Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


8.7 Reaction Mechanism 349
Let us now concentrate on these energy barriers separating the various intermedi-
ates.The barriers are made up of the same quantities, enthalpy and entropy, that define
the energy of any chemical species. Recall that ΔG° ΔH° TΔS° [Eq. (8.6)].
Transition state theory assumes that we can treat a transition state as if it were a real
molecule occupying a potential energy minimum. It is important to emphasize that it
is not, however. It occupies an energy maximum and can never be bottled and exam-
ined as real molecules can.Nonetheless it has an energy,as indeed does every other point
in the energy diagrams on the preceding pages.The energy of the transition state, and
thus the height of the energy barrier in the reaction, can be calculated using Eq. (8.8).
ΔG
‡
ΔH
‡
T ΔS
‡
(8.8)
Remember: The double daggers (
‡
) simply indicate that we are referring to a transi-
tion state,not to an energy minimum.The energy barrier, ΔG
‡
,is the Gibbs free ener-
gy of activation,ΔH
‡
is the enthalpy of activation,and ΔS
‡
is the entropy of activation.
Clearly, the rate constant for any reaction will depend on the height of the bar-
rier,and thus on ΔG
‡
.The theoretical study of reaction rates gives us the exact equa-
tion relating the rate constant k and ΔG
‡
[Eq. (8.9)].
k ν
‡
or k ν
‡
(8.9)
It is worth dissecting this equation a bit further. The quantity ν
‡
is a frequency—
its units are reciprocal seconds (s
1
) in this case. In qualitative terms, ν is the fre-
quency with which molecules with enough energy to cross the barrier actually do
so. So,the rate constant k is equal to e
ΔG
‡
/RT
(the fraction of molecules with enough
energy to cross the barrier) times ν
‡
(the frequency with which such molecules actu-
ally do cross the barrier).
e
¢S
‡
/R
e
-¢H
‡
/RT
e
-¢G
‡
/RT
Summary
Reaction rates are determined not by the relative energies of the starting material
and product, but by the height of the transition state separating them. Even
though the transition state is an energy maximum, a transient species that can-
not be isolated, its energy is determined by enthalpy and entropy factors, just as
is the energy of any stable compound.
8.7 Reaction Mechanism
Throughout this book we will frequently be concerned with the determination of
reaction mechanisms.A mechanism is nothing less than the description of the struc-
tures and energies of the starting materials and products of a reaction, as well as of
any reaction intermediates. In addition, all of the transition states (energy maxima)
separating the stable molecules lying in energy minima must be described. It is rel-
atively easy to deal with energy minima—we can often isolate the molecules them-
selves, take their spectra, and measure their properties. As we can by definition never
isolate a transition state, it is exceptionally difficult to make a direct measurement
on it. Yet if we are to have a good picture of a reaction, we must arrive at good
descriptions of the transition states involved.
We can usually measure the rate law, which tells us the number and kinds of
molecules involved in the transition state. The rate law does not tell us anything
about the orientation of the molecules in the transition state, however. Usually,
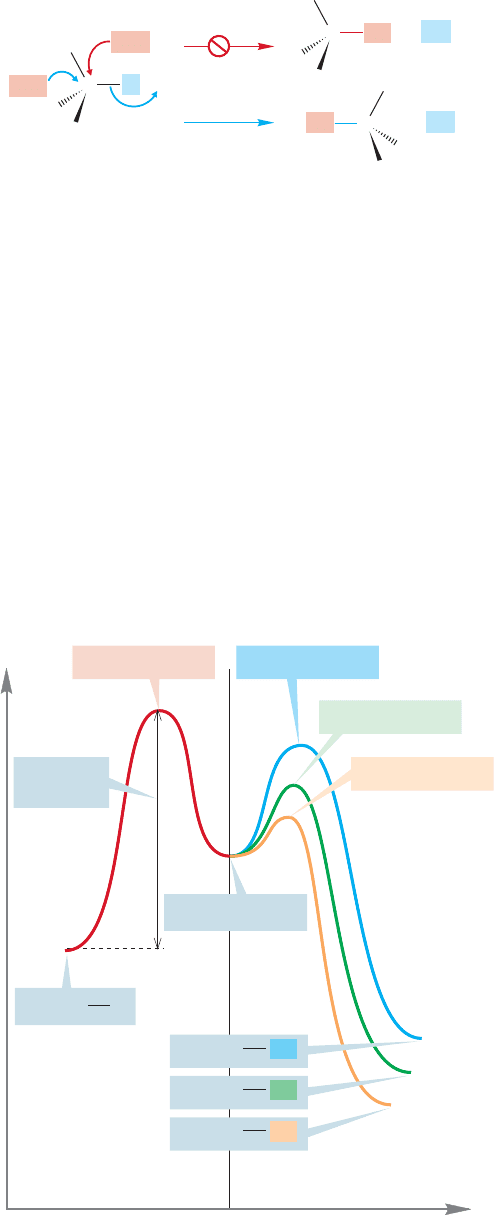
350 CHAPTER 8 Equilibria
stereochemical experiments do that. Recall the S
N
2 reaction (p.268), in which inver-
sion of configuration is observed, thus specifying the direction of approach of the
entering nucleophile in the transition state (Fig. 8.25).
