Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

8.10 Summary 359
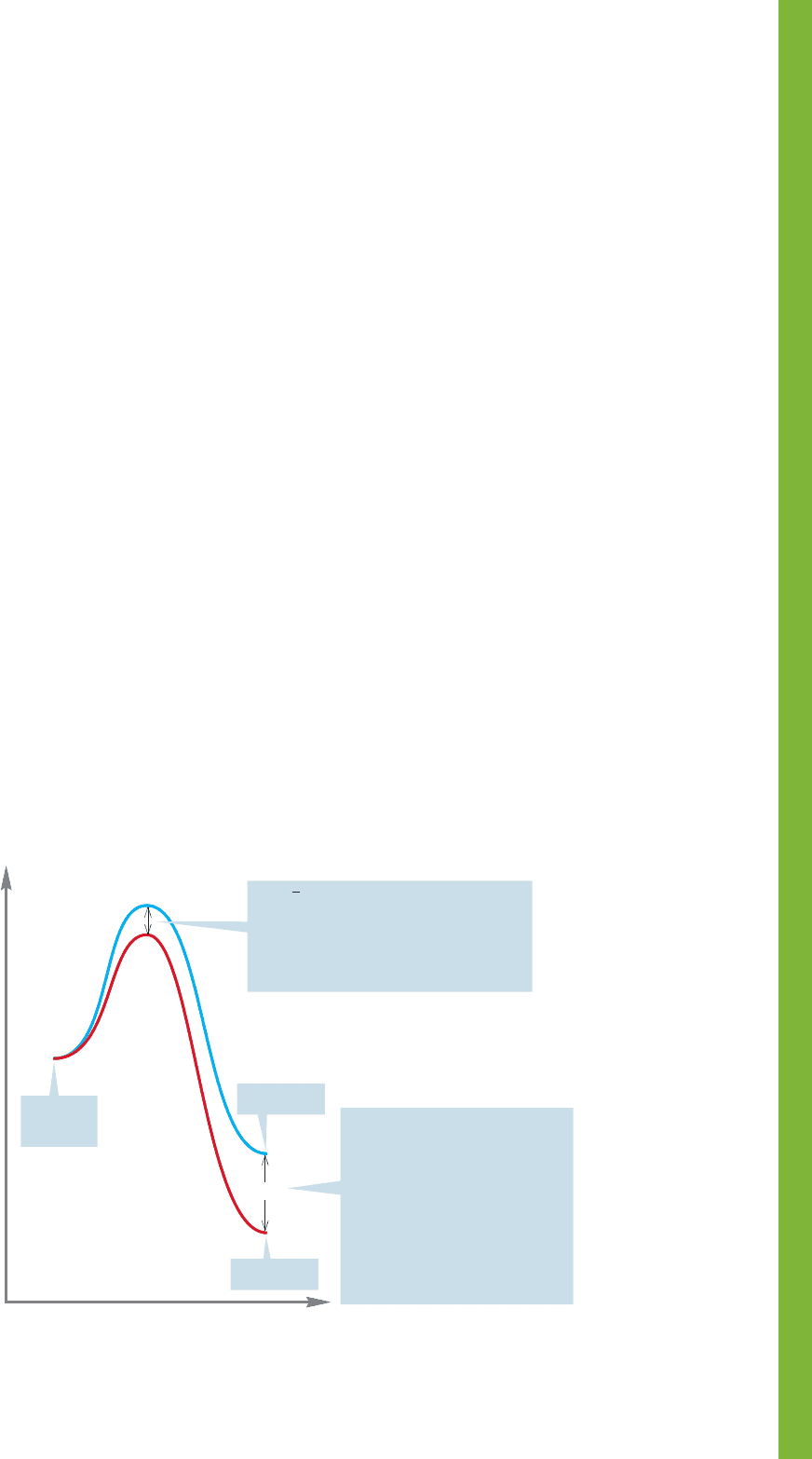
FIGURE 8.36 Kinetic control of product distribution.
Key Terms
Boltzmann distribution (p. 340)
endergonic (p. 335)
entropy change (ΔS°) (p. 335)
equilibrium constant (K ) (p. 334)
exergonic (p. 335)
first-order reaction (p. 340)
Gibbs free energy change (ΔG°) (p. 335)
Hammond postulate (p. 355)
kinetic control (p. 353)
kinetics (p. 351)
Le Châtelier’s principle (p. 336)
microscopic reversibility (p. 346)
pseudo-first-order reaction (p. 340)
rate constant (k) (p. 341)
second-order reaction (p. 340)
thermodynamic control (p. 353)
thermodynamics (p. 351)
Energy
Reaction
p
ro
g
ress
Starting
material
Product X
Product Y
⌬G⬚
ΔΔG
, the energy difference between
the two transition states; if X and Y
cannot revert to starting material, it is
this difference that solely determines
the relative amounts of products
ΔG⬚ is the energy difference
between the products; this
energy can have no effect
on the product distribution
unless X and Y can return to
starting material; if not, it will
be the difference between the
two activation energies that
solely influences the product
distribution
†
Therefore, a small amount of energy difference (ΔG°) has a
great influence on the equilibrium constant K.
The ratios of products formed in a reaction depend either
on the energy difference between the products ΔG° (thermo-
dynamic control) or on the relative heights of the transition
states leading to products (kinetic control).
Differences in G°, the Gibbs free energy, whether they
are ΔG°, the difference in energy between starting material
and product, or ΔG
‡
, the difference in energy between
starting material and the transition state (activation energy),
are made up of entropy (ΔS°) and enthalpy (ΔH°) terms
(ΔG° ΔH° T ΔS° or ΔG
‡
ΔH
‡
T ΔS
‡
).
