Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

7.9 The Bimolecular Elimination Reaction: E2 309
Why is there a preference for the less-substituted alkene in a Hofmann elimi-
nation? And why does the leaving group make a difference? To answer these ques-
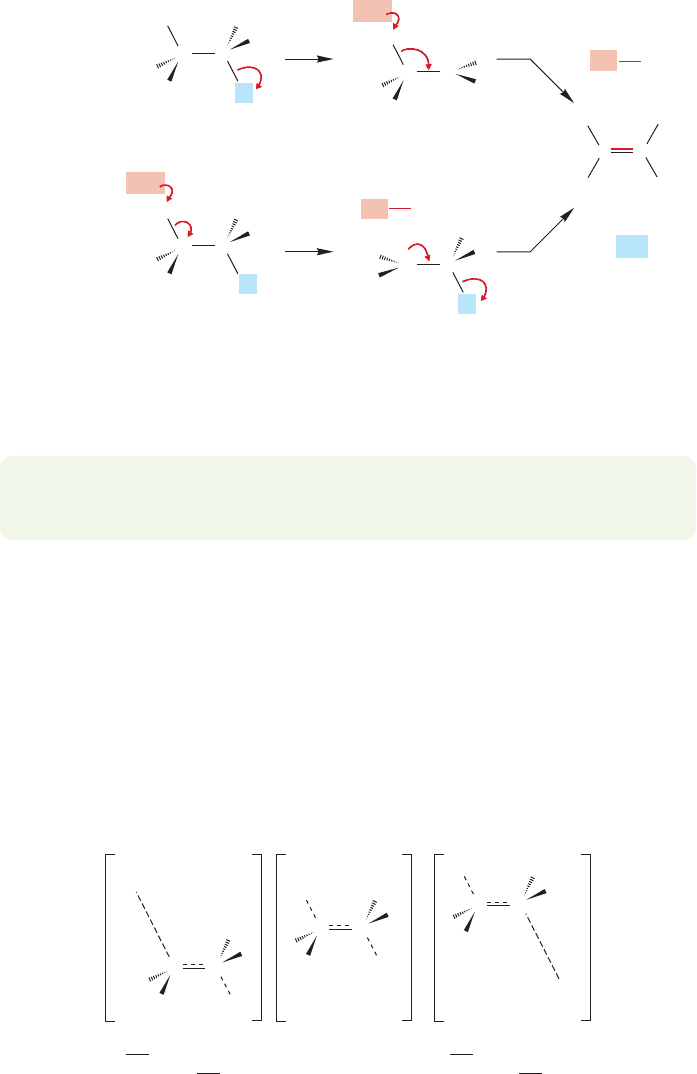
tions, we first have to mention another kind of loss of , the E1cB reaction
(elimination, unimolecular, conjugate base). In this reaction, in essence the opposite
of the E1 elimination, the hydrogen is lost first to generate an anion. Subsequent
ejection of the leaving group as an anion generates the alkene. In the E1 reaction,
the leaving group is lost first to give the carbocation, and a proton lost in the sec-
ond step to give the alkene (Fig. 7.89).
H
O
L
+
–
..
..
H
E1 reaction
E1cB reaction
H
C
C
H
C
C
H
C
C
C
C
+
+
C
H
C
Nu
Nu
L
L
L
L
Nu
..
–
–
..
Nu
..
–
FIGURE 7.89 The E1 and E1cB
reactions contrasted.
E1cB-Like,
C H more broken
than C L
Central,
pure E2
Transition states
E1-Like,
C L more broken
than C H
H
C
C
δ
+
δ
–
H
C
C
δ
+
δ
–
H
C
C
L
L
L
FIGURE 7.90 Three transition states
for an E2 elimination.
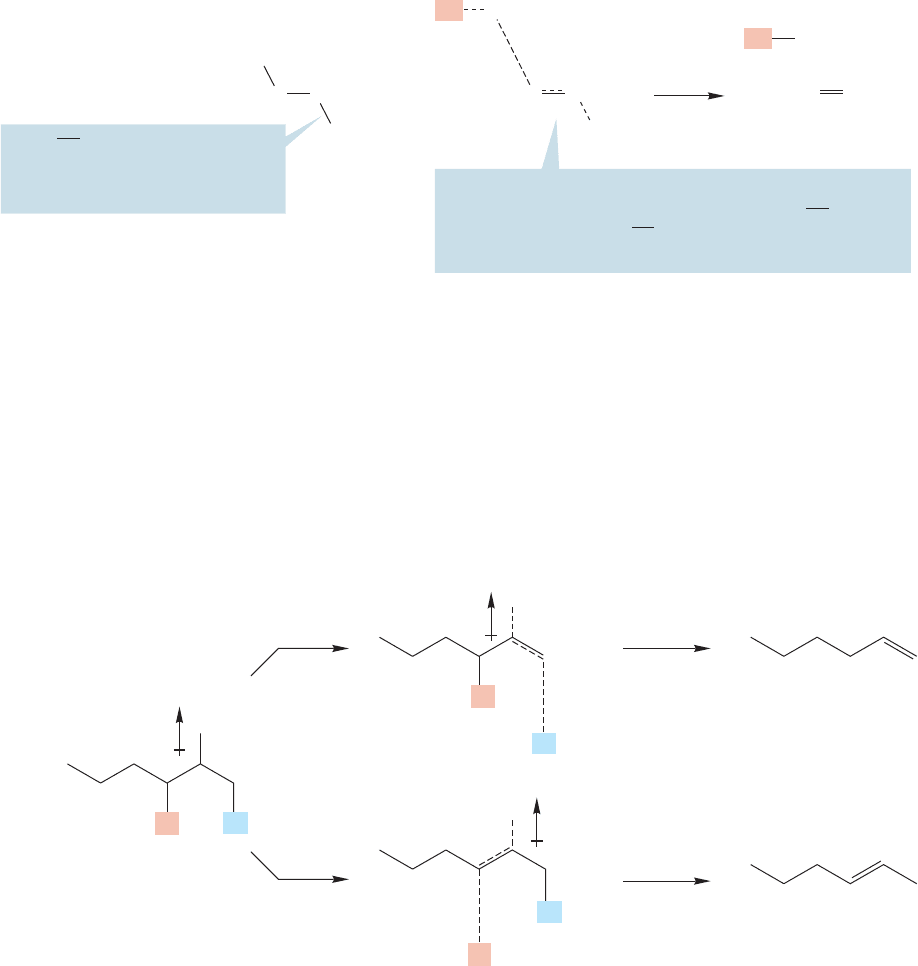
The E1cB reaction is relatively uncommon,but some examples are known.The
requirements for the E1cB reaction are a poor leaving group and a rather easily
lost proton.
