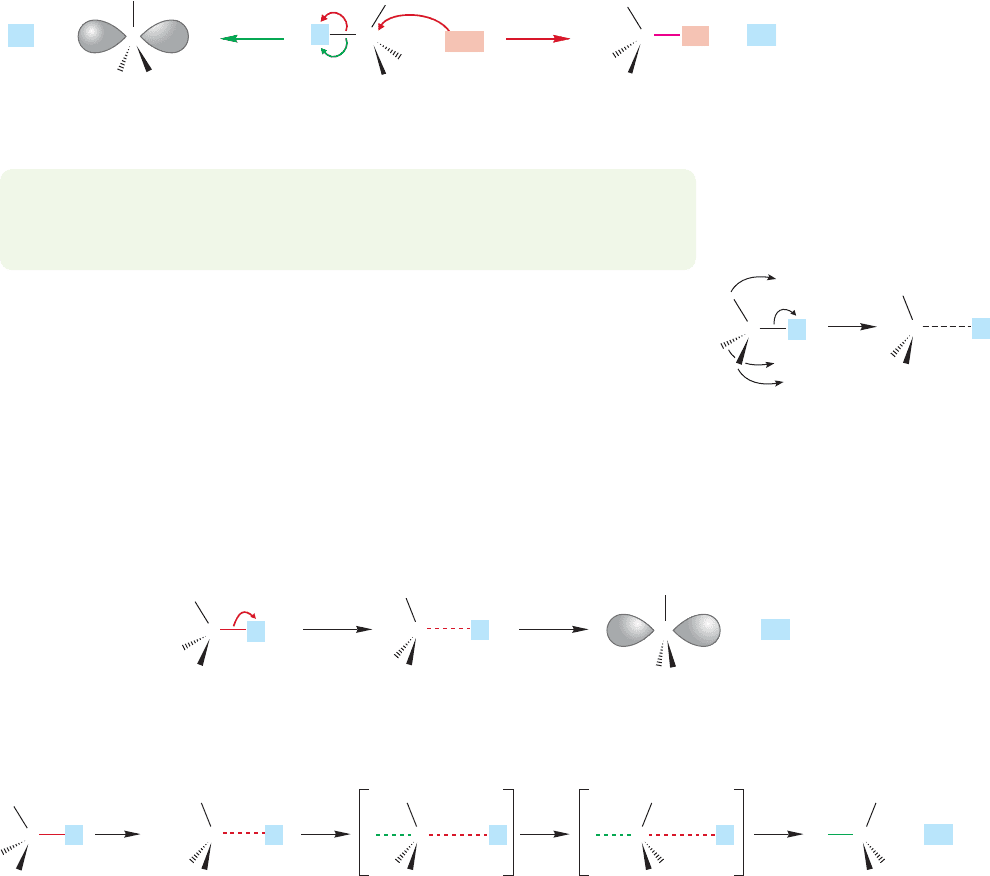
reaction is most effective (Fig. 7.63).