The rate of a reaction depends on temperature, the
concentration of the reactant(s), and the rate constant k for
the reaction. The rate constant is a property of the reaction
and depends on temperature, pressure, and solvent, but not
on the concentrations of the reactants.
The rate-determining step of a reaction is the step with the
highest-energy transition state. It may or may not be the same
as the product-determining step. For example, in the S
N
2
reaction it is the same, but in the S
N
1 reaction it is not.
The principle of microscopic reversibility says that the
lowest-energy path in one direction of an equilibrium reaction
is the lowest-energy path for the reverse reaction.
The Hammond postulate says that the transition state for
an endothermic reaction resembles the product, or, equivalently,
that the transition state for an exothermic reaction resembles
the starting material.
The most common problem experienced with the material in this
chapter is the confusion between thermodynamics and kinetics.
Rates of reactions (kinetics) are determined by the relative
heights of the available transition states leading to products.
If there is insufficient energy available for the products to
re-form starting materials, it will be the relative heights of the
transition states that determine the mixture of products, no mat-
ter what the energy differences among those products (Fig. 8.36).
Common Errors
360 CHAPTER 8 Equilibria
Energy
Reaction
p
ro
g
ress
Starting
material
Product X
Product Y
⌬G⬚
ΔΔG
, the energy
difference between the
two transition states
The energy difference
between the products;
if X and Y can
equilibrate by returning
to starting material, it
will be this ⌬G⬚
that
determines the relative
amounts of X and Y
†
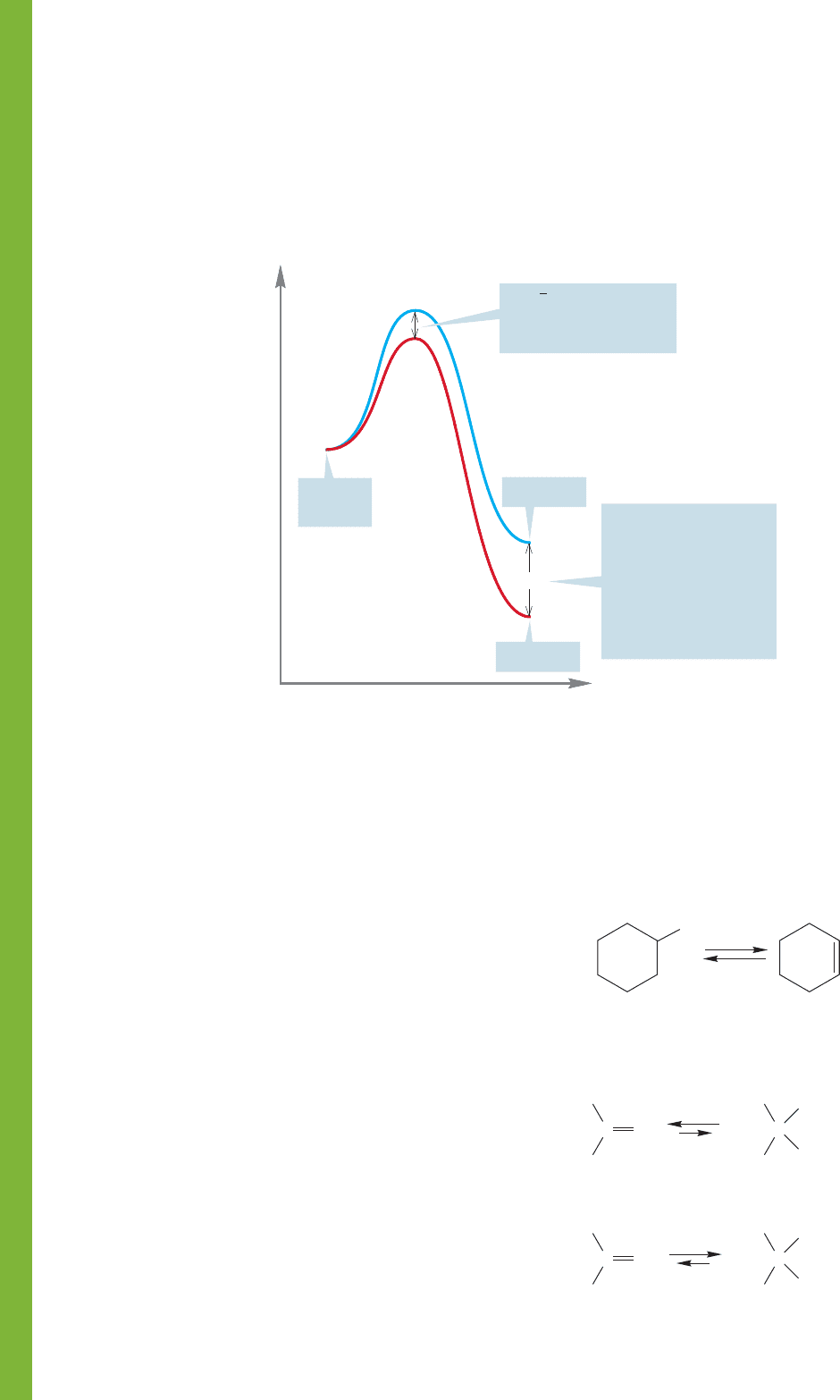
FIGURE 8.37 Thermodynamic control of product distribution.
PROBLEM 8.11 The Finkelstein reaction:
X Cl or Br
is often used to prepare alkyl iodides from alkyl chlorides or
bromides through an S
N
2 reaction.
(a) Suggest a simple way to drive this equilibrium toward the
desired iodide.
