Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


7.10 What Can We Do with These Reactions? How to Do Organic Synthesis 319
PROBLEM 7.35 Predict the products of the following reactions:
(a) HO
CH
3
Br
(b) HO
(CH
3
)
3
CBr
(c) H
2
O (CH
3
)
3
CBr
PROBLEM 7.36 Devise syntheses for the following molecules. You may use any
inorganic reagent, organic iodides containing up to four carbons, and the special
reagents potassium cyanide and propyne.
U
U
U
PROBLEM 7.37 Indicate which member of the following pairs of compounds
will react faster in (1) the S
N
1 reaction and (2) the S
N
2 reaction. Explain your
reasoning.
(a) 1-Bromobutane and 2-bromobutane
(b) 1-Chloropentane and cyclopentyl chloride
(c) 1-Chloropropane and 1-iodopropane
(d) tert-Butyl iodide and isopropyl iodide
(e) tert-Butyl iodide and tert-butyl chloride
PROBLEM 7.38 Devise synthetic routes that will produce the following com-
pounds as the major products from each of the indicated starting materials.
CN
(a) (b) (c) (d) (e)
OCH
3
(CH
3
)
3
C
N
3
(CH
3
)
3
C
N
3
CH
2
Br
from or
CN
(b)
H
3
C H
3
C
CH
CH
2 CH
3
Br
CH
CH
2
H
3
C
CH
2
CH
3
CH
CH
2
from
or
(c)
H
3
C
Br
H
3
C
OH
H
3
C OH from ororC
CH
3
CH
3
H
3
CBrC
CH
3
CH
3
H
3
CCH
CH
3
CH
2
Br
(a)
H
3
C
H
3
C
CCH
2
320 CHAPTER 7 Substitution and Elimination Reactions
PROBLEM SOLVING
Keeping track of synthetic methods can be difficult.You are right at the beginning
of synthetic chemistry now, and here is a hint on how to keep track of it. Make a
4 6-inch file card for every structural type and then keep a short list of the
available synthetic reactions on the card.To help in ordering things, and to keep as
far as possible from the dreaded mindless memorization, you might make a
mechanistic notation for each reaction as well. The reaction of acetylides with
alkyl halides to make new acetylenes might get an “S
N
2 with primary substrates”
beside it, for example. You can start your catalog with the reactions in Figure 7.94.
Be sure to keep up to date; synthetic ability is the kind of thing that sneaks up on
you, like whatever it was that worried Mr. Paige, as quoted at the beginning of this
chapter. A sample file card for alkyl bromides is shown in Figure 7.113.
PROBLEM 7.39 Use the following pK
a
data to decide whether sodium amide would
be effective at producing vinyl anions ( ). pK
a
: ammonia, 38; alkene, 50.
'
R
2
C
P
CH
-
Reaction Mechanism
Br
–
S
N
2
Notes
Needs good leaving group
+
Br
–
S
N
1
tert R group, polar solvent
+
L
L
R
R
E1, E2 compete
Primary or secondary R
E2 competes
Alkyl Bromides
FIGURE 7.113 A sample file card for
alkyl bromides.
7.11 Summary
New Concepts
The most important idea in this chapter is the continuing
expansion of the concept of Brønsted acidity and basicity to
embrace the species known as Lewis acids and Lewis bases.
The relatively narrow notion that acids and bases compete for
a proton (Brønsted acids and bases) is extended to define all
species with reactive pairs of electrons as Lewis bases and all
molecules that will react with Lewis bases as Lewis acids. The
key word here is reactive. All bonds are reactive under some
circumstances, and therefore all molecules can act as Lewis
bases at times.
Figure 7.94 fails to show the potential complications we have talked about.
Remember: There is always a competition between the substitution reactions, S
N
1
and S
N
2, and the E1 and E2 elimination reactions. Under some circumstances the
major products of the reactions summarized in Figure 7.94 will be alkenes. Your file
card catalog should include the synthesis of alkenes through the E1 and E2 reac-
tions. The identity of the leaving group, so conveniently avoided by using the sym-
bol L in the figure, makes a difference,and the structure of R,also deliberately vague
in the figure, is vital. In order to design a successful synthesis, you have to think about
all the components of the reaction.
7.11 Summary 321
Another way to describe the reaction of Lewis bases with
Lewis acids is to use the graphic device showing the stabiliz-
ing interaction of a filled orbital (Lewis base) with an empty
orbital (Lewis acid) (Fig. 7.5). This idea can be generalized if
you recall that the strength of orbital interaction depends on
the energy difference between the orbitals involved. The closer
the orbitals are to each other in energy, the stronger is their
interaction and the greater is the resulting stabilization.
Therefore the strongest interactions between orbitals are likely
to be between the highest occupied molecular orbital
(HOMO) of one molecule (A, a Lewis base) and the lowest
unoccupied molecular orbital (LUMO) of the other (B,a
Lewis acid) (Fig. 7.7). This notion is used in this chapter to
explain the overwhelming preference for backside attack in
the S
N
2 reaction.
Stereochemical experiments are used here for the first
time to probe the details of reaction mechanisms. The
observation of inversion of configuration in the S
N
2
reaction shows that the incoming nucleophile must approach
the leaving group from the rear, the side opposite the
leaving group. Similarly, if the stereochemistry of the E2
reaction is monitored, it can be shown that there is a
strong preference for the anti (180°) E2 reaction in acyclic
molecules.
The curved arrow formalism is used extensively in this
chapter. This bookkeeping device is extremely useful in keeping
track of the pairs of electrons involved in the making and
breaking of bonds in a reaction.