PROBLEM 7.29 Design a good E1cB reaction. Hint: What kind of leaving group
do you need? What kind of carbon–hydrogen bond?
The E2 reaction of alkyl fluorides, ammonium ions,and sulfonium ions does not
go by the E1cB mechanism but the transition state is E1cB-like. In the transition
state for any E2 reaction, the bonds to hydrogen and the leaving group are both
breaking. In the E2 reaction, the transition state may or may not have the bonds to
H and L breaking roughly simultaneously. If breaking of the bond is fur-
ther advanced in the transition state than is breaking of the bond, the tran-
sition state will resemble the E1cB reaction. If the bond is more broken in
the transition state than is bond, the transition state is E1-like (Fig. 7.90).C
O
H
C
O
L
C
O
L
C
O
H
310 CHAPTER 7 Substitution and Elimination Reactions
..
..
..
CH
2
δ
–
The C F bond is polarized;
the very electronegative fluorine
bears a partial negative charge,
leaving the carbon partially positive
H
H
2
C
H
2
C
F
..
..
..
δ
–
_
δ
–
F
..
..
..
..
F
δ
+
CH
2
H
2
C
CH
2
δ
+
H
H
The partial positive charge on carbon favors a E1cB-like
transition state for elimination, in which the C H bond
breaks ahead of the C L bond, because this kind of
transition state places a
δ
–
near the existing δ
+
Nu
Nu
FIGURE 7.91 An E1cB-like transition state is favored by strongly electron-withdrawing groups, such as fluorine
and ’onium ions.
In the example shown in Figure 7.92, the transition state for Hofmann elimina-
tion has a partial negative charge on a primary carbon, whereas the transition state for
Saytzeff elimination has a partial negative charge on a secondary carbon (Fig. 7.92).
In an E1cB-like transition state, the carbon adjacent to the carbon bearing the
leaving group becomes partially negatively charged (Fig. 7.90). When the leaving
group is highly electronegative, as with fluoride, sulfonium, and ammonium, there
will be a partial positive charge on the carbon to which the leaving group is attached,
adjacent to that partial negative charge. For an E1cB-like transition state, this sit-
uation is favorable (Fig. 7.91).
In this transition state, there is
a partial negative charge on a
primary carbon
cis/trans Isomers
Saytzeff
Hofmann
H
b
H
a
δ
–
δ
–
F
..
..
..
H
a
H
b
H
a
F
..
..
..
F
..
..
..
H
b
This transition state, which
leads to the more substituted
alkene, has a partial negative
charge on a secondary carbon
FIGURE 7.92 In the E1cB-like transition state for Hofmann elimination, a partial primary carbanion (less
substituted, more stable) is formed. In the E1cB-like transition state for Saytzeff elimination, it is a partial
secondary carbanion (more substituted, less stable).
7.9 The Bimolecular Elimination Reaction: E2 311
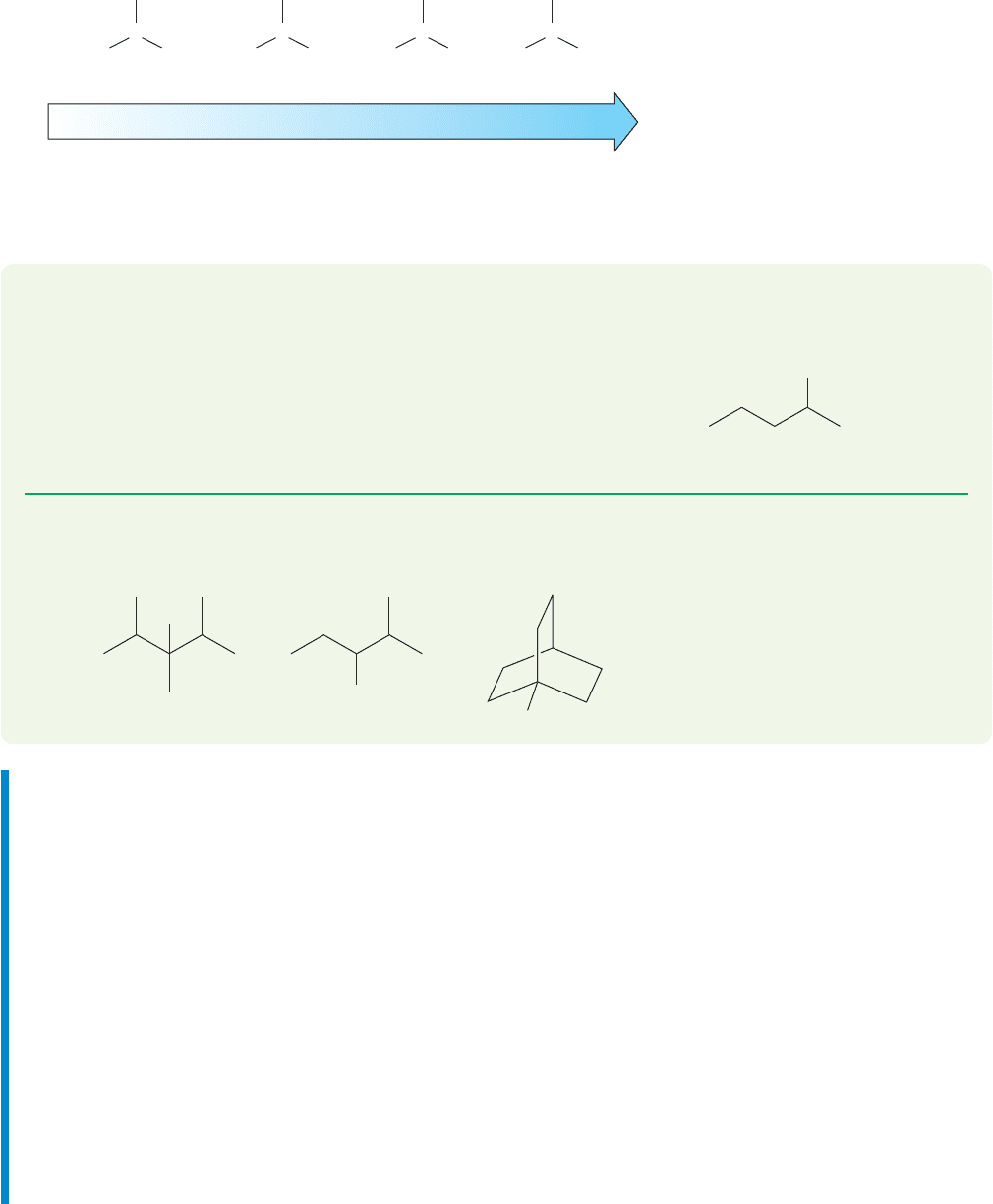
In contrast to carbocations and radicals,less substituted carbanions are more stable
than more substituted carbanions.Methyl and primary carbanions are more stable than
secondary carbanions, and secondary are more stable than tertiary (Fig. 7.93).