(b) In practice, the Finkelstein reaction is usually run in acetone
solvent, because sodium iodide, but not sodium chloride or
sodium bromide, is soluble in acetone. Explain carefully how
this difference in solubilities drives the equilibrium toward
the desired iodide.
PROBLEM 8.12 The alcohol dehydration and alkene hydration
shown below is an equilibrium process.
(a) Suggest experimental conditions that will favor cyclohexene.
R
O
X + NaI
U
Z
R
O
I + NaX
(b) Suggest experimental conditions that will favor cyclohexanol.
PROBLEM 8.13 Calculate the energy differences between the
carbonyl ( ) compounds and their hydrates at 25 °C.
K = 2.8 ⳯10
4
(b)
H
Cl
3
C
C
H
Cl
3
C
CO
OH
OH
H
3
O
+
H
2
O
(a)
H
3
C
H
3
C
C
H
3
C
H
3
C
C
K = 1.4 ⳯10
–
3
O
OH
OH
H
3
O
+
H
2
O
C
P
O
OH
+
H
3
O
..
+
..
..
H
2
O
8.11 Additional Problems
On the other hand, if equilibrium is established, if the
products can revert to starting materials, it will be the relative
energies of the product molecules that determine how much of
each is formed (Fig. 8.37).
Remember also to be careful to distinguish between the rate of
a reaction and the rate constant for that reaction.The rate depends
on the concentration(s) of the reacting molecules; the rate con-
stant does not, and is a fundamental property of the reaction.
8.11 Additional Problems 361
PROBLEM 8.14
Under certain highly acidic conditions, the
three bicyclooctanes shown below can be isomerized. Given the
ratios at equilibrium (3.66 32.95 63.35), calculate the relative
energies of the three isomers at 25 °C.
PROBLEM 8.15 It is possible to equilibrate the following two
ethers. Compound B is favored over A by 1.51 kcal/mol. What
is the equilibrium constant for this interconversion at 25 °C?
PROBLEM 8.16 Use the BDE of Table 8.2 (p. 337) to estimate
ΔH° for the following reactions. Reaction mechanisms are not
necessary!
(a)
FF
FF
H
2
CCH
2
H
2
CCH
2
+
(b)
II
II
H
2
CCH
2
H
2
CCH
2
+
(c)
I
(CH
3
)
2
CI CH
2
(CH
3
)
2
CCH
2
+
(d)
Cl
H
HCl ClCH
2
+
+
.
Cl
(CH
3
)
2
C (CH
3
)
2
CCH
2
.
.
.
A
B
Fe(CO)
5
CH
3
O
CH
3
O
Bicyclo[2.2.2]octane
(3.66%)
Bicyclo[3.3.0]octane
(32.95%)
Bicyclo[3.2.1]octane
(63.35%)
AlBr
3
25 ⬚C
AlBr
3
25 ⬚C
::
PROBLEM 8.17 Use the data in Table 8.2 (p. 337) to design an
endothermic reaction. Don’t worry about mechanism! This
problem does not ask you to design a reasonable reaction—it
merely asks you to find reactions that would be endothermic if
they occurred.
PROBLEM 8.18 For the equilibrium , calculate:
(a) ΔG° at 25 and 200 °C given ΔH° 14.0 kcal/mol and
ΔS° 15.8 cal/deg
·
mol.
(b) ΔG° at 25 and 200 °C given ΔH° 6.0 kcal/mol and
ΔS° 15.8 cal/deg
·
mol.
PROBLEM 8.19 Draw the complete reaction mechanism for
the E1 and S
N
1 reactions of tert-butyl alcohol with a catalytic
amount of H
2
SO
4
and solvent ethanol.
PROBLEM 8.20 Because the E1 and S
N
1 reactions are
reversible, the selection between the two can often be driven
by increasing the temperature. In this fashion the more stable
product is favored. Use data in Table 8.2 to choose the more
stable products in the reaction of tert-butyl alcohol in catalytic
H
2
SO
4
and ethanol.
PROBLEM 8.21 Draw Energy versus Reaction progress dia-
grams for E1 and E2 reactions that are overall (a) exothermic,
(b) endothermic. Use a tertiary iodide as a sample substrate
molecule.
