Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

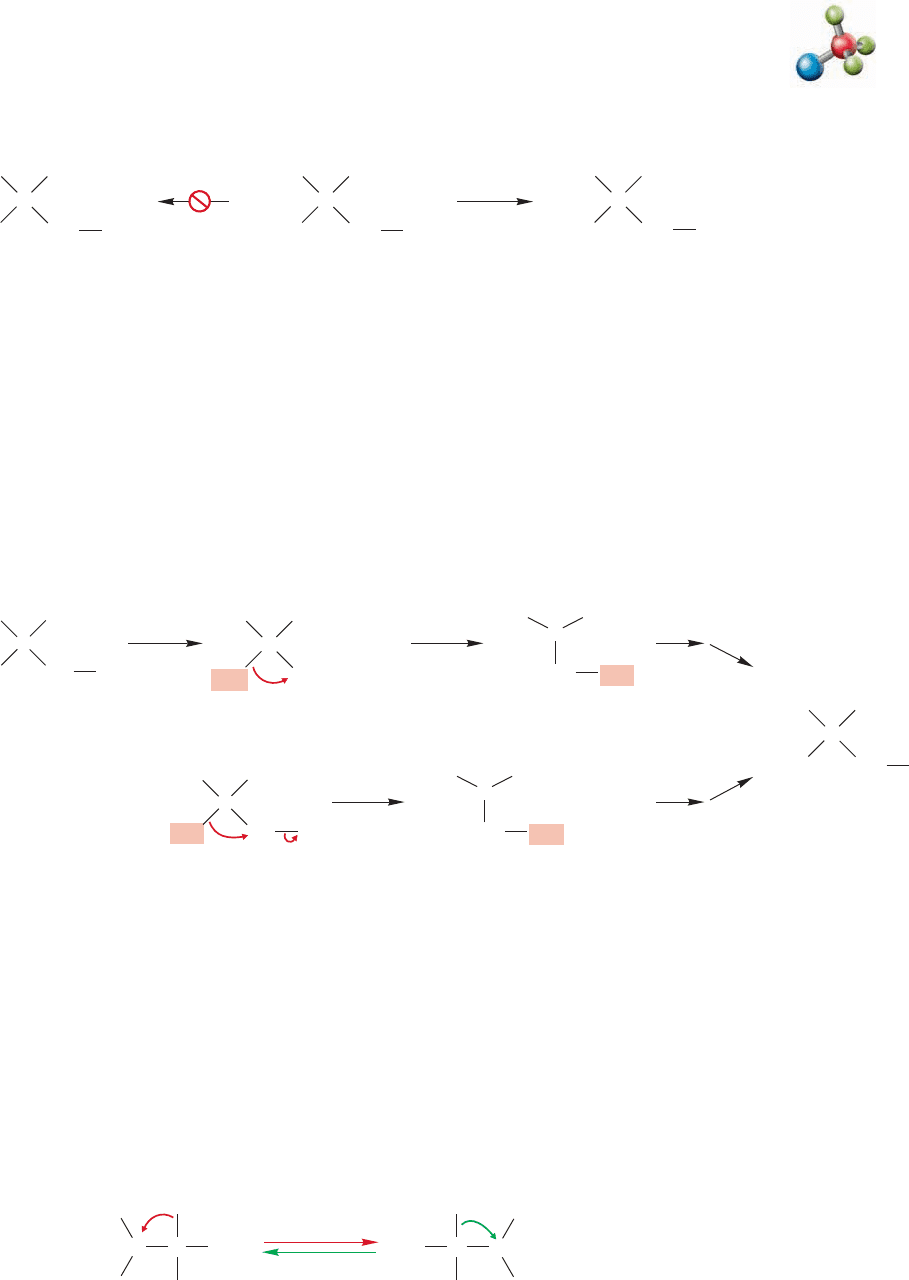
9.9 Rearrangements during HX Addition to Alkenes 389
H
2
O
..
..
..
OH
..
..
H
2
O
..
HO
..
..
I
..
..
..
AgNO
3
AgNO
3
H
3
C
H
3
C
C
CH
2
CH
3
H
3
C
H
3
C
C
CH
2
CH
3
CH
3
H
3
C
C
CH
2
CH
3
Neopentyl alcohol 2-Methyl-2-butanol
(97%)
Neopentyl iodide
FIGURE 9.48 The solvolysis of neopentyl iodide does not take a “normal” course.
AgI
+
+
path a
H
2
O
..
..
path b
H
2
O
..
..
(S
N
1)
H
2
O
..
..
I
..
..
..
H
3
C
H
3
C
C
CH
2
AgNO
3
AgNO
3
CH
3
I
..
..
..
H
3
C
H
3
C
C
CH
2
CH
3
H
3
C
H
3
C
C
CH
2
+
+
CH
3
Neopentyl iodide
Primary carbocation
Tertiary carbocation
Direct formation of the tertiary
carbocation; the methyl group
migrates as iodide leaves
2-Methyl-2-butanol
CH
3
H
3
C
C
CH
2
CH
3
CH
3
HO
..
..
H
3
C
C
CH
2
CH
3
+
H
3
C
C
CH
2
CH
3
CH
3
(S
N
1)
H
2
O
..
..
AgI
FIGURE 9.49 Rearrangements are common in many reactions in which carbocations are involved. Path a shows the
stepwise process and path b is the concerted process.
Such rearrangements are so common that they have come to be diagnostic for reac-
tions involving carbocations. Molecules prone to such rearrangements are used to test
for carbocation involvement. Here is an example: Attempted S
N
1 solvolysis of neopentyl
iodide (1-iodo-2,2-dimethylpropane) in water leads not to neopentyl alcohol, as might
be anticipated, but instead to a rearranged alcohol, 2-methyl-2-butanol (Fig. 9.48).
Carbocation rearrangement E1
These results imply the scenario outlined in path a of Figure 9.49, in which an
unstable primary carbocation rearranges to a much more stable tertiary carbocation
through the shift of a methyl group. In fact, the presence of the primary ion is
so unsettling—they are most unstable and not likely to be formed—that a variant
of this mechanism has been proposed in which the methyl group migrates in a con-
certed fashion as the iodide departs (path b, Fig. 9.49). A concerted reaction is one
that has no intermediates.