Tertiary
Carbanion stability
Secondary
Primary Methyl
HHHHH
H
RRR
RRR
C
–
..
C
–
..
C
–
..
C
–
..
FIGURE 7.93 The order of carbanion
stability is the opposite of that for
carbocations and radicals, partly
because of the greater ease of
solvation for the smaller carbanions.
So,the more favorable transition state is the one with the developing negative charge
on the primary carbon,the one leading to the less substituted,less stable alkene (Fig.7.92).
PROBLEM 7.30 How else can the nature of the leaving group determine the struc-
ture of the alkene? With the exception of F, the “Hofmann” leaving groups are all
large.Consider the possible products of an E2 reaction from the compound in the
figure at the right. First show what the possible products are and identify the dif-
ferent hydrogens that must be lost to produce them.Then draw Newman projec-
tions for the arrangements leading to the two products. Remember the preference
for 180° anti elimination in the E2 process. Finally, use the Newman projections
to explain why a large leaving group might favor Hofmann elimination.
Summary
We have already been introduced to addition reactions in Chapter 3 (p. 132).The
opposite of addition is elimination, in which two groups or atoms are lost with
the introduction of a π bond. There are two general mechanisms for elimination
reactions. First, there is a bimolecular E2 process in which starting material goes
to product over a single transition state.The E2 reaction resembles (and competes
with) the bimolecular S
N
2 reaction but is favored by a strong Brønsted base rather
than a strong Lewis base.The E2 elimination “prefers”an anti arrangement of the
groups being eliminated. The regiochemistry of the product alkene depends on
the nature of the leaving group. Most leaving groups favor the more substituted
alkene (Saytzeff elimination), but a few large and/or highly electronegative leav-
ing groups lead mainly to the less substituted alkene (Hofmann elimination).
Second, there is a unimolecular E1 elimination reaction that parallels (and
competes with) the S
N
1 substitution reaction. The S
N
1 and E1 reactions share
the same intermediate,the carbocation formed by ionization of the leaving group.
Once this carbocation is formed it can be captured by nucleophiles (S
N
1) or
deprotonated to give an alkene (E1). Saytzeff elimination is the rule.
+
N(CH
3
)
3
PROBLEM 7.31 Predict the products of reaction when each of the following com-
pounds reacts with hydroxide ion. Be careful with the molecule in (c).The answer
here is tricky.
(a)
(b)
(c)
Br
Br
Br
312 CHAPTER 7 Substitution and Elimination Reactions
7.10 What Can We Do with These Reactions?
How to Do Organic Synthesis
Your ability to make molecules has just increased enormously. From rather easily
accessible compounds, the alkyl halides and inorganic materials,myriad other struc-
tural types are suddenly available. Figure 7.94 summarizes the synthetic potential of
the substitution reaction. Not all reactions will work for all molecules and
elimination reactions will surely also occur.
R
O
L
Acetylide addition
+
OH
–
OH
NR
2
Alcohols
Tertiary amines
Ethers
OR
NR
3
Ammonium ion
s
SH
–
SH
CN
–
CN
Mercaptans
Cyanides
SR
N
3
–
N
3
Sulfides
Azides
SR
2
Sulfonium ions
Halides
NH
2
–
NH
2
CC
Primary amines
Acetylenes
NHR
HR
H
–
Secondary amines
Hydrocarbons
–
OR
–
SR
–
NR
2
NR
3
R
CC
–
R
–
NHR
R
2
S
R
L
R
R
R
R
R
R
R
R
R
R
R
R
R
–
X
X
+
FIGURE 7.94 The synthetic potential of the substitution reaction.
Beginning in the 1940s, the term
“organic”in connection with food
took on the extra meaning of “lacking
use of man-made pesticides,
herbicides, fertilizers, growth
regulators, or genetically modified
organisms.”This is clearly a misuse of
the word “organic”because all food is
“organic.”“Organic synthesis”deals
with the formation of organic
molecules, whether man-made or
Nature-made.
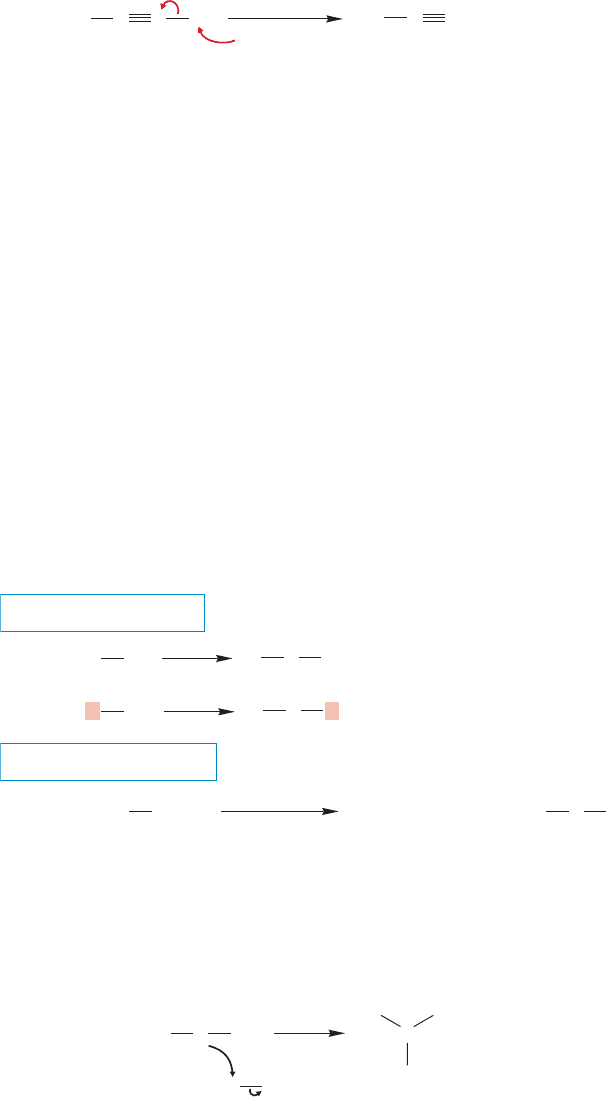
Figure 7.94 adds several reactions to the ones we have discussed specifically. For
example, it sneaks in a brand-new synthesis of substituted alkynes by using the
acetylide ion as a nucleophile (usually effective only with primary com-
pounds). We mentioned the acidity of acetylenes in Chapter 3 (p. 129) and even
R
O
L
7.10 What Can We Do with These Reactions? How to Do Organic Synthesis 313
warned that more was coming about this reaction. Monosubstituted (terminal)
alkynes are decent acids (pK
a
~25), which means that treatment of a terminal alkyne
with a strong base can result in removal of a proton and formation of the acetylide.