C
C
frontside
backside
C
+
+
L
L
L
Nu
Nu
..
..
Nu
..
–
Nu
..
–
–
–
FIGURE 8.25 In the S
N
2 reaction,
kinetics cannot tell frontside from
backside displacement. It takes a
stereochemical experiment to do that.
This observation of inversion in the S
N
2 reaction tells us that the incoming nucleo-
phile must approach from the rear of the departing leaving group. The rate law,
,does not tell us that. It shows only that both the nucleophile
and the substrate are involved in the transition state. As we work through the impor-
tant reactions of organic chemistry, we will see many similar experiments designed
to determine the stereochemistry of the reaction. The energy of the transition state
can be measured if good quantitative kinetic experiments can be performed. If the
rate constant for the reaction is determined at a variety of temperatures, the height
of the transition state can be calculated. But we can do this only for the step that
has the highest-energy transition state. In a multistep reaction, we can only meas-
ure the rate for the slowest step in the sequence.
This slowest step is the rate-determining step, sometimes called the rate-limiting
step. It is always the step in the reaction with the highest-energy transition state. We
cannot measure the rates of faster steps in the reaction.In the S
N
1 reaction,for exam-
ple, the slowest step is the ionization of the substrate to a carbocation. This ion may
then be captured by a host of nucleophiles in following faster steps. There will be a
different product for every capturing nucleophile (p. 295; Fig. 8.26).
rate = k[Nu
:
-
][R
O
L]
Energy
Reaction progress
Transition state 1 Transition state 2
Transition state 3
Transition state 4
(CH
3
)
3
C
+
+
(CH
3
)
3
C
(CH
3
)
3
C
(CH
3
)
3
C
(CH
3
)
3
C
Activation
energy
Slow step
ionization
Fast steps
capture by nucleophiles
–
I
..
..
..
..
I
..
..
..
Nu
Nu
Nu
FIGURE 8.26 In this S
N
1 reaction,
the rate-determining step is the
ionization of the starting iodide to
give the tert-butyl cation.The faster,
product-determining steps follow, but
their rates cannot be measured
kinetically (unless the intermediate
carbocation can be isolated and
investigated independently).
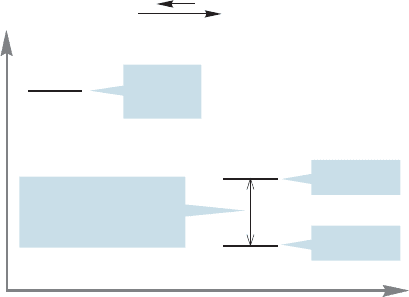
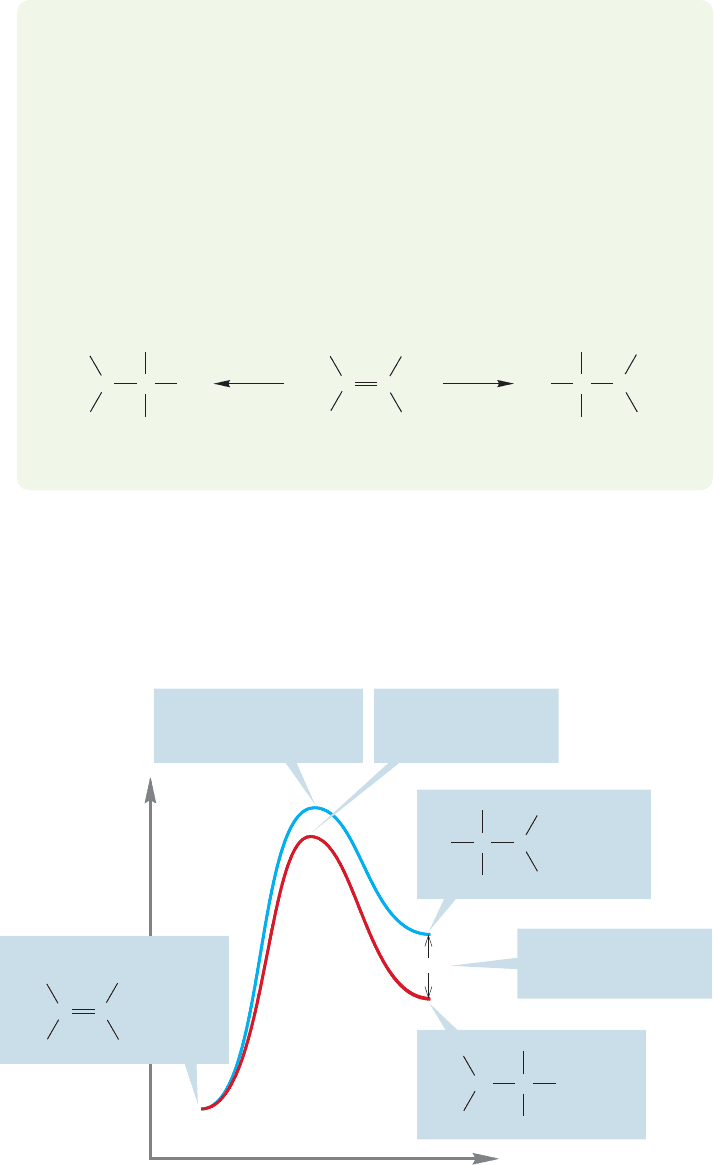
8.8 The Hammond Postulate: Thermodynamics versus Kinetics 351
If we determine the rate of this process by measuring the disappearance of
tert-butyl iodide over time, we can ultimately find the height of transition state 1.
