Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

9.3 Effects of Resonance on Regiochemistry 369
Figure 9.9 illustrates a reaction that we will return to in some detail later but can
examine quickly now. Hydrogen chloride adds to 1,3-butadiene very easily to yield
two products. One product you may be able to predict, but the other will probably
not be immediately obvious (Fig. 9.9).
1
3
2
4
H
H
1,4-Addition
(20–25%)
C
CH
2
H
2
C
C
Cl
..
..
..
H
H
H
H
C
CH
2
H
2
C
C
C
CH
2
H
2
C
C
Cl
..
..
1,2-Addition
(75–80%)
..
H
H
HCl
..
..
..
HCl
..
..
WEB 3D
WEB 3D
FIGURE 9.9 1,2- and 1,4-Addition
of HCl to 1,3-butadiene.
H
H
H
H
A primary carbocation
An allylic cation
or
C
CH
2
H
2
C
C
H H
H
H
H
C
CH
2
H
2
C
C
C
CH
2
H
2
C
C
+
–
Cl
..
..
..
..
+
–
Cl
..
..
..
..
1,3-Butadiene
C
CH
2
H
2
C
C
H
H
+
+
+
HCl
..
..
..
HCl
..
..
..
FIGURE 9.10 The two possible
carbocations from protonation of
1,3-butadiene.
+
Protonation at
the end carbon
gives a delocalized
allylic cation
Protonation at an
internal carbon
gives a localized
primary cation
CH
2
CH
CH
H
3
C
+
CH
2
CH
2
CH
H
2
C
FIGURE 9.11 In the allylic cation, the positive charge is shared by two carbons; in the
primary cation it is localized on a single carbon.
Look at the possibilities.There are two cations that can arise from protonation of
1,3-butadiene by hydrogen chloride (Fig.9.10). In the allylic cation,the positive charge
is on the carbon adjacent to the double bond; in the other, it is isolated on a terminal
primary carbon. The choice between these two ions becomes clear if we draw orbital
pictures of the two carbocations. In the allylic cation, the p orbital on the positively
charged carbon atom overlaps the π orbitals of the double bond (Fig. 9.11). We have
seen the system of three parallel overlapping p orbitals before (Problems 1.16c and 1.61).
Here we have an allylic cation again, and the molecular orbital description is the same
as before.The three 2p atomic orbitals can be combined to yield three molecular orbitals.
There are only two electrons in an allylic cation and they will occupy the lowest, most
stable molecular orbital (Fig. 9.12).The presence of the double bond is highly stabiliz-
ing for this cation, because the charge is delocalized over the allyl system.
Allyl cation
FIGURE 9.12 The molecular orbitals
of the allylic cation.
370 CHAPTER 9 Additions to Alkenes 1
–
Cl
..
..
..
..
+
Protonation to give this localized
cation will be disfavored
Protonation to give this delocalized allylic
cation will be favored
C
CH
2
H
2
C
C
H
H
H
H
H
C
CH
2
H
2
C
C
+
H
C
CH
2
H
2
C
C
H H
H
H
H
C
CH
2
H
2
C
C
+
–
Cl
..
..
..
..
+
+
HCl
..
..
..
HCl
..
..
..
FIGURE 9.13 Preferential formation
of the lower energy, delocalized
cation.
H
CH
2
H
2
C
+
+
H
CH
2
H
2
C
=
–
Cl
..
..
..
..
Cl
..
..
..
–
Cl
..
..
..
..
H
H
2
C
H
CH
2
H
2
C
Notice the partial (dashed) bonds used
to indicate the delocalization
In an alternative
formalism, the
positions sharing the
charge are shown by
(+) placed at the
appropriate sites
H
C
CH
2
H
2
C
C
H H
H
C
H
C
H
C
H
H
H
C
H
C
H
C
H
C
CH
2
H
2
C
C
+
+
H
H
C
CH
2
C
(+)
Cl
..
..
..
FIGURE 9.14 In the allylic cation, the
positive charge is shared by two
carbons. Note the summary
structures in which dashed bonds or
charges in parentheses are used to
indicate delocalization without
writing out all the resonance forms.
PROBLEM 9.3 Derive the π orbitals of the allyl system shown in Figure 9.12.