At very low temperature in highly polar but nonnucleophilic solvents, some
carbocations can be observed spectroscopically. Under such conditions it is possi-
ble to determine that many carbocations are rapidly rearranging as we observe
them. For example, the two tertiary carbocations in Figure 9.50 rapidly intercon-
vert at 60 °C.
CH
3
FSO
2
OH
CH
3
CH
3
C
H
3
C
H
3
C
+
C
CH
3
CH
3
CH
3
CH
3
C
H
3
C
C
+
– 60 ⬚C
FIGURE 9.50 Rearranging tertiary
carbocations.

390 CHAPTER 9 Additions to Alkenes 1
9.10 Hydroboration
Now we pass on to another addition reaction, hydroboration, which is the addition
of hydrogen and boron across a π bond. The mechanism is not simple, and it will
take some effort for you to become comfortable with all the details. The mechanis-
tic discussion does make several worthwhile points,so it merits your attention for that
reason alone. But there is another, more compelling reason to master the hydrobora-
tion reaction—it is one of the most useful of synthetic reactions.Indeed H. C. Brown
(1912–2004) won a Nobel Prize in 1979 for the development of hydroboration.
Consider the following seemingly simple synthetic task outlined in Problem 9.13.
Borane
BH
H
H
Boron trifluoride
BF
F
F
FIGURE 9.51 Both borane and boron
trifluoride are Lewis acids and will
react like carbocations.
WORKED PROBLEM 9.13 Try to provide a synthesis of 2-methyl-1-propanol. You
may start from any alkene.
ANSWER Well, there isn’t one (yet). At this point in your study of organic chemistry,
you have no way to make this simple alcohol. If we start from the obvious alkene,
2-methylpropene, the hydration reaction we learned in this chapter (p. 380) can
only give the product of Markovnikov addition, tert-butyl alcohol.This nonanswer
to such a simple question points out how limited our synthetic skills are so far.
2-Methyl-1-propanol
CH
2
OH
CH
H
3
C
H
3
C
WEB 3D
Alkene hydroboration
The hydroboration reaction solves both Problem 9.13 and the general diffi-
culty, so let’s see how it works.
In the previous sections, we saw both the protonation of alkenes and the addi-
tion of a carbocation to an alkene to generate a new carbocation.The familiar theme
of overlap between filled (alkene π) and empty (carbon 2p) orbitals was recapitulat-
ed: “Lewis bases react with Lewis acids.”Other reagents containing empty p orbitals,
all good Lewis acids, might also be expected to add to alkenes, and so they do.
We know another kind of molecule that has an empty 2p orbital, the trigonal
boranes, BF
3
and BH
3
(Fig. 9.51).There is no positive charge on these compounds,
but they should still be strong Lewis acids (electrophiles).
PROBLEM 9.14 Verify that boron in BF
3
and BH
3
is neutral and that each boron
atom has an empty 2p orbital.
Although BF
3
is known and even commercially available, free BH
3
is unavailable
because it spontaneously dimerizes to diborane (B
2
H
6
),an unpleasant-smelling,flam-
mable, and toxic gas. Diborane ( ) does not have a structure similarH
3
B
O
BH
3
9.10 Hydroboration 391
WORKED PROBLEM 9.15 Propose a structure for the ether–borane complex, and
suggest a mechanism for its formation.
ANSWER Borane is a Lewis acid because the boron atom has an empty 2p orbital.
An ether, here tetrahydrofuran (THF), is a Lewis base.The reaction between the
Lewis base and Lewis acid produces the borane–THF complex.
B
H
Lewis acid
(electrophile)
Lewis base
(nucleophile)
Borane–THF complex
H
H
H
3
B
–
+
O
..
..
O
..
This structure cannot be B
2
H
6
because
there are only 12 electrons available
for bonding in this molecule, and this
formulation requires 14
HH
H
H
B
H
H
B
H
H
H
H
H
H
B
B
H
H
H
H
AA'
H
H
B
B
H
H
H
H
H
H
B
B
=
FIGURE 9.52 The real structure of
diborane.
H
C
C
+
+
Borane–THF
complex
O
..
..
CC
H
3
B
–
+
O
..
BH
2
FIGURE 9.53 A schematic
hydroboration. The elements of
hydrogen and boron have been added
across the double bond.
to ethane, . An ethane-like structure requires 14 electrons to make
7 boron–hydrogen and boron–boron σ bonds. The 6 hydrogens in diborane con-
tribute 6 electrons and the 2 borons another 6 to give a total of 12, so we are 2 elec-
trons shy of the required number. The real structure is shown in Figure 9.52, along
with a resonance formulation. The molecule is a hybrid of the two resonance forms
A and A′. In this molecule, partial bonds (shown as dashed bonds in Fig. 9.52) are
used to connect the atoms.This kind of bonding is more common than once thought.
It abounds in inorganic chemistry, and in the organic chemistry of electron-
deficient species, such as carbocations.
H
3
C
O
CH
3
When diborane dissolves in diethyl ether ( ) a
borane–ether complex is formed.This complex can be used as a source of BH
3
.Often
the cyclic ether tetrahydrofuran is used. Some borane–ether complexes are so stable
that they can even be distilled.