The rates of the faster captures of the carbocation do not affect the rate of ioniza-
tion of tert-butyl iodide. The carbocation is gobbled up by the nucleophiles at a
rate faster than that at which it is formed. In the limit, each molecule of the tert-
butyl cation is captured before another is produced from tert-butyl iodide. The
rate of formation of product depends on how fast the tert-butyl cation is pro-
duced in the slow, rate-determining step but not on the rate of the fast capture
by a nucleophile.
Imagine a cage of especially ravenous rats into which we are slowly dropping
incredibly delicious food pellets. At first, the pellets are gobbled up by the hun-
gry rats at a rate far faster than they are delivered to the cage.The slowest step in
this reaction, the step that determines the rate of disappearance of the food, is the
rate at which the pellets are delivered to the cage. This process is analogous to a
reaction in which the first step (food dropping into the cage) is slower than the
second step (the rats eating the food). Later, however, a different situation obtains.
Even these rats can become satiated, and the food pellets accumulate in the cage
as the no-longer-starving rodents largely ignore them, only taking a nibble now
and then. The second step is now slower than the first step. Now the step that
determines the rate of disappearance of the food is the rate at which the rats eat
the pellets. This second scenario has a different rate-determining step than the
first scenario.
Energy
Reaction progress
Product X
Product Y
Product X
+ Product Y
Starting
material
Starting material
ΔG⬚, the energy
difference between
X and Y
FIGURE 8.27 The energy difference
between X and Y does not necessarily
affect their rates of formation.
8.8 The Hammond Postulate:
Thermodynamics versus Kinetics
We are used to saying, “Product Y is more stable than product X and therefore
is formed faster.” This statement has a comfortable sound to it and intuitively
makes sense. But it need not be true! The first half of the statement describes
thermodynamics, the relationship of the energies of product X and product Y.
The second half speaks of the rates of formation of X and Y, which is a
statement about kinetics. Despite the good intuitive feel of the original state-
ment, there need be no direct connection between thermodynamic stabilities and
kinetics.
Figure 8.27 shows the relative energies of two products, X and Y, being formed
from a starting material. One might expect that it is the ΔG° between the two
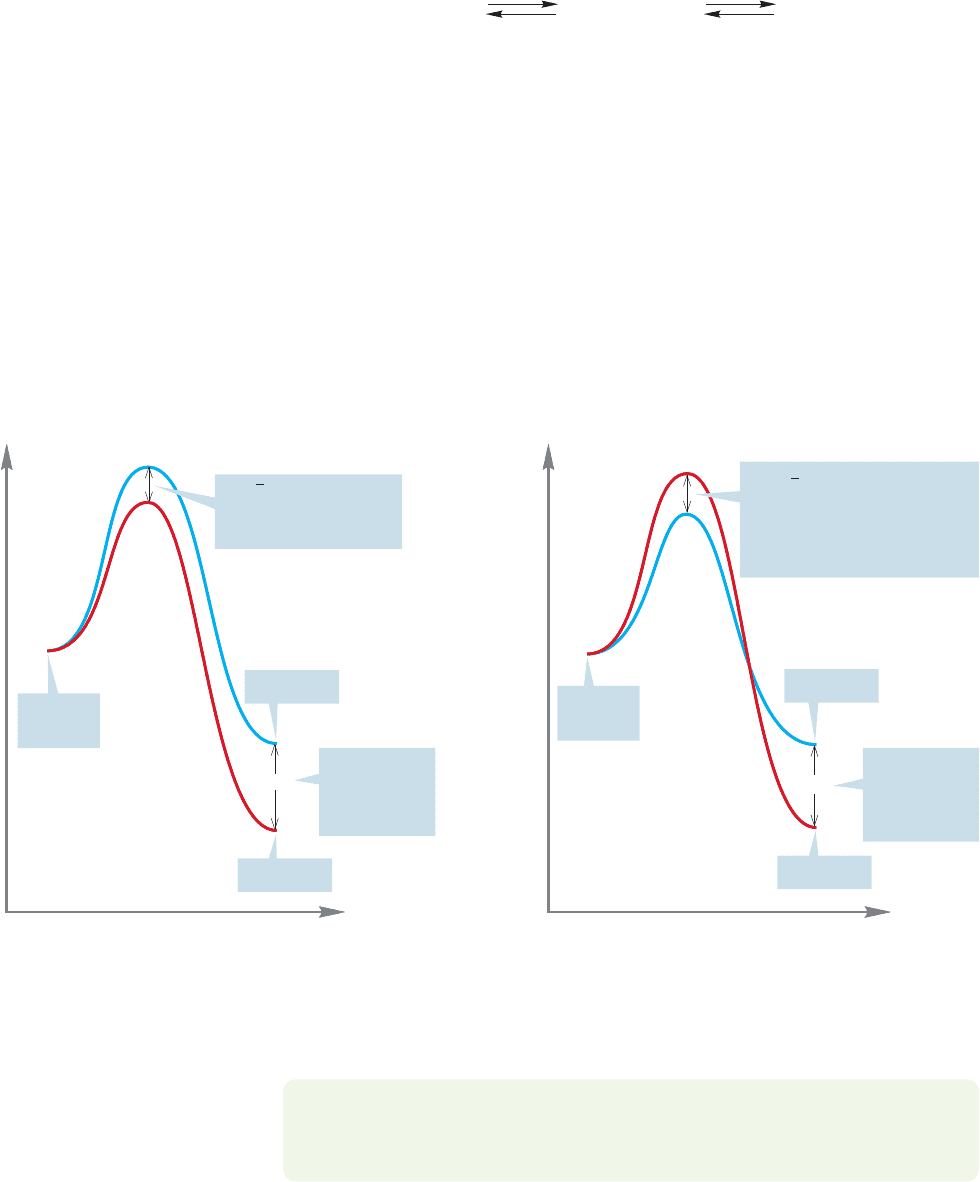
352 CHAPTER 8 Equilibria
products X and Y that determines the amount of the two compounds formed, but
this notion is true only if X and Y are in equilibrium via the starting material
(Fig. 8.28).