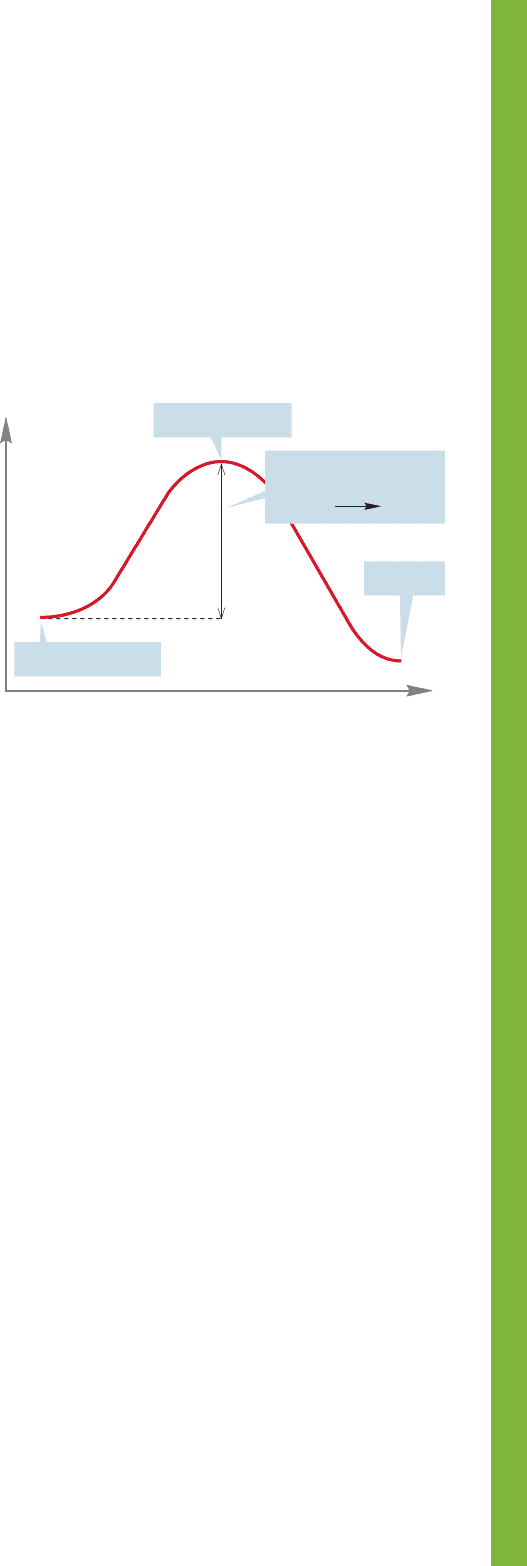
The terms transition state (the structure representing
the high-energy point separating starting material and
products) and activation energy (the height of the transition
state above the starting material) appear several times in
this chapter. Chapter 8 will continue a detailed discussion
of these terms (Fig. 7.114).
Energy
Reaction progress
Transition state
Product
Starting material
Activation energy
for starting
material product
FIGURE 7.114 A typical Energy versus Reaction progress diagram.
Key Terms
anti elimination (p. 305)
E1cB reaction (p. 309)
E1 reaction (p. 298)
E2 reaction (p. 301)
elimination reaction (p. 298)
epoxide (p. 317)
Hofmann elimination (p. 308)
inversion of configuration (p. 269)
leaving group (p. 267)
nucleophilicity (p. 278)
oxirane (p. 317)
product-determining step (p. 294)
rate-determining step (p. 294)
reaction mechanism (p. 263)
regioselective reaction (p. 307)
retention of configuration (p. 269)
Saytzeff elimination (p. 300)
S
N
1 reaction (p. 289)
S
N
2 reaction (p. 268)
solvolysis reaction (p. 290)
substitution reaction (p. 262)
sulfonium ion (p. 313)
syn elimination (p. 305)
thionyl chloride (p. 284)
Williamson ether synthesis (p. 315)
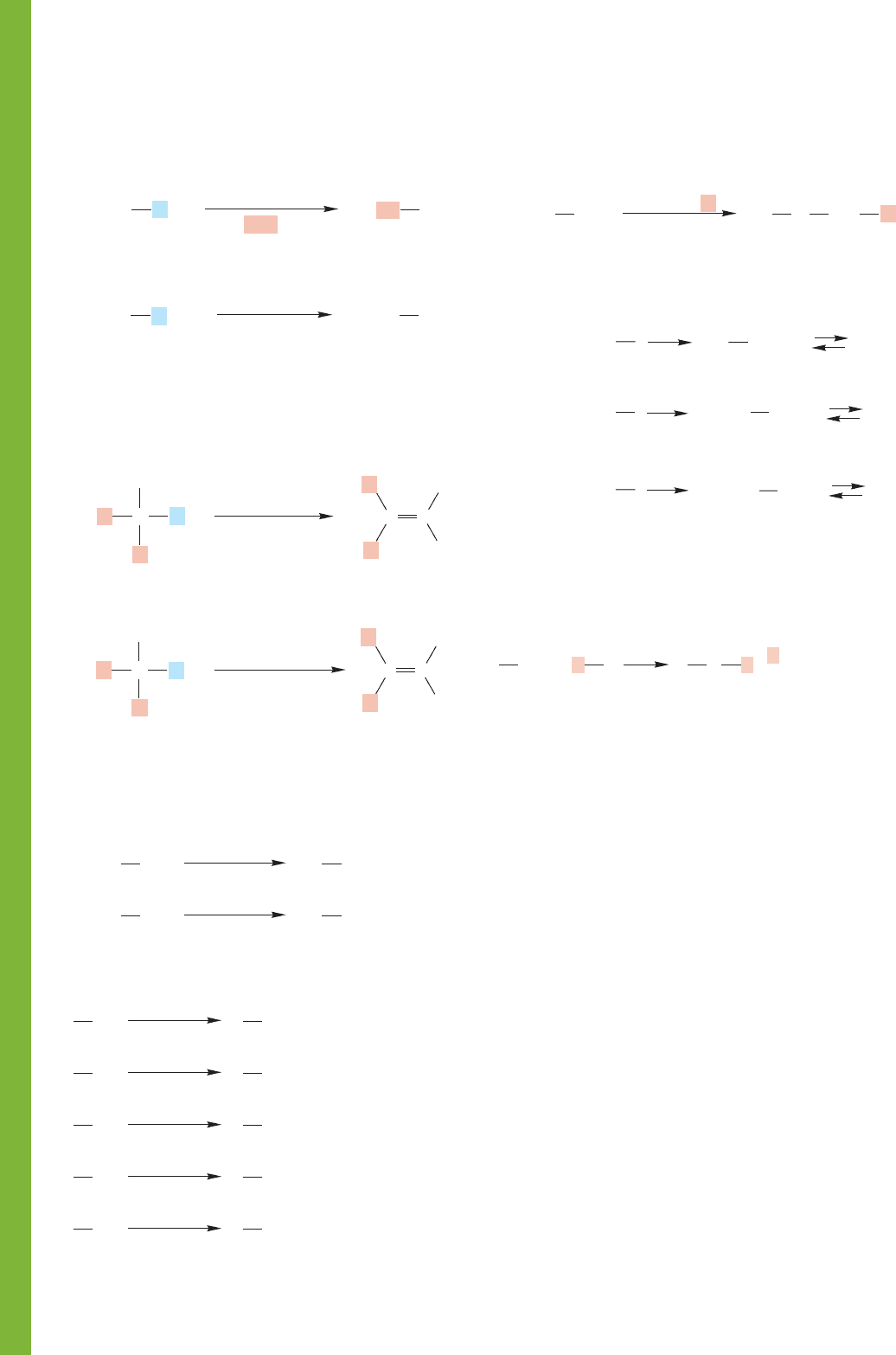
Reactions, Mechanisms, and Tools
Four exceptionally important prototypal reactions are
introduced in this chapter: the S
N
1, S
N
2, E1, and E2
reactions. The E1 and S
N
1 reactions involve the same
carbocationic intermediate. Addition of a nucleophile com-
pletes the S
N
1 reaction; removal of a proton finishes the E1
(Fig. 7.70).
