Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

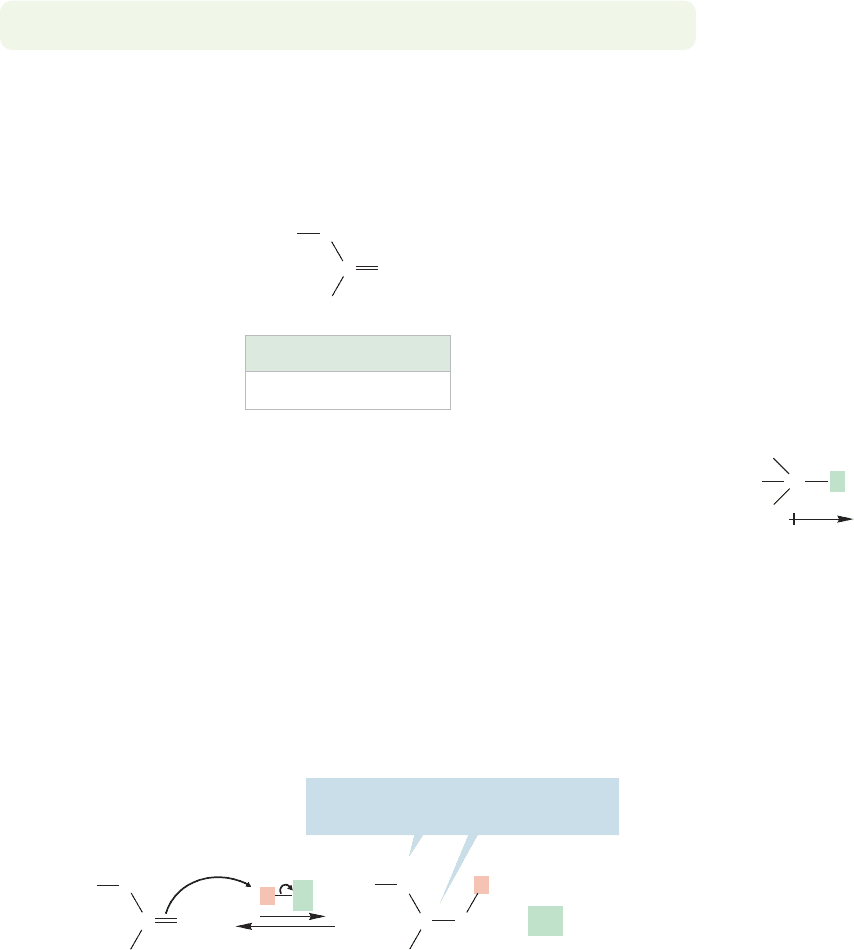
9.6 Inductive Effects on Addition Reactions 379
CH
2
CH
2
C
H
R
=
H
R
Br Cl
1Rate 10
–
4
10
–
4
FIGURE 9.28 Rates of addition to
three alkenes.
which shows typical rates of addition for a series of substituted ethylenes.The reaction
is, in fact, a hydration reaction in which HOH adds across the double bond.
Ethylene itself will protonate only very slowly. Propene will surely protonate so as
to give the more stable secondary cation,and the methoxyl group of methyl vinyl ether
will provide resonance stabilization to an adjacent positive charge. Hyperconjugative
stabilization by the methyl group of propene and resonance stabilization by the
methoxyl group of methyl vinyl ether, respectively, have a great accelerating effect on
the rate of protonation, the rate-determining step in this reaction. Figure 9.27 shows
the rates of protonation compared to that of unsubstituted ethylene itself.
PROBLEM 9.9 Why is the rate of protonation of ethylene especially slow?
Substituents can slow reactions as well, especially if they are not in a position to
provide resonance stabilization.Figure 9.28 shows typical rates for addition to some
substituted alkenes. To see why bromine and chlorine so dramatically slow the rate
of addition,we must again look more closely at the carbon–halogen bond (see p. 225
for an earlier look).
δ
+
δ
–
= Br or Cl
C
XX
FIGURE 9.29 The polar bond.C
O
X
δ
+
δ
–
CH
2
CH
2
C
H
HX
X
H
–
Notice the bad electrostatic interaction
between
δ
+
and the + charge
δ
+
+
δ
–
CH
2
CH
2
+
C
Br
Br
H
..
..
..
..
..
..
..
FIGURE 9.30 Protonation gives an
intermediate destabilized by the
electrostatic interaction between the
newly formed full positive charge and
the partial positive charge (δ
).
Wouldn’t the methoxyl group of methyl vinyl ether act in the same way? Oxygen
is also more electronegative than carbon, and wouldn’t this difference in electroneg-
ativity act to discourage any addition that puts more positive charge on the already
The two atoms involved in the bond, carbon and halogen, are different, so the
bond between them must be polarized.Therefore, the electrons in the bond cannot
be equally shared. Bromine and chlorine are far more electronegative than carbon
(see Table 1.8, p. 15) and they will attract electrons strongly. The carbon–halogen
bond is polarized as shown in Figure 9.29. The carbon will bear a partial positive
charge (δ
) and the more electronegative halogen a partial negative charge (δ
).
For the molecules in Figure 9.28, protonation in the Markovnikov sense places
a full positive charge on the carbon adjacent to the already partially positive carbon
(Fig.9.30).This interaction will surely be destabilizing and accounts for the decrease
in rate for addition reactions to the bromine- and chlorine-substituted molecules
shown in Figure 9.28. Effects such as these, which are transmitted through the σ
bonds, are called inductive effects.