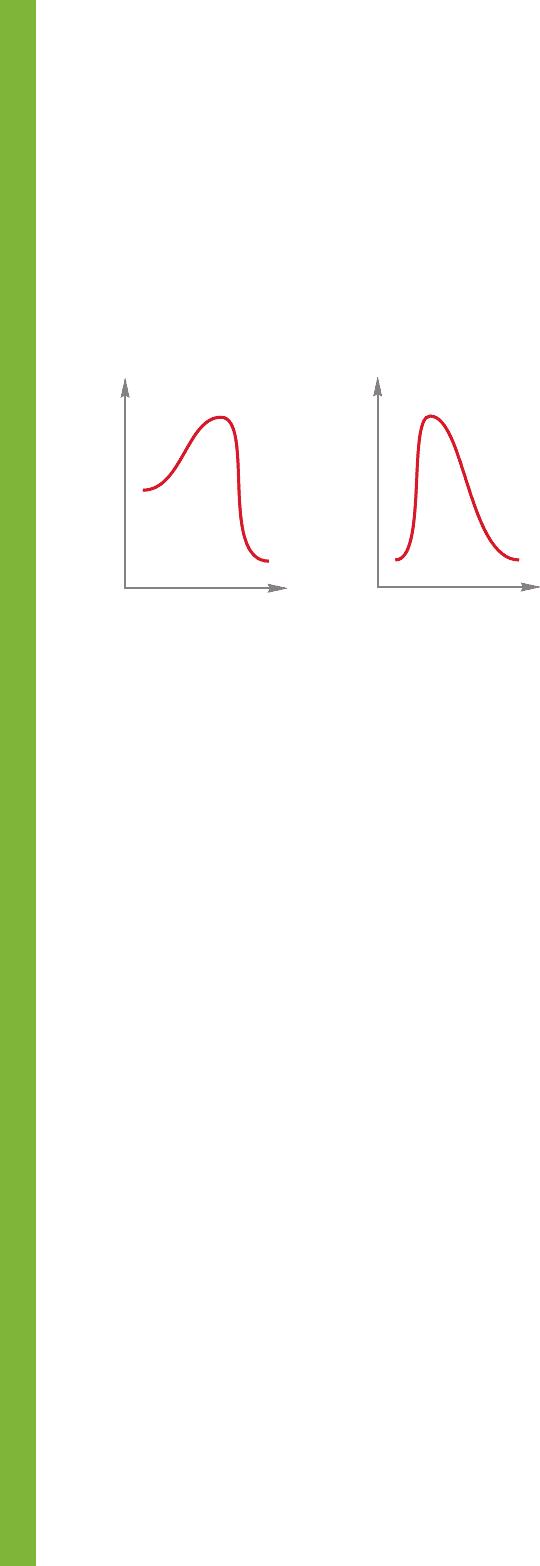
PROBLEM 8.22 For the following two reactions, described
by the two Energy versus Reaction progress diagrams,
(1) and (2):
(a) In each case, which compounds will be present at the end
of the reaction?
(b) In each case, which compound will be present in the largest
amount? Which will be present in the smallest amount?
(c) What is the rate-determining step for the left-to-right
reaction in each case?
(d) Use the diagrams to point out in each case the activation
energy for the following reactions:
AB, AC, CB, and CA
(e) In each case, what is the rate-determining step for the
reaction AC?
U
UUUU
Energy
Reaction progress
(1)
(2)
A
D
B
C
E
Energy
Reaction progress
A
D
B
C
E
A + B
U
Z
C
362 CHAPTER 8 Equilibria
PROBLEM 8.23 Explain in painstaking detail why one is
justified in saying, “The reaction of 2-methyl-2-butene
with HCl leads to the tertiary chloride because a tertiary
carbocation is more stable than a secondary carbocation.”
You might start with the construction of Energy versus
Reaction progress diagrams and good drawings for the
transition states.
PROBLEM 8.24 Find the errors in each diagram.
Energy
Reaction progress
(a)
Energy
Reaction progress
(b)
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 8.25 Select the animation titled “Unimolecular elimi-
nation: E1” and observe the energy diagram. Do you suppose this
reaction is reversible? Use the energy diagram to answer this ques-
tion. Use the nature of the products to answer the same question.
PROBLEM 8.26 Compare the E1 reaction with the “Bimolecular
elimination: E2” in terms of the energy diagram. Do you sup-
pose the E2 reaction is more or less reversible than the E1
reaction? Use the energy diagram to answer this question. Use
the nature of the products to answer the same question.
PROBLEM 8.27 If you needed to make an alkene, would you
prefer to use the E1 reaction or the E2 reaction? Why?
PROBLEM 8.28 Observe the “Hofmann elimination” reaction.
Draw the energy diagram shown for the reaction. Draw a new
reaction curve for the same reaction run in a more polar solvent.
Draw the curve for a reaction run in a less polar solvent. Which
solvent would likely give the faster Hofmann elimination?

Additions to Alkenes 1
363
9.1 Preview
9.2 Mechanism of the Addition of
Hydrogen Halides to Alkenes
9.3 Effects of Resonance on
Regiochemistry
9.4 Brief Review of Resonance
9.5 Resonance and the Stability of
Carbocations
9.6 Inductive Effects on Addition
Reactions
9.7 Addition Reactions:
Hydration
9.8 Dimerization and
Polymerization of Alkenes
9.9 Rearrangements during
Addition to Alkenes
9.10 Hydroboration
9.11 Hydroboration in Synthesis:
Alcohol Formation
9.12 Special Topic: Rearrangements
in Biological Processes
9.13 Summary
9.14 Additional Problems
HX
HX
TWO PATHS Depending on the nature of the alkene, addition of an electrophile to
the alkene can follow more than one path.The path taken and the final product
depend on the nature of the starting alkene and the reagents encountered along
the way.