Starting materialProduct X
Product
Y
FIGURE 8.28 Only if X and Y are in
equilibrium does ΔG° determine their
ratio.
It is conceivable that the products are not in equilibrium with each other. For
instance, there might not be enough energy for the products to go back to the start-
ing material. Under these conditions, the amounts of X and Y are determined not
by their relative energies but only by the heights of the transition states leading to
them. Figure 8.29a shows a conventional picture in which the transition state lead-
ing to the more stable product, Y, is lower in energy than the transition state lead-
ing to the higher-energy product, X. Under such conditions, the major product will
be the more stable compound,Y.We have seen many examples of reactions like this.
The scenario of Figure 8.29a is not the only possibility. It could be the case that the
transition state leading to the more stable compound, Y, is actually higher in energy
than the transition state leading to the less stable product, X (Fig. 8.29b).
(a)
Energy
Reaction progress
The energy
difference
between
the products
Starting
material
Product X
Product Y
ΔΔG
, the energy
difference between the
two transition states
ΔG⬚
†
(b)
Energy
Reaction progress
The energy
difference
between
the products
Starting
material
Product X
Product Y
ΔG⬚
ΔΔG
, the energy difference
between the two transition
states; the transition state
for formation of Y is higher
in energy
†
FIGURE 8.29 (a) The usual picture is this: The higher transition state leads to the less stable product. The lower transition state
leads to the more stable product. (b) The situation could be otherwise; the higher transition state could lead to the lower-energy
product, and the lower-energy transition state to the higher-energy product.This second scenario is rare, but it does happen.
PROBLEM 8.9 In Figure 8.29a what is the activation energy for the reaction of X
going to starting material? What is the activation energy for the reaction of Y
going to starting material?
8.8 The Hammond Postulate: Thermodynamics versus Kinetics 353
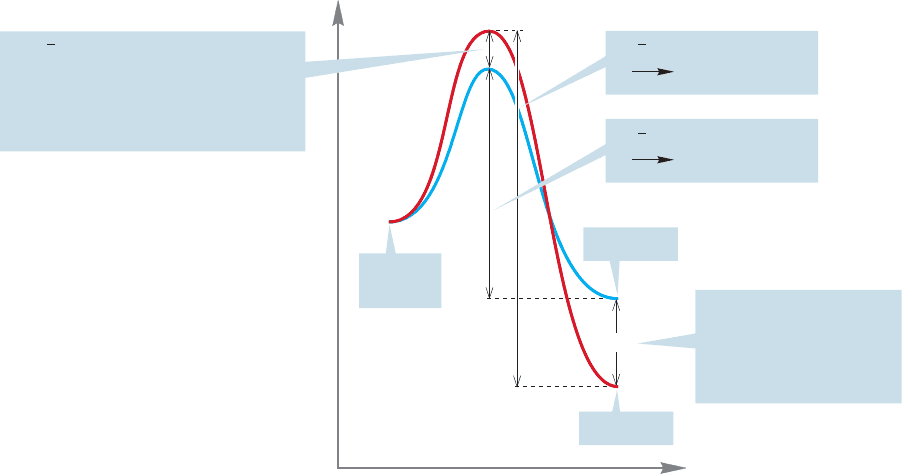
This inversion will not matter if X and Y equilibrate via the starting material.
In such a case, the greater stability of Y will win out eventually, and the ratio of X
and Y depends only on the ΔG° between them. Such a reaction is said to be under
thermodynamic control. But what if the two products do not equilibrate? What if there
is enough energy available so that the forward reactions are possible but the backward
reactions from X or Y to starting material are not? In the exothermic processes shown,
the forward reaction is easier—has a lower activation barrier—than the backward
reaction (Fig. 8.30).