380 CHAPTER 9 Additions to Alkenes 1
partially positive carbon adjacent to oxygen? Why does addition to methyl vinyl
ether occur so rapidly (Fig. 9.27) in the Markovnikov sense? Figure 9.31 frames the
question, and answers it as well. The answer is simply that the resonance effect of
oxygen overwhelms the inductive effect.The two effects are in competition, and the
stronger resonance effect wins out. Now let’s summarize, then look at a number of
related addition reactions.
..
..
δ
+
δ
–
CH
3
O
CH
3
O
CH
2
C
H
H
H
CH
2
C
H
X
X
X
..
H
CH
2
C
H
..
..
..
–
..
..
..
..
..
..
CH
3
O
H
CH
2
C
H
This intermediate is strongly
stabilized by resonance and
the stabilizing resonance
effect outweighs the
destabilizing inductive effect
The C
O σ
bond in
methyl vinyl ether is
polarized so that a
partial positive charge
is on carbon; this
makes protonation on
the adjacent carbon
more difficult
+
CH
3
O
+
FIGURE 9.31 Stabilizing resonance
effects and destabilizing inductive
effects can “fight” each other.
Generally, resonance stabilization
is more important.
+
(97%)
I
..
..
..
–
I
..
..
..
..
H
H
H
I
..
..
..
FIGURE 9.32 Addition of HI to 1-octene in Markovnikov fashion.
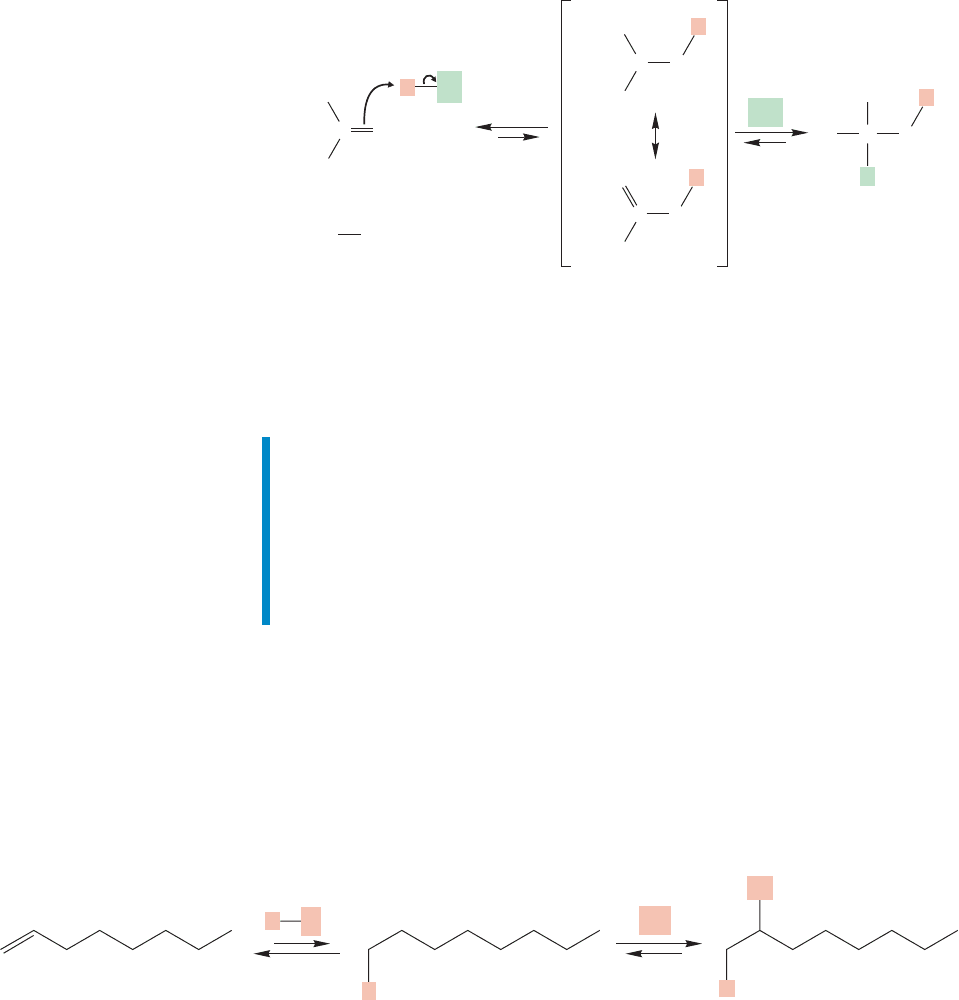
9.7 Addition Reactions: Hydration
If and add to double bonds, it should not be surprising that other acidic
compounds undergo similar addition reactions. Hydrogen iodide is a simple
example, and addition rather closely resembles the reactions we have already
studied in this chapter. Markovnikov’s rule is followed, for example (Fig. 9.32).
HI
HX
HClHBr
HX
Summary
Protonation (or addition of any Lewis acid) to an unsymmetrical double bond
leads preferentially to the more stable carbocation.The energy of carbocations is
strongly influenced by stabilizing resonance effects. Substituents bearing a lone
pair of electrons are especially effective at stabilization, but even alkyl groups can
donate electrons to the empty 2p orbital of a carbocation. In cases in which induc-
tive and resonance effects oppose each other, resonance usually wins.
Water ( ) is an reagent, but, as we saw on p. 139 it is not a strong
enough acid to undergo addition to simple alkenes at a useful rate.If one mixes water
and a typical alkene, no reaction occurs. The difference between and isHXHOH
HXHOH
9.7 HX Addition Reactions: Hydration 381
that and are strong enough acids to protonate the alkene and start things
going.Water is not.The pK
a
values of , ,and are 9,8,and 15.7,
respectively, which means that water is a weaker acid than and by factors
of about 10
25
and 10
24
! Acid must be added to protonate the alkene and thus gen-
erate a carbocation that can continue the reaction.The acid catalyst in this reaction
sets things going. The catalyst has no effect on the position of the final equilibri-
um,but it does lower the energy requirement for generating product.As Figure 9.33
illustrates, the catalyst provides a new mechanistic pathway for product formation.
