Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

7.8 The Unimolecular Elimination Reaction: E1 299
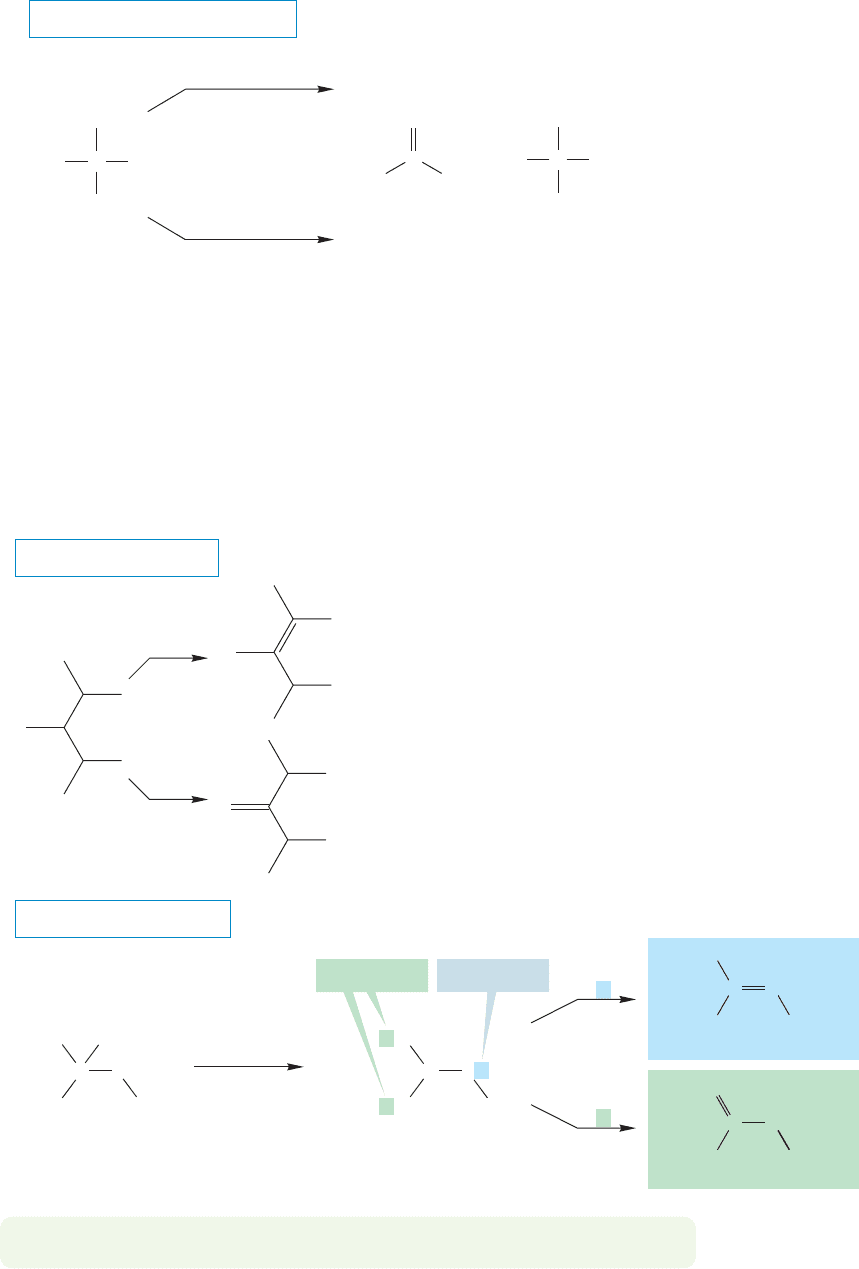
7.8b Selectivity in the E1 Reaction In many E1 reactions,more than one hydro-
gen can be removed from the carbocationic intermediate to give an alkene.When there
is a choice, the major product is the more substituted, more stable alkene (Chapter 3,
p. 115). Of course, statistical effects will also influence the product mix, but the over-
whelming factor is alkene stability.The more substituted alkene is favored strongly in
the E1 reaction. For example, 2-bromo-2-methylbutane reacts in a solution of ethyl
alcohol and water to give a mixture of 2-methyl-2-butene and 2-methyl-1-butene in
which the more substitued alkene predominates by a factor of 4 (Fig. 7.72).
high affinity for a proton.Good nucleophiles will be less effective in the elimination reac-
tion,which requires removal of a proton,but more effective in the S
N
1 reaction (Fig.7.71).
SOME SPECIFIC EXAMPLES
%S
N
1
C
–
H
3
C
Na
+
CH
3
CH
3
CH
2
OH
OCH
2
CH
3
20 80
93 7
%E1
CH
3
C
H
3
C
CH
2
CH
3
Br
..
..
..
..
CH
3
CH
2
OH
..
..
CH
3
CH
2
O
..
..
..
..
..
..
H
3
C
CH
3
CH
3
C
FIGURE 7.71 The elimination and
S
N
1 reactions compete. In pure
ethyl alcohol, the ratio of alkene
(2-methylpropene) to S
N
1 product
(tert-butyl ethyl ether) is 0.25. In the
presence of the much stronger base
sodium ethoxide, this ratio increases
to about 13. A strong base (ethoxide)
favors the elimination reaction.
THE GENERAL CASE
A SPECIFIC EXAMPLE
More substituted
(major product)
Less substituted
(minor product)
CH
2
CH
3
H
3
C
H
3
C
C
Br
+
CH
3
CH
2
OH
CH
3
C
CH
CH
3
H
3
C
H
3
C
6 Hydrogens 2 Hydrogens
C
CH
3
2-Methyl-2-butene (32%)
2-Methyl-1-butene (8%)
H
3
C
H
2
C
H
2
O
– H
+
+
– H
+
CH
2
C
..
..
..
H
3
C
H
3
C
CH
2
2-Bromo-2-methylbutane
FIGURE 7.72 The more substituted
(more stable) alkene is favored in the
E1 reaction, even when statistical
factors favor the less substituted
isomer. This reaction is known as
Saytzeff elimination.
PROBLEM 7.21 Draw arrow formalisms for the reactions of Figure 7.72.
300 CHAPTER 7 Substitution and Elimination Reactions
PROBLEM 7.22 The specific example in Figure 7.72 also produces two products of
the S
N
1 reaction: one C
5
H
12
O and the other C
7
H
16
O. What are these products
and how are they formed?
