Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

7.12 Additional Problems 329
(b) When menthyl chloride is treated with 80% aqueous ethyl
alcohol with no ethoxide present, once again, two products
are formed in the ratio shown. Explain mechanistically.
Steric effects are important in solvolysis reactions. Problems
7.75 and 7.76 illustrate this point.
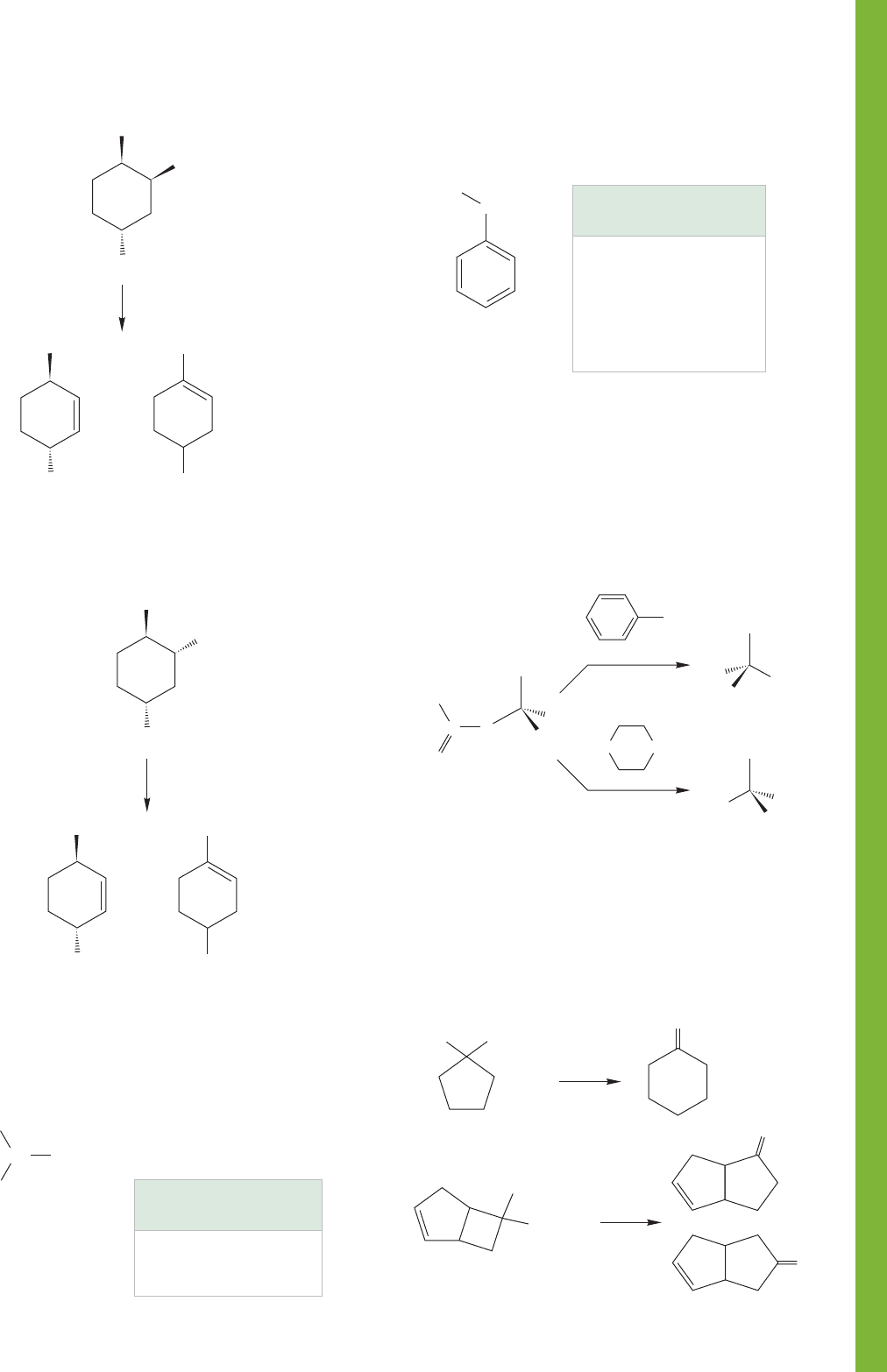
PROBLEM 7.75 Explain the following rates for S
N
1 solvolysis.
Construct an Energy versus Reaction progress diagram showing
the two reactions.
CF
3
COOH, 25 ⬚C
CH OTs
R
R
Rate
CH
3
(CH
3
)
3
C
1
10
5
R
CH
3
Cl
CH(CH
3
)
2
CH
3
(
32%
)(
68%
)
CH(CH
3
)
2
H
2
O
..
..
CH
3
CH(CH
3
)
2
+
..
..
CH
3
CH
2
OH
CH
3
CH(CH
3
)
2
CH
3
CH(CH
3
)
2
(25%) (75%)
+
Na
..
..