It is able to carve a new pathway along a lower slope of the mountain we first encoun-
tered in Figure 8.20 (p. 346).
HClHBr
HOHHClHBr
HBrHCl
Alkene hydration
The lowest energy
path from A to B
A catalyst provides a lower energy path but it does not alter the
energy of starting material and product, A and B; rather, it changes
the energy of the transition state, or transition states, in the reaction
Village A Village A
Village BVillage B
FIGURE 9.33 A catalyst does not change the energy of either starting material or product but does provide
a lower energy path between them.
As can be seen in Figure 9.34, the catalyst H
3
O
is used up in the first step of the
reaction and is regenerated in the last step. A number of acids can be used in the cat-
alytic role; sulfuric (H
2
SO
4
or ) or nitric (HNO
3
or ) are
commonly used to promote the addition reaction. In water containing a little sulfu-
ric acid for instance,2,3-dimethyl-2-butene is protonated to generate the tertiary car-
bocation. This intermediate is then attacked by (water, not hydroxide,
OH)
to give the oxonium ion, , which can be deprotonated by water to
regenerate the catalyst, H
3
O
,and produce a molecule of an alcohol, .Thus,
the outcome of the hydration reaction is addition of across a π bond.HOH
R
O
OH
R
O
OH
2
+
HOH
HONO
2
HOSO
2
OH
WEB 3D
OH
2
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
+
+
..
An alcoholAn oxonium ion
C
H
HH
C
H
C
..
..
..
..
H
3
C
H
3
C
H
2
O
..
..
H
2
O
..
H
2
O
..
..
H
3
O
..
H
3
O Catalyst regenerated in this step
..
H
3
O Catalyst used up in this step
C
C
H
3
C
H
3
C
C
+
H
3
C
H
3
C
H
OH
CH
3
CH
3
C
H
C
..
H
3
C
H
3
C
+
O
+
+
+
FIGURE 9.34 The mechanism of
hydration of 2,3-dimethyl-2-butene.
382 CHAPTER 9 Additions to Alkenes 1
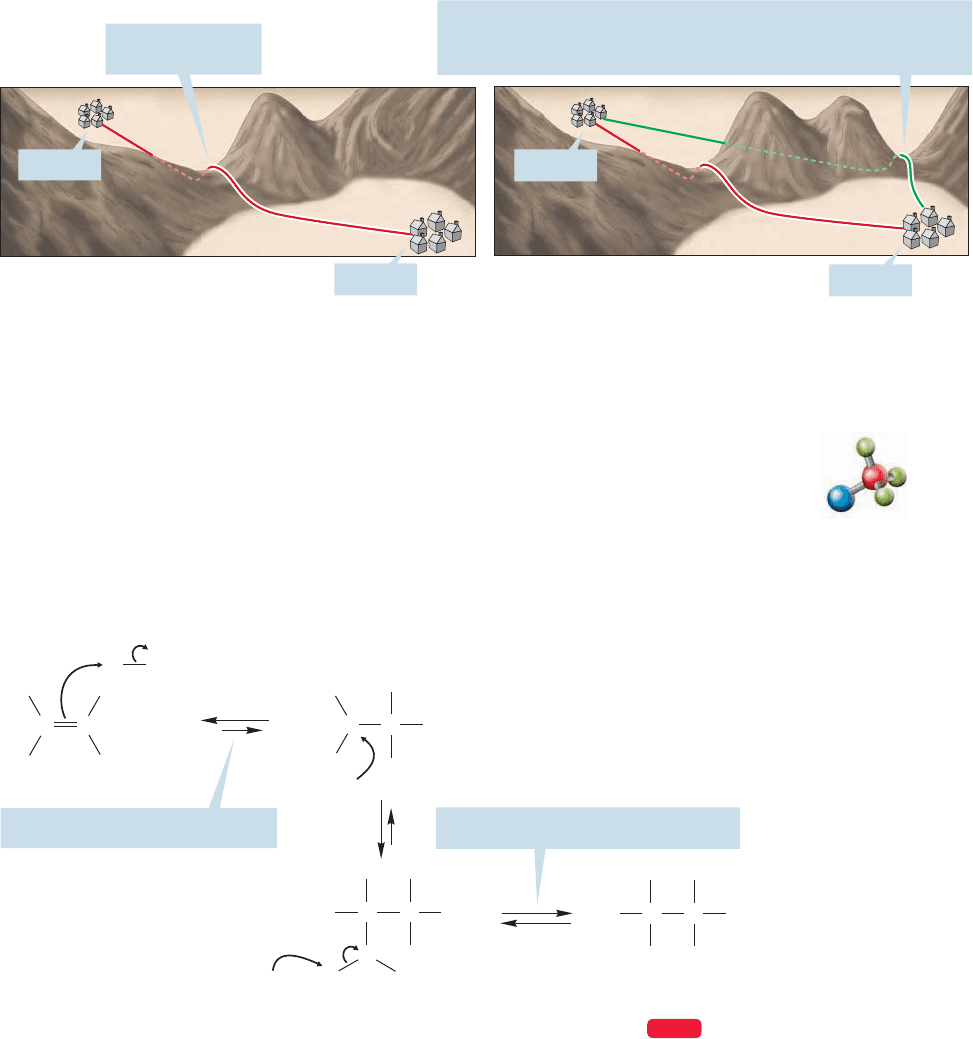
The mechanism shown in Figure 9.34 predicts, indeed requires, that the
Markovnikov rule be followed, and that sets up a mechanistic test. We will exam-
ine the hydration of 2-methylpropene to probe the regiochemistry of the reaction.