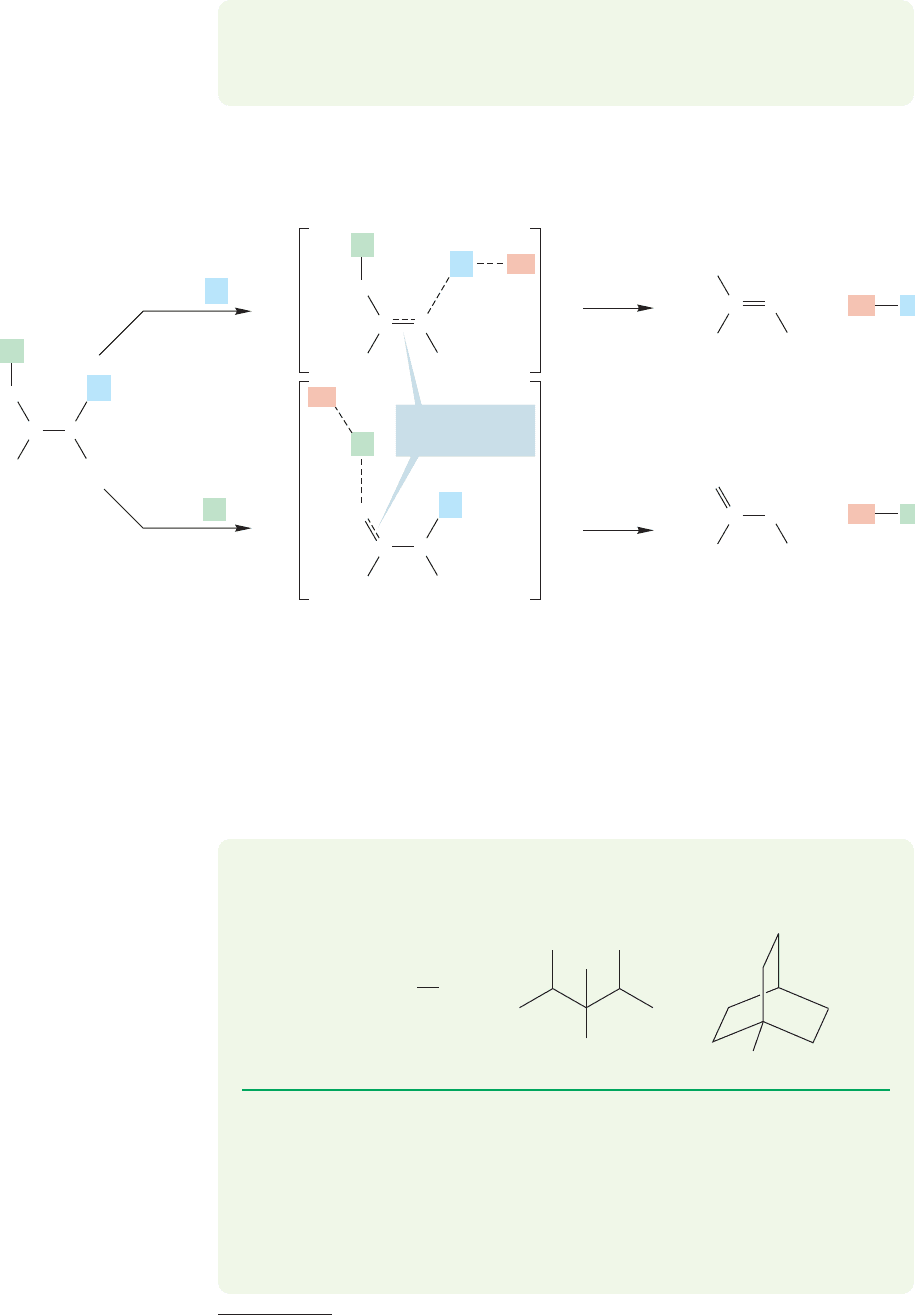
To see why the more substituted alkene is favored, look at the transition state
for proton removal. In the transition state the carbon–hydrogen bond is lengthened
and the double bond of the alkene is partially formed (Fig. 7.73).
5
Saytzeff can be transliterated from the Cyrillic as Zaitzev, Saytzev, or Saytseff.
+
CCH
H
2
C
H
3
CCH
3
Partially formed
double bonds
Transition states for proton removal by a
base to give the alkene and NuH
loss of
H
b
H
b
H
a
H
a
loss of
CCH
H
2
C
H
3
CCH
3
H
b
H
a
CCH
H
2
C
H
3
CCH
3
CCH
H
3
C
H
3
CCH
3
CCH
2
H
2
C
H
3
CCH
3
H
b
H
a
–
Nu
..
Nu
Nu
Nu
+
H
+
Nu H
+
+
FIGURE 7.73 In the transition state, a
double bond is partially formed. The
increased stability of more substituted
double bonds will be felt, and the
transition state for formation of the
more substituted alkene (loss of H
a
)
will be lower in energy than the
transition state for formation of the
less substituted alkene (loss of H
b
).
The factors that operate to stabilize alkenes with fully formed double bonds are
also operative in the transition states in which there are partially formed double bonds.
So, the more alkyl groups, the better, and the E1 reaction preferentially produces the
most substituted alkene possible.This phenomenon is called Saytzeff elimination,after
Alexander M. Saytzeff (1841–1910), the Russian chemist who discovered it.
5
PROBLEM 7.23 Predict the products of the E1/S
N
1 reactions of the following
molecules in water:
PROBLEM 7.24 It was noticed as early as 1862 that treatment of isobutyl halides,
, with silver acetate, Ag
OAc (
OAc is the acetate ion, a
weak nucleophile, and Ag
is a strong Lewis acid, especially toward chloride),
produced tert-butyl acetate, , and isobutene. Use the stability
order of carbocations (p. 292) to provide an explanation for this behavior. Hint:
Reason backward from the structure of the product. What ion must be present to
form this product?