9
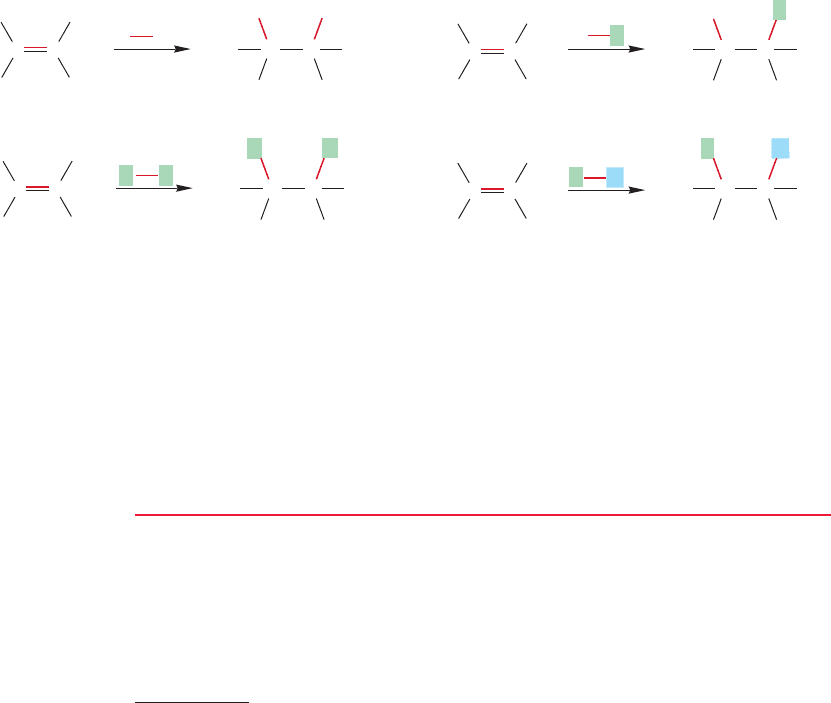
364 CHAPTER 9 Additions to Alkenes 1
H
3
C
H
3
C
H
Br
CH
3
CH
3
HBr
C
C
H
3
C
H
3
C
H
CH
3
CH
3
C C
Y
Y
H X
X
X
X
H
3
C
H
3
C
CH
3
CH
3
C C
H
3
C
H
3
C
CH
3
CH
3
CC
H
3
C
H
3
C
CH
3
CH
3
CC
H
3
C
H
3
C
CH
3
CH
3
CC
H
3
C
H
3
C
CH
3
CH
3
C C
X
XX
X
H
3
C
H
3
C
CH
3
CH
3
CC
FIGURE 9.1 A variety of additions to alkenes. Many , X
2
, and reagents undergo the addition reaction.XYHX
In the last third of his life, there came over Laszlo Jamf—so it seemed to
those who from out in the wood lecture halls watched his eyes slowly
granulate, spots and wrinkles grow across his image, disintegrating it
towards old age—a hostility, a strangely
personal
hatred, for the covalent
bond.
—THOMAS PYNCHON,
1
GRAVITY’S RAINBOW
9.1 Preview
In this chapter, we revisit one of the building-block reactions of organic chemistry—
addition to alkenes. We first saw this useful process in Chapter 3, where we used
it to introduce reactivity. Here and in Chapter 10, we review very briefly the
material from Chapter 3 and then expand on the addition process, using the style of
theme and variations. By all means, go back and reread the material in Chapter 3
(pp. 132–139) if this introduction is not completely obvious to you.
In Chapter 3, we restricted ourselves to a few examples of the addition of HX
reagents to alkenes. Now we expand to a variety of old and new HX reagents, and
in Chapter 10, we add X
2
and XY reagents (Fig. 9.1). Our growing catalog of reac-
tions will continue to add to your expertise in synthesis.
ESSENTIAL SKILLS AND DETAILS
1. Many addition reactions, especially HX additions, proceed through formation of a
carbocation that is captured in a second step by X
. You must be able to analyze the
possibilities for cation formation and pick out the most stable one possible. In practice,
this task is not especially difficult.
1
Thomas Pynchon is an American author born in 1937.
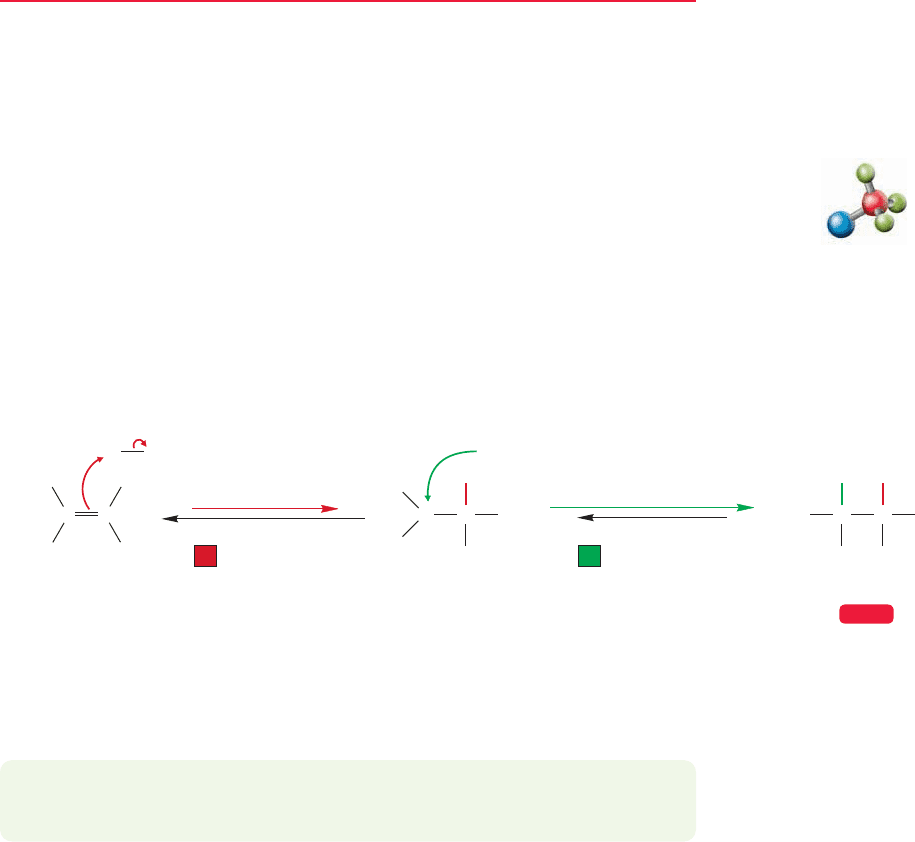
The kinds of molecules we know how to make will increase sharply as a result
of material in Chapters 9 and 10. Addition of hydrogen bromide and hydrogen
chloride to alkenes provides access to alkyl halides. We already have the S
N
1 and
S
N
2 reactions available for transforming the alkyl halides into other compounds
(Fig. 7.94, p. 312).