Before we even start, it is worth thinking a bit about the results.What will we know
after we have done the experiment? There are only two general results possible:
Either the Markovnikov rule is followed or it is not (Fig. 9.35). If it is not, we can
say with certainty that the mechanism we have suggested for this reaction is wrong.
CH
2
CH
2
C
H
3
C
H
3
C
..
..
OH
..
..
H
2
O
This compound is the
product of Markovnikov
addition, and is formed
in the reaction
Mixture
This compound is the
product of a path that does
not follow Markovnikov
addition (anti-Markovnikov
addition), and is not formed
in the reaction
C
H
3
C
H
3
C
H
CH
2
C
..
..
OH
H
3
C
H
3
C
H
CH
2
C
..
..
OH
H
3
C
H
3
C
H
CH
2
C
..
..
OH
H
3
C
H
3
C
H
+
+
..
H
3
O
+
+
path a
path b
path c
FIGURE 9.35 Three possible
regiochemical results from the
hydration of 2-methylpropene.
WEB 3D
H
H
O
OH
2
CH
2
CH
2
+
+
..
tert-Butyl alcohol2-Methylpropene More stable, tertiary
cation intermediate
C
C
..
H
3
C
H
3
C
..
..
H
2
O
..
..
H
3
O
protonation addition deprotonation
+
+
CH
2
C
H
3
C
H
3
C
H
3
C
H
3
C
H
H
H
H
..
H
2
O
..
O
CH
2
C
..
H
3
C
H
3
C
H
+
FIGURE 9.36 The mechanism for Markovnikov addition of water to 2-methylpropene.The product must be tert-butyl alcohol
if this is indeed the mechanism.
A crucial step in our mechanism involves the formation of a carbocation. When
there is a choice, as there certainly is with 2-methylpropene, the more stable car-
bocation must be formed. Once formed, this cation must ultimately give the
product in which the hydroxyl group is attached to the more substituted carbon.
If our mechanism postulated in Figure 9.36 is correct, there is no other possible
result. So if we get either the “wrong” alcohol or a mixture of alcohols (paths b
and c in Fig. 9.35), we know the mechanism is wrong. It may need only a minor
adjustment, or it could require complete scrapping, but it cannot be correct as
written.
More interesting, and less obvious, is what we know if our prediction turns
out to be correct. Have we proved the mechanism? Absolutely not. Our mecha-
nistic hypothesis remains just a hypothesis: a guess at how the reaction goes that

9.7 HX Addition Reactions: Hydration 383
is consistent with all experiments that have been done so far.There may be some
other test experiment that would not work out as well. The sad fact is that no
number of experiments we can do will suffice to prove our mechanism. We are
doomed to be forever in doubt, and our mechanism to be forever suspect. There
is no way out. Indeed, all the mechanisms in this book are “wrong,” at least in
the sense that they are broad-brush treatments that do not look closely enough
at detail.
Organic chemistry has come a long way since the days of the rectangular
hypothesis, which was little more than a bookkeeping device. In olden times it was
common practice to write a reaction such as the S
N
1 reaction of tert-butyl iodide
with water in the way shown in Figure 9.37. To use the rectangular hypothesis,
one simply surrounds the appropriate groups with a rectangle, which somehow
removes the captured atoms and reconstitutes them, in this case as hydrogen iodide.
CH
3
CH
3
C I OHH HI
H
3
C
CH
3
CH
3
COH
H
3
C
+
FIGURE 9.37 The rectangular
hypothesis applied to the reaction
of tert-butyl iodide with water.
This archaic device is scarcely a reasonable description of how the reaction occurs
and has almost no predictive value. If you know one rectangle, you do not know
them all! But let’s not get too smug. We do know more than chemists of other gen-
erations. We know, for example, that carbocations are involved in the reactions we
are currently studying,and molecular orbital theory gives us additional insight into
the causes of these reactions. But we are still very ignorant. We are certain that
much of what we “know” is wrong, at least in detail. Chemists of the future will
regard us as primitives, and they will be quite right. It doesn’t matter, of course.
Our job is to do the best we can; to add what we can to the fabric of knowledge
and to provide some shoulders for those future chemists to stand on, as our earlier
colleagues did for us.
In this case, things turn out well for the mechanistic hypothesis shown in
Figure 9.36, and the Markovnikov rule is followed in hydration reactions.
There are variations on this reaction. Ethylene, for example, is hydrated only
under more forcing conditions—concentrated sulfuric acid must be used—and the
initial product is not the alcohol, but ethyl hydrogen sulfate, which is converted in
a second step into the alcohol (Fig. 9.38). It is not easy to be sure of the mechanism
of this reaction.The concentrated sulfuric acid may be required to produce the very
unstable primary carbocation, or a different, perhaps cyclic cation may be involved
(see Problem 9.9).
OSO
2
OH
H
Ethyl hydrogen sulfate Ethyl alcohol
sulfuric acid
H
2
O , 0 ⬚C
HO SO
2
OH
H
2
C CH
2
H
2
C CH
2
OH
H
3
C CH
2
H
3
O
+
..
..
..
..
..
..
..
H
2
O
..
..
..
..
..
..
..
..
FIGURE 9.38 Ethyl hydrogen sulfate
is an intermediate in the hydration of
ethylene.