(CH
3
)
3
C
O
OAc
(CH
3
)
2
CHCH
2
O
X
Br
(a)
(b)
(c)
Br
(CH
3
CH
2
)
3
C
Br
7.9 The Bimolecular Elimination Reaction: E2 301
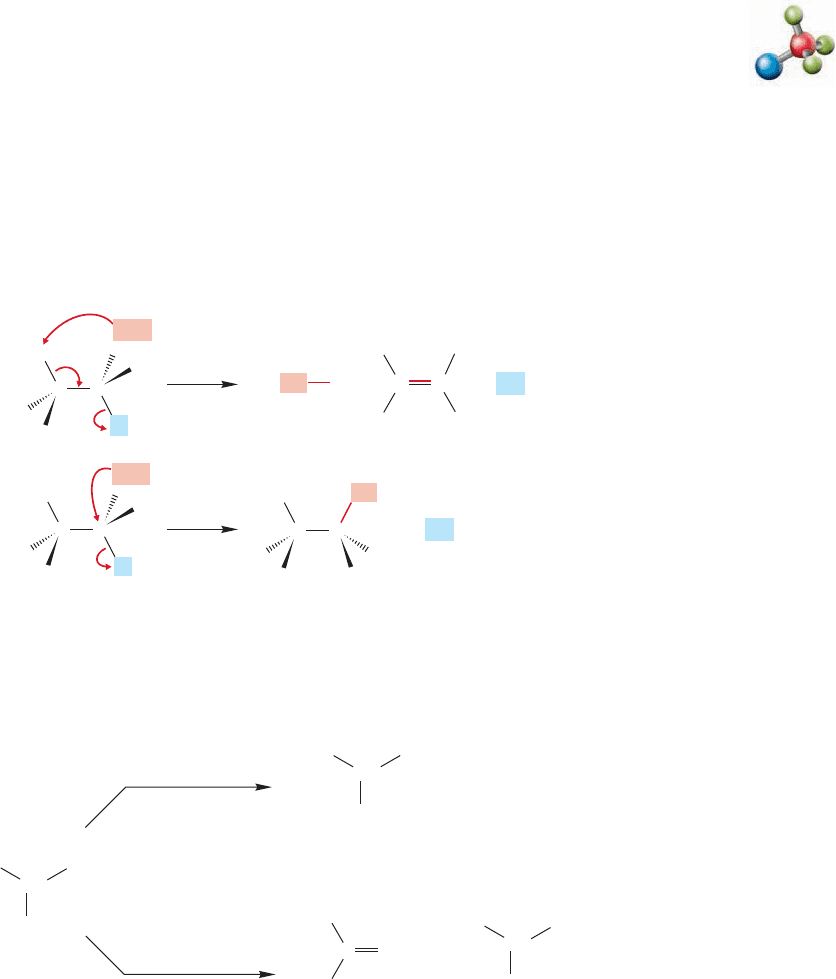
7.9 The Bimolecular Elimination Reaction: E2
As with the substitution reaction, there are two common mechanisms for the elim-
ination reaction. We have just explored the first-order process, the E1 reaction.Now
let’s look at the bimolecular reaction, the E2 reaction.
7.9 a Rate L aw The S
N
2 reaction is also complicated by alkene formation. Just
as we saw for the S
N
2 reaction, the typical rate law for the bimolecular E2 reaction
shows that the alkene is formed in a second-order process that is first order in both
substrate and nucleophile:
ν k[substrate] [nucleophile]
The mechanism involves attack by the base at a hydrogen attached to a carbon
adjacent to the carbon bearing the leaving group. Figure 7.74 shows the arrow
formalisms for the competing E2 and S
N
2 reactions.
Bimolecular elimination: E2
E2
..
S
N
2
+
+
+
CC
H
CC
H
H
H
CC
CC
Nu
Nu
L
L
L
–
Nu
..
–
Nu
..
–
..
L
–
FIGURE 7.74 The competing
E2 and S
N
2 reactions.
This picture gives us an immediate clue as to how to influence the product mix-
ture from competing E2 and S
N
2 reactions. As in the E1–S
N
1 competition (p. 298),
strong Brønsted bases (high affinity for hydrogen 1s orbital) should favor the E2
reaction and strong nucleophiles (high affinity for carbon 2p orbital) should favor
the S
N
2 reaction (Fig. 7.75).
+
–
–
Br
..
..
..
..
Cl
..
..
..
..
OCH
2
CH
3
OCH
2
CH
3
..
..
..
HOCH
2
CH
3
..
H
3
C
acetone
Bu
4
N
Na
CH
CH
3
Cl
..
..
..
H
3
C
CH
CH
3
H
3
C
CH
CH
3
H
S
N
2 Product
E2 Product
(75%)
S
N
2 Product
(25%)
CCH
2
H
3
C
+
+
..
..
FIGURE 7.75 The E2 and S
N
2
reactions compete. In the examples
shown, the relatively good
nucleophile chloride gives exclusively
the S
N
2 reaction, whereas the much
more basic ethoxide ion gives mostly
the E2 reaction. Bu means butyl.
7.9b Effect of Substrate Branching on the E2–S
N
2 Mix The rate of the
S
N
2 reaction depends strongly on the structure of the substrate. The more highly
branched the substrate, the more hindered is the obligatory approach from the rear of
the leaving group and the slower is the S
N
2 reaction.This effect reaches a limit with
tertiary substrates, in which the S
N
2 rate is effectively zero.The slower the S
N
2 reac-
tion, the more important is the competing E2 reaction. With a typical tertiary sub-
strate such as tert-butyl bromide, the E2 reaction is the only effective reaction under
302 CHAPTER 7 Substitution and Elimination Reactions
E2–S
N
2 conditions.The steric requirements for deprotonation are far less severe than
for attack at the rear of the carbon–bromine bond of tert-butyl bromide (Fig. 7.76).
Br
..
..
..
..
..
E2
–
H
3
C
H
3
C
C
H
C
H
3
C
CH
2
CH
3
+
+
Br
..
..
..
H
H
3
C
CH
2
H
3
C
C
Br
..
..
..
Nu
Nu
..
–
Nu
..
–
No S
N
2
H
3
C
FIGURE 7.76 For tertiary substrates, the S
N
2 reaction does not proceed, but the E2 reaction is relatively unhindered.
Attack from the rear in S
N
2 fashion is blocked by the three methyl groups.The E2 reaction is relatively easy.
S
N
2
(49%)
E2
(51%)
–
Bu
4
N
+
acetone
Cl
..
..
..
..
–
Bu
4
N
acetone
Cl
..
..
..
..
With a secondary substrate, the S
N
2 and E2 reactions are competitive
With a primary substrate, the S
N
2 reaction is often the only process
(CH
3
)
2
CH (CH
3
)
2
CH
CH
3
Br
..
..
..
CH
CH
3
H
(CH
3
)
2
C
C
Cl
..
..
..
CH
3
CH
+
Cl
..
..
..
+
Br
..
..
..