CH
3
CH
2
OH
CH
3
CH
2
O
–
..
..
..
+
Neomenthyl
chloride
CH
3
Cl
CH(CH
3
)
2
PROBLEM 7.76 Explain the relative rates of the following
compounds in the S
N
1 reaction:
PROBLEM 7.77 Rationalize the following curious experimental
results. When the optically active chlorosulfite 1 is heated in
toluene, the chloride with inverted configuration is obtained.
However, when 1 is decomposed in 1,4-dioxane (2), retention
of configuration in the resulting chloride is observed. Hint:
See Figure 7.43, and also think about the differences between
toluene and 1,4-dioxane. A good Lewis structure for
1,4-dioxane may also be helpful.
For Problems 7.78 and 7.79 you need to know that treatment of
amines, , with nitrous acid, HONO, gives diazonium
ions, .
PROBLEM 7.78 Provide arrow formalism mechanisms for the
following reactions:
HONO
HONO
+
CH
2
NH
2
CH
2
NH
2
HO
OH
O
O
(a)
(b)
O
R
O
N
2
+
R
O
NH
2
CH
3
toluene
Δ
Δ
*
SO
1
2
H
Bu
CH
3
Cl
O
*
H
Bu
Retention
Inverted
CH
3
*
H
Bu
H
3
C
OO
Cl
Cl
CHCl
R
Relative Rate
CH
3
CH
3
CH
2
(CH
3
)
2
CH
(CH
3
)
3
C
540
125
27
1
R
330 CHAPTER 7 Substitution and Elimination Reactions
PROBLEM 7.79 Rationalize the different products formed on treat-
ment of the stereoisomeric aminocyclohexanols 1–4 with nitrous
acid.
PROBLEM 7.80 You are given a supply of 2-bromo-1-methoxybi-
cyclo[2.2.2]octane. Your task is to prepare 1-methoxybicyclo-
[2.2.2]oct-2-ene (A) from this starting material. In your first
attempt, you treat the bromide with sodium ethoxide in ethyl alco-
hol. Although a small amount of the desired alkene is formed, most
of the product is 2-ethoxy-1-methoxybicyclo[2.2.2]octane (B).
Na
+
A
(minor)
B
(major)
Br
HOCH
2
CH
3
–
OCH
2
CH
3
H
3
CO
H
3
COH
3
CO
OCH
2
CH
3
+
HONO
O
C(CH
3
)
3
NH
2
O
OH
C(CH
3
)
3
HONO
C(CH
3
)
3
NH
2
1
2
OH
C(CH
3
)
3
C
HONO
HONO
C(CH
3
)
3
NH
2
CHO
3
OH
C(CH
3
)
3
NH
2
4
OH
C(CH
3
)
3
Suggest a simple experimental modification that should
result in an increase in the amount of the desired alkene,
and a decrease in the amount of the unwanted substitution
product, B.
PROBLEM 7.81 A common procedure to increase the rate of
an S
N
2 displacement is to add a catalytic (very small) amount of
sodium iodide. Notice that even though the rate increases, the
structure of the product is not the iodide. Explain the function
of the iodide. How does it act to increase the rate, and why is
only a catalytic amount necessary?
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 7.82 Select the animation titled “Halide formation”
in the panel of reactions labeled Semester 2A. Write out the
reaction (see Fig. 7.45). How many intermediates are included
in the animation? According to the energy diagram shown for
the reaction, which step is the fastest?
PROBLEM 7.83 The “Halide formation” reaction has several
intermediates formed that aren’t shown in the animation.
Predict what they are. It might help to know that one of the
final products in the reaction is P(OH)
3
.
PROBLEM 7.84 After you watch the “Halide formation”
reaction, you should be able to describe the mechanism
of the second step. What kind of reaction would you call it?
Do you suppose a tertiary alcohol goes through the same
mechanism?
PROBLEM 7.85 Select the “Bimolecular elimination: E2” reac-
tion. You know about the stereochemistry of the reaction from
Section 7.9c. Select the LUMO track for the reaction and
observe the starting alkyl halide. Which hydrogens have
LUMO character? Why?
PROBLEM 7.86 If you were to add the methoxide base to
propane, would propane have a hydrogen acidic enough to react
with the base? In other words, would you predict a reaction?
See Table 6.4 (p. 235) if you need pK
a
information.
Na
+
I
–
Na
+
Cl
R
R
+
(small amount)
acetone
(solvent)
Nu
–
Nu

Equilibria
331
8.1 Preview
8.2 Equilibrium
8.3 Entropy in Organic Reactions
8.4 Rates of Chemical Reactions
8.5 Rate Constant
8.6 Energy Barriers in Chemical
Reactions: The Transition
State and Activation Energy
8.7 Reaction Mechanism
8.8 The Hammond Postulate:
Thermodynamics versus
Kinetics
8.9 Special Topic: Enzymes and
Reaction Rates
8.10 Summary
8.11 Additional Problems
8
ENERGY BARRIERS Reactions have energy barriers that must be crossed to go from
reactants to products. A reasonable analogy is the mountain range that must be
crossed to go from your starting point to the other side of the mountain.There may
be intermediates in the reaction path, just like the valleys that separate the peaks.