Under these circumstances, the relative amounts of X and Y depend only on the
relative heights of the transition states leading to the two products.The product for
which the transition state is lower is the product that is formed in greater amount.
Such a reaction is said to be under kinetic control.
There is no necessary connection between thermodynamics and kinetics. In our
original statement, “Product Y is more stable than product X and therefore is
formed faster,” we are equating thermodynamics and kinetics. We tacitly assume
that it will always be the case that the product of greater stability (lower energy)
will be formed at a faster rate via the lower-energy (more stable) transition state
(Fig. 8.29a).
In principle, it could be that the transition state for the reaction leading to the
more stable product is actually higher in energy than the transition state leading to
the less stable product. Such a situation can lead to the preferred formation of the
less stable product, if the reaction is under kinetic control (Fig. 8.29b).
Although there are a few reactions for which the scenario of Figure 8.29b applies,
fortunately they are rare because there are factors that operate to preserve a paral-
lelism between the energies of products and the transition states leading to them.
The rest of this section will try to show you why the parallelism between product
energy and transition state energy exists for many reactions. Consider, for a start,
Worked Problem 8.10.
Energy
Reaction progress
ΔG
, activation energy for
Y starting material
Product X
Product Y
ΔG⬚
ΔΔG
, the energy difference between
the two transition states; this quantity
will determine the relative amounts
of Y and X if these products cannot
go back to starting material
The energy difference
between the products;
this makes no difference
if the products cannot go
back to starting material
ΔG
, activation energy for
X starting material
Starting
material
†
†
†
FIGURE 8.30 If there is enough
energy so that all barriers can be
crossed, the ratio of products will be
determined by ΔG°, and the reaction
is said to be under thermodynamic
control. If, however, there is not
enough energy to cross the high
barriers for the reverse reactions
leading from X and Y to starting
material, the ratio of products is
determined by the relative energies
of the two transition states, and the
reaction is said to be under kinetic
control.
354 CHAPTER 8 Equilibria
Before we accept the comfortable reasoning laid out in the answer to Worked
Problem 8.10, we had better think it through. Look first at the Energy versus
Reaction progress diagram (Fig. 8.31).
Energy
Reaction progress
The energy difference
between the two cation
products
Transition state for
formation of the
secondary carbocation B
⌬G⬚
Transition state for
formation of the
tertiary carbocation A
+
C
+
H
C
H
H
3
C
CH
3
H
3
C
C
+
CH
H
3
C
CH
3
H
H
3
C
+
..
..
H
2
O
..
..
H
2
O
Starting material
C
H
C
H
3
C
CH
3
H
3
C
+
H
3
O
..
+
FIGURE 8.31 The more substituted
cation is at lower energy than the less
substituted cation. Note also that the
transition state for formation of the
tertiary cation is lower than the
transition state for formation of the
secondary cation. Why?
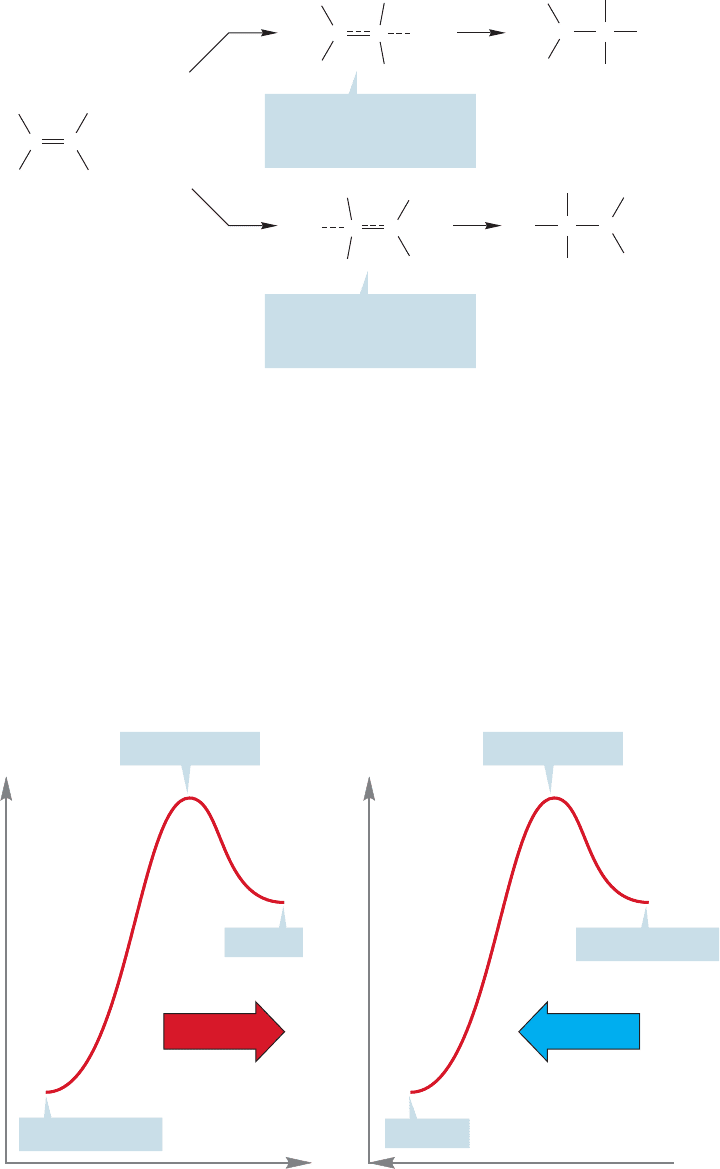
WORKED PROBLEM 8.10 As we saw in Chapter 3, alkenes can be protonated to
give carbocations. Unsymmetrical alkenes, such as 2-methyl-2-butene, could be
protonated at either end of the double bond to give two different carbocations.