9.2 Mechanism of the Addition of Hydrogen Halides to Alkenes 365
2.
One of the important factors influencing cation stability is delocalization of
charge through the phenomenon we call resonance. Writing resonance forms
well is an essential skill, and this chapter provides a review of how to do it
properly.
3. Hydration and hydroboration/oxidation give you the ability to add water across an
unsymmetrical double bond in either a Markovnikov or an anti-Markovnikov
fashion, respectively. Although the mechanistic details of the hydroboration process
are complex, the synthetic outcome is decidedly not. Be sure you know how to use
these reactions to good effect in making alcohols.
4. Less stable carbocations rearrange to more stable ones through hydride (H:
) shifts.
Stay alert—from now on, every time you see a carbocation, you have to consider
whether it will rearrange into a lower-energy carbocation. Once again, you have to
be able to judge and predict stability.
2,3-Dimethyl-2-butene 2-Chloro-2,3-dimethylbutane
H
3
C
H
3
C
H
3
C
H
3
C
Cl
..
..
..
Cl
..
..
..
H
C
+
H
CH
3
CH
3
C C
H
CH
3
CH
3
C
–
Cl
..
..
..
..
..
Protonation
1
Addition of Cl
–
2
H
3
C
H
3
C
CH
3
CH
3
CC
WEB 3D
FIGURE 9.2 The first step in this two-step reaction is the protonation of the alkene to give a carbocation. In the
second step, a chloride ion adds to the cation to give the final product.
PROBLEM 9.1 Use the bond dissociation energies of Table 8.2 (p. 337) to estimate
the exothermicity or endothermicity of the reaction in Figure 9.2.
Alkene hydrohalogenation
9.2 Mechanism of the Addition of Hydrogen
Halides to Alkenes
Consider once again the reaction of 2,3-dimethyl-2-butene with hydrogen chlo-
ride to give 2-chloro-2,3-dimethylbutane (Fig. 9.2). This process is called an
alkene hydrohalogenation because it is an addition of hydrogen and halogen
across a π bond. The arrow formalism maps out the process for us and develops
the picture of a two-step mechanism. In the first step, the alkene, with the filled
π orbital acting as base, is protonated by hydrogen chloride to give a carbocation
and a chloride ion. In the second step, chloride acts as a nucleophile and adds to
the strongly Lewis acidic cation to give the product.
The protonation of the alkene to give a carbocation is the slow step in the
reaction. The rate-determining step, the one with the highest-energy transition
state, is this addition of a proton. Because this step is endothermic, the transition
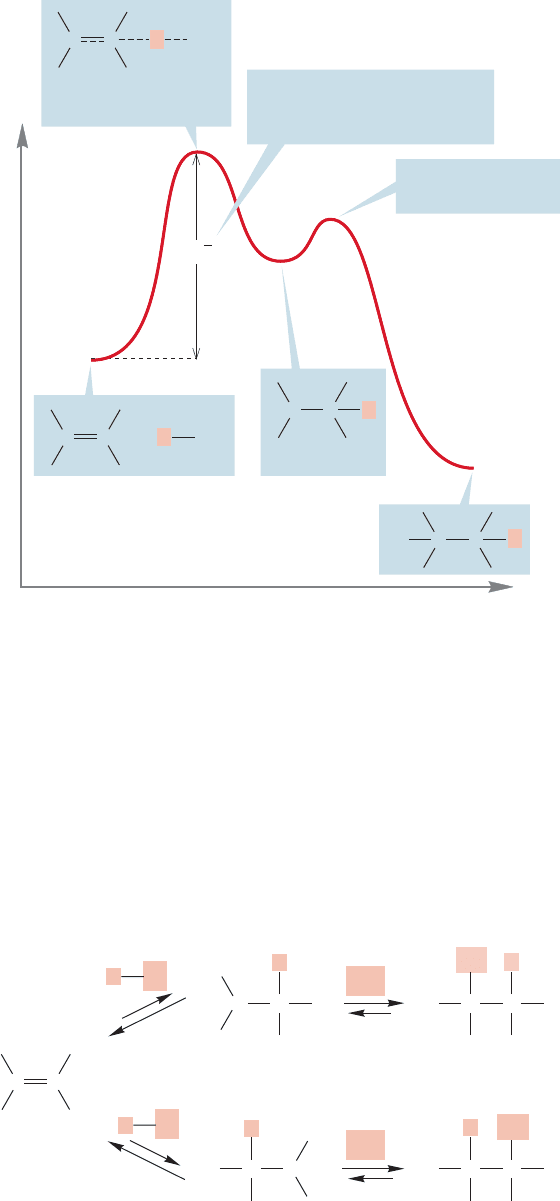
366 CHAPTER 9 Additions to Alkenes 1
Reaction
p
ro
g
ress
Energy
+
⌬G
–
+
Cl
..
..
..
..
Cl
..
..
..
Cl
..
..
..
H
H
H
CC
CC
CC
The activation energy for
the first, and rate-determining,
step of the addition
Transition state for
the second step
Transition state
for the first step
δ
+
Cl
..
..
H
CC
δ
–
..
†
FIGURE 9.3 In the addition of
hydrogen chloride to an alkene, the
first step, the endothermic formation
of a carbocation, is the slow, rate-
determining step. In the transition
state for this step, the positive charge
accumulates on one of the carbons of
the starting alkene.
state will resemble the carbocation intermediate, and it will have substantial
positive charge developed on carbon (Fig. 9.3). That notion leads directly to the
next topic.