..
+
+
–
Transition state
Energy
Reaction progress
Nu
L
L
R
+
LR
R
+
Nu
R
Nu
R
Nu
..
–
–
L
Nu
..
–
..
–
L
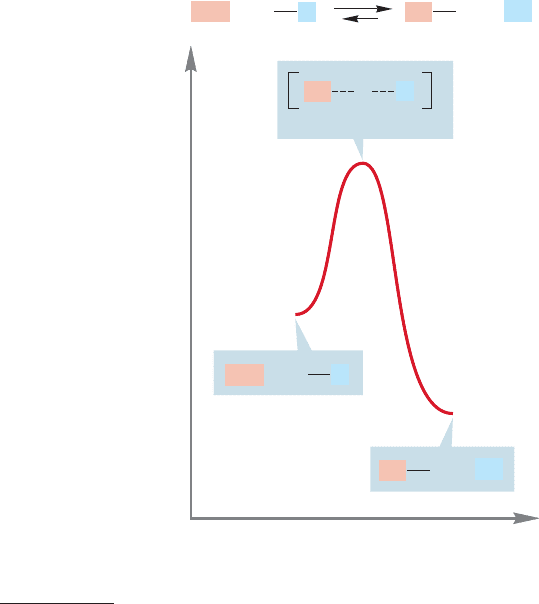
FIGURE 8.1 The S
N
2 reaction
is an equilibrium in which two
nucleophiles (the Lewis bases Nu
−
and L
−
) compete for a Lewis acid.
:
:
“I hope,” the Golux said, “that this is true. I make things up, you know.”
—JAMES THURBER,
1
THE 13 CLOCKS
8.1 Preview
The study of how reactions occur depends on an analysis of the structures of the
starting materials, the products, and the transition states separating them. We have
spent much time on structure in the last several chapters. However, there are other,
nonstructural factors that are important. To understand chemical reactivity, and to
be able to use our knowledge in a predictive way, we also need to know something
of the energetics involved in the reaction. Which are more stable, starting materi-
als or products? Of course those designations—starting materials and products—
are arbitrary. Nature doesn’t care about such synthetic labels! How high is the energy
of the transition state separating “starting materials and products”? If the transition-
state energy is too high, no reaction will occur. If there are several available low-
energy pathways—several accessible transition states—there will be a mixture of
products, and the synthetic chemist’s longed-for specificity will be impossible. So
we need to know about energy.
In Chapter 7, we saw some examples of chemical equilibria. For instance, the
S
N
2 reaction involves an equilibrium in which two nucleophiles (Nu
and L
)
compete for the R group (Fig. 8.1).
::
1
James Grove Thurber (1894–1961) was an American writer and cartoonist, much of whose work first
appeared in The New Yorker magazine. The 13 Clocks is a fairy tale of surpassing charm and wit written in
Bermuda.
332
CHAPTER 8 Equilibria
8.1 Preview 333
In practice, thermodynamics may dictate that one side of an equilibrium be
overwhelmingly favored over the other. Nevertheless, we can still apply techniques
for studying equilibria to the reaction, deriving information about the position
of the equilibrium (thermodynamics) and the rate of reaction (kinetics). The
concepts we will develop in this short chapter are extremely general and very
important.