FIGURE 7.77 For secondary
substrates, the E2 and S
N
2 reactions
are competitive. For primary
substrates, the S
N
2 reaction
dominates.
7.9c Stereochemistry of the E2 Reaction When we explored the
mechanism of the S
N
1 and S
N
2 reactions, an examination of the stereochemical
outcome of the reactions was crucial to our development of their mechanisms.
Stereochemical experiments are common in organic chemistry and are one of the
reasons we spent so much time and effort earlier on stereochemical analysis. In the
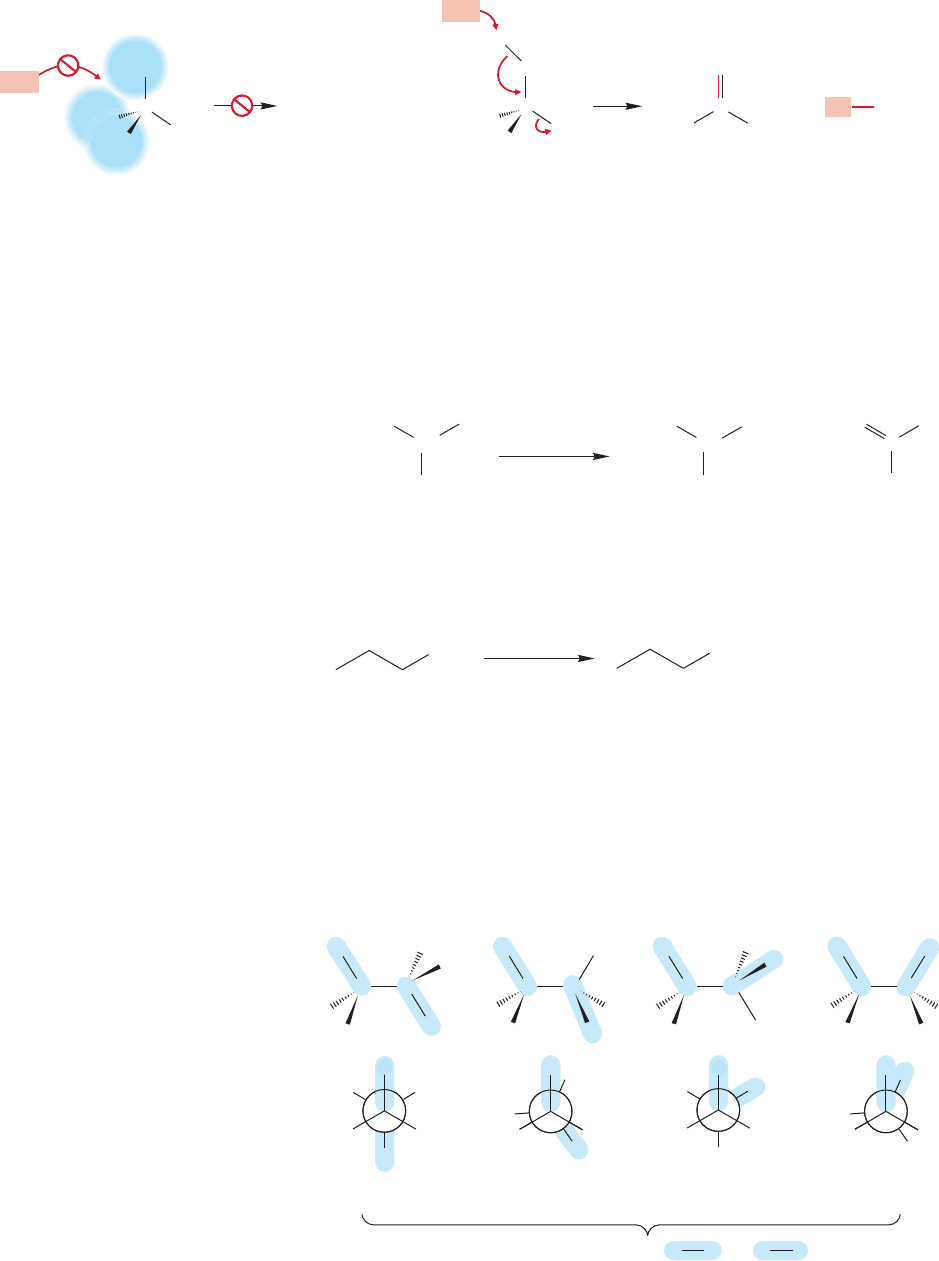
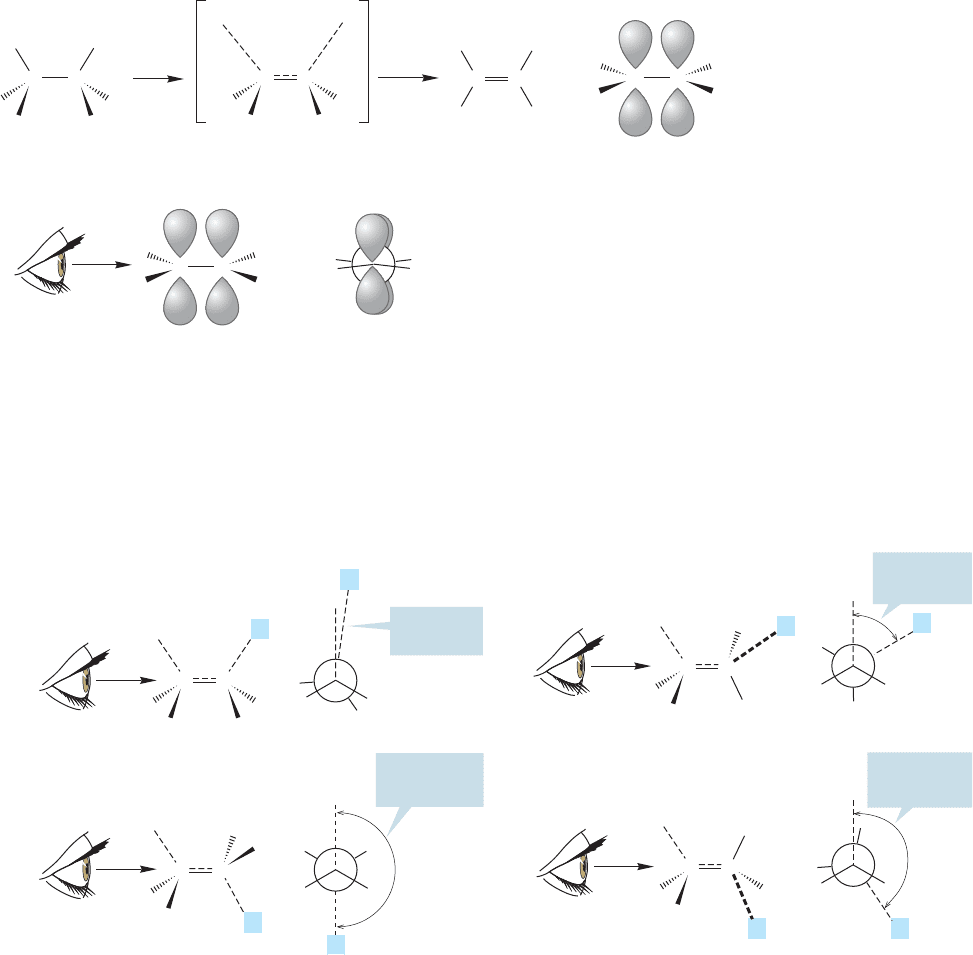
E2 reaction, there are four possible extreme orientations. They can be classified by
noting the dihedral angle between the two groups that come off, the hydrogen and
the leaving group L (Fig. 7.78).