The S
N
2 reaction and the E2 reaction are also competitive
processes. In the S
N
2 reaction, a nucleophile attacks the sub-
strate at the rear of a carbon–leaving group bond. In the E2
reaction, it is the adjacent hydrogen that is removed along with
the leaving group (Fig. 7.74).
For primary substrates, it is the S
N
2 and E2 reactions that
compete. For tertiary substrates in polar solvents with weak
bases, it is the S
N
1 and E1 reactions that are important. If
strong bases are employed in nonionizing solvents, E2 reac-
tions are most important. For secondary substrates all four
reactions compete.
It is absolutely vital to have these four building block reac-
tion mechanisms under complete control.
322 CHAPTER 7 Substitution and Elimination Reactions
Syntheses
5. Alkyl Sulfonates
OH
These sulfonates are good substrates for further
conversions using S
N
1, S
N
2, E1, and E2 reactions
Cl—SO
2
SO
2
O
R
—
R
R
R
2. Alkenes
polar solvent
E1 reaction. Must be a tertiary or secondary halide. Saytzeff
elimination. Competes with the S
N
1 reaction
CH
2
R
CCC
strong base
E2 reaction. Competes with S
N
2, which dominates for
primary substrates. anti Elimination favored, regiochemistry
depends on leaving group
CH
2
R
H
CC
L
R
R
L
R
H
C
R
R
R
R
R
R
R
7. Ethers
1. Substituted Alkanes
S
N
2 reaction. The R must be methyl, primary, or secondary
Always goes with inversion. The E2 reaction is competitive
HOS
SO
strong nucleophile
S
N
1 reaction. The R must be tertiary or secondary.
Stereochemical result is largely racemization.
The E1 reaction competes. HOS is
a polar, protic solvent
polar solvent
L
L
R
R
R
R
Nu
Nu
..
–
3. Alkoxides
Na
+ –
H
OHR O
–
Na
+
H
2
+R
Na
OHR O
–
Na
+
H
2
+R
6. Amines
4. Alkyl Halides
OH
This reaction can be S
N
1 or
S
N
2, but the intermediate is
always the protonated alcohol
This reaction can be S
N
1 or
S
N
2, but the intermediate is
a chlorosulfite ester
Inversion is the usual result
HX
R
R
X
OH
SOCl
2
R
R
Cl
OH
CCl
4
(Ph)
3
P
R
R
Cl
OH
PCl
5
R
R
Cl
OH
PBr
3
R
R
Br
H
3
N
S
N
2
+ CH
3
Primary
amine
I
CH
3
I
–
H
3
N CH
3
I
–
H
2
NCH
3
HN(CH
3
)
2
CH
3
NH
2
Restrictions on S
N
2 reaction apply;
overalkylation is a serious problem
+
+
CH
3
NH
2
S
N
2
+ CH
3
Secondary
amine
I
CH
3
N(CH
3
)
3
(CH
3
)
2
NH
+
(CH
3
)
2
NH
S
N
2
+ CH
3
Tertiary
amine
I
I
–
O
–
R XR OR R
Williamson ether synthesis.
R must be able to undergo
the S
N
2 reaction—it cannot
be tertiary
+
The S
N
1 and S
N
2 reactions allow the conversion of alkyl
halides into a host of new structures. These reactions were
summarized earlier in Figure 7.94. Remember that the S
N
2
reaction works only for primary and secondary halides and
the S
N
1 reaction works only for tertiary and secondary
molecules.
Alcohols can also be used as starting materials in the S
N
1
and S
N
2 reactions, but the OH group is a very poor leaving
group and must be transformed either by protonation into
water or by other reactions into some other good leaving
group.

7.12 Additional Problems 323
Common Errors
Students of organic chemistry long for certainty. They have not
yet learned to love the messiness of the subject or at least to
accommodate to it. That the answer to the question, What
happens if we treat A with B? might be “All sorts of things” can
be profoundly unsettling. In this chapter, we meet four basic
building blocks: the S
N
1, S
N
2, E1, and E2 reactions. It is a sad fact
that in many instances they compete with one another. We can usu-
ally adjust reaction conditions to favor the reaction we desire, but it
will be a rare joyous occasion when we achieve perfect selectivity.
There is much detail in this chapter, but that is not usually
a problem for most students. Good nucleophiles can be distin-
guished from good bases, good leaving groups can be identified,
and so on. What is hard is to learn to adjust reaction conditions
(reagents, temperature, and solvent) so as to achieve the desired
selectivity, to favor the product of just one of these four reac-
tions. That’s hard in practice as well as in answering a question
on an exam or problem set. To a certain extent, we all have to
learn to live with this problem!