All chemical reactions can be regarded as equilibria. Multistep reactions are
simply series of equilibria in which species occupying energy minima are sepa-
rated by barriers, the tops of which are transition states. The S
N
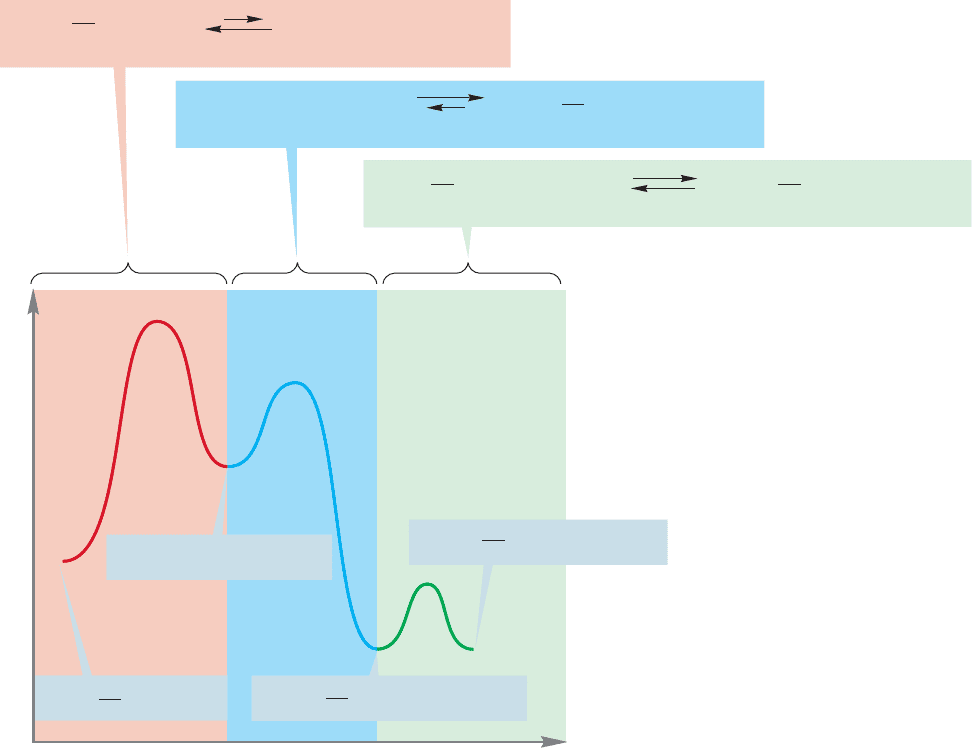
1 solvolysis
reaction of tert-butyl iodide in water makes a good example. Here we have three
equilibria.The first is between tert-butyl iodide and the tert-butyl cation and iodide
ion. This equilibrium is highly unfavorable, because the cation–anion pair is far
less stable than the covalent iodide. The second equilibration is the exothermic
capture of the cation by water to give the protonated alcohol, an oxonium ion.
Finally, the oxonium ion is deprotonated to give the ultimate product, tert-butyl
alcohol (Fig. 8.2).
Energy
Reaction progress
+
OH
2
..
+
(CH
3
)
3
C
+
++
(CH
3
)
3
C
+
+
+
(CH
3
)
3
C
++
+
(CH
3
)
3
C
+
(CH
3
)
3
C
Equilibrium 1
Equilibrium 2
Equilibrium 3
(CH
3
)
3
C
+
+
OH
2
..
++
(CH
3
)
3
C
++
(CH
3
)
3
C
..
..
++
(CH
3
)
3
C
(CH
3
)
3
C
I
..
..
..
..
..
2 H
2
O
..
..
2 H
2
O
–
I
..
..
..
..
I
..
..
..
–
I
..
..
..
..
–
I
..
..
..
..
++
..
..
2 H
2
O
–
I
..
..
..
..
–
I
..
..
..
..
–
I
..
..
..
..
–
I
..
..
..
..
..
..
H
2
O
+
–
I
..
..
..
..
..
..
H
2
O
..
..
2 H
2
O
..
..
H
2
O
..
..
2 H
2
O
+
OH
2
..
H
3
O
..
+
OH
..
..
OH
H
3
O
..
+
FIGURE 8.2 S
N
1 reactions—and all
multistep reactions—are series of
equilibria.

334 CHAPTER 8 Equilibria
8.2 Equilibrium
For a general chemical reaction at equilibrium,
(8.1)
the concentrations of the species on the two sides of the arrows are related to each
other by an equilibrium constant, K:
(8.2)
or, more familiarly:
(8.3)
The small letter superscripts are the coefficients in the balanced equation for
the reaction [Eq. (8.1) in this case], and the capital letters enclosed in brackets rep-
resent the molar concentrations of the products and reactants at equilibrium. The
concepts of starting materials and products are arbitrary ones, arising from practical
considerations—we generally are interested in making the reaction run one way or
the other, and by convention the desired reaction is written left to right. But it need
not be; it is just as valid to look at the reaction in the other direction.
A reaction will be a successful one if the compounds we seek, the products, are
strongly favored at equilibrium. So we are very interested in what determines
the magnitude of the equilibrium constant (K ): the difference in the free energies
(ΔG° ΔH° T ΔS°; see below for a fuller discussion) of the compounds involved
in the reaction.The exact relationship between K and the relative free energies of
the starting materials and products is shown in Eq. (8.4):
(8.4)¢G
ⴰ
=-RT ln K
K =
[C]
c
[D]
d
[A]
a
[B]
b
[C]
c
[D]
d
= K [A]
a
[B]
b
aA + bB
U
Z
cC + dD
ESSENTIAL SKILLS AND DETAILS
1. Thermodynamics. Understanding the importance of the relative energies of starting
materials and products, as well as the heights of the barriers separating these species,
is critical to understanding reactivity. The relative energies of starting materials and
products determine the ratio of products under conditions that allow all energy barriers
to be passed.
2. Kinetics. The heights of the barriers separating starting materials from products
determine the rates of formation of products. If there is not sufficient energy to return
to starting material from products, it is the heights of energy barriers—topped by
transition states—that will determine the ratio of products.
3. A little bit of energy goes a long way at equilibrium.The outcome of a reaction at
equilibrium is determined by the energy difference between the starting materials
and products: ΔG RT ln K is the operative equation.