RX
X
R
C
+
CX
X
C
X
X
C
C
+
C C
X
X
C
CC
R
R
R
RR
R
R
R
X
X
H
–
Cl
..
..
..
..
–
Cl
..
..
..
..
H
H
H
..
..
..
Cl
Cl
..
..
..
ClH
..
..
..
ClH
..
..
..
FIGURE 9.4 For an unsymmetrical
alkene, there are two possible
intermediate cations, and therefore
two possible chloride products.
9.3 Effects of Resonance on Regiochemistry
The reaction of hydrogen chloride with a symmetrically substituted alkene such as
2,3-dimethyl-2-butene is relatively simple.Protonation is followed by chloride addi-
tion to give the alkyl chloride. There are no choices to be made about the position
of protonation, and once the carbocation is made, chloride formation is easy to
rationalize. What happens if the ends of the alkene are differently substituted? In
such an instance, two products are possible (Fig. 9.4). We discussed this question
briefly in Chapter 3 (p. 137), and the answer is that it depends on how the ends of
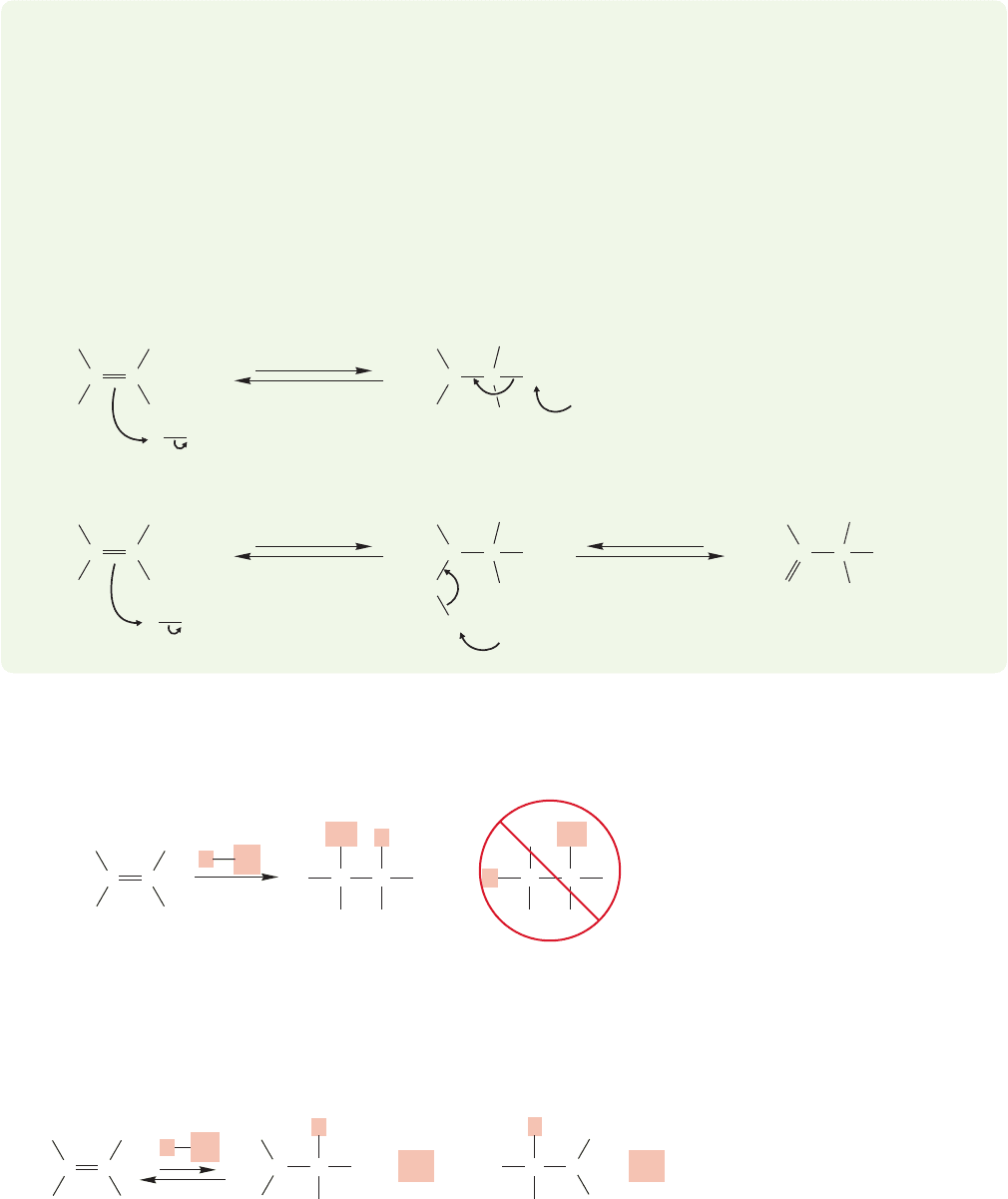
9.3 Effects of Resonance on Regiochemistry 367
WORKED PROBLEM 9.2 Chloride formation isn’t the only result possible in the
reaction of Figure 9.2. In the early stages of this reaction, a small amount of an
alkene isomeric with the 2,3-dimethyl-2-butene is produced. What is this other
alkene, and what is the mechanism of its formation?
ANSWER Remember the E1 reaction! Just because we are starting a new subject
does not mean that old material disappears. Cations will undergo the E1 and S
N
1
reactions as well as the addition reactions encountered in this chapter. The cation
formed on protonation of 2,3-dimethyl-2-butene can undergo an E1 elimination
in two ways. Path a simply reverses to give starting compounds back, whereas
path b leads to the isomeric alkene, 2,3-dimethyl-1-butene.