A favorite base for doing this is the amide ion,
NH
2
(Fig. 7.95). There are some
other new reactions in Figure 7.94. Go over each of them carefully and be sure you
see how each works.
–
..
..
..
..
..
CC
Acet
y
lide ion
R
CCH
Na
+
Na
NH
2
/ NH
3
..
NH
3
R
+
+
–
FIGURE 7.95 Formation of an
acetylide from a terminal alkyne.
Sulfides have two lone pairs of electrons, and these relatives of ethers are quite
nucleophilic. S
N
2 reactions of sulfides give sulfonium ions (Fig. 7.97).
THE GENERAL CASE
A SPECIFIC EXAMPLE
HS
..
..
..
IR
..
..
..
I
..
..
..
..
CH
3
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
S
N
2
–
–
++RS
..
..
H
RS
..
..
..
Br
..
..
..
..
..
..
S KBrK
+
..
..
..
Br
..
..
..
..
S
N
2
–
–
–
++S
..
..
S
(~100%)
..
..
R
CH
3
CH
2
Br
25 ⬚C
+
RR
FIGURE 7.96 The S
N
2 reactions
of mercaptide and substituted
mercaptides lead to thiols and
sulfides.
+ I
..
..
..
..
I
..
..
..
CH
3
CH
3
CH
3
S
..
..
S
A sulfonium ion
..
H
3
C
H
3
C
H
3
C
S
N
2
+
–
FIGURE 7.97 Sulfides can also be
alkylated to give sulfonium ions.
It is relatively easy (note that we didn’t say it was easy!) to keep track of
the available reactions and therefore not too difficult to think up one-step trans-
formations. Once synthetic chemistry goes beyond one-step reactions, however,
it gets much harder. Now, the intellectual challenge of synthetic chemistry
increases sharply, and synthesis problems, whether in the real world or in this
book, become not only more challenging, but much more fun. One can begin to
see why the art of making molecules fascinates so many people. Let’s look at a
few examples.
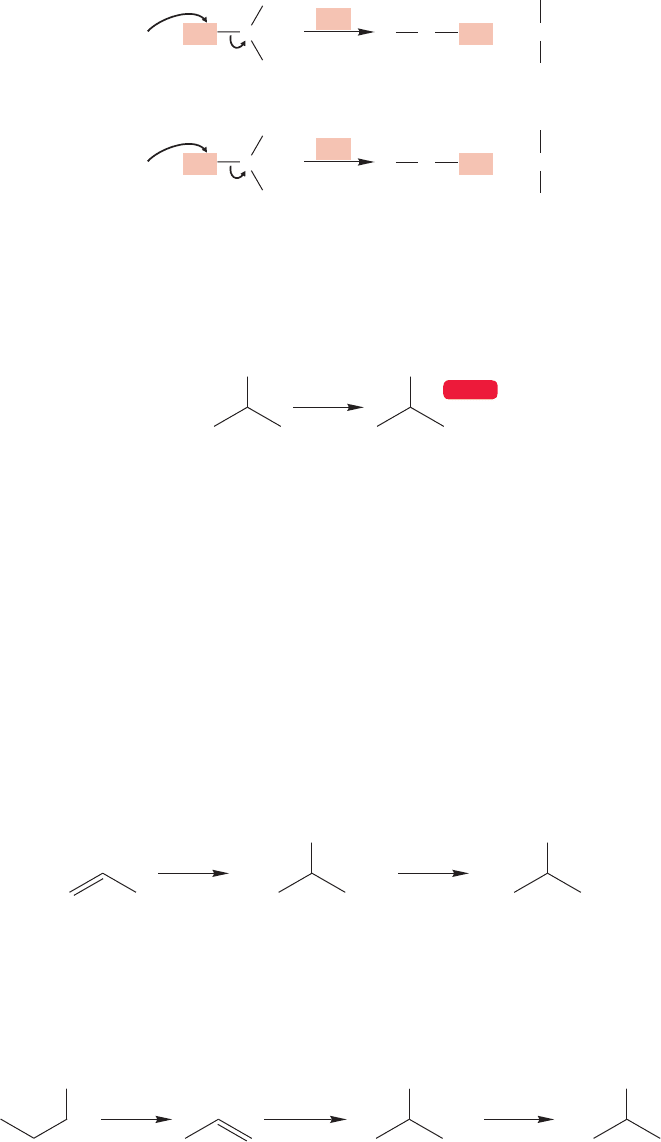
7.10a Sulfur as Nucleophile Suppose we are set the task of making a thiol
from an alkyl halide. We will use the nucleophilicity of sulfur to accomplish this
transformation. The mercaptide ion itself (
SH) and alkyl mercaptides (
SR) are
powerfully nucleophilic and can be used to great effect in the S
N
2 reaction. This
nucleophilicity leads to the most common synthesis of thiols and sulfides. The
only serious restriction is that the alkyl halide must be active in the S
N
2 reaction
(Fig. 7.96).
314 CHAPTER 7 Substitution and Elimination Reactions
+
–
RO
..
..
..
CH
3
CH
3
CH
3
CH
3
SSH
3
C
+
S
N
2
CH
3
O
..
..
R
..
..
..
+
–
RO
..
..
..
CH
3
CH
3
CH
3
CH
3
OOH
3
C
+
S
N
2
CH
3
O
..
..
R
..
..
..
FIGURE 7.98 Alkylations by
sulfonium and oxonium ion.
Sulfonium ions and the related, less stable, oxonium ions are used as alkylating
agents. Dimethyl sulfide and dimethyl ether are excellent leaving groups and can be
displaced by nucleophiles in S
N
2 reactions (Fig. 7.98). As we have already seen
(p. 288), Nature uses a variant of this reaction,displacement on the amino acid deriv-
ative S-adenosylmethionine, to do many methylation reactions.
So, if we were set the task of synthesizing a specific thiol, say, propane-2-thiol,
we have an answer right at hand: Just treat isopropyl bromide with thiolate, HS
.