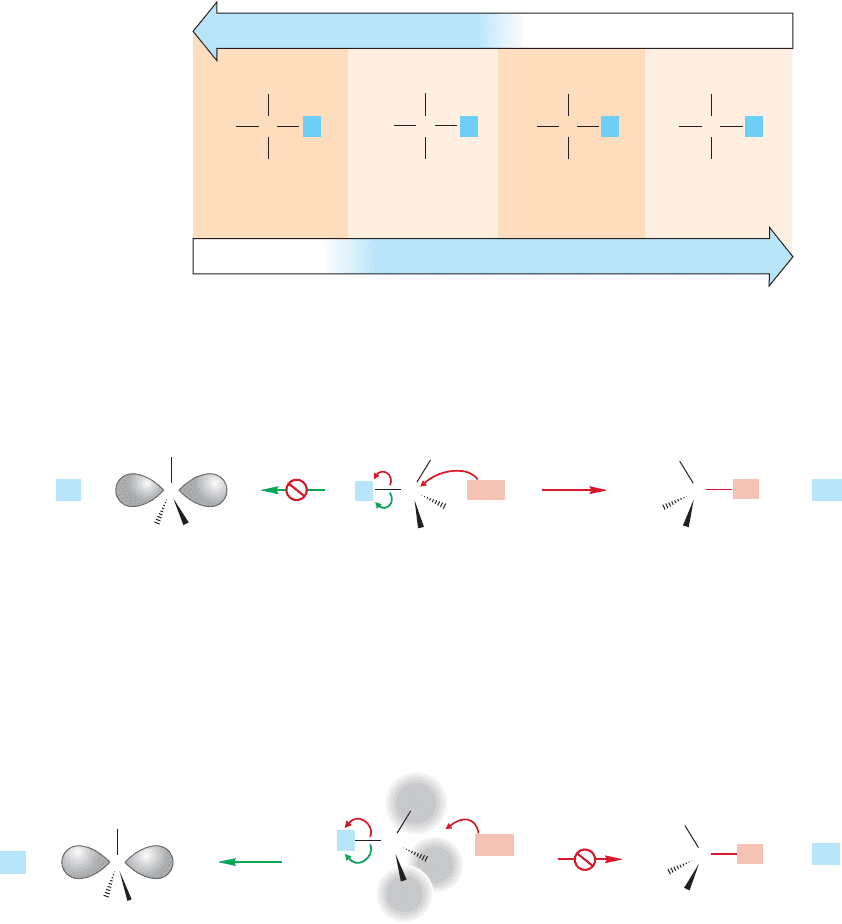
So, at the extremes the situation is clear.The S
N
2 reaction dominates for methyl
and primary substrates because there is little steric hindrance to the displacement
of the leaving group and because the competing S
N
1 reaction is disfavored by the
necessity of forming the highly unstable methyl or primary carbocations (Fig. 7.64).
The S
N
1 reaction dominates for tertiary substrates because ionization can produce
the relatively stable tertiary carbocation and because the competing S
N
2 reaction is
strongly disfavored by the steric hindrance to the nucleophile approaching from the
rear (Fig. 7.65).
Tertiary
Secondary
Primary Methyl
H
3
C
CH
3
CH
3
C
H
3
C
CH
3
H
C
H
CH
3
H
C
H
H
H
C
Efficiency of the S
N
1 reaction
No S
N
1 No S
N
1
Efficiency of the S
N
2 reactionNo S
N
2
L L L L
FIGURE 7.63 The S
N
2 and S
N
1
reactions are complementary.
+
C
H
R
R
R
H
S
N
1
S
N
2
C
H
H
A difficult ionization to the hideously
unstable primary carbocation
C
H
H
+ +
A relatively easy displacement
at the unhindered primary carbon
Nu
LL L
..
..
–
Nu
..
–
..
..
–
FIGURE 7.64 A comparison of the S
N
1 and S
N
2 reactions for primary substrates.
+
C
S
N
1
S
N
2
An easy S
N
1 reaction
as the relatively stable
tertiary carbocation is
formed
C
+
+
The S
N
2 is thwarted
by the difficulty of
displacement at the
sterically hindered
tertiary carbon
C
R
R
R
R
R
R
L
Nu
R
R
R
L
L
..
..
–
Nu
..
–
..
..
–
FIGURE 7.65 For tertiary systems, the S
N
1 reaction dominates; the ionization produces the relatively stable
tertiary carbocation and the S
N
2 reaction is hindered by the three R groups that thwart approach of the
nucleophile from the rear.
7.7 Summary and Overview of the S
N
2 and S
N
1 Reactions 297
In the middle ground—secondary substrates—we might expect to be able to find
both reactions. Small R groups, use of a powerful nucleophile, and a solvent of proper
polarity for the charge type of the reaction favor the S
N
2 reaction. Large R groups, a
weak nucleophile, and a polar solvent favor the S
N
1 reaction (Fig. 7.66).