4. Rates.The rate of a reaction has nothing to do directly with the energy difference
between starting materials and products. Rates are determined solely by the energy
difference between the starting molecules and the transition states separating those
molecules from products.
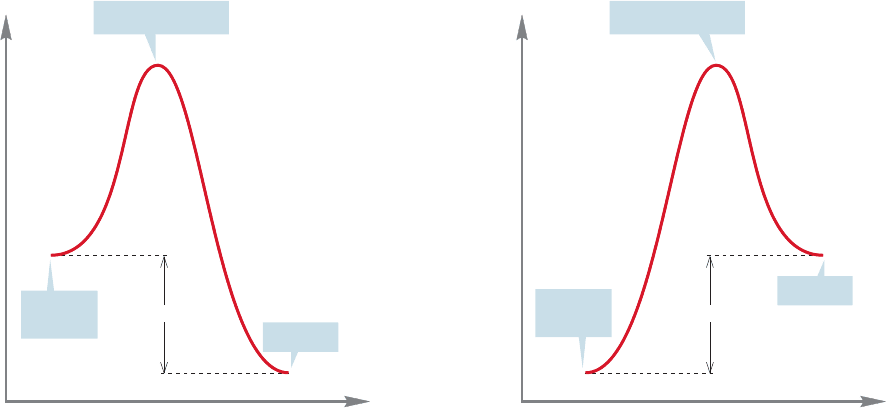
8.2 Equilibrium 335
Energy
Reaction progress
ΔG⬚ or ΔH⬚
Starting
material
Product
Exothermic (ΔH⬚) left-to-right
Exergonic (ΔG⬚) left-to-right
Transition state
FIGURE 8.3 An exothermic (exergonic) reaction.
Energy
Reaction progress
ΔG⬚ or ΔH⬚
Starting
material
Product
Endothermic (ΔH⬚) left-to-right
Endergonic (ΔG⬚) left-to-right
Transition state
FIGURE 8.4 An endothermic (endergonic) reaction.
or, if we want to use base 10 rather than natural logarithms, in Eq. (8.5):
(8.5)
R is the gas constant, 1.986 × 10
3
kcal/deg
·
mol, T is the absolute temperature, and
ΔG°, the Gibbs free energy change, is the difference in energy between starting
materials and products in their standard states (Figs. 8.3 and 8.4).
¢G
ⴰ
=-2.3 RT log K
Up to now, we have more or less equated energy with H°, the enthalpy. The
enthalpy change ΔH°, or the heat of reaction,is a measure of the bond energy changes
in a reaction.The Gibbs free energy (ΔG°) is more complicated than this, as we will
see in Section 8.3, and includes not only ΔH°, but entropy change (⌬S°) as well.
The entropy of a reaction is a measure of the change in amount of order in starting
materials and products.The higher the degree of ordering of reactants necessary, the
greater are the energy requirements for the reaction. Conversely, an increase in
disorder will require less energy for the reaction.
A number of times in Chapter 7 we drew diagrams that described the course
of a reaction. We can now label those diagrams a bit more completely. The dia-
grams are plots of energy versus something called,most often,“reaction progress.”
Figures 8.3 and 8.4 show an exothermic reaction (products more stable than
starting materials) and an endothermic reaction (starting materials more stable
than products).
The terms “exothermic” and “endothermic” really refer to the enthalpy change,
ΔH°,which may or may not be in the same direction as the free energy change,ΔG°,
which involves entropy, ΔS°, as well as enthalpy, ΔH°. If we really mean free ener-
gy, ΔG°, the proper terms are exergonic and endergonic.
For an exothermic reaction, the difference in enthalpy between products and
starting materials is negative and the equilibrium constant (K ) is greater than 1. For
an endothermic reaction (read the first reaction backward to produce an endother-
mic process), the enthalpy difference is positive and K is less than 1.
336 CHAPTER 8 Equilibria
More stable products are favored at equilibrium. By how much? Note that the
relationship between the difference in free energy between starting material and
products (ΔG°) and the equilibrium constant K is logarithmic. We can rewrite
Eqs. (8.4) and (8.5) to emphasize that point:
(8.4)
or, in base 10:
(8.5)
These equations show that a small difference in energy between starting materials and
products results in a large excess of one over the other at equilibrium. Let’s do a few
calculations to show this. First,let’s take a case in which products are more stable than
starting materials by the seemingly tiny amount of 1 kcal/mol, ΔG° 1 kcal/mol,
2
so K 10
1/(1.364)
10
0.7331
5.4.
An equilibrium constant of 5.4 translates into 84.4% product at equilibrium! One
would very often be quite happy to find a reaction that gives about 85% product, and
a mere 1 kcal/mol suffices to ensure this. Conversely, if we are “fighting”a 1-kcal/mol
endothermic process, it will be a futile fight indeed because equilibrium will settle out
at 84.4% starting material. A little bit of energy goes a long way at equilibrium.Table 8.1
summarizes a number of similar calculations.