You might get a little elimination product as well,but the reaction should work quite
well. That’s an easy problem, at least if you know the reaction in Figure 7.99.
WEB 3D
Br SH
2-Bromopropane Propane-2-thiol
HS
–
FIGURE 7.99 The conversion of
2-bromopropane into propane-2-thiol.
Br
Propene 2-Bromopropane
HBr
SH
Propane-2-thiol
HS
–
FIGURE 7.100 The two-step
conversion of propene into propane-
2-thiol.
BrBr
Propene 2-Bromopropane
HBr
1-Bromopropane
(CH
3
)
3
CO
–
SH
Propane-2-thiol
HS
–
(addition)(E2) (S
N
2)
FIGURE 7.101 A three-step sequence
for converting 1-bromopropane into
propane-2-thiol.
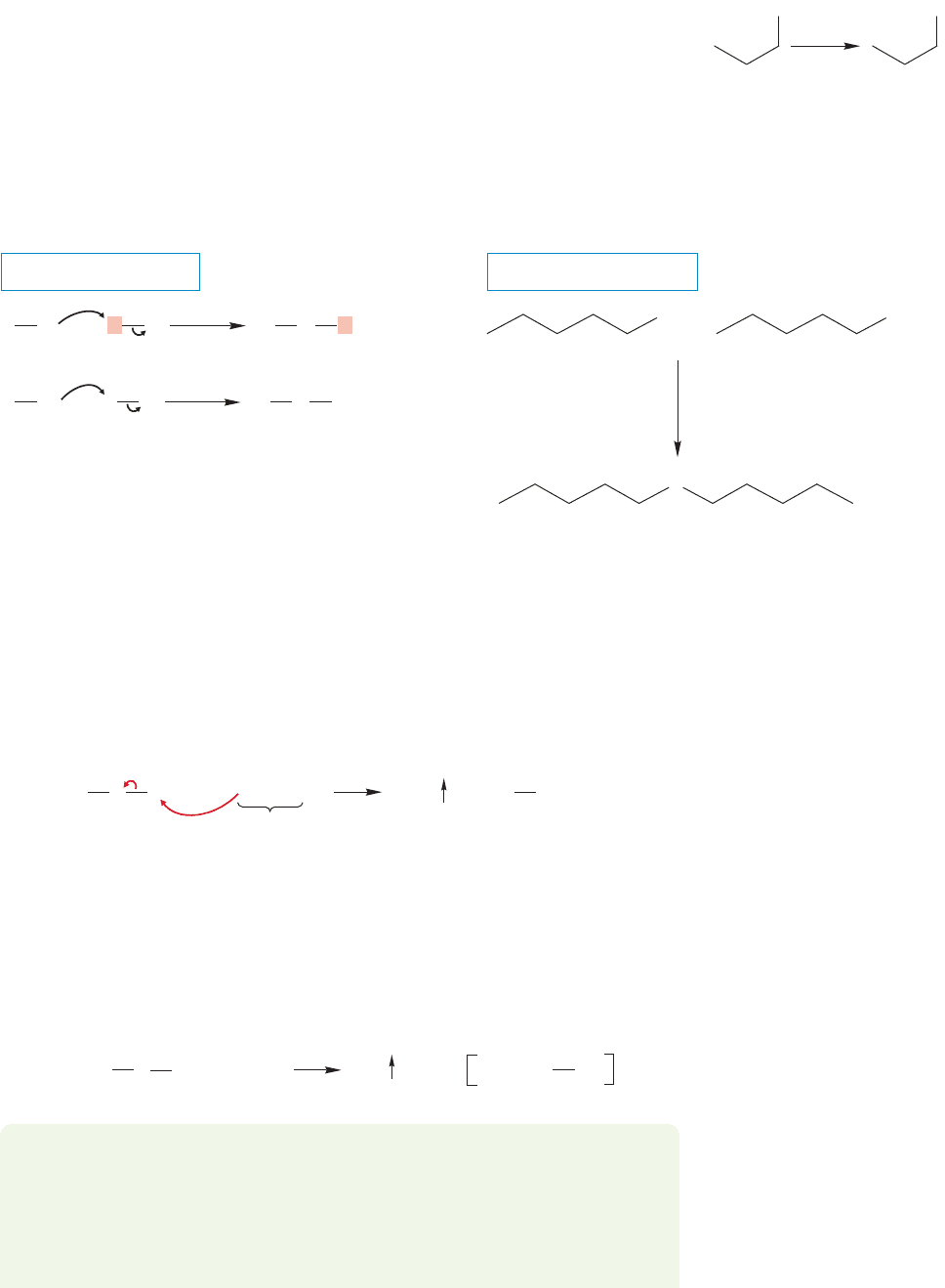
Now, however, suppose we had to make propane-2-thiol not from an alkyl halide,
but from propene. We have no direct way to do this transformation, and Figure 7.94 is
no help. But we do have a way to convert propene into 2-bromopropane (Chapter 3,
p. 132). So, a two-step synthesis is possible (Fig. 7.100), but it is much harder to con-
jure up than the simple one-step method. By far the best way to find it is to work
backward from the desired product, asking yourself the question, From what imme-
diate precursor molecule or molecules can I get the ultimate product? In the reaction
we are looking at here,the answer to that question should lead you to 2-bromopropane,
among others. Now ask again, From what immediate molecule or molecules can I
get 2-bromopropane? The answer to that question should include propene plus HBr.
Working backward in this way is the only successful route to solving problems in
synthesis, and all successful chemists, both beginners and pros, do it this way.
Now let’s make the sequence even harder and ask how to make propane-2-thiol from
1-bromopropane. Now a three-step sequence is necessary, and one suggestion is shown
in Figure 7.101.Be sure you go through the process of working backward for Figure 7.101.
7.10 What Can We Do with These Reactions? How to Do Organic Synthesis 315
7.10b Oxygen as Nucleophile: The Williamson Ether Synthesis Here’s
another synthetic challenge that uses propyl bromide: Make methyl propyl ether.
Given our discussion of Figure 7.99, you have almost certainly come up with the
idea of displacing bromide ion from 1-bromopropane with methoxide in a straight-
forward S
N
2 sequence (Fig. 7.102). Good idea! In fact, you have just invented a
good way to make ethers—well, not exactly, because A. W. Williamson
(1824–1904) discovered this process, now called the Williamson ether synthesis,
over 100 years ago (Fig. 7.103). There are both limitations and opportunities in this
synthesis, and they rather nicely illustrate some of the kinds of thinking that syn-
thetic chemists have to go through. So let’s explore the Williamson synthesis a bit.