7.12 Additional Problems
PROBLEM 7.42 Explain with crystal clarity why treatment
of propyl alcohol with bromide ion fails to give propyl bromide,
but treatment with HBr succeeds.
PROBLEM 7.43 Propose a synthesis of each of the following
compounds starting with 1-butene:
(a) 2-chlorobutane
(b) 2-methoxybutane
(c) 2-butanamine
(d) 2-butene (mixture of cis and trans)
(e) butane
PROBLEM 7.44 Propose a synthesis of each of the following
compounds starting with 2-butanol:
(a) 2-chlorobutane
(b) 2-methoxybutane
(c) 2-butanamine
(d) 2-butene (mixture of cis and trans)
(e) 2-butanethiol
B
r
Br
OH
HBr
Na
+
Br
–
This chapter expands the topic of organic synthesis. We still
have very few reactions for making molecules at our disposal,
basically just additions and the E1, E2, S
N
1, and S
N
2 reactions.
Nonetheless, it is possible to devise simple routes to target
molecules and even to craft a few multistep sequences. Organic
synthesis is one of the most creative (and fun) parts of this
science, and so it is important to start early. Here we group
several synthesis-related problems before going to more
mechanistic material. In just a few chapters, we will have many
more reactions under control, and be able to do much fancier
work. Stay tuned.
PROBLEM 7.40 The S
N
2 reaction occurs between an alkyl halide
and a nucleophile. It is a very important process in organic chem-
istry. For this reason it is necessary to recognize the nucleophilicity
of various reagents. In the following pairs of compounds, indicate
the more nucleophilic reagent. Explain your reasoning.
(a) (CH
3
CH
2
)
3
N (CH
3
CH
2
)
3
P
(b) (CH
3
CH
2
)
3
N (CH
3
CH
2
)
2
N
(c) CH
3
CH
2
SNa CH
3
CH
2
OK
PROBLEM 7.41 Which nucleophiles would serve to effect the
following conversions of 1-iodopropane?
(a)
(b)
(c)
SH
(d)
CN
(e)
O
(f)
O
NH
3
N
3
I
??
–
+
I
324 CHAPTER 7 Substitution and Elimination Reactions
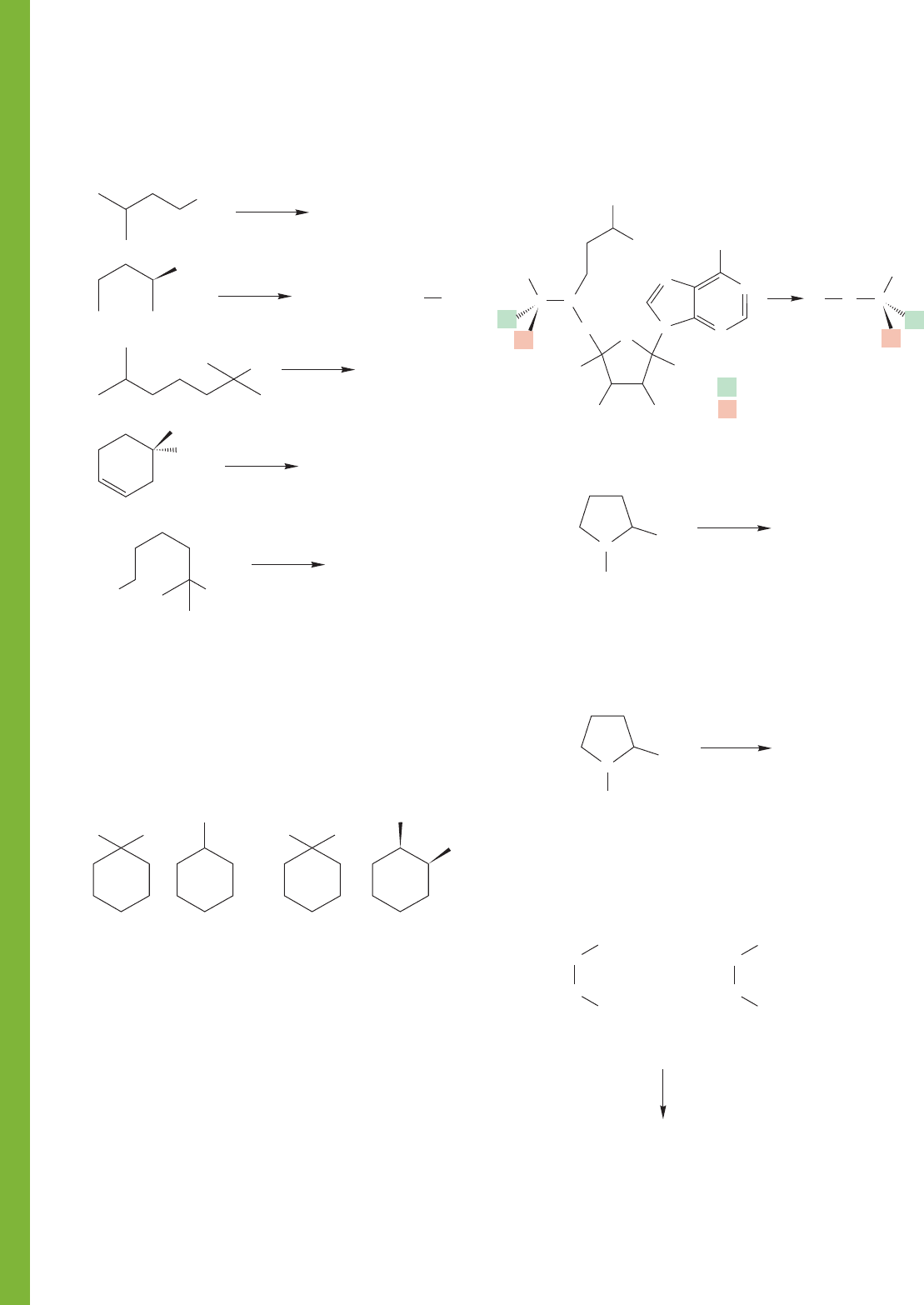
PROBLEM 7.45 Predict the product(s) for each of the reactions
shown. Indicate the pathway (S
N
1, S
N
2, E1, or E2) for forma-
tion of each product.