Hint: Do this task by placing a carbon 2p orbital between the two p orbitals of
ethylene. Another Hint: Watch out for “net zero,” orthogonal interactions!
For the primary carbocation in the reaction shown in Figure 9.10, the positive
charge is localized at the end of the molecule and has no resonance stabilization.
Protonation at the end carbon gives an ion (the allylic cation) that can be represent-
ed by more than one resonance structure. The choice is easy once we have looked
carefully at the structures. Formation of the lower energy delocalized allylic cation will
be favored over formation of the higher energy localized primary cation (Fig. 9.13).
The real structure of the delocalized cation is best represented as a combination
of the two resonance forms.This way of looking at the allylic cation is especially help-
ful because it points out simply and clearly which carbons share the positive charge.
When the chloride ion approaches the allylic cation it can add at either of the two
carbons that share the positive charge to give the two products shown in Figure 9.14.
9.3 Effects of Resonance on Regiochemistry 371
CONVENTION ALERT
Energy
⌽
2
⌽
2
⌽
1
⌽
3
C(3)
C(1)
C(2)
For allyl, the LUMO is ⌽
2
.
In this orbital, there are
only two places where
the filled orbital of
chloride can overlap, C(1)
and C(3); there is a node
at the middle carbon,
C(2), and there can be no
interaction with another
orbital there
CH
2
H
3
C
add at C(1)
add at C(3)
CH
2
H
3
C
Cl
..
..
..
–
Cl
..
..
..
..
Cl
..
..
..
–
Cl
..
..
..
..
1,4-Product
1,2-Product
H
CH
2
H
2
C
+
C
H
C
H
H
C
H
C
H
C
C
H
C(3) C(1)
C(2)
Filled, nonbonding
orbital of chloride
HOMO
The lowest energy
unoccupied orbital
of the allylic cation
LUMO
FIGURE 9.15 In this reaction, the HOMO–LUMO
interaction is between a filled nonbonding orbital
on chloride and the lowest energy unoccupied
orbital of allyl, in this case, .
£
2
Whether one uses resonance forms or molecular orbital descriptions is largely a
matter of taste.Resonance forms are the traditional way, and they are extremely good
at letting you see where the charge resides. We will use them extensively. It’s worth
taking time now to review the whole resonance system.
Be careful not to think of this resonance-stabilized structure as spending part of its
time with the charge on carbon 1 and part of the time with the charge on carbon 3!
The cation has a single structure in which two carbons of the allylic cation share the
positive charge.
This single, summary resonance structure is often shown using dashed bonds to
represent the bonds that are double in one resonance form and single in the other.
The charge is placed at the midpoint of the dashes as shown in the structure at the
top right of Figure 9.14. Alternatively, one resonance form is drawn out in full and
the other atoms sharing the charge are shown with a () or (), as also shown in
Figure 9.14.
Now let’s ask if the molecular orbital description can predict both products. Of
course it can, or it would hardly be very useful. What happens as the chloride ion,
with its filled nonbonding orbitals,begins to interact with the allylic cation? Stabilizing
interactions occur between filled orbitals and empty orbitals. Chloride bears the filled
orbital; therefore we must look for the lowest unoccupied molecular orbital (LUMO)
of allyl. Figure 9.15 shows it, . There are two points at which chloride can add
to , and they lead to the two observed products. Note that the middle carbon,
through which the node passes,cannot be attacked. So the molecular orbital descrip-
tion also explains the regiochemistry of the addition.
£
2
£
2

372 CHAPTER 9 Additions to Alkenes 1
Summary
Addition of HX acids to alkenes really is straightforward. An initial protonation
to give the most stable carbocation possible is followed by capture by X
.
The only difficulty is figuring out which carbocation intermediate is the lowest
energy. Here, an analysis of the “usual suspects” is necessary. You have to consider
the substitution pattern, resonance effects, and, as we shall soon see, inductive
effects as well.
H
H
CCH
2
Cl
H
H
CCH
2
Cl
H
CCH
2
Cl
–
+
+
Cl
..
..
..
..
..
..
..
..
..
..
..
..
+
c
2
c
1
ClH
..
..
..
FIGURE 9.16 In the chlorine-
substituted carbocation, the charge
is shared by carbon and chlorine.