THE GENERAL CASE
A SPECIFIC EXAMPLE
++ORR R
..
..
..
..
XX
S
N
2
OR
..
..
–
–
+
+
+ORR
..
..
O
B
r
70 ⬚C, 6 h
(53%)
DMSO
..
..
..
..
XX
S
N
2
ORR
..
..
–
–
..
..
O
..
..
..
–
FIGURE 7.103 Alkoxides can displace halides in
an S
N
2 reaction to make ethers.This reaction is
the Williamson ether synthesis.
First of all,we need to be able to make the necessary alkoxide.As we saw in Chapter
6 (p. 236), when an alcohol is treated with a much stronger base, it can be converted
entirely into the alkoxide. Note that the base used must be much stronger than the
alkoxide. If that is not the case, an equilibrium mixture of the base and the alkoxide
will result. A favorite reagent for forming alkoxides is sodium hydride (Fig. 7.104).
Sodium hydride has the advantage of producing hydrogen gas, which can easily be
removed from the reaction mixture, thus driving the equilibrium toward the alkoxide.
Na
H
H
2
R
H
+
+
O
..
..
..
..
..
–+
Na
+
–
Sodium
h
y
dride
R
O
..
..
FIGURE 7.104 Sodium hydride
irreversibly removes a proton to give
the alkoxide, the conjugate base of
the alcohol.
Just as water reacts with metallic sodium or potassium to give the corresponding
metal hydroxide,alkoxides can be made through a reaction in which sodium or potas-
sium metal is dissolved directly in the alcohol. Hydrogen is liberated in what can be
a most vigorous reaction indeed.Potassium is particularly active in all cases, and the
reaction of sodium metal with smaller alcohols, such as methyl and ethyl alcohol, is
also rapid and exothermic (Fig. 7.105).
2 Na
H
2
2
+
.
Na
+
–
H
+
2 CH
3
H
CH
3
O
..
..
O
..
..
..
FIGURE 7.105 Alkoxides can be
made through the reaction of metallic
sodium or potassium with alcohols.
Br OCH
3
CH
3
O
–
S
N
2
FIGURE 7.102 The Williamson ether
synthesis.
WORKED PROBLEM 7.32 Is a molar equivalent amount of sodium hydroxide an
effective reagent for forming sodium ethoxide from ethyl alcohol? Explain your
answer using Table 7.4.
CH
3
CH
2
O
O
:
.
.
.
.
-
Na
+
+ H
2
O
.
.
:
U
Z
CH
3
CH
2
O
O
.
.
.
.
O
H + Na
+
-
:
O
.
.
.
.
H
(continued)
316 CHAPTER 7 Substitution and Elimination Reactions
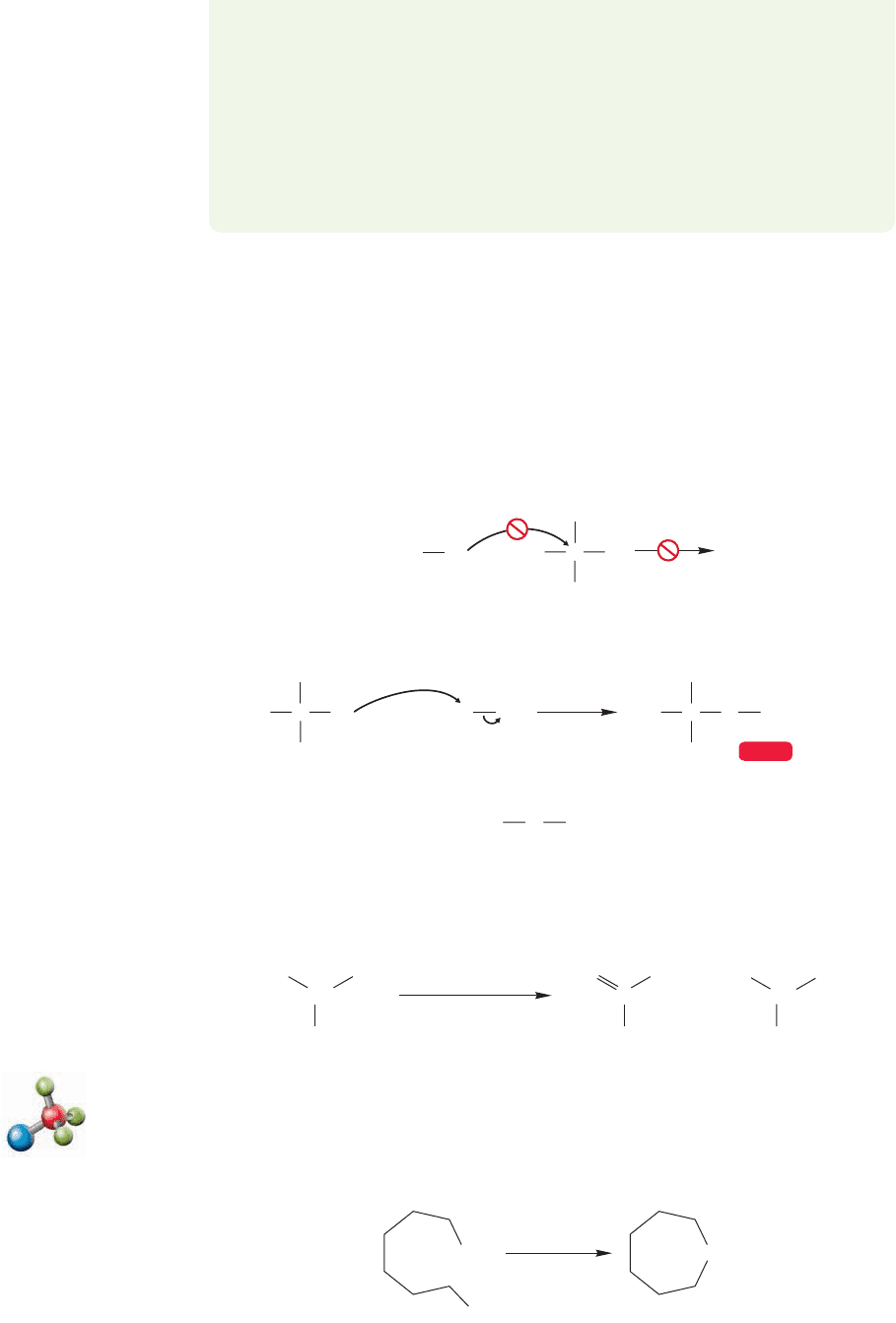
Now let’s use alkoxides to make ethers—the Williamson ether synthesis. Several
modifications of the original procedure have made this venerable method quite use-
ful, although there are some restrictions. The reaction works only for alkyl halides
that are active in the S
N
2 reaction.Therefore, tertiary halides cannot be used. They
are too hindered to undergo the crucial S
N
2 reaction. Sometimes there is an easy
way around this problem, but sometimes there isn’t. For example, tert-butyl methyl
ether cannot be made from tert-butyl iodide and sodium methoxide, but it can be
made from tert-butoxide and methyl iodide (Fig. 7.106). However, there is no way
to use the Williamson ether synthesis to make di-tert-butyl ether.