Predict the direction of protonation of 2-methyl-2-butene and explain your
answer.
ANSWER The choice is between the formation of secondary carbocation B and
tertiary carbocation A. It is surely tempting to predict that the more stable ter-
tiary carbocation will be formed faster than the less stable secondary carbocation.
However, this comfortable reasoning equates thermodynamics (more stable)
with kinetics (will be formed faster), and this equation is not necessarily valid.
Read on.
Carbocation A Carbocation B2-Methyl-2-butene
C
+
H
C
H
H
3
C
CH
3
H
3
C
C
+
H
C
H
H
3
C
CH
3
H
3
C
C
H
C
H
3
C
CH
3
H
3
C
H
3
O
..
+
H
3
O
..
+
The tertiary cation is more substituted than the secondary cation and thus is more
stable by virtue of hyperconjugative overlap of a sigma bond with the empty 2p
orbital (p.292). Substitution stabilizes the positive charge on carbon in these species.
8.8 The Hammond Postulate: Thermodynamics versus Kinetics 355
In the transition states for formation of the carbocations, the new bond from
carbon to hydrogen is partially formed and the carbons have developed partial
positive charges (Fig. 8.32).
C
H
C
H
δ
+
δ
+
δ
+
δ
+
H
3
C
CH
3
H
3
C
C
+
H
C
H
CH
3
C
+
C H
H
3
C
H
3
C
C
H
3
C
H
3
C
H
A
B
H
C
H
H
3
C
CH
3
CH
3
H
3
C
C
H
C
H
3
C
CH
3
H
3
C
Transition state contains
a partial positive charge
on a tertiary carbon
Transition state contains
a partial positive charge
on a secondary carbon
+
H
3
O
..
+
FIGURE 8.32 In the two transition
states, partial positive charge has built
up on carbon. A partial positive
charge on a tertiary carbon is more
stable than a partial positive charge
on a secondary carbon.
Energy
Reaction pro
g
ress
Starting material
Product
Endothermic
Energy
Reaction pro
g
ress
Starting material
Transition state
Product
Exothermic
Transition state
FIGURE 8.33 Equivalent energy
diagrams for endothermic and
exothermic reactions.
The factors that operate to stabilize a full positive charge also operate to stabi-
lize a partial positive charge. We therefore expect the transition state for the forma-
tion of a tertiary carbocation to be lower in energy than the transition state for
formation of a secondary carbocation, although the difference will not be as great
as that for the fully developed cations (Fig. 8.31).
This specific situation has been generalized in what is called the Hammond pos-
tulate after its originator, G. S.Hammond (1921–2005): “For an endothermic reac-
tion, the transition state will resemble the product.” Note immediately that this
statement can be turned around. “For an exothermic reaction, the transition state
will resemble the starting material.” Figure 8.33 shows an endothermic reaction,
but we know that all we have to do to see an exothermic reaction is look at the
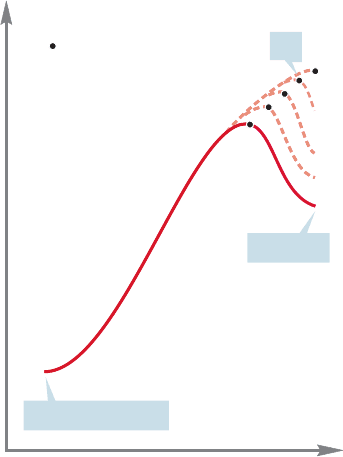
356 CHAPTER 8 Equilibria
endothermic reaction in the other direction! So these two versions of the Hammond
postulate are really the same.
In the endothermic protonation of 2-methyl-2-butene, we rationalized the
faster formation of the more stable tertiary cation rather than the less stable
secondary carbocation by pointing out that in the transition state for carbocation
formation partial positive charge builds up on a tertiary, rather than secondary
carbon. The tertiary carbocation intermediate and the transition state leading to it
are more stable than their secondary counterparts. Because the transition state for
tertiary carbocation formation is lower energy than the transition state for
secondary carbocation formation, the tertiary carbocation will be formed faster.
The key here is that the transition states resemble the final products, just as the
Hammond postulate would have it.