¢G
ⴰ
=-2.3RT log K
or
K = 10
-¢G
ⴰ
>2.3RT
¢G
ⴰ
=-RT ln K
or
K = e
-¢G
ⴰ
>RT
TABLE 8.1 The Relationship between ⌬G° and K at 25 °C
G° (kcal/mol) K Equilibrium Ratio
0.1 1.2 54.5/45.5
0.5 2.3 69.7/30.3
1 5.4 84.4/15.6
2 29.3 96.7/3.3
5 4631 99.98/0.02
10 2.1 10
7
99.999996/0.000004
2
At 25 °C, 2.3RT 1.364 kcal/mol. This number is useful to remember because with it one can do calcula-
tions easily at odd moments without having to look up the value of R and multiply it by T.
PROBLEM 8.1 Calculate the energy difference between starting material and prod-
ucts at 25 °C given equilibrium constants of 7 and 14.
3
The principle is named for Henri Louis Le Châtelier (1850–1936). The mathematics of this principle had
been worked out earlier by the American Josiah Willard Gibbs (1839–1903), who donated the G in Gibbs to
ΔG. Le Châtelier knew of Gibbs’s contributions and was a great popularizer of Gibbs’s work in France.
In practice, there often is something we can do to drive a reaction in the direc-
tion we want.Le Châtelier’s principle
3
states that a system at equilibrium responds
to stress in such a way as to relieve that stress. This statement means that if we
increase the concentration of one of the starting materials, an equilibrium process
will be driven toward products. In Eqs. (8.1–8.3), if we increase [A]
a
, the quantity
[C]
c
[D]
d
/[B]
b
must also increase to maintain K, and we will have more product.
Similarly, if we can remove one product as it is formed, the equilibrium will be driv-
en toward products. Perhaps either C or D is a solid that crystallizes out of solution
or is a gas that can be driven off. As the concentration of this compound drops, the
equilibrium will be disturbed and more C or D will be formed.
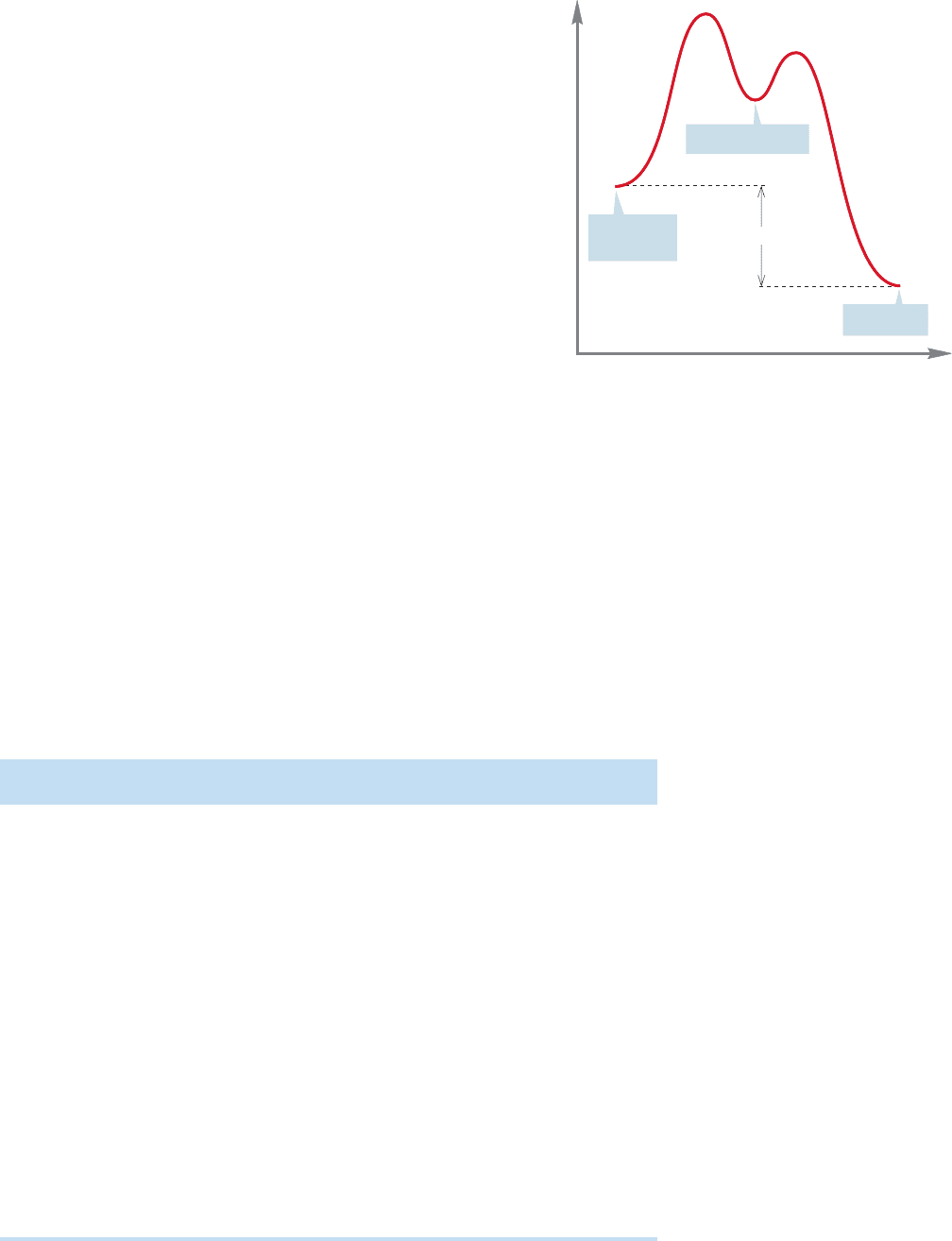
8.3 Entropy in Organic Reactions 337
In a multistep reaction,it often does not matter that an early
step involves an unfavorable equilibrium, as long as the overall
reaction is exergonic.As long as all barriers can be traversed,the
ratio of C and A will be governed by the ΔG° between C and
A. So, A B is endergonic, but, overall, A C is exergonic
(ΔG° 0). Figure 8.5 shows such a process for the conversion
of A into C via an intermediate B.