9.4 Brief Review of Resonance
This subject was treated in detail in Chapter 1. Here we only pick up the highlights
of that treatment. If anything seems unfamiliar or strange go back to Chapter 1! A
good way to check that this important material is under control is to see if you can
do Problems 1.18–1.21.
Remember that a molecule that is best described as a resonance hybrid, does not,
repeat not,spend part of its time as one form and part as another.That is chemical equi-
librium not resonance.The two are very different phenomena. Resonance is always
indicated by the special double headed arrow , whereas equilibrium is shown by
two arrows pointing in opposite directions .
As we pointed out as early as Chapter 1, the real structure of a resonance-
stabilized molecule is a hybrid, or combination, of the resonance forms contribut-
ing to the structure.These forms represent different electronic descriptions of the
molecule. Resonance forms differ only in the distributions of electrons and never in
the positions of atoms. If you have moved an atom, you have written a chemical
equilibrium.
It is important to be able to estimate the relative importance of resonance
forms in order to get an idea of the best way to represent a molecule. To do
so, we can assign a weighting factor, c, to each resonance form. The weighting
factor indicates the percent contribution of each resonance form to the overall
structure. Some guidelines for assigning weighting factors are summarized
below.
1. Equivalent resonance forms contribute equally.
2. The more bonds in a resonance form, the more stable the form is.
3. Resonance forms must have the same number of paired and unpaired electrons.
4. Separation of charge is bad.
5. In ions, delocalization is especially important. As we have mentioned a number of
times, small charged atoms are usually most unstable. Delocalization of electrons
that allows more than one atom to share a charge is stabilizing.The protonation of
vinyl chloride is a good example. Vinyl chloride is protonated to give the cation in
which the charge is shared by carbon and chlorine. The resonance forms show this
sharing clearly (Fig. 9.16).
Z
U
Z
U
9.4 Brief Review of Resonance 373
H
2
C
O
=
C
H
–
H
2
C
C
H
–
H
2
C
C
H
–
..
..
..
..
..
..
c
2
c
1
AB
OO
In this case, c
1
> c
2
FIGURE 9.17 Two resonance forms
for the enolate anion.
WEB 3D
O
..
..
..
..
..
=
90⬚
Rotation
–
O
C
..
C
O
..
..
..
–
O
C
C
..
–
Bridgehead
position
..
–
..
Overlap very poor
FIGURE 9.18 In this enolate-like
anion, the orbital containing the pair
of electrons does not overlap well
with the p orbitals of the .
There is no resonance, no matter
what the deceptive two-dimensional
surface says.
C
P
O
7. Now that we have a good deal of structure under our belts, we can look at one more
phenomenon relevant to evaluating good (low energy) resonance forms.We can tell
you to “Watch out! Geometry is important.” Is the “enolate” anion in Figure 9.18
resonance stabilized? On paper it certainly looks like it is, and there is at least a
typographical relationship to the enolate anion of Figure 9.17. If you answered
yes, however, you have been fooled by the two-dimensional surface. The three-
dimensional structure of the cage molecule prevents substantial overlap of the
orbitals. Even though the paper doesn’t protest when you draw the second reso-
nance form, in reality, it does not contribute. The negative charge is borne on only
one carbon—the bridgehead—and is not shared by any other atom. Build a model
and you will see it easily.
PROBLEM 9.4 Are the two structures shown below resonance forms? Explain why
or why not?
+
+
6. Electronegativity is important. In the enolate anion, which has two resonance
forms, the two do not contribute equally because they are not equivalent (Fig.9.17).
Each has the same number of bonds, so we cannot choose the better representation
that way. However, form A has the charge on the relatively electronegative oxygen
while form B has it on carbon. Form A is the better representation for the enolate
anion, although both forms contribute. Mathematically, we would say that the
weighting factor for A,c
1
, is larger than that for B,c
2
.
374 CHAPTER 9 Additions to Alkenes 1
PROBLEM 9.5 How many signals will the following species show in their
13
C
NMR spectra? In fact, not all of these species are stable enough to be detected
by NMR, but in each case it is possible to predict what would be (will be?)
seen.
(a)
(d)
H
2
C
C
H
CH
2
(b) (c)
(e) (f)
H
2
C
C
H
CH
2
–
+
+
+
+
..
–
..