PROBLEM 7.46 The contents of four flasks containing the
compounds shown in (a), (b), (c), and (d) are treated with
. In two cases, a product C
7
H
12
is formed that shows only five signals in its
13
C NMR spectrum.
In two other cases, a product of the same formula, C
7
H
12
,is
formed that shows seven signals in the
13
C NMR spectrum.
What are the products, and which of the compounds, (a), (b),
(c), and (d), leads to which product? Explain.
PROBLEM 7.47 Why are sulfonates (p. 283) such good partici-
pants in the S
N
1 and S
N
2 reactions?
PROBLEM 7.48 Write a mechanism for the formation of alkyl
bromides from the reaction of alcohols and PBr
3
.
PROBLEM 7.49 You may be familiar with the compound
tert-butyl methyl ether. Its common acronym is MTBE. It has
been an additive for gasoline since 1979, although its use has
declined in the United States since 2003. How would you
make MTBE if you have bottles of tert-butyl alcohol and
methyl alcohol? You may use any other inorganic reagents
needed. Your proposed route should avoid mixtures containing
undesired products.
(a)
H
3
C
F
(b)
CH
2
Br
(d)
CH
3
(c)
H
3
C
Cl
I
(CH
3
)
3
C
O
O
-
/(CH
3
)
3
C
O
OH
(d)
OTs
CH
3
H
2
O
H
2
O
(b)
OH
catalytic
H
2
SO
4
(c)
CH
3
ONa
Br
CH
3
OH
(e)
HO OTs
(a)
NaSCH
3
Br
THF
PROBLEM 7.50 The reaction shown below was used to help clarify
the S
N
2 nature of the alkylation reactions of S-adenosylmethionine.
Explain what the results tell us about the mechanism.
COO
–
NH
3
+
2
H
+
3
H
C
H
–
OR
..
..
..
..
S
+
O
..
..
R
N
N
N
2
H
2
H
= Tritium (T)
= Deuterium (D)
3
H
C
H
NH
2
3
H
O
N
CH
2
OHHO
H
H
PROBLEM 7.51 (a) Reaction of 1,2-dimethylpyrrolidine
(1) with ethyl iodide leads to two salts, C
8
H
18
IN. Explain.
(b) However, when 2-methylpyrrolidine (2) undergoes a similar
reaction with ethyl iodide, followed by treatment with weak
base, analysis of the product by
1
H NMR spectroscopy
reveals only one set of signals for C
7
H
15
N. Explain.
PROBLEM 7.52 Treatment of 1,2-dibromoethane with the dithio-
late shown below leads to two products, C
4
H
8
S
2
and C
6
H
12
S
2
Br
2
.
Write structures for these products and explain how they are
formed.
H
2
C
H
2
C
Br
Br
A dithiolate
H
2
C
H
2
C
+
S
+
–
S
–
Na
+
Na
+
C
4
H
8
S
2
C
6
H
12
S
2
Br
2
(b)
N
H
CH
3
CH
2
I1.
weak base2.
One
C
7
H
15
N
2
CH
3
(a)
N
CH
3
CH
3
CH
2
I
Two
C
8
H
18
IN
1
CH
3
7.12 Additional Problems 325
PROBLEM 7.53 Devise a synthetic route that will convert
(R)-sec-butyl alcohol into (S)-sec-butyl alcohol.
PROBLEM 7.54 Provide reagents that will effect the following
changes:
PROBLEM 7.55 Devise S
N
2 reactions that would lead to the
following products:
PROBLEM 7.56 Predict the products of the following S
N
2
reactions:
N
I
..
..
..
..
+
(a)
CH
3
CH
2
?
I
–
I
–
(a) (b)
(c) (d) (e)
ON
+
H
H
3
C
NH(CH
3
)
2
+
CN
N
3
Br
Br
D
??
(c)
??
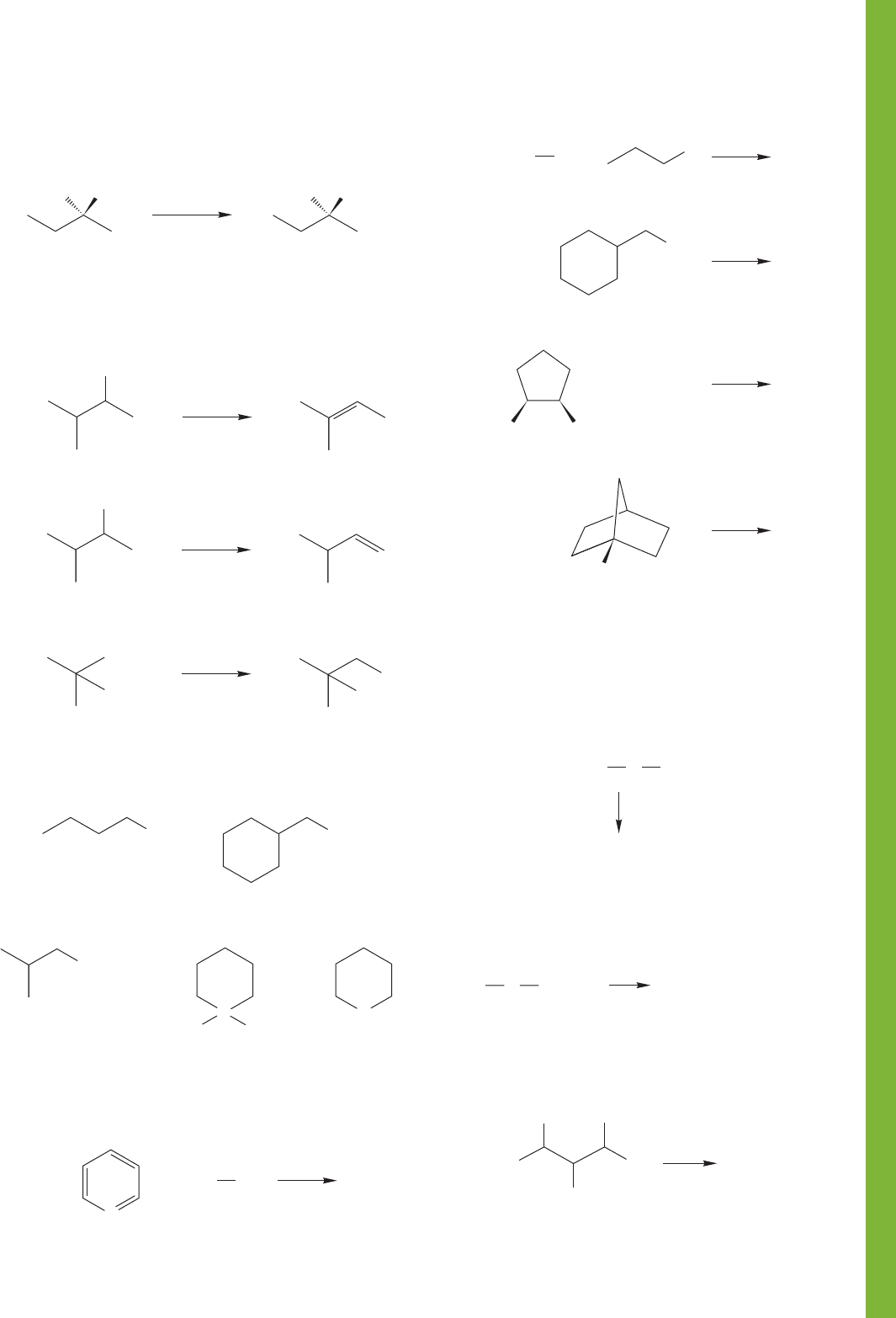
Br
(b)
??