As long as some B is present in equilibrium with starting
material A, the reaction will be successful. As the equilibrium
is established, B will be converted into the much more
stable final product C. As the small amount of B present in
equilibrium with A is depleted, the equilibrium will be
reestablished and more B will be formed from A. In the
long run, A will be largely converted into C, and as long as
there is enough energy to reach the intermediate B, the final
mixture of A and C will depend on the ΔG° between these
two compounds.
A
U
Z
B
A
U
Z
B
UU
8.3 Entropy in Organic Reactions
The parameter ΔG° is composed of enthalpy (ΔH°, the change in bond strengths in
the reaction) and entropy (ΔS°, the change in freedom of motion in the reaction).
The exact relationship is
(8.6)
where T is the absolute temperature.We can make rather accurate estimates of the ΔH°
term by knowing the bond dissociation energies for a variety of bonds. We know a
few of these values already from our discussion of alkanes.Table 8.2 gives several more.
¢G
ⴰ
=¢H
ⴰ
- T ¢S
ⴰ
TABLE 8.2 Some Homolytic Bond Dissociation Energies (BDE)
Bond BDE Bond BDE
(kcal/mol) (kcal/mol)
36.1 101.1
46.1 98.6
59.0 96.5
38.0 57.6
51 72.1
38 83.7
104.2 115
71.3 92.1
87.5 83.2
103.2 85.2
136.3 90.1
118.8 89.0
104.6 66
107.6 174.1
105.0 230.7
178.8H
2
C
P
O (total)
HC
q
CH (total)H
O
CH
3
H
2
C
P
CH
2
(total)H
O
NH
2
H
2
C
P
CH
2
(π bond only)H
O
OCH
3
H
3
C
O
CH
2
CH
3
H
O
OH
H
3
C
O
CH
3
H
O
F
H
2
N
O
CH
3
H
O
Cl
CH
3
O
O
CH
3
H
O
Br
HO
O
CH
3
H
O
I
F
O
CH
3
H
O
H
Cl
O
CH
3
(CH
3
)
3
CO
O
OC(CH
3
)
3
Br
O
CH
3
HO
O
OH
I
O
CH
3
F
O
F
H
O
C(CH
3
)
3
Cl
O
Cl
H
O
CH(CH
3
)
2
Br
O
Br
H
O
CH
2
CH
3
I
O
I
Energy
Reaction progress
ΔG⬚
Starting
material A
Product C
Intermediate B
FIGURE 8.5 As long as equilibrium is reached between
A and B, it does not matter that formation of B from A
is endergonic, compound C will be the major product.
338 CHAPTER 8 Equilibria
PROBLEM 8.2 Estimate the ΔH° for the following reactions:
(a)
(b)
(c)
(d)
PROBLEM 8.3 The bond dissociation energies for the carbon–hydrogen bonds in
Table 8.2 show a steady decline as the breaking carbon–hydrogen bond becomes
more substituted. What can you infer from these data about the stability of the
neutral species, , called “free radicals”?R
.