CH
3
CH
2
O
O
O
CH
2
CH
3
When the borane–ether complex is allowed to react with an alkene, there is a
rapid addition of the borane across the double bond. The initial product is an
alkylborane (Fig. 9.53). As in the addition of a carbocation to a double bond, the
initial interaction is between the empty 2p orbital (in this case on neutral boron,
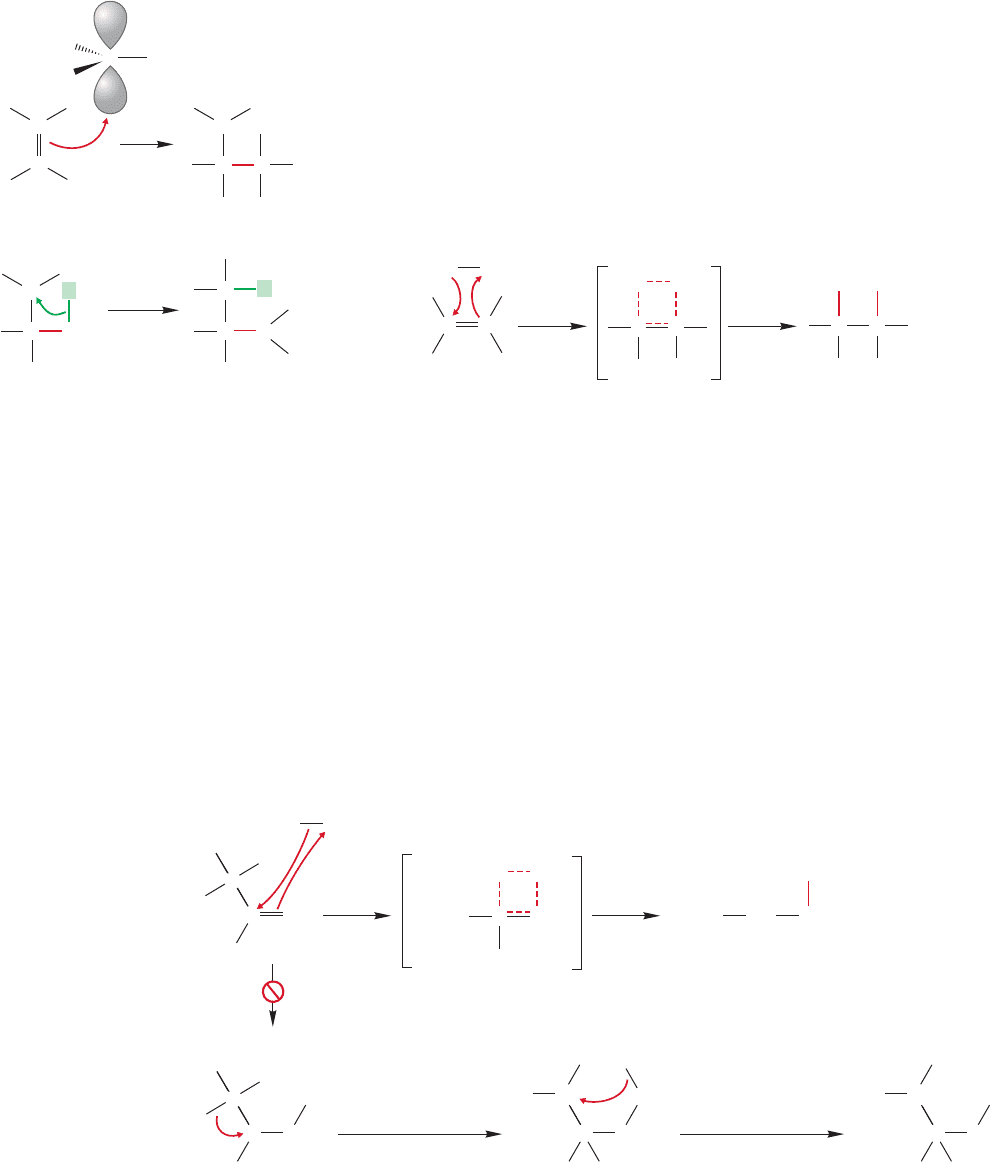
392 CHAPTER 9 Additions to Alkenes 1
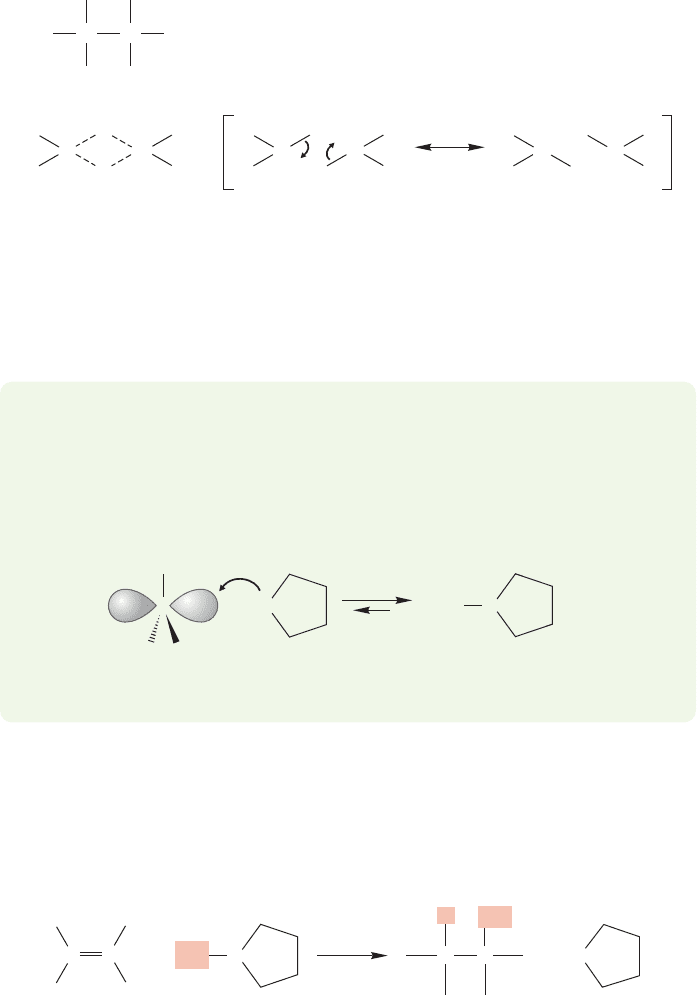
not positive carbon) and the filled π orbital of the alkene (Fig. 9.54a). If the mech-
anism were directly parallel to that of cation addition, we would form the charge-
separated molecule shown in Figure 9.54a.A hydride (H
) could then be delivered
from the negative boron to the positively charged carbon to complete the addition
(Fig. 9.54b). However, the mechanism is not exactly parallel to what we saw before.