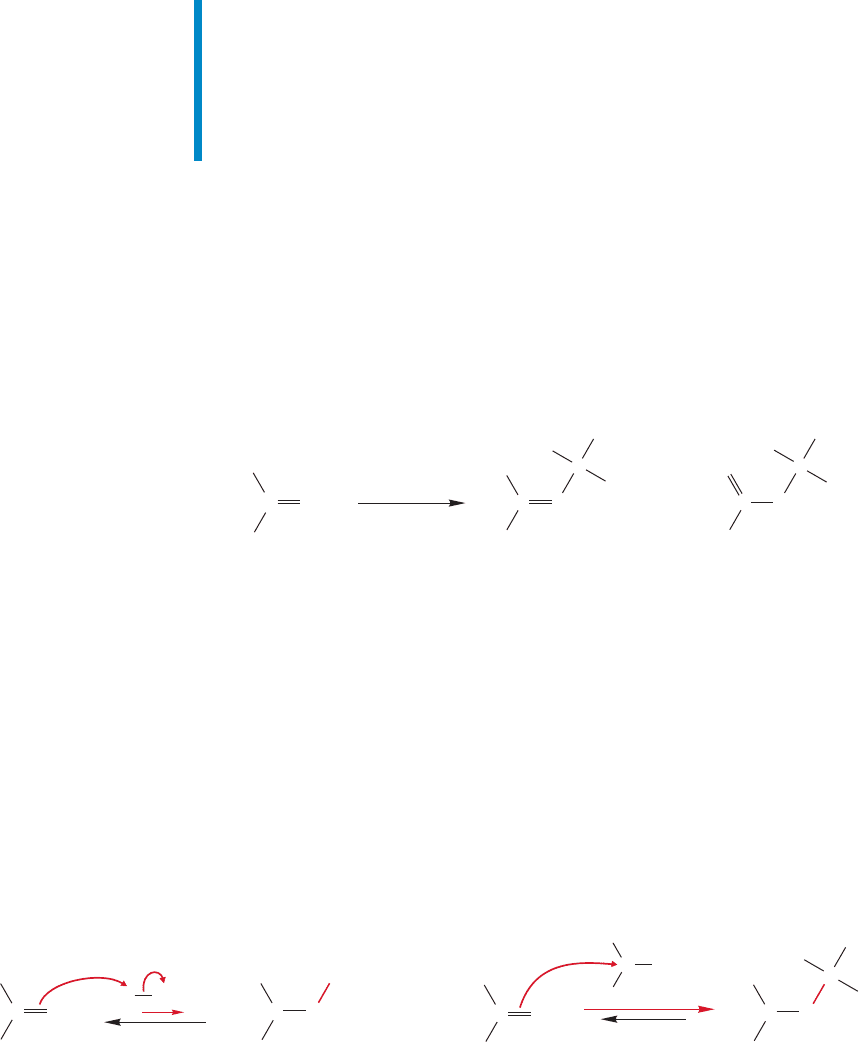
384 CHAPTER 9 Additions to Alkenes 1
Now we have a structure that contains eight carbons, as do the two new prod-
ucts. It looks as though we are on the right track.The only remaining trick is to see
how to lose the “extra” proton and form the new alkenes. Losing H
a
as shown in
H
3
C
CH
3
CH
3
C
+
HOSO
2
OH
H
2
O
CCH
2
H
3
C
H
3
C
CCH
H
3
C
H
3
C
H
3
C
CH
3
CH
3
C
CCH
2
H
3
C
H
2
C
FIGURE 9.39 Dimeric products
formed from 2-methylpropene.
9.8 Dimerization and Polymerization of Alkenes
Let’s look again at the hydration of 2-methylpropene. What if we change the con-
ditions a bit from what they were in Figure 9.36? What if both the temperature and
concentration of sulfuric acid are increased? Figure 9.39 shows that two new prod-
ucts,each containing eight carbons,are formed.These are dimeric products (C
8
H
16
),
formed somehow by the combining of two molecules of 2-methylpropene (C
4
H
8
).
+
+
..
Brønsted acid
The nucleophilic
alkene acts as
a Brønsted base
H
+
C
CH
2
H
3
C
H
3
C
C
CH
2
H
3
C
H
3
C
+
+
Lewis acid
The nucleophilic
alkene acts as
a Lewis base
C
CH
3
H
3
C
H
3
C
H
3
C
CH
3
CH
3
C
CCH
2
H
3
C
H
3
C
CCH
2
H
3
C
H
3
C
..
..
H
2
O
HOH
2
FIGURE 9.40 Many different acids add to 2-methylpropene (and other alkenes). Here we see H
3
O
and the tert-butyl
cation adding to 2-methylpropene to give tertiary carbocations. In each case, addition occurs in the Markovnikov sense, as
the more stable carbocation is formed preferentially.
Surely, increasing the acid concentration can only make protonation of the alkene
more probable, so the first step of the reaction is again formation of the tertiary car-
bocation intermediate shown in Figure 9.36. Now, however, the initially formed car-
bocation finds itself in the presence of fewer water molecules than it did in the reaction
we looked at before. Accordingly,formation of the alcohol through reaction with water
is less likely.We might ask what other Lewis bases are available for the strongly Lewis
acidic carbocation.The most obvious one is the starting alkene itself. The tert-butyl
cation intermediate can react with it, just as the proton did. Both the proton and the
carbocation are Lewis acids and can add to the double bond of 2-methylpropene to
give a new cation (Fig. 9.40). There is no essential difference in the two reactions,
and once more it is the more stable, tertiary carbocation intermediate that is formed.