C CC
H
C
H
CC
H
CC
H
180⬚ 120⬚ 60⬚ 0⬚
Dihedral angle between C H and C L
L
L
L
L
L
L
L
L
H
H
H H
FIGURE 7.78 Four possible
arrangements for the E2 reaction.
The Newman projections emphasize
the dihedral angle between the
and bonds involved in
the reaction.
C
O
LC
O
H
With less hindered substrates, the S
N
2 reaction can compete much more
effectively with the E2 reaction, and with primary substrates the S
N
2 reaction is
dominant (Fig. 7.77).
7.9 The Bimolecular Elimination Reaction: E2 303
As the and bonds lengthen in this reaction, the molecule flat-
tens out as the 2p orbitals that will constitute the π system of the alkene are formed.
Remember (p. 105): In the product alkene the 2p orbitals are perfectly lined up,
eclipsed, with a 0° dihedral angle (Fig. 7.79).
C
O
LC
O
H
Transition state
Newman projection
C
C
H
CCCC
=
=
H
C C
C C
L
L
FIGURE 7.79 The generation of a π bond as the E2
reaction occurs. In the E2 reaction, the
and bonds lengthen as the π bond between
the carbons is created. Note especially the
requirement for orbital lineup as the π bond is
generated.The dihedral angle between the two
p orbitals in the product alkene is 0°.
C
O
L
C
O
H
0⬚ syn
180⬚ anti
CC
Dihedral
angle
= 180⬚
Dihedral
angle = 0⬚
H
H
H
H
Dihedral
angle = 60⬚
Dihedral
angle = 120⬚
CC
H
H
60⬚ gauche
CC
H
120⬚ gauche
CC
H
L
L
L
L
L
L
L
(a) (c)
(d)(b)
L
FIGURE 7.80 Four views of the transition state for the E2 reaction. In examples (a) and (b), where the dihedral angle
is 0° or 180°, the π bond can be generated without an extra rotation. In examples (c) and (d), where the dihedral angle
is 60° or 120°, an extra rotation must take place in order for the π bond to be made.
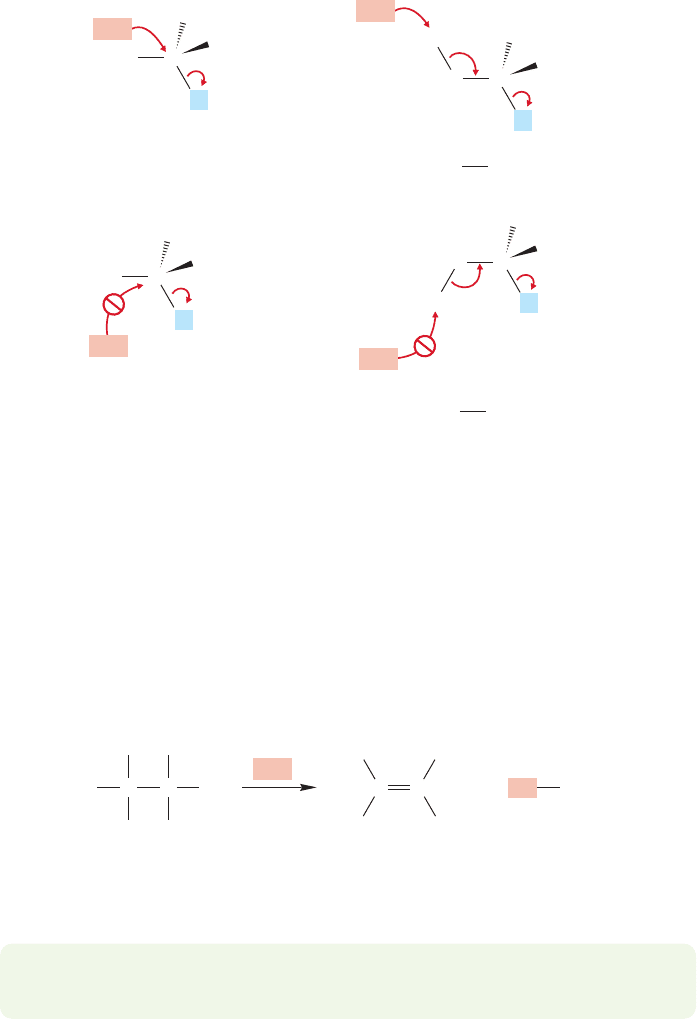
Note that only the 0° (syn) and 180° (anti) arrangements shown in Figure 7.78
can lead smoothly to an alkene with perfectly overlapping 2p orbitals.The 120° and
60° forms cannot produce alkene without an additional rotation to line up the orbitals
(Fig. 7.80).
Thus, we can anticipate that the 60° and 120° gauche arrangements for
elimination should be disfavored. It is much more difficult to decide which of
the remaining two possibilities, 0° (syn) or 180° (anti) will be preferred in the E2
reaction.
304 CHAPTER 7 Substitution and Elimination Reactions
C
The favored backside
displacement in the
S
N
2 reaction
The disfavored
frontside displacement
in the S
N
2 reaction
C
C
The 180⬚ E2 reaction:
the electrons in the C
H bond displace
the leaving group from the rear
C
H
C
C
The 0⬚ E2 mimics the frontside S
N
2 reaction:
the electrons in the C
H bond displace
the leaving group from the front
H
L
L
L
L
Nu
..