In this case, the hydride is transferred as the carbon–boron bond is made (Fig. 9.55).
This reaction occurs in a single step.There is no intermediate.In the transition state,
partial bonds from carbon to boron and carbon to hydrogen are formed. Let’s see
how we know this mechanism satisfies the experimental data and, as we do this,
why the more conventional mechanism is inadequate.
:
(a)
(b)
BH
C
C
+
C H
H
C
BH
H
H
Nucleophile—
π bond
Electrophile (empty 2p orbital)
–
B
C
H
C
BH
2
C
C
H
H
H
+
–
FIGURE 9.54 (a) A possible first step in
addition of BH
3
to an alkene.The π
bond of the alkene acts as nucleophile
and reacts with the strong Lewis acid,
BH
3
. (b) A possible second step in the
hydroboration reaction is transfer
of a hydride (H
).
:
C
Transition state with
partial bonds (dashed)
BH
2
BH
2
BH
2
H
H
C
CC
CC
CC
H
CC
FIGURE 9.55 A one-step or concerted mechanism for
hydroboration.
First of all,there are no rearrangements during hydroboration.The concerted mech-
anism outlined in Figure 9.55 cannot lead to rearrangements, but the intermediate car-
bocation of Figure 9.54 would surely do so. For example, the hydroboration of
3,3-dimethyl-1-butene leads exclusively to borane A, shown in path a of Figure 9.56.
Were a carbocation intermediate involved, rearrangement of the secondary carbocation
to the more stable tertiary carbocation would seem inevitable, and product B should be
observed (path b of Fig.9.56).But the reaction yields a regiospecific hydroboration.Only
one of two possible product boranes is formed.This phenomenon is general—in hydro-
boration the boron becomes attached to the less substituted carbon of the double bond.
Our one-step mechanism must be able to explain this regiochemical preference.
(CH
3
)
3
C
A
B not observed
Transition state with
partial bonds (dashed)
Tertiary carbocation
(more stable)
Secondary carbocation
(less stable)
rearrangement hydride shift
BH
2
CC
BH
2
H
C CH
2
CH
2
CH
3
(CH
3
)
3
C CH
2
CH
2
BH
2
H
H
C
H
3
C
H
3
C
H
C CH
2
CH
3
C
H
3
C
H
3
C
H
+
+
–
–
BH
3
CCH
2
H
3
CH
H
BH
2
C
H
3
C
CH
3
CCH
2
H
3
CH
BH
2
CH
H
3
C
CH
3
path b
path a
FIGURE 9.56 An intermediate carbocation of path b should lead to rearrangements in hydroboration, but
no evidence of such can be found, which suggests that the concerted path a is the correct mechanism.
9.10 Hydroboration 393
There are at least two factors, one simple, the other more complicated, that
explain the regiospecific addition in the hydroboration reaction. The simple analy-
sis is a steric argument. Consider the reaction of BH
3
with 2-methyl-1-butene.There
are steric differences in the two possible concerted additions. In the addition lead-
ing to the observed product (Fig. 9.57a), the larger end of the borane molecule, the
BH
2
portion, is nearer to the smaller hydrogens than to the larger alkyl groups. In
the path leading to the product that is not observed (Fig. 9.57b), the larger end
opposes the relatively large methyl and ethyl groups.
(a)
(b)
Transition state
The sterically less
congested arrangement
leads to the observed
product
This sterically more
congested arrangement
leads to the product that
is not observed
CH
2
CH
2
CH
2
BH
2
BH
2
BH
2
CH
3
CH
2
H
3
C
C
H
H
C
H
CCH
2
CH
2
CH
2
H
2
B
CH
3
CH
2
H
3
C
H
2
B
C
H
H
2
B
H
H
C
Transition state
Observed product
Not observed
C
CH
3
CH
2
H
3
C
CH
3
CH
2
H
3
C
CH
3
CH
2
H
3
C
CH
3
CH
2
H
3
C
FIGURE 9.57 (a) Steric factors favor
addition of the larger BH
2
group to
the less congested end of the alkene.
(b) Steric interactions could hinder
formation of the nonobserved
product.
δ
+
δ
–
Tertiary δ
+
(more stable)
Observed product
This product is not formed
CH
2
CH
2
BH
2
BH
2
CH
3
CH
2
H
3
C
C
H
C
H
δ
+
δ
–
Primary δ
+
(less stable)
CH
2
CH
2
H
2
B
C
H
H
2
B
H
C
CH
3
CH
2
H
3
C
CH
3
CH
2
H
3
C
CH
3
CH
2
H
3
C
FIGURE 9.58 In the two possible
transition states for the concerted
addition, there will be partial positive
charge developed on carbon. This δ
will be more stable at the more
substituted position.
The more complicated factor has to do with the way in which the two new
bonds are made in the transition state for the hydroboration reaction. Two bonds
are formed in the same step, without a cationic intermediate, but there is no need
for the new bonds to develop to the same extent as the reaction proceeds. Indeed,
in a philosophical sense, they cannot, because they are different. In the transition
state, one bond will be further along in its formation than the other. This differ-
ential progress can be symbolized by bonds of different lengths, as in Figure 9.58.