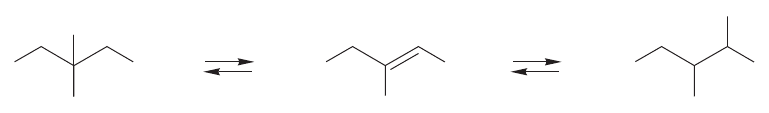
We can also do a thought experiment to see that the more endothermic a reac-
tion is, the more the transition state will look like the product. Imagine an
endothermic reaction with the energy profile shown in Figure 8.34. Now apply a
pair of molecule-grabbing chemical tweezers and raise the energy of the product
without changing the energy of anything else. As we magically increase the ener-
gy of the product, it becomes less and less stable. Eventually, it becomes the
transition state, as it reaches a point where it is so unstable that it no longer lies
in an energy minimum. Now think about the moment just before this happens.
At this point (ε in Fig. 8.34), the product and the transition state are so close to
each other that the slightest increase in energy in the product makes them the
same (Fig. 8.34).
Energy
Reaction
p
ro
g
ress
Starting material
Product
Transition
state
=
ε
FIGURE 8.34 A thought experiment
in which we raise the energy of the
product without changing the energy
of the starting material.
By reflecting on this limiting situation,we can see how the transition state and the
product approach each other as the energy of the product increases and the reaction
becomes more endothermic.
8.9 Special Topic: Enzymes and Reaction Rates 357
It is a simple matter to turn this all around and examine the other side of the
Hammond postulate, “The more exothermic a reaction, the more the transition
state resembles starting material.” Figure 8.34 will do it for you if you simply read
it backward, from right to left. See what happens to the transition state as the
right-to-left reaction becomes less and less exothermic.
The take-home lesson of all this discussion is that although one cannot
take the correspondence between thermodynamics and kinetics as a given—there
will be counterexamples—in general, the statement “Y is more stable and thus
formed faster” will be true. There is more to it than it appears at first. This
statement is tricky indeed, and it is worth stopping and examining it every time
we make it.
8.9 Special Topic: Enzymes and Reaction Rates
In this chapter, we have discussed the interconversion of compounds by passing
from starting material to product over a high-energy point called the transition
state. The higher the transition state, the slower is the reaction. In the laboratory,
we affect the rate of reaction by adding acid or base catalysts,by changing solvents,
or sometimes simply by supplying lots of energy. Our bodies run on the same chem-
istry found in the laboratory. For example, the substitution and elimination reac-
tions described in Chapter 7 are all reasonably common in the transformations of
biomolecules occurring in each of us.
Nature can’t overcome activation barriers to reactions important to us by adding
catalysts such as sulfuric acid,and turning up the heat in our bodies to provide ener-
gy becomes uncomfortable very quickly indeed. So,Nature does it another way, and
it is most successful. Nature uses enzymes, which are polyamino acid chains with
catalytic activity, to lower the barriers to chemical transformations. Aconitase, an
enzyme of molecular weight 89,000 (!), catalyzes the conversion of citrate into cis-
aconitate, an elimination reaction. Aconitate is then hydrated to give isocitrate. So,
this enzyme makes possible one of the key reactions of Chapter 7, elimination, and
then reverses the process, but putting the OH on the other alkene carbon, to give
the isocitrate (Fig. 8.35).
Citrate cis-Aconitate Isocitrate
–
OOC
COO
–
OH
COO
–
–
OOC
–
OOC
COO
–
COO
–
COO
–
COO
–
OH
FIGURE 8.35 The interconversions catalyzed by the enzyme aconitase.
The vast molecular architecture of the enormous molecule aconitase serves
to bind the substrates precisely, holding them in perfect position for the side
chains of the amino acids to act as catalysts, speeding the elimination and addi-
tion reactions. Spectacular rate accelerations can be achieved by enzymes, allow-
ing all sorts of reactions to take place at body temperature, 37 °C, that could not
occur otherwise.