H
H
H
H
Cl
..
..
..
Cl
..
..
..
C
H
Cl
..
..
..
H
1,1-Dichloroethane not 1,2-DichloroethaneVinyl chloride
(chloroethylene)
H
C
H
Cl
..
..
..
Cl
..
..
..
C
HH
H
C
CC
ClH
..
..
..
FIGURE 9.5 Addition of hydrogen
chloride to vinyl chloride gives
1,1-dichloroethane as the major
product, not 1,2-dichloroethane.
C
H
H
H
H
Hor
C
Cl
..
..
..
C
H
+
C
+
–
Cl
Cl
..
..
..
..
+
–
Cl
..
..
..
..
H
H
H
Cl
..
..
..
CC
H
Cl
..
..
..
..
..
H
H
+
..
FIGURE 9.6 The two cations that
could be formed by protonation of
vinyl chloride.
the alkene differ from each other.The preferential formation of one isomer in those
cases where a choice is possible is called regioselectivity. The next few sections dis-
cuss factors influencing regiochemistry.
CC
H
H
3
C
H
3
C
CH
3
CH
3
C
+
C H
H
3
C
H
3
C
CH
3
CH
3
(a)
protonation
elimination
Cl
..
..
..
..
Cl
..
..
..
–
CC
H
H
3
C
H
3
C
CH
3
CH
3
C
+
H
C H
H
2
C
H
3
C
CH
3
CH
3
(b)
protonation
elimination
protonation
elimination
path a
path b
Cl
..
..
..
..
Cl
..
..
..
–
CCH
H
2
C
H
3
C
CH
3
CH
3
When hydrogen chloride adds to vinyl chloride (chloroethylene), the major product
is 1,1-dichloroethane, not 1,2-dichloroethane (Fig. 9.5).The best way to rationalize the
regiospecificity of this reaction is to examine the two possible intermediate carbocations
(Fig. 9.6) and see which is more stable. Remember: The molecule has an empty p orbital
on the carbon bearing the positive charge. In the first carbocation shown in Figure 9.6,
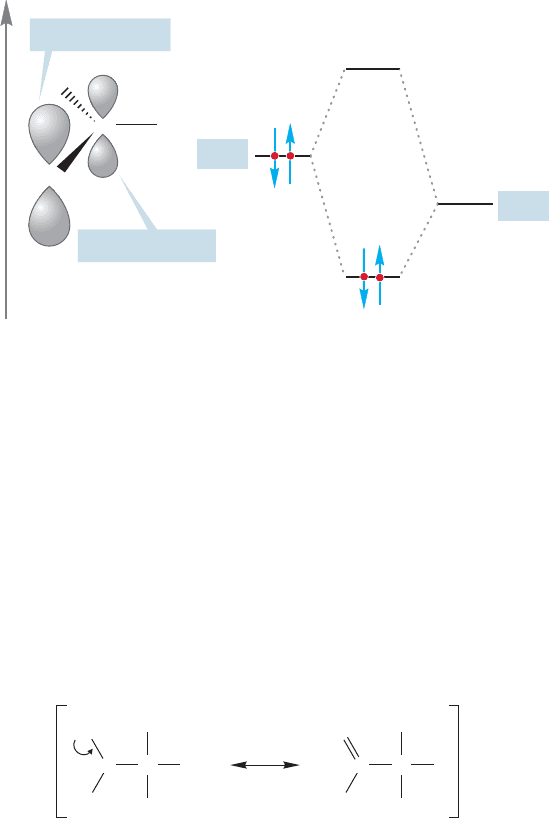
this empty orbital overlaps with a p orbital on chlorine, which contains two electrons.
368 CHAPTER 9 Additions to Alkenes 1
This overlap of filled and empty orbitals produces strong stabilization (Fig. 9.7).
In this ion, the chlorine atom helps bear the positive charge. Only when the charge
is adjacent to the chlorine is there stabilization through overlap of the empty 2p
orbital on carbon with the filled 3p orbital on chlorine. Because this stabilization
is not available to the second carbocation shown in Figure 9.6, it is much harder
to form.
Energy
CH
3
C 2p
Cl 3p
Empty 2p orbital
Filled 3p orbital
H
C
+
Cl
..
FIGURE 9.7 Stabilization of a cation
through overlap of a filled and empty
orbital.
H
H
In this resonance
form, the carbon bears
the
p
ositive char
g
e
In this resonance
form, the chlorine bears
the
p
ositive char
g
e
HC
H
C
+
Cl
H
H
+
HC
H
C
Cl
..
..
..
..
..
FIGURE 9.8 Resonance stabilization
of a carbocation.
Another way to represent this stabilization is through a resonance formulation.
(For an earlier treatment of resonance see Section 1.4, p. 22.) There are two rea-
sonable ways to draw a Lewis structure for the cation next to chlorine (Fig. 9.8).
In one, the carbon is positively charged, and in the other it is the chlorine that
bears the positive charge.The two representations differ only in their distribution
of electrons,which means they are resonance forms of the same structure. By itself
neither is exactly right, as you learned in Section 1.4. Neither by itself shows that
two atoms share the charge. Taken together they do. The real structure of this
cation is not well represented by either form alone, but the two combined do an
excellent job.
The molecular orbital picture we developed in Figure 9.7 does a good job of
showing the stabilization, but it is not particularly well suited for bookkeeping
purposes. In practice, the ease of bookkeeping—of easily drawing the next chemi-
cal reaction—makes the resonance picture simpler to use.The price is that you have
to either draw two structures (or more in other cases) or adopt some other code that
indicates the presence of more than one representation for the molecule. We will
see many examples in the next pages and chapters.