C
H
Cl
H
H
+
+
H
Less stable
More stable
(delocalized)
C
H
..
..
..
C
H
H
H
C
H
H
H
+
H
C
C
H
H
H
C
H
–
Cl
..
..
..
..
Cl
..
..
..
Cl
..
..
..
..
..
..
Cl
C
..
..
Cl
H
H
H
CC
Vinyl chloride
..
..
..
Cl
..
..
..
HCl
+
FIGURE 9.19 In the addition of hydrogen chloride to vinyl chloride, it is formation of the more stable,
resonance-stabilized carbocation that determines the product.
9.5 Resonance and the Stability of Carbocations
We saw in Figure 9.5 one example of how an atom capable of delocalizing electrons
can help share a charge and influence the regiochemistry of an addition reaction.
Addition of hydrogen chloride to vinyl chloride gives 1,1-dichloroethane, not
1,2-dichloroethane. Protonation produces a carbocation adjacent to the chlorine,
which is far more stable than the alternative in which the positive charge sits local-
ized on a primary carbon (Fig. 9.19).
In Section 9.2, we looked at the reaction of symmetrically substituted
2,3-dimethyl-2-butene with hydrogen chloride (Fig. 9.2). In the formation of the
carbocation, there was no choice to be made—only one cation could be formed.
When the less symmetrical alkene 2-methyl-1-butene reacts with hydrogen
9.5 Resonance and the Stability of Carbocations 375
C
C
H
+
+
In an unsymmetrical system, it is the more stable carbocation that leads to product:
C
H
H
3
C
H
3
C
H
3
C
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
CH
2
CH
2
CH
2
C
H
–
Cl
..
..
..
..
–
Cl
..
..
..
..
Cl
..
..
..
CH
2
H
3
C
CH
3
CH
2
C
H
CH
2
Primary carbocation
(less stable)
Tertiary carbocation
(more stable)
Cl
..
..
..
H
H
3
C
CH
3
CH
2
Cl
..
..
..
FIGURE 9.20 The regiospecific
addition of HCl to 2-methyl-1-
butene. It is the more stable
carbocation that leads to product.
chloride, there are two possible carbocations (Fig.9.20).The only chloride formed
is the one produced from the more stable, tertiary carbocation.This product results
from the hydrogen adding to the less substituted end of the alkene and the chlo-
rine to the more substituted end (Fig. 9.20).
2
Markovnikov states in his 1870 paper (Ann. 1870, 153, 228): “wenn ein unsymmetrisch constituirter
Kohlenwasserstoff sich mit einer Haloïdwasserstoffsäure verbindet, so addirt sich das Haloïd an das weniger
hydrogenisirte Kohlenstoffatom, d.h. zu dem Kohlenstoff, welcher sich mehr unter dem Einflusse anderer
Kohlenstoffe befindet.”Which is to say “when an unsymmetrical alkene combines with a hydrohalic acid, the
halogen attaches itself to the carbon atom containing the fewer hydrogen atoms—that is to say, the carbon
that is more under the influence of other carbons.”
2-Pentene
–
+
Cl
..
..
..
..
–
Cl
..
..
..
..
H
H
(45–48%)
(52–55%)
H
+
H
Secondary
carbocations
H
+
Cl
..
..
..
..
..
..
Cl
Cl
..
..
..
FIGURE 9.21 Two products are
formed from the reaction of HCl
and 2-pentene.
These phenomena have been known a long time and were first correlated by the
Russian chemist Vladimir V. Markovnikov (1838–1904) in 1870. The observation
that the more substituted halide is formed now bears his name in Markovnikov’s
rule.
2
Rules are useless (or worse) unless we understand them, so let’s see why
Markovnikov’s rule works.
Unsymmetrical alkenes such as 2-pentene, from which two secondary carbocat-
ions of roughly equal stability can be formed, do give two products in comparable
amounts (Fig. 9.21).
376 CHAPTER 9 Additions to Alkenes 1
Figures 9.19–9.21 show that the mechanism of HX addition must involve for-
mation of the more substituted cation,because the less substituted cation would lead
to a product that is either not observed or is only formed in minor amounts.In turn,
a reasonable presumption is that the more substituted a carbocation is,the more sta-
ble it is (Fig. 9.22). Remember: These differences in stability are large. In practice,
reactions in which tertiary and secondary carbocations are intermediates are com-
mon, but primary and methyl carbocations cannot be formed.