–
Nu
..
–
Nu
..
–
Nu
..
–
FIGURE 7.81 The 0° E2 reaction
resembles a frontside S
N
2 reaction,
whereas the 180° E2 reaction looks
like the favored, backside S
N
2
displacement reaction.
The E2 is a kind of extended S
N
2 reaction. The Lewis base (Nu ) attacks the
hydrogen, and the pair of electrons in the carbon–hydrogen bond does the displac-
ing of the leaving group. If we keep this analogy in mind we can see that the 180°
elimination involves backside displacement, as does the S
N
2 reaction,but the 0° elim-
ination requires frontside displacement. We might therefore expect the 180° E2 to
be favored (Fig. 7.81).
:
-
2-Chloro-2,3-dimethylbutane
H
H
3
C
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
3
H
3
C
C
CCCCl
++
–
H
Nu
Cl
..
..
..
..
..
..
..
Nu
..
–
FIGURE 7.82 In this reaction there is
only one possible tetrasubstituted
alkene product.
Now the problem is to find an experiment that will let us see this level of detail.
As usual, we will use a stereochemical analysis, and this recquires a certain com-
plexity of substitution in the substrate molecule. For example, there is no use in
examining the stereochemistry of the E2 reaction of a molecule as simple as
2-chloro-2,3-dimethylbutane because there is only one tetrasubstituted alkene
possible (Fig. 7.82).
PROBLEM 7.25 What other alkene could be formed in the reaction of
Figure 7.82?
Molecules more highly substituted than 2-chloro-2,3-dimethylbutane can reveal
the stereochemical preference, however. For example, consider the molecules of
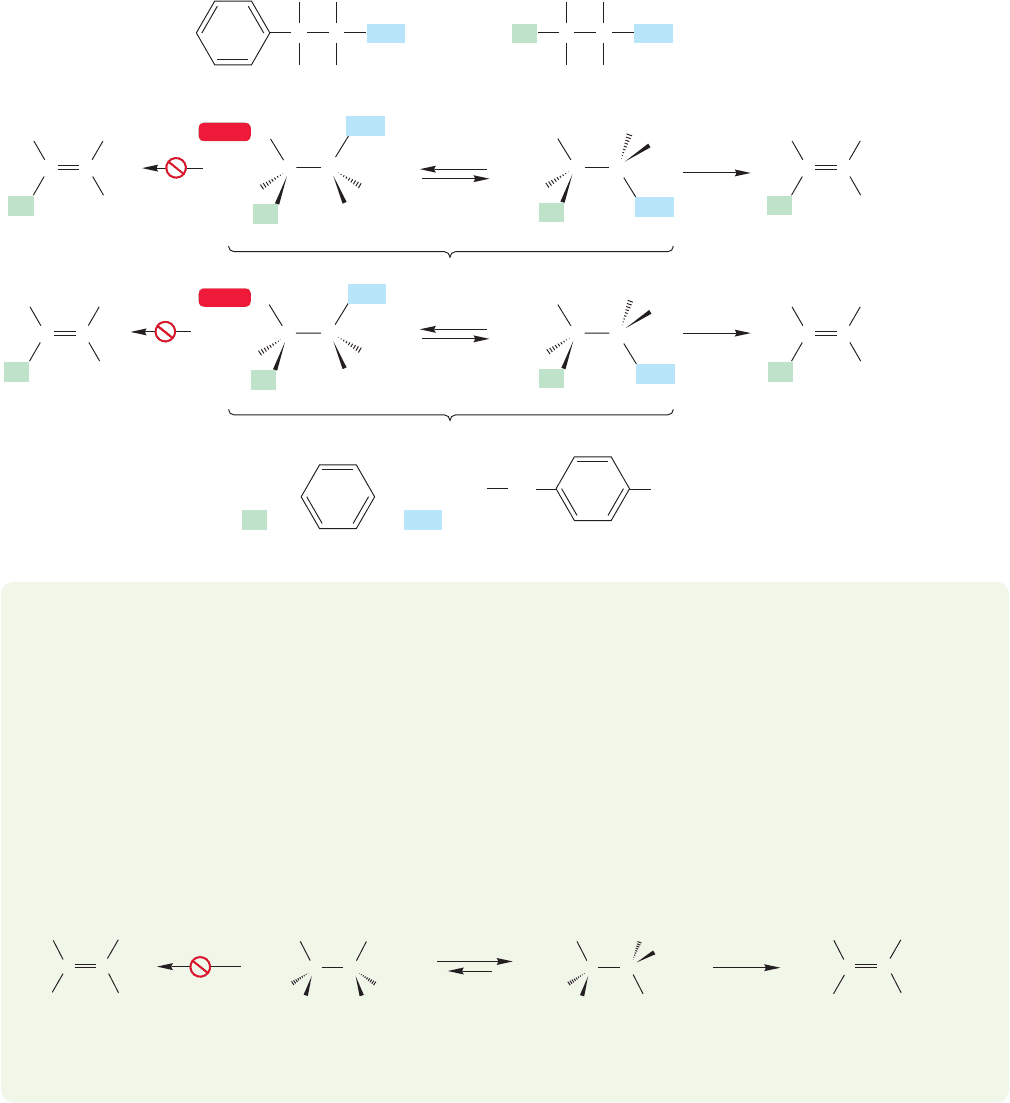
7.9 The Bimolecular Elimination Reaction: E2 305
Figure 7.83. In diastereomer A,syn (0°) elimination would lead to a product in which
the methyl groups are cis [this is,however, the (E) alkene], whereas anti (180°) elimina-
tion would give the compound with trans methyls [the (Z) alkene]. In B, these prefer-
ences are reversed:syn elimination would produce the (Z) alkene,and anti elimination would
give the (E) alkene. Remember: The syn form is an eclipsed, energy-maximum structure
and the anti form is a staggered,energy-minimum structure.Many tests of this kind have
been carried out,and it is certain that in acyclic molecules anti (180°) elimination is favored
over the syn process (0°) by very large amounts, typically greater than 10
4
(Fig. 7.83).