Summary
Water is not a strong enough acid to protonate a simple alkene.However, if a trace
of acid catalyst is present, an initial protonation will be followed by addition of
water to produce an oxonium ion.A final deprotonation gives the alcohol.As usual,
you have to be careful to analyze which carbocation is formed, and to consider
structural, resonance, and inductive effects in making that determination.

9.8 Dimerization and Polymerization of Alkenes 385
–H
a
deprotonation
OH
2
..
..
OH
2
..
..
protonation
–H
b
deprotonation
protonation
H
3
C
CH
3
H
a
CH
3
C
C
+
CH
H
3
C
H
3
C
H
3
C
CH
3
CH
3
C
C CH
H
3
C
H
3
C
+
OH
2
H
..
+
H
3
C
CH
3
H
b
CH
3
C
C
+
CH
2
H
2
C
H
3
C
H
3
C
CH
3
CH
3
C
CCH
2
H
2
C
H
3
C
+
OH
2
H
..
+
FIGURE 9.41 The two products of
Figure 9.39 are formed by
deprotonation of the intermediate
carbocation.
Figure 9.41, will lead to the first product and losing H
b
gives the second. We have
seen similar deprotonations before in our discussion of the E1 reaction (p. 298), and
we have also seen the reverse reaction. Loss of these protons is just the reverse of
protonation of an alkene (Fig. 9.41).
Alkene polymerization
The proton can’t just leave, however. It must be removed by a base. In concentrated
sulfuric acid, strong bases do not abound.There are some bases present, however, and
the carbocation is a high-energy species, and thus rather easily deprotonated. Under
these conditions, both water and bisulfate ion (HSO
4
) are capable of assisting the
deprotonation.
Note the exact correspondence of the steps involved in alkene protonation and
deprotonation.The two reactions are just the two sides of an equilibrium (Fig. 9.42).
OH
2
..
protonation
loss of a proton
(deprotonation)
CH
2
R
2
C
H
OH
2
CH
2
+
..
..
H
R
2
C
+
+
FIGURE 9.42 Protonation and
deprotonation are just two sides
of an equilibrium.
If the concentration of acid is even higher, the base concentration will be even
lower and both alcohol formation and deprotonation will be slowed (both require water
to act as Lewis base). There is no reason why the dimerization process cannot con-
tinue indefinitely under such conditions, leading first to a trimer (a molecule formed
from three molecules of 2-methylpropene) and, eventually, to a polymer (Fig. 9.43).
C
CH
3
H
3
C
H
3
C
C
CH
3
CH
3
H
2
C
H
3
C
H
3
C
CH
2
CH
3
C
C
CH
3
CH
3
H
2
C
C
CH
3
CH
3
H
2
C
C
+
CH
3
CH
3
Repeat
H
3
C
H
3
C
CH
2
CH
2
CH
3
C
C
+
CH
3
CH
3
CH
3
CH
3
C
+
FIGURE 9.43 The beginning stages of the cationic polymerization of 2-methylpropene.

386 CHAPTER 9 Additions to Alkenes 1
PROBLEM 9.10 Treating 2-ethyl-1-butene with H
3
O
/H
2
O leads to three prod-
ucts each having the formula C
12
H
24
. Draw the three structures and provide a
mechanism for their formation.
H
3
C
HCl
Cl
Cl
C
CCH
2
CH
3
CH
CH
2
CH
3
H
3
C
H
CH CH
3
CH
3
CH
3
CH
H
3
C
+
2-Chloro-3-methylbutane
(40%)
3-Methyl-1-butene 2-Chloro-2-methylbutane
(60%)
FIGURE 9.44 Reaction of HCl with
3-methyl-1-butene gives a new
product in addition to the expected
one.
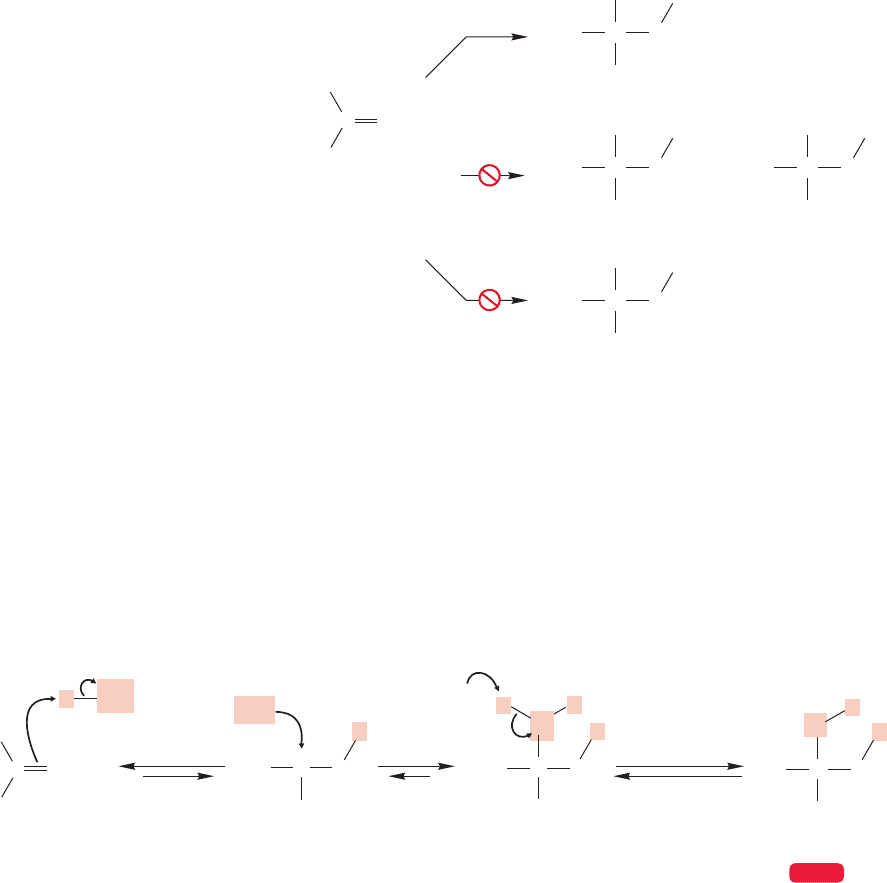
The obvious question is, Why? Will our mechanism for addition have to be
severely changed to accommodate these new data? In fact, we will be able to graft
a small modification onto our general mechanism for addition reactions and avoid
drastic changes. The way to attack a problem of this kind—explaining the for-
mation of an unusual product—is to write the mechanism for the reaction as we
have developed it so far and then see if we can find a way to rationalize the new
product. In this case, protonation of 3-methyl-1-butene can give either a secondary
Teflon is the polymer formed from
polymerization of tetrafluoroethene.