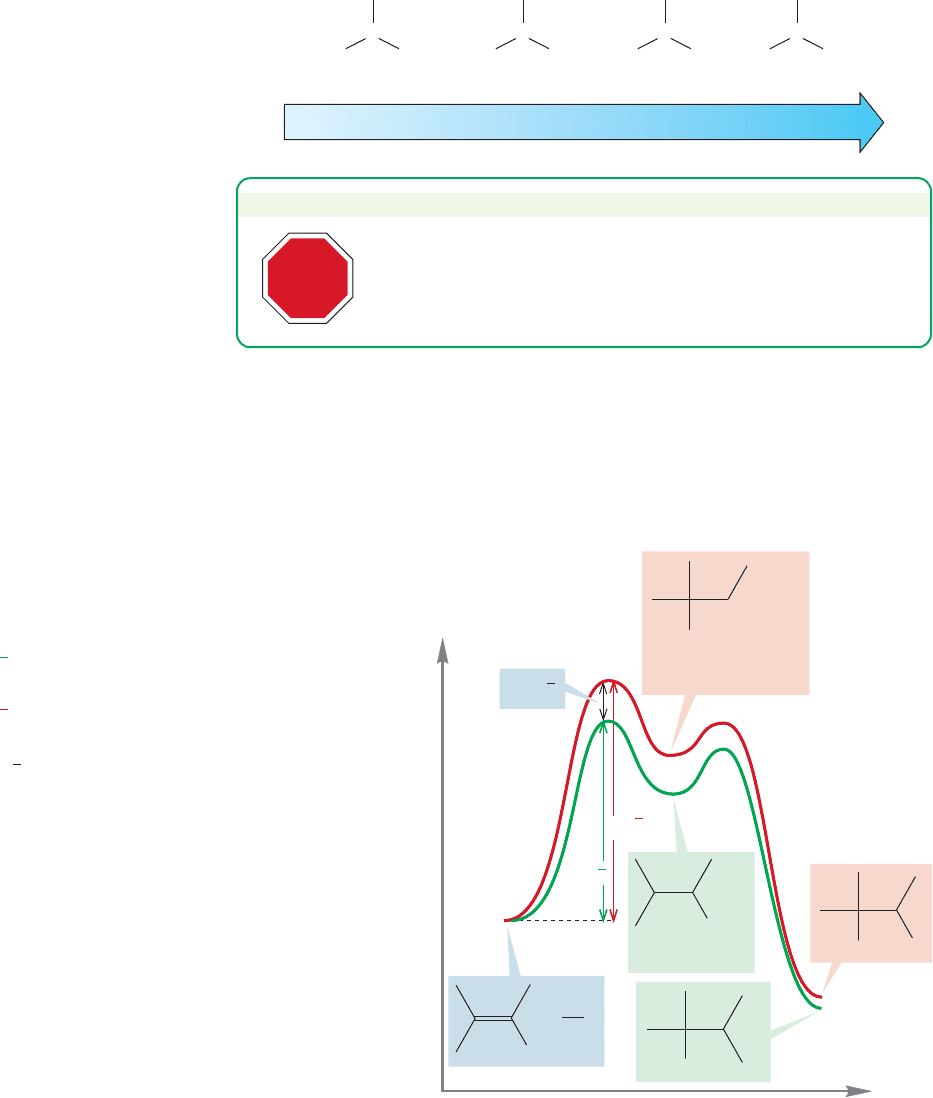
Tertiary
Cation stability (R = alkyl group)
Secondary
H
C
+
C
+
Primary
HH
RRR
RR
R
C
+
Methyl
HH
H
C
+
FIGURE 9.22 Stability order for
carbocations.
PROBLEM SOLVING
STOP
Energy
Reaction
p
ro
g
ress
⌬G
⌬G
⌬G
⌬G
HX
+
X
–
+
X
–
+
Tertiary
carbocation
Secondary
carbocation
+
⌬⌬G
⌬⌬G
Activation energy for formation of more stable
tertiary carbocation
Activation energy for formation of less stable
secondary carbocation
Difference in energies of transition states is
the difference in activation energies
H
H
H
X
H
X
+
H
†
†
†
†
†
†
FIGURE 9.23 The transition states for formation of
the high-energy carbocations will contain partial
positive charges (δ
).The same order of stability
that holds for full positive charges applies to partial
positive charges. A δ
on a tertiary carbon is more
stable than a δ
on a secondary carbon, and so on.