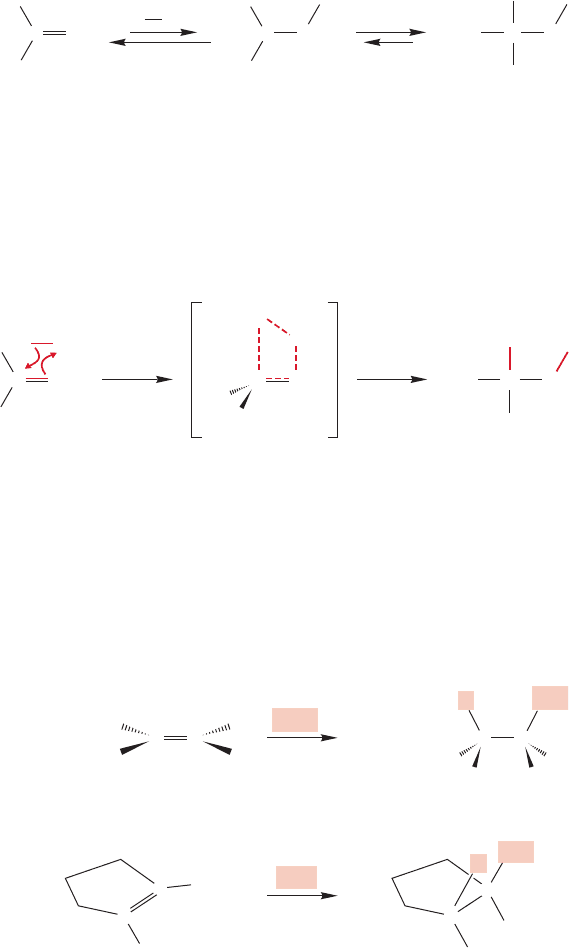
394 CHAPTER 9 Additions to Alkenes 1
In such a mechanism, a full positive charge is not developed on carbon, but a partial
one is. All the factors that operate to stabilize a full positive charge also operate to sta-
bilize a partial positive charge. A more-substituted partial positive charge is more sta-
ble than a less-substituted partial positive charge,and this difference favors the observed
product. So, the initial product of hydroboration of an alkene is the monoalkylborane
formed by addition in which boron becomes attached to the less substituted end of
the double bond (Fig. 9.58). Does this result violate Markovnikov’s rule? No!
Here is an instance where rules can be confusing. When we say “Markovnikov
addition,” we are used to seeing the hydrogen attached to the less substituted car-
bon of the double bond and the X group to the other carbon (Fig. 9.59).
H
3
CC
HX
X
X
H
H
C
CH
2
H
3
C
H
3
C
H
3
C
C
CH
2
H
3
C
H
3
C
CH
2
..
–
+
FIGURE 9.59 Typical Markovnikov
addition in which the more stable
cation is formed as an intermediate to
give the product in which H is
attached to the less substituted
carbon of the original double bond,
and X is attached to the more
substituted end.
When adds across a π bond, it is the boron that is the positive end of
the dipole, the electrophile in the addition. It is the boron, not the hydrogen, that
becomes attached to the less substituted end of the double bond. As Figure 9.60 tries
to show,it is the terminology that gets complicated,not the reaction mechanisms.There
is danger in learning rules; it is much safer to understand the reaction mechanism.
H
O
BH
2
Transition state
H
BH
2
H
BH
2
H
3
C
H
3
C
C
CCH
2
H
3
C
H
3
C
CCH
2
H
3
C
H
3
C
CH
2
BH
2
δ
+
δ
–
H
FIGURE 9.60 This reaction is still
Markovnikov addition.The
electrophilic boron in this case adds
to the less substituted end of the
alkene. Positive charge develops on
the more substituted carbon.
There is another reason we know that the concerted mechanism for hydrobora-
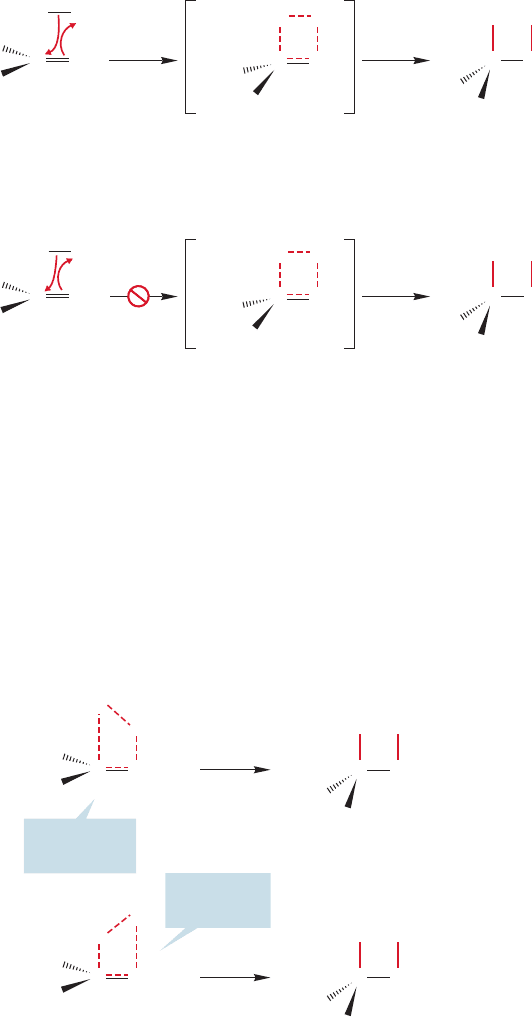
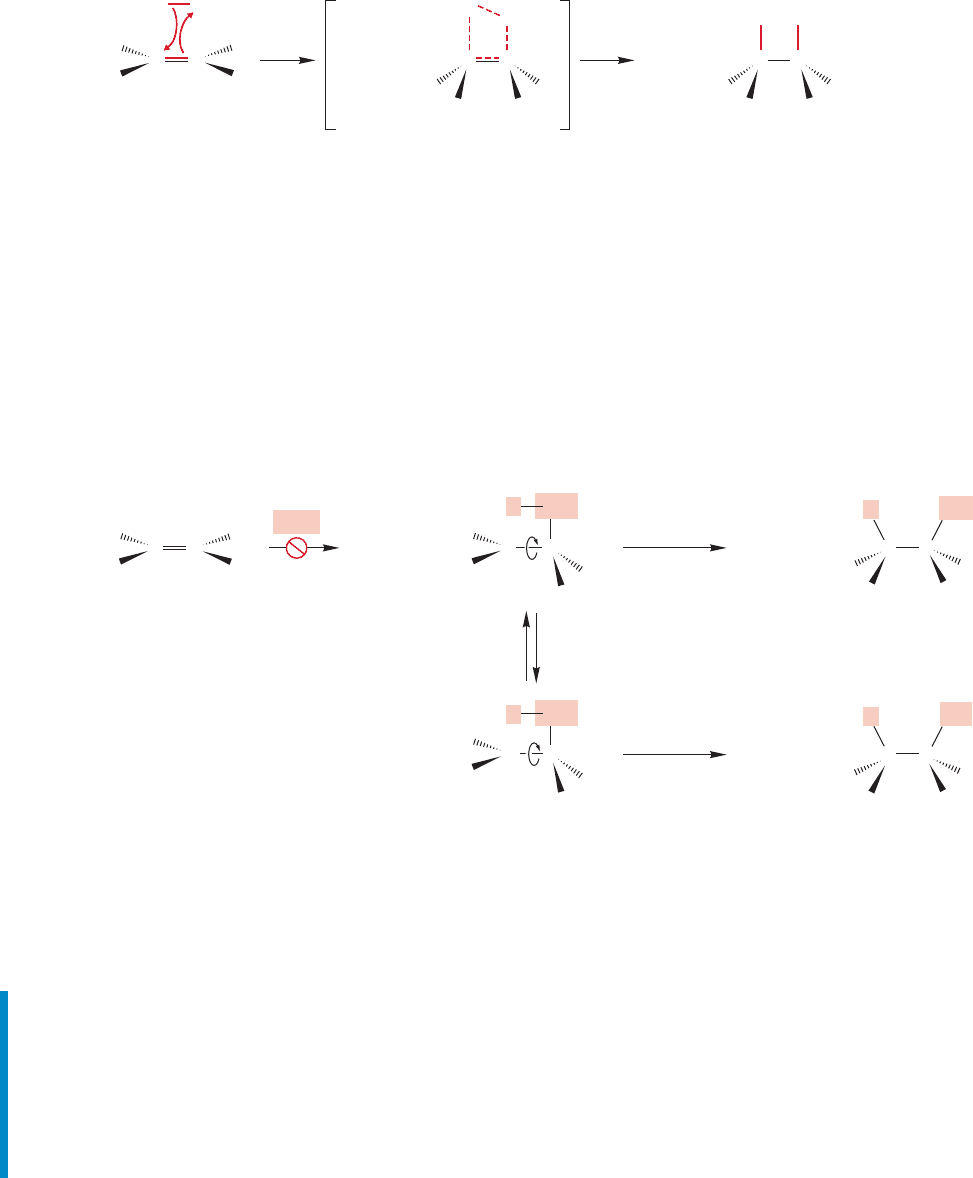
tion is correct, and it involves the stereochemistry of the addition reaction.
Hydroboration can be shown to proceed in syn fashion. A syn addition describes