Tosylate, an excellent
leaving group (see Fig. 7.41)
= =
=
CC
H
CC
H
(Z)
(E)
syn
= 0⬚ anti = 180⬚
(E)(Z) syn
= 0⬚
B
A
anti
= 180⬚
CC
H
H
CC
H
H
CC
H
CC
H
CC
H
H
CC
H
H
H
3
C
H
C
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
CH
3
H
C
H
C
H
C
O SO
2
CH
3
C
6
H
5
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
Ph
OTs
OTs
OTs
OTs
OTs
OTs
OTs
WEB 3D
WEB 3D
FIGURE 7.83 The possibilities for 0°
and 180° E2 reactions for the two
diastereomers A and B shown.
WORKED PROBLEM 7.26 In chemistry, it is customary to report one’s research
results by submitting papers to the chemical literature.As a kind of quality control,
these papers are sent to experts in the field for comment and review before publi-
cation. Imagine that you are such a referee looking over the experiment described
in Figure 7.83. Do the data absolutely require the conclusion that anti (180°) elim-
inations are preferred to syn (0°)? Obviously, we think not. What do you think?
ANSWER There is a problem. The analysis of Figure 7.83 shows a 180° elimina-
tion proceeding from a staggered, energy-minimum form. By contrast, the 0°, syn
elimination of Figure 7.83 proceeds from a fully eclipsed arrangement, which is a
transition state, not an energy minimum (p. 67; Fig. 7.22, p. 273). This compari-
son is scarcely fair! In order to make a real comparison of the syn and anti E2
processes, we will have to be more clever.
H
3
C
H
3
C
CH
3
CH
3
syn = 0⬚ anti = 180⬚
Eclipsed, energy maximum
form—no lifetime!
Staggered, energy
minimum form
H
C
Ph
C
H
3
C
CH
3
H
CC
H
H
OTs
OTs
Ph
CC
H
3
C
CH
3
H
H
Ph
Ph
CC
306 CHAPTER 7 Substitution and Elimination Reactions
H
D
OTs
(98%)
H
H
Na
+
–
OR
H
H
H
H
OTs
syn, – DOTs
Na
+
The dihedral angle between H and
OTs is 120⬚, far from a perfect 180⬚
anti elimination
anti, – HOTs
D
syn, Perfect 0⬚ angle
between D and OTs
–
OR
D
WEB 3D
FIGURE 7.84 Sometimes syn elimination can be preferred.
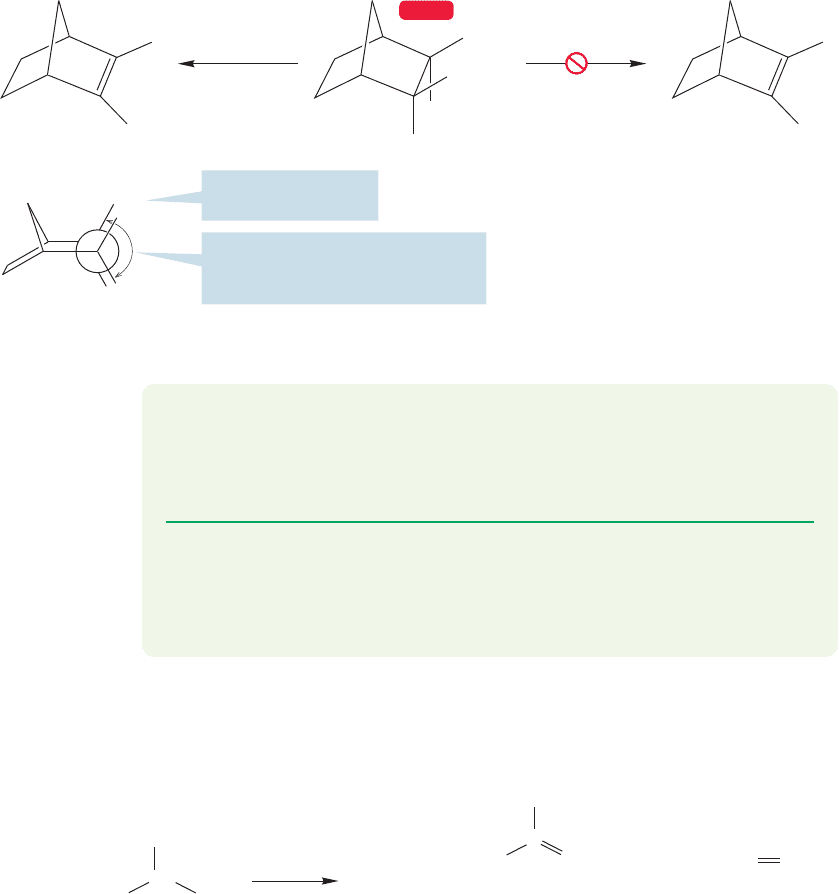
It is important to remember that orbital overlap is vital in the E2 elimination
reaction,and a syn elimination in which the orbitals overlap well may compete effec-
tively with an anti elimination in which the perfect 180° dihedral angle cannot be
attained. For example, some polycyclic molecules give syn elimination.In the exam-
ple shown in Figure 7.84,the 2-bicyclo[2.2.1]heptyl, or 2-norbornyl, system, the ideal
anti (180°) elimination (loss of HOTs) is impossible, because only a 120° arrange-
ment of the departing groups can be achieved. By contrast, the syn elimination (loss
of DOTs) has a perfect 0° dihedral angle between the two departing groups. The
result is that elimination is very slow and the perfect syn elimination is preferred to
the 120° anti elimination (Fig. 7.84).
PROBLEM 7.27 Draw all nine possible stereoisomers of 1,2,3,4,5,6-hexachloro-
cyclohexane. First, draw the molecules flat using some schematic device to indi-
cate the stereochemistry of the chlorine atoms. Next, make three-dimensional
drawings of these molecules.
PROBLEM 7.28 One of the isomers you drew in Problem 7.27 reacts much more
slowly in the E2 reaction than all the others.Which one is it, and why is it so slow
to undergo an E2 reaction? Hint: Remember the steric requirements for the E2
reaction.
–
..
..
..
..
..
CH
OR
Na
CH
3
HOCH
3
(19%)
C
CH
2
H
Both cis and trans
isomers are formed
CH
3
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
+
Br
..
..
..
CH
3
CH
2
CH
2
CH CHCH
3
(81%)
+
FIGURE 7.85 In the E2 reaction, it is the more substituted alkene that is usually formed
preferentially. Both cis and trans isomers are formed in this example.