Br
(a)
(R)(S)
HO
H
??
OHH
PROBLEM 7.57 Treatment of butyl ethyl ether with HI
leads to both butyl iodide and ethyl iodide, along with the
related alcohols. By contrast, when tert-butyl ethyl ether is
treated the same way, only ethyl iodide and tert-butyl alcohol
are formed. tert-Butyl iodide and ethyl alcohol are not
produced. Explain.
PROBLEM 7.58 Write all the possible products of the reaction
of alcohol 1 with HCl/H
2
O.
?
HCl
H
2
O
R'R
1
CH
3
CH
3
OH
+
CH
3
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
I CH
3
CH
2
I
+CH
3
CH
2
CH
2
CH
2
OH CH
3
CH
2
OH
O
CH
2
CH
3
HI
and
(CH
3
)
3
C +(CH
3
)
3
COH CH
3
CH
2
I
O CH
2
CH
3
HI
–
–
–
I
..
..
..
I
..
..
..
..
..
..
+
+
+
+
(c)
(d)
(e)
(b)
CH
3
CN
–
..
..
CN
O
..
..
..
CH
3
CH
2
I
..
..
..
Br
..
..
..
I
..
..
..
?
?
?
?

326 CHAPTER 7 Substitution and Elimination Reactions
PROBLEM 7.59 Predict the products of the following S
N
1
reactions:
PROBLEM 7.60 You are given a supply of ethyl iodide, tert-
butyl iodide, sodium ethoxide, and sodium tert-butoxide. Your
task is to use the S
N
2 reaction to make as many different ethers
as you can. In principle, how many are possible? In practice,
how many can you make?
PROBLEM 7.61 The following molecules can form conjugate
bases by loss of a proton from more than one position (arrows).
Start by drawing a good Lewis structure (electron dots are
deliberately left out) and then choose which proton will be lost
more easily. Explain your choice.
PROBLEM 7.62 The following molecules can accept a proton
to form their conjugate acids by addition of a proton at more
than one possible place (arrows). Choose the position at which
protonation will be preferred and explain your choice. Once
again, it makes sense to start by drawing a good Lewis structure
for the molecule in question.
(a) (b) (c)
CH
3
H
H
3
C
H
3
C
OH
C
(CH
3
)
3
C
C
O
O
H
3
C
H
3
C
C
C
(a) (b) (c)
CH
3
CH
2
HO
OR
CN
CR
2
CH
3
CH
2
C
O
H
(a)
(b)
(c)
(d)
CH
3
H
3
C
H
..
..
CH
3
CH
2
OH
I
..
..
..
I
..
..
..
?
?
?
?
Br
..
..
..
Br
..
..
..
..
..
H
2
O
..
..
H
2
O
..
..
H
2
O
..
..
CH
3
CH
2
OH
..
..
CH
3
OH
PROBLEM 7.63 Write arrow formalisms for the following
reactions. Charges and electron dots are deliberately left out of
the reagents and products, so you must start by putting them in.
PROBLEM 7.64 The S
N
2 reactions at the allyl position
are especially facile. For example, allyl compounds are even
more reactive than methyl compounds (Table 7.1, p. 275).
Explain this rate difference. Why are allyl compounds
especially fast? Hint: This, like all “rate” questions, can be
answered by examining the structures of the transition
states involved.
PROBLEM 7.65 In the following pairs of compounds, rank
the molecules in order of rate of reaction in the S
N
2 process.
Explain your reasoning.
(a)
(b)
(c)
(d)
Br
Br
B
r
Br
Br
I
I
I
H
2
C
CH CH
2
H
2
C CH CH
2
..
S
N
2
+
–
X
Nu
X
Nu
..
–
H
3
C
H
3
C
C
+
–
OH
OH
+
(a)
(b)
(c)
H
3
C
H
2
C
OH
C
(CH
3
)
3
C
C
O
OH
(CH
3
)
3
C
C
OH
O
–
OH
HOH
2
HO
H
3
C
H
3
C
C
OO
H
3
C
H
2
C
C
OH
OHH
H
7.12 Additional Problems 327
PROBLEM 7.66 In the following pairs of compounds, rank the
molecules in order of rate of reaction in the S
N
1 process.
Explain your reasoning.