Impossible on tertiary halide
O
..
..
..
I
..
..
..
H
3
CH
3
C
S
N
2
CH
3
CH
3
C
–
+
The Williamson ether synthesis cannot
be used to make di-tert-butyl ether
I
..
S
N
2
H
3
C
CH
3
CH
3
C OH
3
C
CH
3
CH
3
CH
3
C
(CH
3
)
3
C O C(CH
3
)
3
..
..
..
IH
3
C
–
O
..
..
..
–
..
..
..
..
..
WEB 3D
FIGURE 7.106 How to use the
Williamson ether synthesis.
Even secondary halides are of little use in the Williamson reaction. Alkoxides
are strong bases (high affinity for a hydrogen 1s orbital) and the E2 reaction is a
prominent side reaction when a secondary halide is used (Fig. 7.107).
+
CH
3
Na
+ –
OCH
2
CH
3
HOCH
2
CH
3
H
3
C
CH
Br
CH
3
H
2
C
C
H
E2
(75%)
CH
3
H
3
C
CH
OCH
2
CH
3
S
N
2
(25%)
FIGURE 7.107 Secondary halides
undergo substantial E2 elimination
with alkoxides.
ANSWER The pK
a
values for water (15.7) and ethyl alcohol (15.9) are very close,
and the equilibrium concentration of sodium ethoxide will be slightly less than
that of hydroxide ion. Hydroxide ion is a slightly weaker base than ethoxide ion
in solution. Thus, hydroxide will not be effective in making large amounts of
ethoxide. At equilibrium, both species will be present:
pK
a
15.9 pK
a
15.7
CH
3
CH
2
O
O
-
+ H
O
O
O
H
Z——
U
CH
3
CH
2
O
O
O
H +
-
OH
Be alert for intramolecular, ring-forming versions of the Williamson ether synthe-
sis. Cyclic ethers can be formed by intramolecular S
N
2 reactions.There is no mystery in
this reaction;it is simply the acyclic process applied in an intramolecular way (Fig.7.108).
Intramolecular S
N
2
p-xylene
140 ⬚C, 7 h
(33%)
OH
KOH
Br
O
FIGURE 7.108 An intramolecular
Williamson ether synthesis.
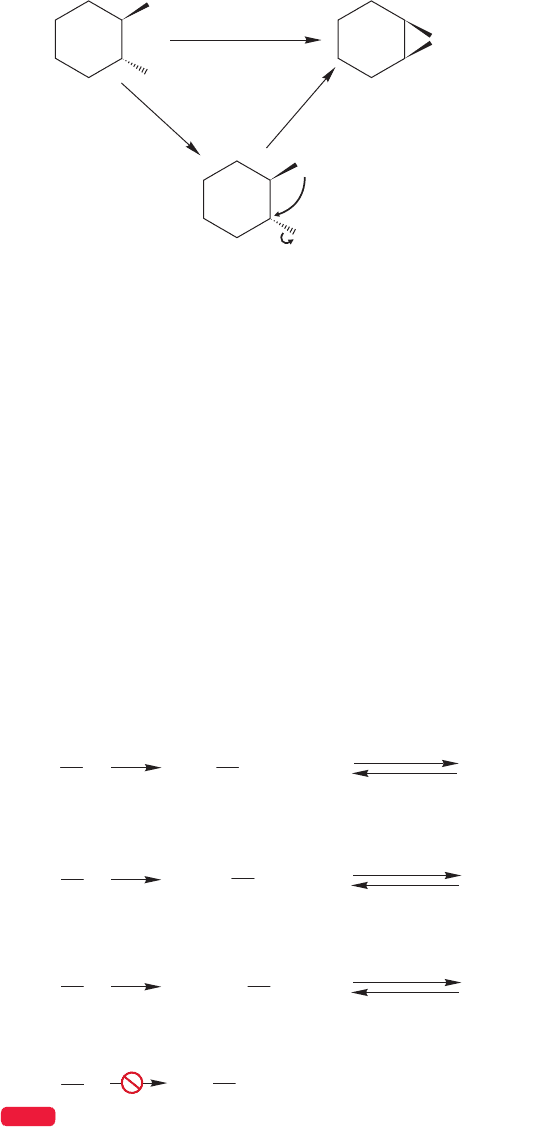
7.10 What Can We Do with These Reactions? How to Do Organic Synthesis 317
+
+
O
An oxirane
(epoxide)
(73%)
..
..
OH
NaOH/H
2
O
NaOH/H
2
O
A
chlorohydrin
S
N
2
25 ⬚C, 1 h
..
..
O
..
..
..
Cl
..
..
..
..
Cl
..
..
..
Cl
..
..
..
–
–
..
..
H
2
O
FIGURE 7.109 A common epoxide
synthesis takes advantage of an
intramolecular S
N
2 reaction.