the result when two pieces (H and BH
2
in this case) are delivered to the same side of an
alkene. Figure 9.61 shows the hydroboration of cis-1,2-dideuterio-1-hexene and of
1-methylcyclopentene.These reactions exclusively produce the products of syn addition.
cis -1,2-Dideuterio-1-hexene
1-Methylcyclopentene
CC
CH
3
BH
2
BH
3
HBR
2
CH
3
CH
2
CH
2
CH
2
C
C
CH
3
H
H
H
C
C
H
D
D
H
C
DD
C
CH
3
CH
2
CH
2
CH
2
H
BR
2
FIGURE 9.61 These two
stereochemical experiments show
that addition of BH
3
proceeds in a
syn fashion.The H and BH
2
groups
are delivered to the same side of the
alkene (R cyclohexyl).=
9.10 Hydroboration 395
Summary
The current mechanistic hypothesis for hydroboration, a concerted, one-step syn
addition to alkenes in which the two new bonds are partially formed to different
extents in the transition state, nicely rationalizes all the experimental data. A
mechanism involving formation of an open cation followed by hydride transfer
fails to account for the observed syn stereochemistry of addition, which is
demanded by the one-step mechanism.
Transition state
CC
CH
3
CH
2
CH
2
CH
2
H
D
D
CC
CH
3
CH
2
CH
2
CH
2
H
H
D
DD
D
BR
2
H
BR
2
CC
CH
3
CH
2
CH
2
CH
2
H
H
BR
2
FIGURE 9.62 Syn addition is accommodated by a mechanism in which both new bonds are
forming in the transition state.There are no intermediates and therefore no chance for
rotation and scrambling of stereochemistry.
H
H
..
H
H
D
D
D
D
transfer
rapid
rotation
CC
CH
3
CH
2
CH
2
CH
2
H
D
D
D
D
H
CC
CH
3
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
BR
2
H
D
C
CC
CH
3
CH
2
CH
2
CH
2
H
H
D
C
CH
3
CH
2
CH
2
CH
2
BR
2
C
C
+
–
H
..
transfer
–
+
H
HBR
2
BR
2
–
BR
2
–
FIGURE 9.63 Rotation about carbon–carbon single bonds is very fast. A two-step
mechanism predicts that two products should be formed from the hydroboration of cis-1,2-
dideuterio-1-hexene. Both syn and anti addition products should be formed. However, only
the product of syn addition is found, indicating that there is no carbocation intermediate.
Of course,the one-step addition process must involve syn addition because both
new bonds, the one to boron and the one to hydrogen, are made at the same or nearly
the same time. Figure 9.62 shows the observed syn addition to deuterated 1-hexene.