We have given a picture of these reactions in which extremes are emphasized,
in which an “either S
N
2 or S
N
1”view is developed. However, the situation is messier
than this simple explanation would suggest, especially for secondary compounds.
Imagine beginning to ionize a bond to a leaving group. As the bond stretches,
positive charge begins to develop on carbon (δ
) and rehybridization toward sp
2
begins as the groups attached to the carbon flatten out (Fig. 7.67).
If we simply continue the process of ionization, we have the pure S
N
1 reac-
tion. If we let a nucleophilic solvent molecule interact with the rear of the depart-
ing bond and ultimately form a bond to carbon, we have the S
N
2 reaction with
inversion (Fig. 7.68).
C
+
H H
C C
S
N
1S
N
2
Nu
L
R
H
R
R
R
R
R
L
L
..
..
–
Nu
..
–
..
..
–
FIGURE 7.66 For secondary substrates, both S
N
1 and S
N
2 reactions may be possible.
C
C
δ
–
δ
+
L
L
FIGURE 7.67 The beginning of an
S
N
1 reaction.The bond to the leaving
group L
has lengthened, and the
tetrahedron has begun to flatten out.
The hybridization of the central
carbon is changing from nearly sp
3
to
the sp
2
of the planar carbocation.
:
C
+
However, solvation at the side away from the old leaving group can be converted into nucleophilic attack (S
N
2) to give inverted produc
t
C
C
δ
–
δ
+
+
..
..
C
S
S
C C
δ
–
δ
+
+ +
In the classic S
N
1 reaction, this ionization continues to produce the planar
carbocation. Notice, however, that the carbocation when first formed is not
symmetrical. The leaving group lurks on one side, partially shielding the cation
from solvent and other nucleophiles
–
SS
C C
–
L
L L
L L L L
–
..
..
–
L
..
..
–
FIGURE 7.68 Especially for secondary substrates, the difference between S
N
2, displacement by solvent, and preferential
solvation at the rear of the departing bond is a subtle one.
How strong an interaction with the rear lobe of the orbital is needed before we call
it bonding? In polar solvents, ions are highly solvated, and in an S
N
1 reaction solvation
will begin to be important as soon as a δ
charge begins to build up on carbon. Solvation
will be especially easy at the rear of the departing group as the leaving group shields the
frontside. We have already seen that the leaving group can hinder reaction at one side
of the developing cation and produce some net inversion in the reaction (Fig. 7.58).
PROBLEM 7.20 We saw at least one S
N
1 reaction before, although it was not
labeled as such because we didn’t yet know the name. Where was it? If you get
this one, you have a great eye and memory for detail. Hint: It is in this chapter.
298 CHAPTER 7 Substitution and Elimination Reactions
Now we begin to see the difficulty of telling the two mechanisms apart in some
situations. When does “preferential solvation from the rear” become “displacement
from the rear”? It becomes painfully hard to sort out in some cases. Organic chem-
istry is frustratingly, if delightfully, messy.
This messiness raises its head in other ways. Almost all substitution reactions are
accompanied by some formation of alkene. In organic chemistry few reactions pro-
ceed in 100% yield, quantitatively producing one molecule from another. A large part
of the business of organic synthesis is the search for specificity. Synthetic chemists seek
reagents and reaction conditions that greatly favor one reaction pathway over others.
In particular, attempts to synthesize molecules through substitution reactions must
take account of competitive reactions called eliminations.A hydrogen atom and a leav-
ing group (L) are lost (eliminated) from the substrate to give an alkene (Fig. 7.69).
Sections 7.8 and 7.9 examine two general mechanisms for these elimination reactions.