PROBLEM 7.67 Each of the following molecules contains two
halogens of different kinds. They are either in different posi-
tions in the molecule or are different halogens entirely. In each
case, determine which of the two halogens will be the more
reactive in the S
N
2 reaction. Explain.
PROBLEM 7.68 Each of the following molecules contains two
halogens at different positions in the molecule. In each case,
determine which of the two halogens will be the more reactive
in the S
N
1 reaction. Explain.
(a)
Br
Br
(a)
(b)
(c)
Br
Br
II
Cl Br
(c)
I
I
(b)
I
Br
(a)
I
I
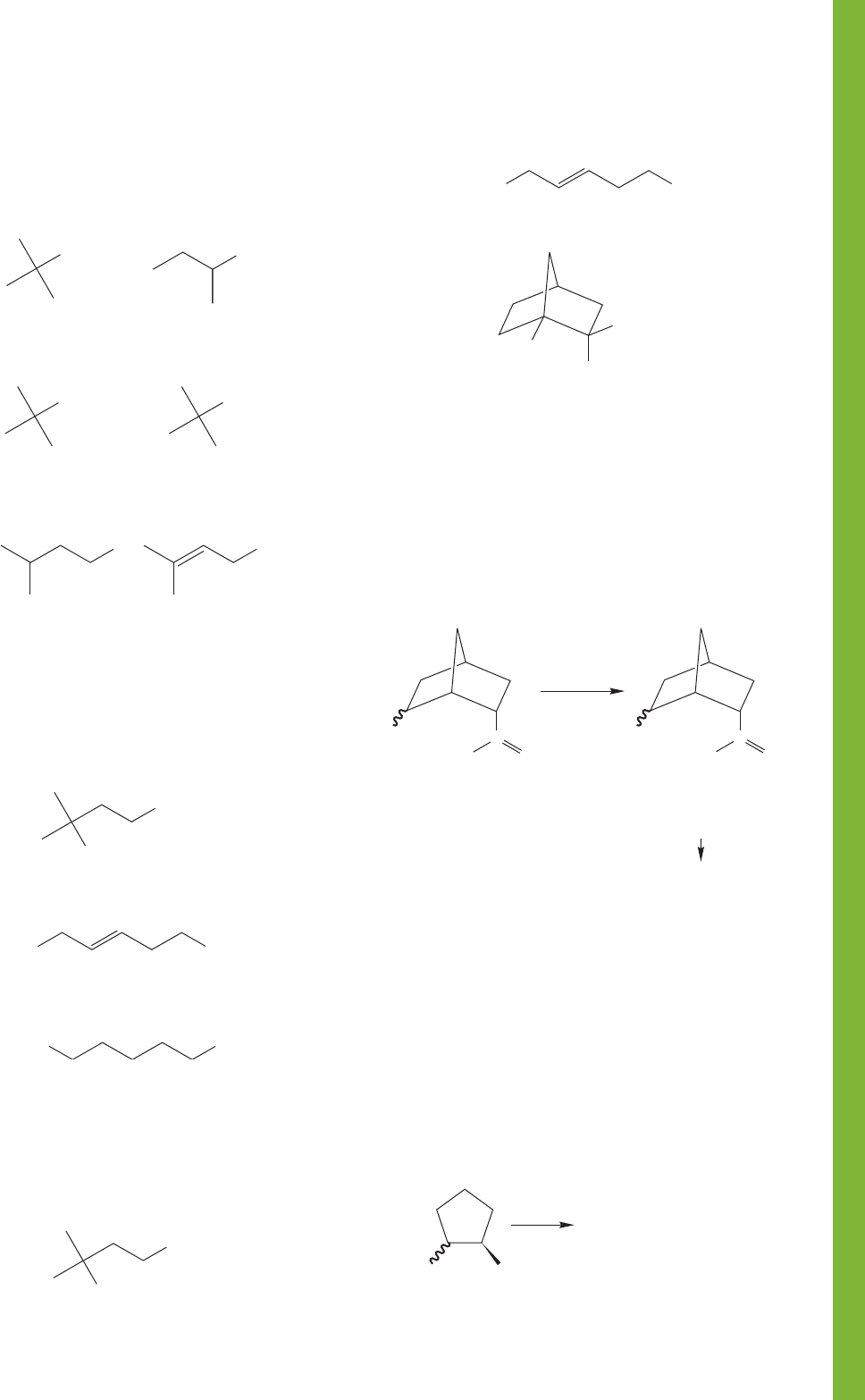
PROBLEM 7.69 You have two bottles containing the two
possible diastereomers of the compound shown below
(C
8
H
11
IO
2
). When the salts of these two carboxylic acids are
made, only one of them is converted into a new compound,
C
8
H
10
O
2
. What is the structure of the new compound, and
how does its formation allow you to determine the stereo-
chemistry of the original two compounds? The squiggly bond
indicates both stereoisomers.
PROBLEM 7.70 You have two bottles containing the two
possible diastereomers of the compound shown below
(C
5
H
9
IO). When the molecules are treated with a strong
base, such as sodium hydride, only one of them is
converted into a new compound, C
5
H
8
O. What is the
new compound, and how does its formation allow you
to determine the stereochemistry of the original two
compounds?
Na
+
+
NaH
OH
C
5
H
8
OC
5
H
8
IO
–
C
5
H
9
IO
..
..
I
..
..
..
H
2
O
..
Na
+
NaHCO
3
C
8
H
10
O
2
One remaining C
8
H
10
IO
2
Na
Two isomers, each
C
8
H
11
IO
2
Two isomers, each
C
8
H
10
IO
2
Na
+
I
..
..
..
I
..
..
..
HO
..
..
O
..
..
O
..
C
..
O
..
..