HCl + H
2
C
P
CH
2
U
ClCH
2
O
CH
3
Br
2
+ H
2
C
P
CH
2
U
BrCH
2
O
CH
2
Br
H
2
+ H
2
C
P
CH
2
U
H
3
C
O
CH
3
CH
3
OH + H
2
C
P
CH
2
U
H
3
C
O
CH
2
OCH
3
The entropy term (ΔS°) is related to freedom of movement. The more restricted
or “ordered”the product(s) of a reaction, the more negative is the entropy change in
the reaction. Since it is the negative of ΔS° that is related to ΔG° [Eq. (8.6)], the
more negative the entropy change, the higher is the free energy change (ΔG°) of
the reaction. Note also that the entropy term is temperature-dependent. Entropy
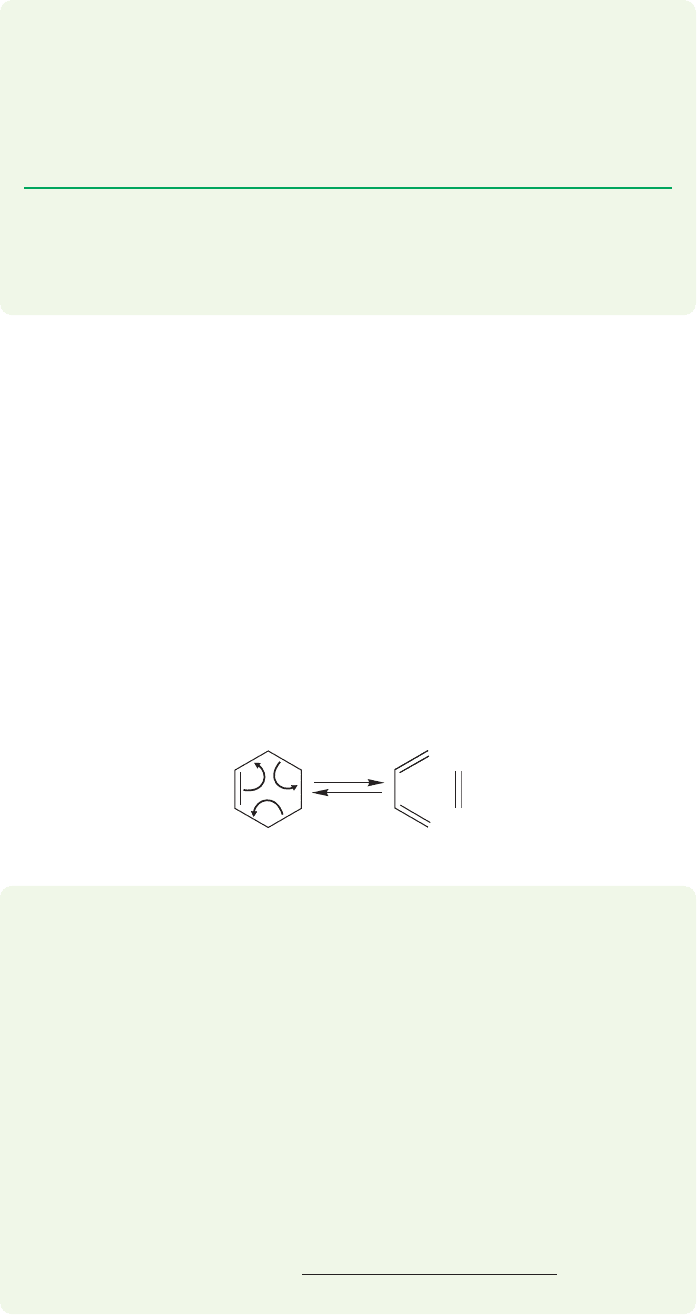
becomes more important at high temperature. Imagine the reaction in Figure 8.6,
in which strong carbon–carbon σ bonds are broken and weaker carbon–carbon π
bonds are made (Table 8.2). The ΔH° term will surely favor the left-hand side. At
the same time, one molecule is made into two other, smaller molecules, and this
change will be favored by the entropy change, ΔS°. This reaction, in which entropy
change favors the right-hand side, can be driven to the right by using a high tem-
perature,at which the T ΔS° term is large.An unfavorable reaction can even become
favorable at high temperature where the entropy term becomes more important in
determining the value of ΔG°.
WORKED PROBLEM 8.4 Use ΔH° values from Table 8.2, as well as the value for
the BDE of of 80 kcal/mol,
to estimate ΔH° for the reaction in Figure 8.6.
ANSWER Compare the bonds that are breaking with those that are being made.
Two carbon–carbon single bonds are broken. As the problem says, each is worth
80 kcal/mol. In addition, a π bond (66 kcal/mol) is broken in the reaction. So, the
bonds broken are worth a total of 226 kcal/mol. Three π bonds are made, each
worth 66 kcal/mol, for a total of 198 kcal/mol. The left-to-right reaction is
endothermic by about 28 kcal/mol, because the bonds made are weaker than
those broken.
¢H
ⴰ
=+28 kcal/mol
3 * C
P
C (π) =-198 kcal/mol (bonds made)
C
P
C (π) =+66 kcal/mol (bond broken)
2 * C
O
C =+160 kcal/mol (bonds broken)
CH
2
P
CH
2
U
H
3
C
#
#
CH
2
CH
P
CH
2
CH
O
H
3
C
+
FIGURE 8.6 This reaction is a process
in which one direction is favored by
enthalpy and the other by entropy.
Although ΔH° favors cyclohexene, at
very high temperature the formation
of two compounds from one can
drive this reaction to the right.