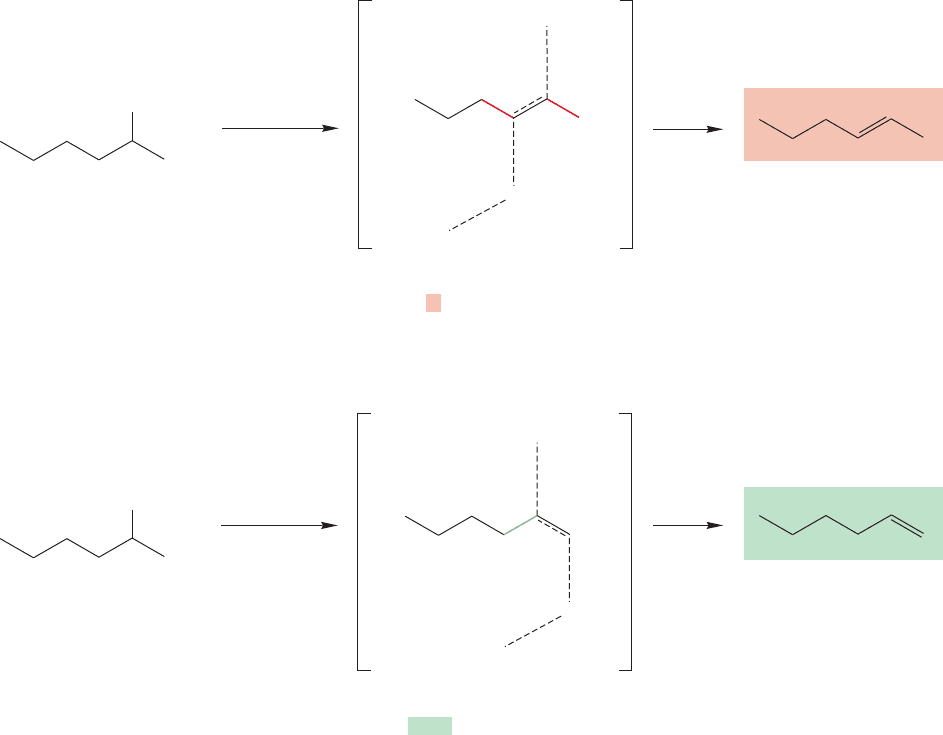
7.9d Selectivity in the E2 Reaction Generally, Saytzeff elimination is the
rule for the E2 elimination. The most substituted, and therefore most stable,
alkene is the major product in this regioselective reaction (Fig. 7.85). Recall that
7.9 The Bimolecular Elimination Reaction: E2 307
regiochemistry simply refers to the outcome of a reaction in which more than
one orientation of substituents is possible in the product, and in a regioselective
reaction, one product predominates.
In the transition state for alkene formation, the π bond is partially developed.
The factors that make more substituted alkenes more stable than less substituted
alkenes will operate on the partially developed alkenes in the transition states as
well. The more substituted the partially developed alkene, the more stable it is,
and this effect inevitably favors the more substituted alkene as the major product
(Fig. 7.86).
–
..
..
..
..
..
OCH
3
HOCH
3
Na
..
..
..
Br
Major product
–
..
CH
3
O
In this transition state for the formation
of the major product, there is a partially
formed disubstituted double bond
+
..
H
..
..
..
Br
..
..
HOCH
3
Minor product
–
In this transition state for the formation
of the minor product, there is a partially
formed monosubstituted double bond
–
..
..
..
OCH
3
Na
+
..
CH
3
O
..
..
..
Br
..
H
..
..
..
Br
Many factors influence the ratio of alkene products, however, and some
are quite surprising at first. The size of the base makes a small difference,
with larger bases favoring the less stable alkene. Although there is only a
very small change in the product distribution when the base is changed
from methoxide to tert-butoxide in the reaction of 2-iodobutane with
FIGURE 7.86 In the E2 reaction, the more stable transition state leads to the major product,
the more substituted alkene.
308 CHAPTER 7 Substitution and Elimination Reactions
THE GENERAL CASE
A SPECIFIC EXAMPLE
Na
4.9
trans/cis
= 3.1
3.8
trans/cis
= 3.0
Saytzeff elimination
1
1
Hofmann elimination
dimethyl sulfoxide
(DMSO)
B
–
OCH
3
..
..
DMSO =
O
S
CH
3
H
3
C
..
..
..
..
K
DMSO
–
OC(CH
3
)
3
..
..
..
Size makes little difference in the approach
to one of the hydrogens whose removal
leads to the less substituted alkene
There are greater steric problems in
approaching one of the hydrogens in the
interior of the molecule; thus size of the
base operates to favor the less
substituted alkene (Hofmann elimination)
H
B
I
H
I
B
H
B
I
H
I
I
+
+
..
..
..
..
H
H
H
H
H
H
FIGURE 7.87 The effect of increasing
base size is to increase the amount of
the less stable “Hofmann” product
somewhat.
alkoxide (Fig. 7.87), when the base is made truly enormous these effects can be magni-
fied. In order to form the more substituted alkene, the base must penetrate farther into
the middle of the substrate. Formation of the less stable alkene requires attack by base
farther toward the periphery of the molecule and thus has lower steric requirements.
2-Alkene
(%)
1-Alkene
(%)
I
Br
Cl
F
81
72
67
30
19 (Saytzeff
preferred)
28 (Saytzeff
preferred)
33 (Saytzeff
preferred)
70 (Hofmann
preferred)
L
(cis and trans isomers)
Hofmann
Major
(98%)
+
Major
(69%)
Saytzeff
Minor
(31%)
–
..
..
..
..
..
OR
HOCH
3
Na
..
..
HOCH
3
..
..
HOCH
3
L
+
–
..
..
..
OCH
3
Na
+
–
..
..
..
OCH
3
Na
+
N(CH
3
)
3
Br
..
..
(cis and trans isomers)
Minor
(2%)
(cis and trans isomers)
..
FIGURE 7.88 The regioselectivity of the E2
reaction depends on the identity of the leaving
group. Leaving groups such as fluoride,
ammonium (R
3
N
), and sulfonium (R
2
S
)
lead to predominant Hofmann elimination, in
which the less stable isomer is the major product.
Hofmann elimination
Formation of the less stable alkene is called Hofmann elimination after the man
who discovered this preference, August W. von Hofmann (1818–1892). Hofmann
elimination contrasts with the more usual Saytzeff process, in which the more sta-
ble alkene is the major product. Hofmann elimination is favored by certain leaving
groups, however. Typical examples of Hofmann leaving groups are fluoride and the
’onium ions, such as ammonium and sulfonium (Fig. 7.88).