Here are several useful applications of
this wonderful material.
The cation-induced polymerization is called, logically enough, cationic poly-
merization and ends only when the alkene concentration decreases sufficiently
to slow the addition step.
9.9 Rearrangements during Addition
to Alkenes
When adds to isopropylethylene (3-methyl-1-butene), a quite surprising thing
happens. We do get some of the product we expect, 2-chloro-3-methylbutane, but
the major compound formed is 2-chloro-2-methylbutane (Fig. 9.44).
HCl
HX
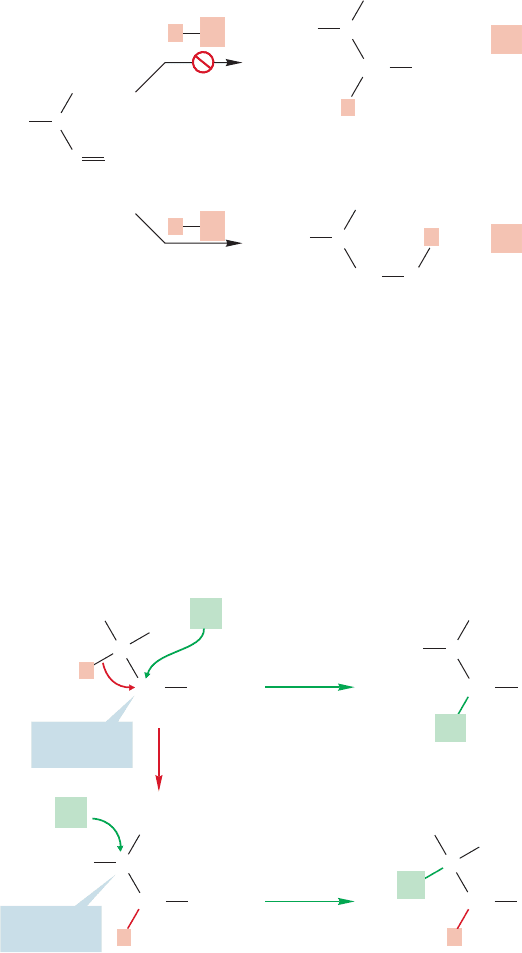
9.9 Rearrangements during HX Addition to Alkenes 387
+
CH
HC
CH
3
CH
2
H
3
C
CH
3
CH
2
H
CH
CH
H
3
C
CH
3
CH
2
H
CH
CH
H
3
C
Less stable primary cation is not formed
3-Methyl-1-butene
More stable secondary cation is formed
Cl
..
..
..
..
+
–
Cl
..
..
..
..
+
–
+
..
..
..
HCl
..
..
..
HCl
FIGURE 9.45 No real choice here—
the more stable secondary
carbocation is formed.
H
Cl
..
..
..
..
–
..
–
Cl
..
..
..
..
–
H
3
C
C
CH
3
CH
3
CH
CH
3
CH
3
H
H
CH
CH
H
3
C
Secondary
carbocation
Minor product
shift of
hydride,
H
Major product
Tertiary
carbocation
H
3
C
C
CH
3
CH
3
CH
CH
3
CH
3
CH
C
+
H
3
C
chloride
adds
path a
path b
chloride
adds
+
..
..
..
Cl
Cl
..
..
..
FIGURE 9.46 In the reaction between
3-methyl-1-butene and HCl the
major product is formed through a
rearrangement mechanism (path b)
involving the migration of hydrogen
with its pair of electrons (hydride,
H
) to give the more stable tertiary
carbocation from the less stable
secondary ion.
:
or primary carbocation (Fig. 9.45). There is no real choice here, because the
secondary carbocation is of far lower energy than the hideously unstable primary
carbocation and will surely be the one formed.
If the chloride ion adds to the positively charged carbon of the secondary carbo-
cation, we get the minor product of the reaction,and the one we surely expected (path
a, Fig. 9.46). To get the major product, we must have a rearrangement, which is a
word describing the relocation of an atom (or atoms) in a molecule as a result of elec-
trons shifting. In this case,a hydrogen must move, with its pair of electrons, from the
carbon adjacent to the cation to the positively charged carbon itself (path b,Fig. 9.46).
Such migrations of hydrogen with a pair of electrons are called hydride shifts.
Why should this rearrangement happen? Note that in such a process we have
generated a lower-energy tertiary carbocation from a higher-energy secondary car-
bocation. The cation is certainly “better off” (lower in energy) thermodynamically
if the hydride moves. In practice, such rearrangements are quite common. Hydride
shifts to produce more stable cations from less stable ones are easy. Indeed, not only
are hydride shifts common, but so are alkyl shifts, called Wagner–Meerwein
rearrangements (Georg Wagner, 1849–1903; Hans Lebrecht Meerwein,1879–1965).