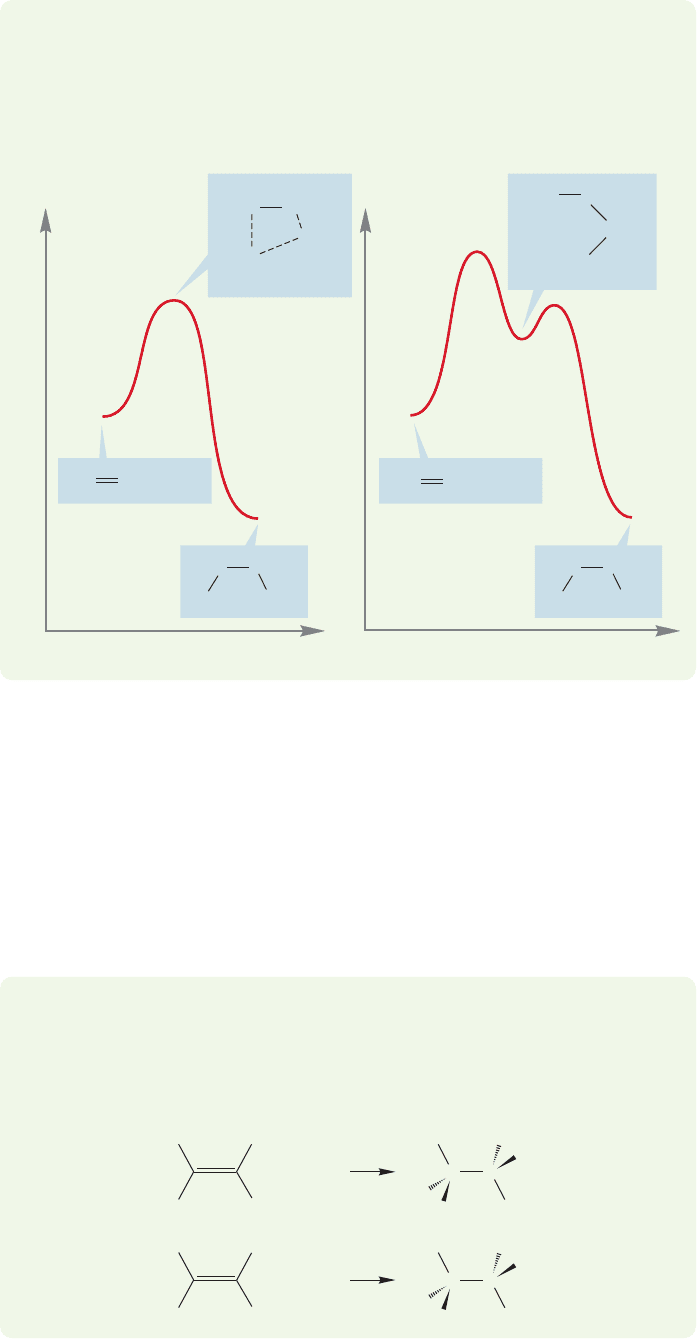
A mechanism involving an intermediate carbocation has great problems account-
ing for syn addition. If a planar cation were formed on addition to the deuterat-
ed 1-hexene, we would expect the rapid ( 10
11
s
1
) rotation about carbon–
carbon single bond to produce the two diastereomeric products shown in
Figure 9.63.
'
396 CHAPTER 9 Additions to Alkenes 1
WORKED PROBLEM 9.16 Draw Energy versus Reaction progress diagrams for the
concerted and two-step mechanisms for the addition of BH
3
to alkenes.
ANSWER The crucial difference is the presence of an intermediate in the two-step
process.The one-step (concerted) reaction simply passes from starting material to
product over a single transition state.
Reaction progress
Energy
Energy
BH
3
+
One-step
(concerted)
Two-step
Reaction progress
BH
3
+
Intermediate
BH
2
BH
2
H
H
CR
2
CR
2
CR
2
R
2
C
CR
2
R
2
C
R
2
C
BH
2
H
CR
2
R
2
C
R
2
C
+
–
Transition state
δ
+
δ
–
BH
2
H
CR
2
R
2
C
This example is typical of the use of stereochemistry in determining reaction mech-
anism. One reason we spent so much time on the stereochemical relationships of mol-
ecules of various shapes (diastereomers, enantiomers, and meso forms) was that we will
use these relationships over and over again as we dig into questions of reaction mech-
anism. If it is not obvious what we are talking about in the last few paragraphs, and if
Figures 9.61–9.63 are at all unclear, be sure to go back to Chapter 4 and work over the
stereochemical arguments again.Otherwise,you run the risk of being lost in further dis-
cussion.The key points to see are the difference between the two hypothetical products
shown in Figure 9.63 and their required formation from the rotating cation.A good test
is to see if you can do Problems 9.17 and 9.18 easily. If you can, you are in good shape.
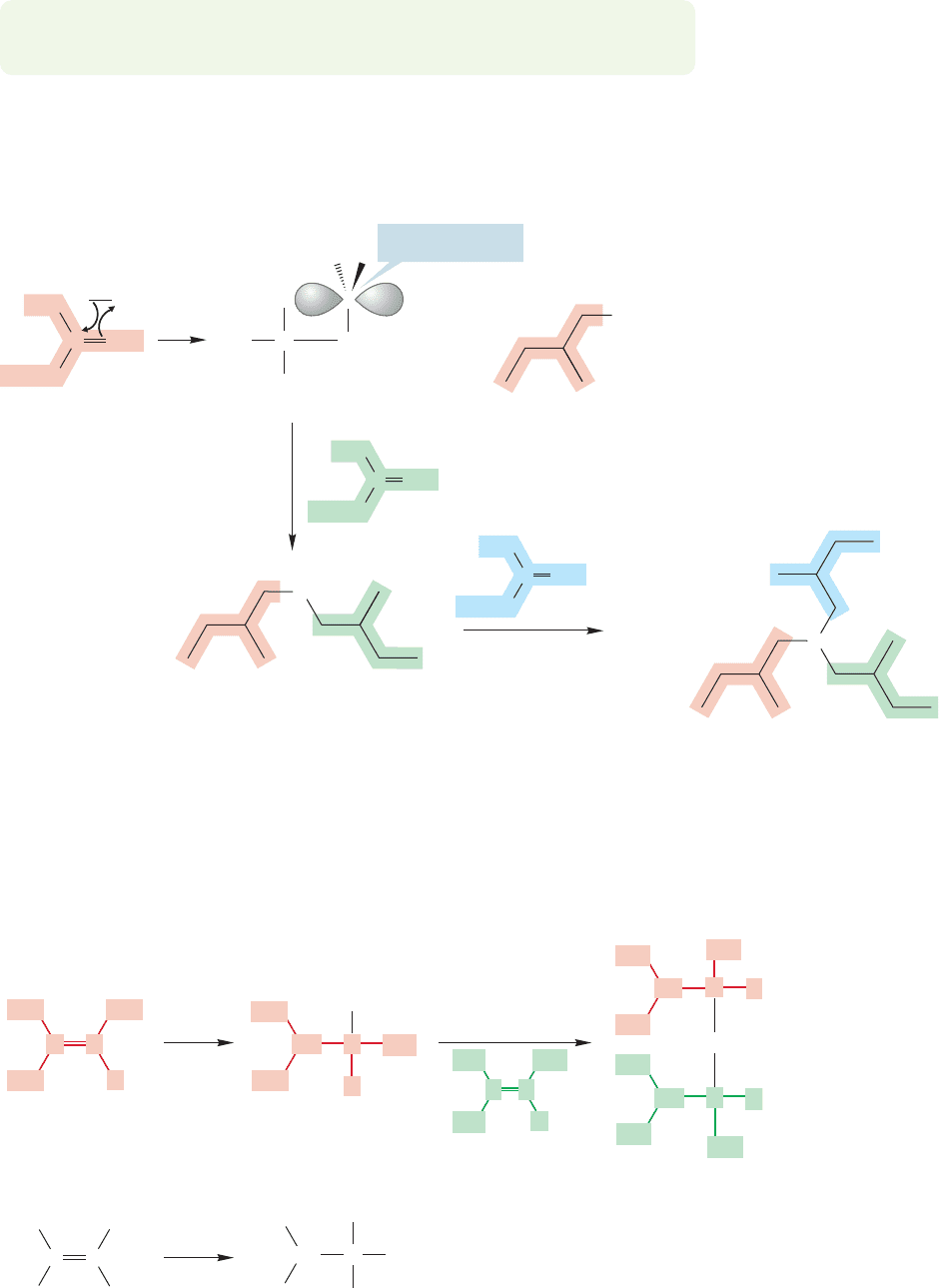
PROBLEM 9.17 Bromine adds to alkenes to give dibromo compounds. Based on
the products from the reaction of bromine with the 2-butenes, determine if the
addition is syn or anti. This problem does not pose a mechanistic question—you
do not have to draw arrow formalisms. You only need to determine from the
structure of the products the direction from which the two bromines have added.
H
H
CH
3
C
H
CH
3
C
Br
Br
H
3
C
H
3
C
+ Br
2
H
C
H
H H
H
C
Br
Br
H
3
C
H
3
C
+ Br
2
CH
3
CH
3
9.10 Hydroboration 397
PROBLEM 9.18 Devise a way to determine the stereochemistry of the addition of hydro-
gen bromide to alkenes. Assume you have access to any necessary starting materials.
B
Still a Lewis acid!
repeat the
hydroboration
A dialkylborane
=
H
BH
2
BH
2
BH
H
3
C
CH
3
CH
2
CH
3
CH
2
C
CCH
2
H
3
C
CCH
2
H
3
C
CH
3
CH
2
repeat the
hydroboration
CH
2
H
HH
R
2
BH
=
RBH
2
=
B