Remember (p. 141) simple primary and methyl carbocations (no
delocalization through resonance) are mechanistic “stop signs.”
They are too high in energy to be formed, and are not known as
intermediates under “normal”conditions.
How does the difference in energy of these two carbocations, the tertiary and sec-
ondary cationic intermediates in the addition reaction, become translated into a pref-
erence for one product over the other? The relative rates of the two possible protonation
steps will not depend directly on the relative energies of the two carbocations them-
selves, but on the energy difference between the two transition states leading to them
(ΔΔG
‡
in Fig. 9.23). Both protonation steps are endothermic and it is likely that the
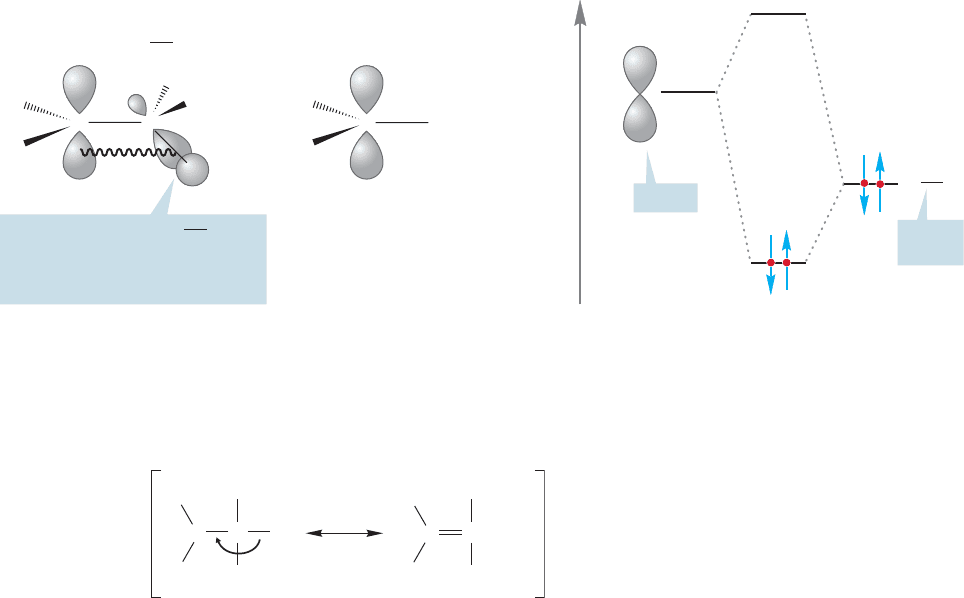
9.5 Resonance and the Stability of Carbocations 377
H
H
H
C
C
H
In the ethyl cation, a C
H
bond can overlap with the empty
2p orbital; this filled–empty
orbital interaction is stabilizing
In the methyl cation, no
such stabilization is
possible!
Methyl cation (CH
3
)Ethyl cation (H
2
CCH
3
)
+
C
H
+
H
H
++
H
Energy
CH
2p
Empty
Filled σ
bond
FIGURE 9.24 The ethyl cation is stabilized by a filled orbital overlapping with an empty orbital.
transition states will bear a strong resemblance to the cations. (Remember the
Hammond postulate, “For an endothermic reaction the transition state will resemble
the product,” p. 355.) In these two transition states, positive charge is building up on
carbon. There are partial positive charges in the transition states. A tertiary partial
positive charge is more stable than a secondary partial positive charge, just as a full positive
charge on a tertiary carbon is more stable than a full positive charge on a secondary carbon.
Once the carbocation intermediate is formed, the structure of the product is deter-
mined. Capture of the tertiary carbocation leads to the observed product, in which X
is attached to the more substituted carbon of the starting alkene (the site of the terti-
ary positive charge in the cation). Similarly, the secondary carbocation would lead
inevitably to a product in which X is attached to the less substituted carbon (Fig. 9.23).