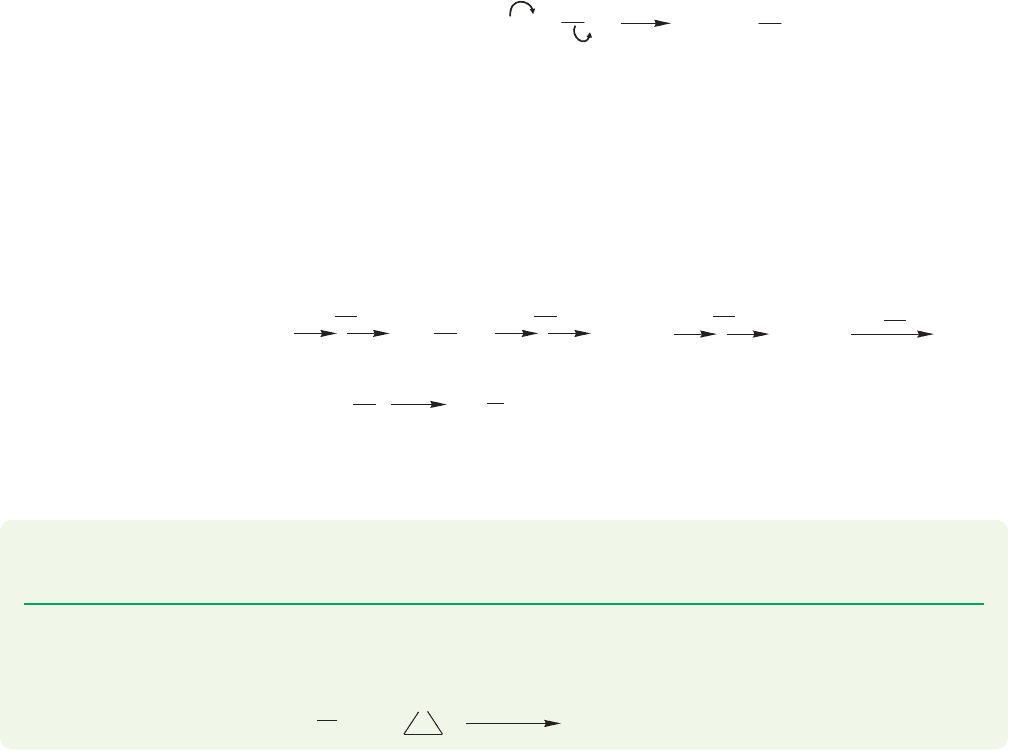
7.10c Nitrogen as Nucleophile: Alkylation of Amines We have just exam-
ined a series of synthetically useful reactions that uses oxygen as the nucleophile.
Nitrogen has a lone pair of electrons, and therefore is also nucleophilic. Let’s look
here at some synthetic reactions in which nitrogen is the nucleophile. Conceptually,
the following reactions are rather closely related to the Williamson ether synthesis.
Amines are good nucleophiles and can displace leaving groups in the S
N
2 reaction.
Of course, the limitations of the S
N
2 reaction also apply—there can be no displace-
ments at tertiary carbons. Successive displacement reactions yield primary, second-
ary, and tertiary amines. The initial products are alkylammonium ions that are
subsequently deprotonated by the amine present. These S
N
2 reactions produce
amines that are also nucleophilic. Further displacement reactions are possible,
which can lead to problems. Indeed, the product amine is often a better participant
in the S
N
2 reaction than the starting material. Therefore it can be difficult to stop
the reaction at the desired point, even if an excess of the starting amine is used to
minimize overalkylation (Fig. 7.110).
An important use of the intramolecular Williamson ether synthesis is in the mak-
ing of oxiranes, ethers with the oxygen atom in a three-membered ring, also called
epoxides.A 2-halo-1-hydroxy compound (a halohydrin) is treated with base to form
the haloalkoxide, which then undergoes an intramolecular backside S
N
2 displace-
ment to give the three-membered ring (Fig. 7.109).
..
CH
3
S
N
2
II
+
NH
3
H
3
N
..
H
3
N
CH
3
++
H
2
NCH
3
Primary amine
–
I
–
..
..
NH
4
(CH
3
)
3
C
S
N
2
II
H
3
N
..
H
3
N
C(CH
3
)
3
+
–
CH
3
S
N
2
II
+
(CH
3
)
2
NH
(CH
3
)
2
NH
(CH
3
)
2
NH
No S
N
2 for tertiary
substrates!
CH
3
++
N(CH
3
)
3
Tertiary amine
But
–
I
–
(CH
3
)
2
NH
2
..
..
..
CH
3
S
N
2
II
+
CH
3
NH
2
CH
3
NH
2
CH
3
NH
2
CH
3
++
HN(CH
3
)
2
Secondary amine
–
I
–
CH
3
NH
3
..
..
WEB 3D
FIGURE 7.110 Alkylation reactions
of amines through S
N
2 displacement
followed by deprotonation.
318 CHAPTER 7 Substitution and Elimination Reactions
Tertiary amines are also strong nucleophiles,and a final displacement reaction can
occur to give a stable quaternary ammonium ion (Fig. 7.111). Thus, the alkylation
process can be pushed to its logical conclusion in an effective synthesis of quaternary
ammonium ions.
CH
3
S
N
2
II
(CH
3
)
3
N
(CH
3
)
3
N
CH
3
+
–
..
Tetramethylammonium
iodide
FIGURE 7.111 The formation of an
ammonium ion through alkylation
of a tertiary amine.
These alkylation reactions of amines are so efficient that it is difficult to stop at
monoalkylation. However, Theodore Cohen (b. 1929) and his co-workers at the
University of Pittsburgh worked out a method that uses ammonia in excess under
pressure to generate primary amines efficiently. The vast excess of ammonia means
that it is difficult for the initial alkylated product, methylamine, to compete with
ammonia for a molecule of methyl iodide (Fig. 7.112).
H
3
N
+
CH
3
HN(CH
3
)
2
N(CH
3
)
4
N(CH
3
)
3
H
2
N
–
I
+
..
..
..
..
H
3
N
..
H
2
N
CH
3
CH
3
Excess
I
..
CH
3
I
CH
3
ICH
3
ICH
3
I
FIGURE 7.112 The synthesis of alkylamines through alkylation reactions (S
N
2 reactions).
PROBLEM 7.33 What product do you expect from an attempt to make tert-butylamine
from the reaction of the amide ion (
NH
2
) with tert-butyl bromide?
PROBLEM 7.34 Another nucleophilic synthesis of amines involves the reaction of
simple amines with epoxides. Write a mechanism for the reaction below.
O
CH
3
CH
2
NHCH
2
CH
2
OH
CH
3
CH
2
N(CH
2
CH
2
OH)
2
CH
3
CH
2
NH
2
H
2
O
+
+
7.10d “Real-World” Difficulties In the real world, the challenges of synthe-
sis are great—one needs not only to find a reaction or, more likely, a sequence of
reactions that will make the desired molecule, but one must do so specifically and,
in industry, economically. Specificity becomes increasingly important these days as
we recognize how critical chirality is in biological action. And, of course, the more
stereogenic centers (p. 152) a molecule has, the more difficult the synthesis. Imagine
making a molecule with only a modest number of stereogenic atoms,say 4.There are
16 possible isomers and you almost certainly can use only 1. Every reaction you use
must lead to the correct stereochemistry and no others.
Although it is easy to look down one’s nose at questions of cost, one would be very
wrong to do so.Chemists in industry must find sophisticated ways to make those stereo-
chemically rich molecules without using superexpensive reagents that might be fine
in very small-scale work but utterly useless in work designed to produce a molecule to
be used by millions of people.Doing clever syntheses with cheap reagents is much more
difficult,both intellectually and practically, than making the molecule regardless of cost.