A trialkylborane
R
3
B
=
CH
2
C
H
3
C
CH
3
CH
2
FIGURE 9.64 When BH
3
is used, the
initially formed alkylboranes are
Lewis acids and can undergo further
hydroborations.
A single addition isn’t the end of the hydroboration story.The initially produced
monoalkylborane still has a trigonal boron; therefore, it has an empty 2p orbital
(Fig. 9.64). It’s still a Lewis acid, and it can participate in a second hydroboration
reaction to give the product of two hydroborations of the alkene, a dialkylborane.
Nor must the reaction stop here. The dialkylborane is also a Lewis acid, and it can
do one more hydroboration to give a trialkylborane. Now, however, all the original
hydrogens that were on the boron are used up and there can be no further hydro-
borations.In practice,the number of hydroborations depends on the size of the alkyl
groups in the alkene. When the groups are rather large, further reaction is retarded
by steric effects—the alkyl groups just get in the way. For example, 2-methyl-
2-butene hydroborates only twice, and 2,3-dimethyl-2-butene only once (Fig. 9.65).
2-Methyl-2-butene
2,3-Dimethyl-2-butene
2-Methyl-2-butene
repeat
A monoalk
y
lborane
A dialkylborane
CC
H
3
C
H
3
C
H
3
C
H
3
C
H
CH
3
CC
H
3
C
H
3
C
CH
3
CH
3
CC
H
3
C
H
3
C
H
CH
3
CH
C
CH
3
BH
2
H
3
C
H
3
C
CH
C
H
3
C
H
3
C
CH
C
CH
3
CH
3
BH
3
BH
3
H
CH
H
3
C
H
3
C
C
CH
3
CH
3
BH
2
BH
H
H
FIGURE 9.65 The number of
hydroborations depends on steric
effects.
398 CHAPTER 9 Additions to Alkenes 1
WORKED PROBLEM 9.19 Provide a mechanism for the following reaction:
ANSWER The first hydroboration goes in normal fashion.
Now a second,intramolecular hydroboration takes place as the remaining dou-
ble bond in the ring attacks the boron.
It takes some unraveling to see the structure of the final product. It is
important to begin to hone your skills at this kind of thing, and this problem
provides practice.
BH
HB
H
H
H
=
BH
BH
3
H
2
B
H
BH
3
BH
Alcohol Boric acid
OH
H
2
O
2
/ HO
R
3
B
..
..
..
..
B(OH)
3
..
..
..
+
–
3 R
FIGURE 9.66 Formation of an alcohol
from a borane.
9.11 Hydroboration in Synthesis:
Alcohol Formation
It would be presumptuous to attempt to summarize fully the utility of the hydro-
boration reaction here.There are many variations of this reaction, each designed
to accomplish a specific synthetic transformation. A book could be written on
the subject. In fact, Professor H. C. Brown has written just such a book. We’ll
only mention one especially important reaction here, one that leads to a new
process for the synthesis of alcohols (recall Problem 9.13, p. 390). When one
molecule of trialkylborane is treated with hydrogen peroxide (H
2
O
2
) and hydrox-
ide ion (HO
), three molecules of an alcohol are formed along with boric acid
(Fig. 9.66).