In Chapters 3 and 7, we avoided the issue, but it is now time to deal with the
question of why alkyl groups stabilize carbocations. Our mechanistic hypothesis is
consistent with the idea that the more substituted halide is produced because it
is the result of the initial formation of the more substituted (and hence more sta-
ble) carbocation (Fig. 9.20). But this consistency doesn’t answer anything; we have
just pushed the question further back.
Why are more substituted carbocations more stable than less substituted ones?
To answer such questions,we start with structure. Carbocations are flat, and the cen-
tral carbon is sp
2
hybridized and therefore trigonal. Figure 9.24 compares the methyl
cation (
CH
3
) with the ethyl cation (
CH
2
CH
3
).Note that in the ethyl cation there
is a filled carbon–hydrogen bond that can overlap with the empty p orbital. This
phenomenon has the absolutely grotesque name of hyperconjugation, but there is
nothing particularly “hyper” about it. It is a simple effect and can be rationalized
in familiar molecular orbital or resonance terms. The orbital mixing is stabilizing
(Fig. 9.24) and nothing like it is available to the methyl cation. The more interac-
tions of this kind that are possible,the more stable the ion.Thus the tert-butyl cation
is most stable, the isopropyl cation next, and so on.
Alternatively, we could write the following kind of resonance form, which shows
the charge delocalization from carbon to hydrogen especially well (Fig. 9.25).
It’s important to see that the second carbon–carbon bond in the newly drawn
C
+
C
H
H
H
C
H
+
C
H
H
H
H
H
H
FIGURE 9.25 A resonance description
of the ethyl cation (
CH
2
CH
3
).
378 CHAPTER 9 Additions to Alkenes 1
H
+
C
C
In the “hyperconjugative” resonance
form for the ethyl cation, the “double”
part of the double bond is not made up
of 2p /2p overlap, but of 2p /sp
3
overlap
The π bond in ethylene is
made up of 2p /2p overlap
C
C
FIGURE 9.26 A detailed look at
hyperconjugative stabilization of the
ethyl cation.
resonance form of Figure 9.25 is not identical to a π bond of an alkene.It is not formed
from 2p/2p overlap but rather from 2p/sp
3
overlap (Fig. 9.26). Be sure you see the
difference between these two fundamentally different,but similar-looking structures.
PROBLEM 9.6 It is often claimed that the tert-butyl cation has 10 resonance
forms, 1 in which the carbon bears the positive charge and 9 in which each hydro-
gen bears the positive charge. Is this claim true?
PROBLEM 9.7 On page 115 we described the stabilization of alkenes by attached
alkyl groups in different terms, focusing on the σ bond system. We could con-
struct a similar treatment here. Provide a justification for the relative stability of
differently substituted carbocations using an analysis of the σ system.
PROBLEM 9.8 Do you think the π part of the double bond in the resonance form
in Figure 9.25 is stronger or weaker than a normal π bond? Explain why.
We have just seen that carbocations are stabilized by delocalization through the
overlap of filled and empty orbitals. One example of this effect is fairly straightfor-
ward; a lone pair of electrons on an adjacent atom interacts with an empty orbital.
Less obvious is the last phenomenon, the overlap of filled σ orbitals of
carbon–hydrogen bonds with empty orbitals, called hyperconjugation. Now we pro-
ceed to other factors influencing the stability of carbocations.
Relative rate of
addition at 25 ⬚C
..
..
CH
3
CH
3
CH
3
CC
HH
H
Ethylene
Propene
Methyl vinyl ether
H
CC
H
CC
+
H
H
H
H
H
H
H X1
HX
HX
HX
X
–
..
X
–
..
X
–
..
CC
HH
H
CC
H X2 ⫻ 10
6
OCH
3
..
..
OCH
3
..
..
OCH
3
CC
HH
H
C
+
..
OCH
3
5 ⫻ 10
14
CC
+
H
H
H
H
CC
+
H
H
H
H
CC
H
H
H
H
H
H
H
H
H
C
H X
H
H
H
FIGURE 9.27 Addition reaction to
three different alkenes.
in this case.
HX = HOH
9.6 Inductive Effects on Addition Reactions
Polar bonds in a molecule can have a major effect on both the rate and the regiochem-
istry of an addition reaction. Let’s look first at the series of ethylenes in Figure 9.27,