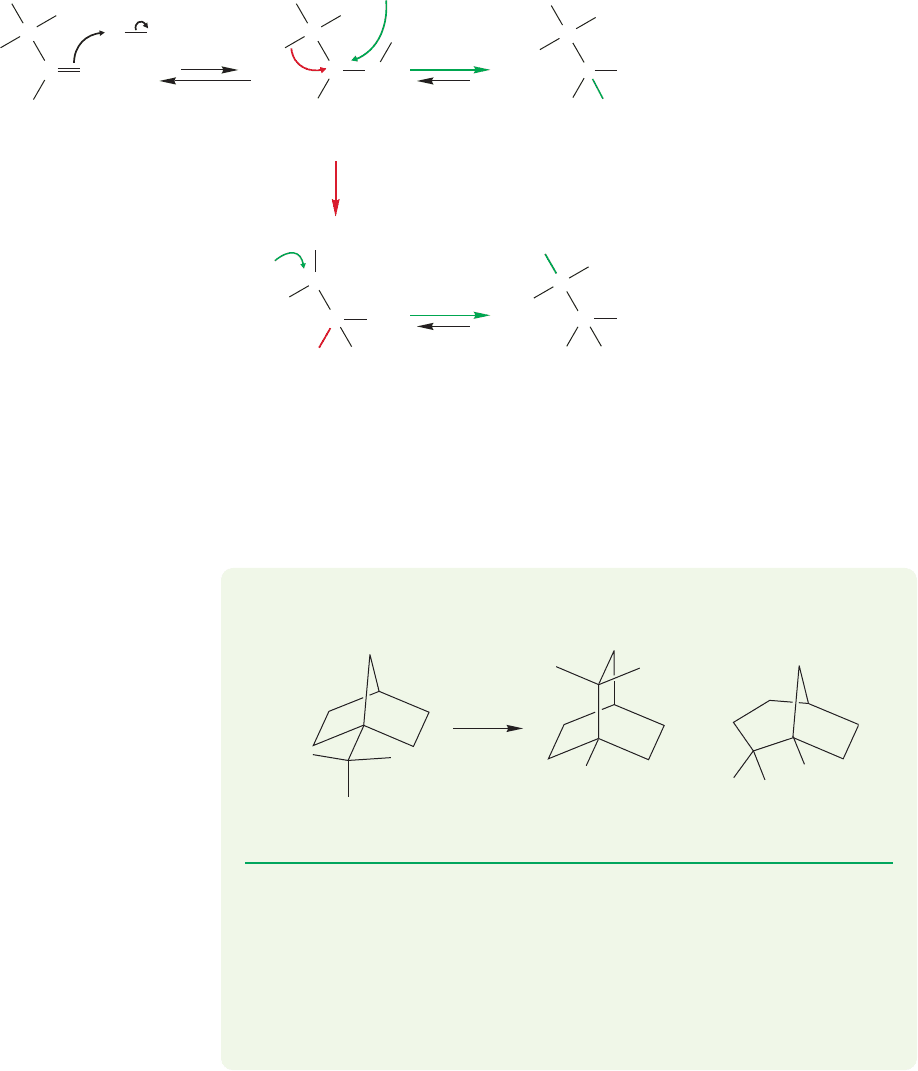
388 CHAPTER 9 Additions to Alkenes 1
For example, 3,3-dimethyl-1-butene adds hydrogen chloride to give not only
2-chloro-3,3-dimethylbutane but also 2-chloro-2,3-dimethylbutane. The new,
rearranged compound results from an alkyl shift—in this case the migration of a
methyl group with its pair of electrons (Fig. 9.47). Why is this reaction called an
“alkyl shift,”when by analogy to “hydride shift” it should be “alkide shift”? We don’t
know, but it is!
PROBLEM 9.11 Write mechanisms that account for the products in the following
reaction:
PROBLEM 9.12 A hydride shift to a positively charged carbon is just another
example of a Lewis base–Lewis acid reaction. Although it looks very different
from the more obvious electrophile–nucleophile reactions we have studied, it
really is quite similar. In the reaction shown in the previous problem, there is a
very strong and strategically located electrophile and a weak, but nonetheless
reactive enough nucleophile. Identify the nucleophile and electrophile in this
reaction.
CH
3
H
3
C
HCl
Cl
..
..
..
..
..
..
CH
3
Cl
+
..
..
H
2
O
..
..
..
H
3
C
H
3
C
CH
3
Cl
..
..
..
Cl
..
..
..
..
–
Secondary carbocation
..
–
CH
3
shift
Tertiary carbocation
H
H
3
C
H
3
C
C
CH
3
CH
2
C
H
H
3
C
H
3
C
C
CH
3
CH
2
C
+
H
path a
path b
2-Chloro-2,3-dimethylbutane
(60–75%)
2-Chloro-3,3-dimethylbutane
(25–40%)
3,3-Dimethyl-1-butene
Cl
..
..
..
..
–
Cl
..
..
..
H
H
3
C
H
3
C
+
C
CH
3
CH
3
C
H
3
C
H
3
C
C
CH
3
CH
3
C
H
Cl
..
..
..
Cl
..
..
..
H
3
C
C
CH
3
CH
3
C
H
H
The minor, unrearranged,
product is formed from the
secondary carbocation
(path a)
The major product is formed
from the rearranged, tertiary
carbocation (path b)
H
3
C
FIGURE 9.47 Formation of the major product involves a rearrangement mechanism in which a shift of a methyl
group with its pair of electrons generates a more stable tertiary carbocation from a less stable secondary
carbocation.