C
–
CH
3
Br
Br
(c)
(b)
II
328 CHAPTER 7 Substitution and Elimination Reactions
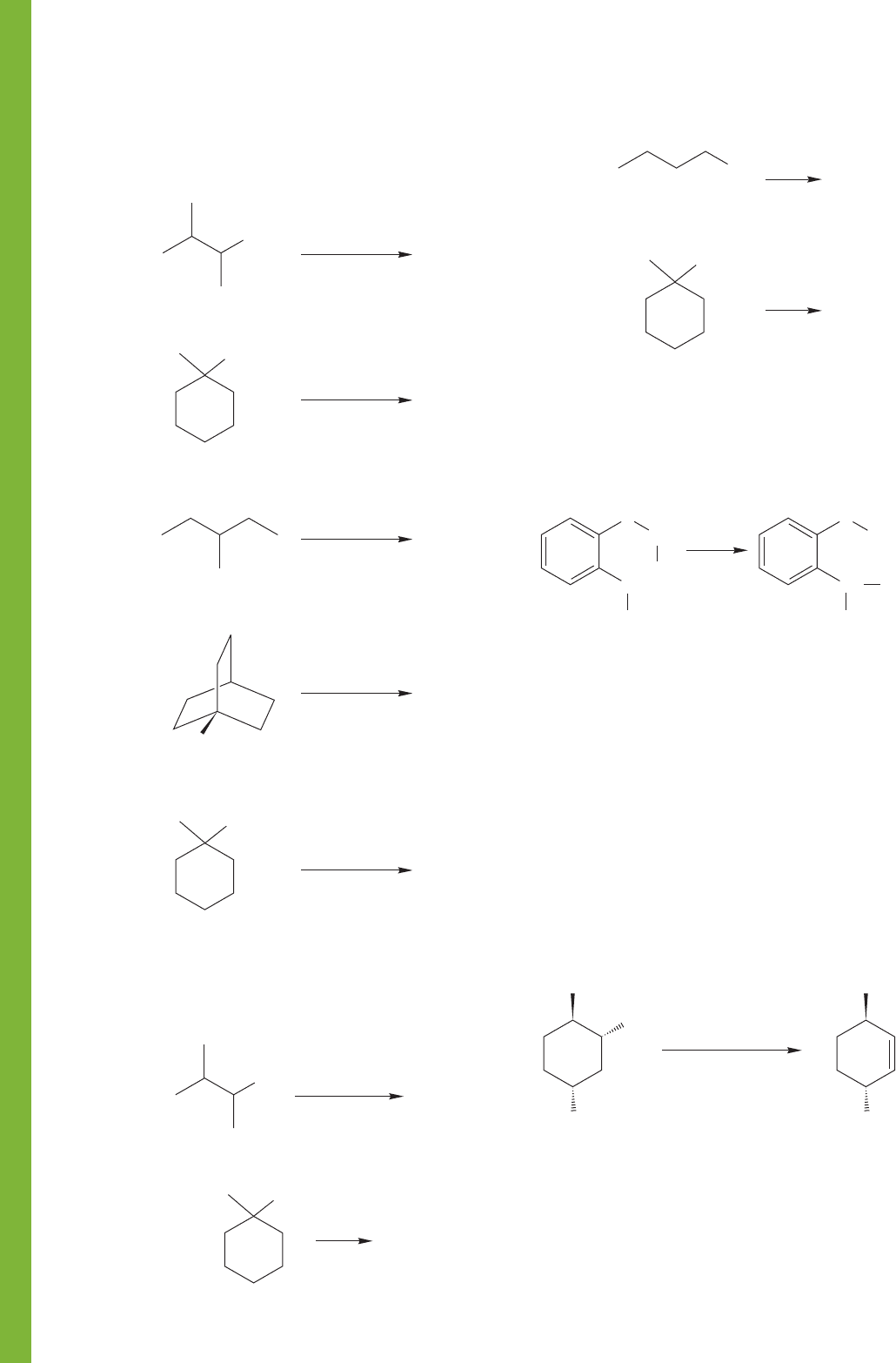
PROBLEM 7.71 Predict the products of the following E2
reactions. If more than one product is expected, indicate which
will be the major compound formed.
PROBLEM 7.72 Predict the products of the following E1 reac-
tions. If more than one product is expected, indicate which will
be the major compound formed.
(b)
..
..
..
Br
H
2
O
..
..
(a)
OH
..
..
CH
3
CH
2
H
2
O
..
Br
..
..
..
..
(e)
..
..
CH
3
CH
2
OH
CH
3
CH
2
O
–
..
..
..
+
S(CH
3
)
2
..
(d)
..
..
CH
3
CH
2
OH
CH
3
CH
2
O
–
..
..
..
I
..
..
..
(c)
..
..
CH
3
CH
2
OH
CH
3
CH
2
O
–
..
..
..
I
..
..
..
(b)
CH
3
CH
2
O
..
..
CH
3
CH
2
OH
–
..
..
..
..
..
..
Br
(a)
..
..
CH
3
CH
2
OH
CH
3
CH
2
O
–
..
..
..
Br
..
..
..
PROBLEM 7.73 (a) Write the “obvious” arrow formalism for
the following transformation. Of course it is going to be wrong;
you are being set up here. But don’t mind, it’s all a learning
experience.
(b) OK, what you wrote is almost certainly wrong. To find the
correct mechanism, you measure the kinetics of this process
and discover that the reaction is bimolecular. Now write
another arrow formalism.
(c) What’s wrong with the mechanism suggested by the origi-
nal arrow formalism? Hint: Think about the geometry of
the transition state for the S
N
2 reaction.
PROBLEM 7.74 (a) Menthyl chloride reacts with sodium
ethoxide in ethyl alcohol to give a single product as shown.
Analyze this reaction mechanistically to explain why
this is the only alkene produced. Hint: Think in three
dimensions.
By contrast, neomenthyl chloride treated in the same
fashion gives an additional, major, product. Moreover,
menthyl chloride reacts much more slowly than
neomenthyl chloride. Explain these observations
mechanistically.
Na
Menthyl chloride
CH
3
Cl
..
..
CH
3
CH
2
OH
CH(CH
3
)
2
CH
3
CH(CH
3
)
2
CH
3
CH
2
O
–
..
..
..
+
O
CH
3
CH
OTs
–
–
..
..
..
O
CH
3
CH
OTs
..
..
..
SO
2
SO
2
(d)
+
S(CH
3
)
2
..
H
2
O
..
..
(c)
I
..
..
..
H
2
O
..
..