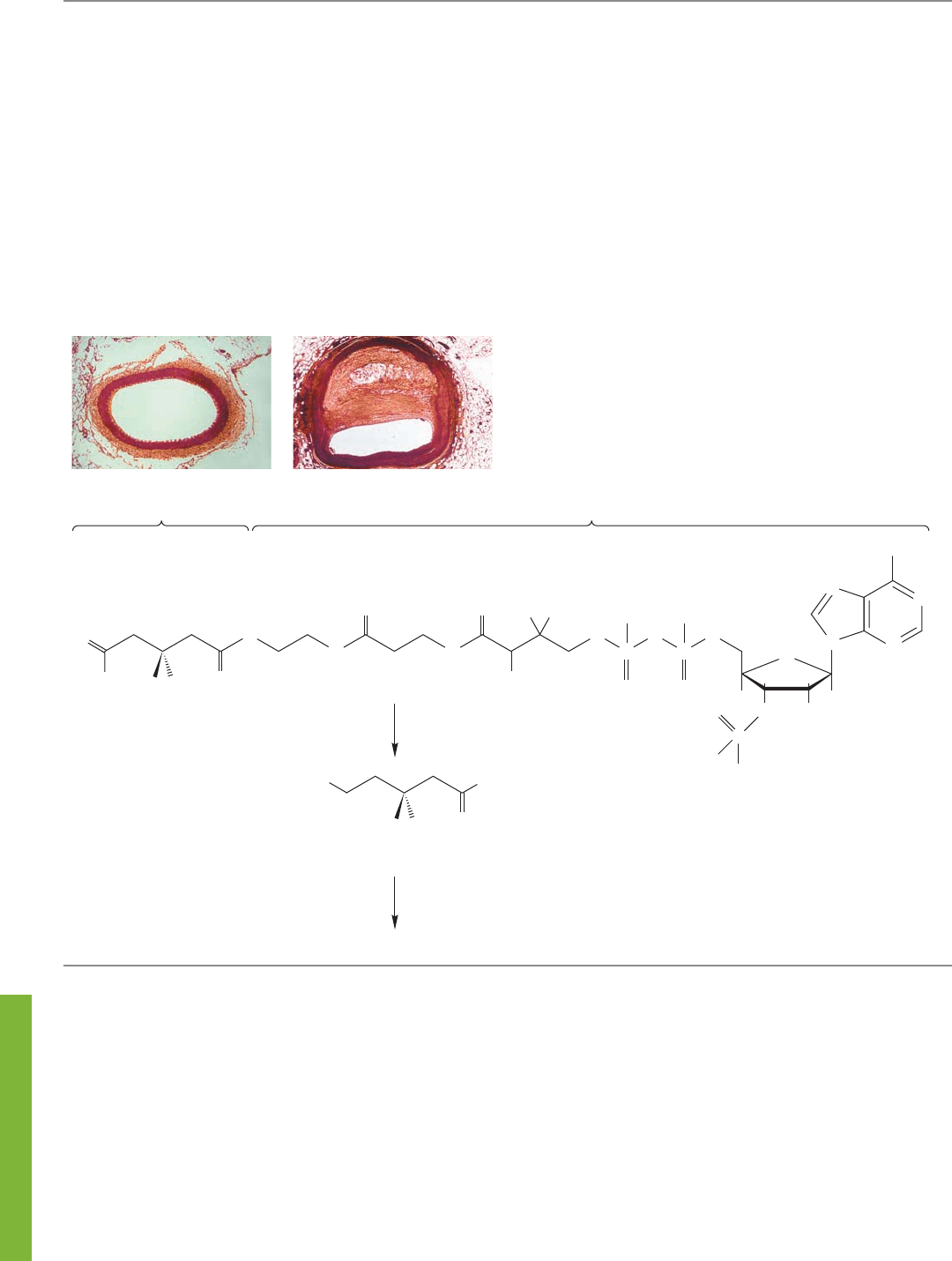
358 CHAPTER 8 Equilibria
3-Hydroxy-3-methylglutaryl
(or HMG)
Mevalonic acid
Cholesterol
CoA
HMG-CoA
reductase
rate-limiting
step
many
steps
O
OH
–
O
–
O
HO
A
NH
2
N
O
–
O OH
S
O
N
H
N
H
OH
N
N
O
O
O O
O
–
P
O
O
O
H H
H H
O
O
–
P
O
OH
O
–
P
O
N
CHOLESTEROL FORMATION
Sometimes, our well-being depends on interfering with
an enzyme-mediated rate acceleration. Cholesterol (p. 121)
is formed in the body by a lengthy process in which the
thioester A is reduced to mevalonic acid, which is then
converted in a series of reactions into cholesterol.
Controlling cholesterol levels in the body is an important
part of healthy living. Recent advances in medicinal and
organic chemistry have allowed the development of the
“statin” drugs (i.e., atorvastatin, fluvastatin, lovastatin, pravas-
tatin, simvastatin, and rosuvastatin), which act to reduce the
level of “unhealthy” low-density lipoprotein (LDL) choles-
terol. Examples of a healthy artery and a partially clogged
artery, which can result from high levels of LDL cholesterol,
are shown below. The benefit of taking a statin drug is a sig-
nificant reduction in the risk of heart attacks and strokes.
The statins function by inhibiting the enzyme HMG-
CoA reductase, which is involved in the rate-limiting or
slowest step in the formation of mevalonic acid, and hence
cholesterol. Without the enzyme, the reduction to mevalonic
acid in the body is much too slow. We have learned about
hydride reagents (p. 315), and we do have biological hydride
sources. You will meet one, NADH, in Chapter 16. But simply
mixing this “natural” hydride reagent with the HMG-CoA
thioester A results in no reaction. However, HMG-CoA
reductase can bring the hydride source and the thioester A
together in a fashion that allows the reaction to occur. The
energy barrier for the reaction is lowered, and mevalonic acid
is formed and goes on to make cholesterol. The statins inter-
fere with the reduction by inhibiting the enzyme necessary—
no reduction, no cholesterol!
This chapter is devoted entirely to concepts. You won’t find a
new mechanism or new synthetic route in it. Yet, it is extremely
important because what we say here about equilibrium and rates
is relevant to all the reactions that will appear in subsequent
chapters.
All reactions are equilibria or, in the case of multistep
reactions, sequences of equilibria between a starting material
and a product. The equilibrium constant K is related to the
difference in energy between starting materials and products
in a logarithmic fashion (ΔG° RT ln K or K e
ΔG°/RT
).
8.10 Summary
New Concepts