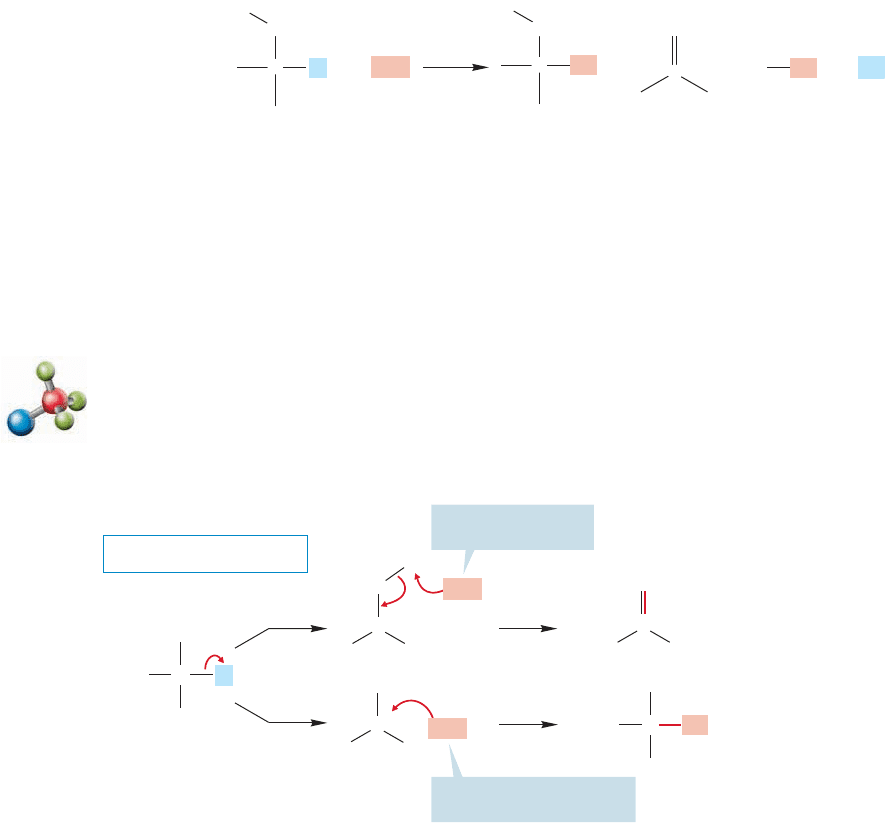
7.8 The Unimolecular Elimination Reaction: E1
7.8a Rate Law If we determine the rate law for the formation of the alkene
accompanying an S
N
1 reaction, we find that this elimination reaction is also a first-
order process, . As in the S
N
1 reaction, the slow step in this reaction is
the ionization of the substrate to give a carbocation, which can do two things: It can
continue the S
N
1 reaction by adding a nucleophile (Nu ) or, if a proton is available,
an alkene can be formed in what is called the E1 reaction. Note that in the elimi-
nation phase of the reaction, the loss of hydrogen, the nucleophile is acting as a
Brønsted base.The carbocation is an intermediate in both processes (Fig. 7.70).
:
-
ν = k[R
O
L]
++++
H
Substitution
reaction
Elimination
reaction
Nu
NuL
L
C
H
C
H
C
CH
2
CH
2
CH
2
Nu
..
–
..
–
FIGURE 7.69 In almost all S
N
1 and
S
N
2 reactions, substitution is
accompanied by some elimination
of to give an alkene.H
O
L
Unimolecular elimination: E1
Here
–
Nu is acting as
Lewis base = nucleophile
Here
–
Nu is acting
as Brønsted base
THE GENERAL CASE
H
E1
S
N
1
C
..
..
Favored by a highly
nucleophilic nucleophil
e
Favored by a highly
basic nucleophile
C
H
3
C
H
3
C
H
2
C
CH
3
CH
3
CH
3
CH
3
C
H
3
C
CH
3
C
CH
2
+
C
+
H
3
C
H
3
C
CH
3
H
3
CCH
3
Nu
L
Nu
..
–
Nu
..
..
–
..
FIGURE 7.70 Competing E1 and S
N
1 reactions.
The ratio of E1 and S
N
1 products depends primarily on the nucleophile’s basicity.
Strong Brønsted bases are more effective at removing the proton than weak Brønsted
bases. That’s exactly what we mean by a strong Brønsted base—something that has a
