Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

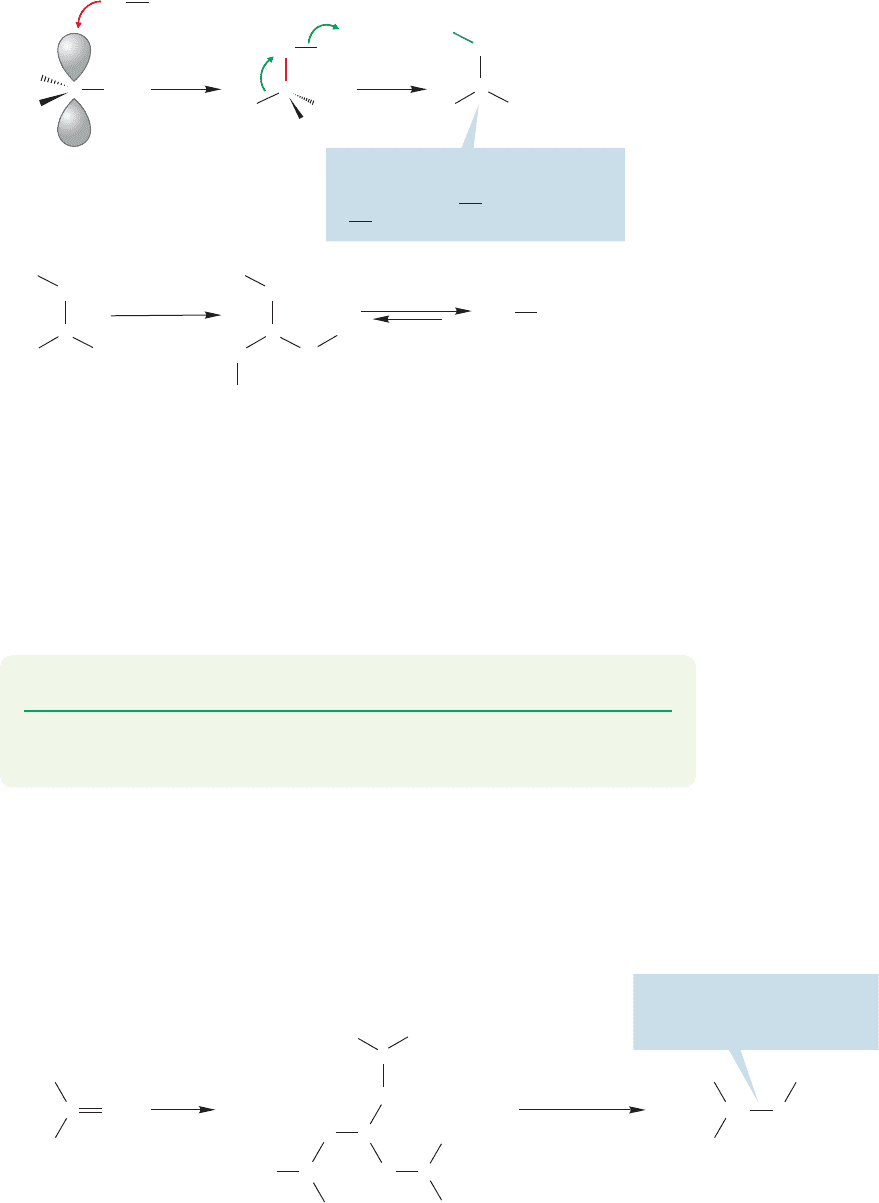
9.11 Hydroboration in Synthesis: Alcohol Formation 399
We are not yet up to a full discussion of the mechanism of this reaction, which
is outlined in Figure 9.67, but a number of important points are within our powers.
–
O
..
..
..
–
OH
..
..
..
repeat the
steps above
two times
B
Boric acid
A boronic ester
OH
..
..
OH
..
..
–
OH
..
..
..
H
2
O
..
..
O
..
..
O
..
..
OH
..
..
B(OH)
3
..
..
+
+
+
B
–
B
O
..
..
B
O
..
O
..
..
..
O
..
..
B
The initial product is another trigonal
boron compound; notice that we
have traded a B
R bond for a
B
OR bond
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
3 R
FIGURE 9.67 The mechanism
of alcohol formation from an
alkylborane.
The first step, addition of peroxidate ion ( ) to the borane, is just
the reaction of a filled orbital on oxygen with the empty 2p orbital on boron. It’s
another nucleophile–electrophile reaction. Notice that the boron becomes oxidized
in this process. A discussion of the later steps is deferred for now, but at least you
can see that the boron–oxygen bonds in the B(OR)
3
(boronic ester) should be espe-
cially strong ones and the reaction will be favored thermodynamically. Finally, in
excess hydroxide the boronic ester equilibrates with boric acid and the alcohol.
H
O
O
O
O
-
PROBLEM 9.20 Why are boron–oxygen bonds especially strong?
PROBLEM 9.21 Write a mechanism for the formation of boric acid, B(OH)
3
,from
hydroxide ion, HO
, and a boronic ester, B(OR)
3
.
In this case,questions of mechanism are secondary to the utility of this reaction.Note
one very important thing: In the overall reaction we are able to produce the less substi-
tuted alcohol from the alkene. The hydroxyl group enters at the position to which the
boron was attached:the less substituted end of the double bond.This reaction is an over-
all anti-Markovnikov addition (Fig. 9.68) because the regioselectivity of the product is
the opposite of the Markovnikov product. But Markovnikov’s rule was not broken.
B
C 3CH
2
CH
3
CH
3
CH
2
CH
3
CH
CH
CH
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
CH
2
CH
3
H
3
C
H
2
C
H
2
C
CH
3
CH
2
CH CH
2
H
3
C
BH
3
OH
H
2
O
2
/ HO
..
..
..
B(OH)
3
..
..
..
..
+
–
Three molecules of alcohol
This alcohol is the product of
overall anti-Markovnikov
addition of water to the alkene
A trialkylborane
FIGURE 9.68 Hydroboration/oxidation accomplishes
the anti-Markovnikov addition of water to an alkene.
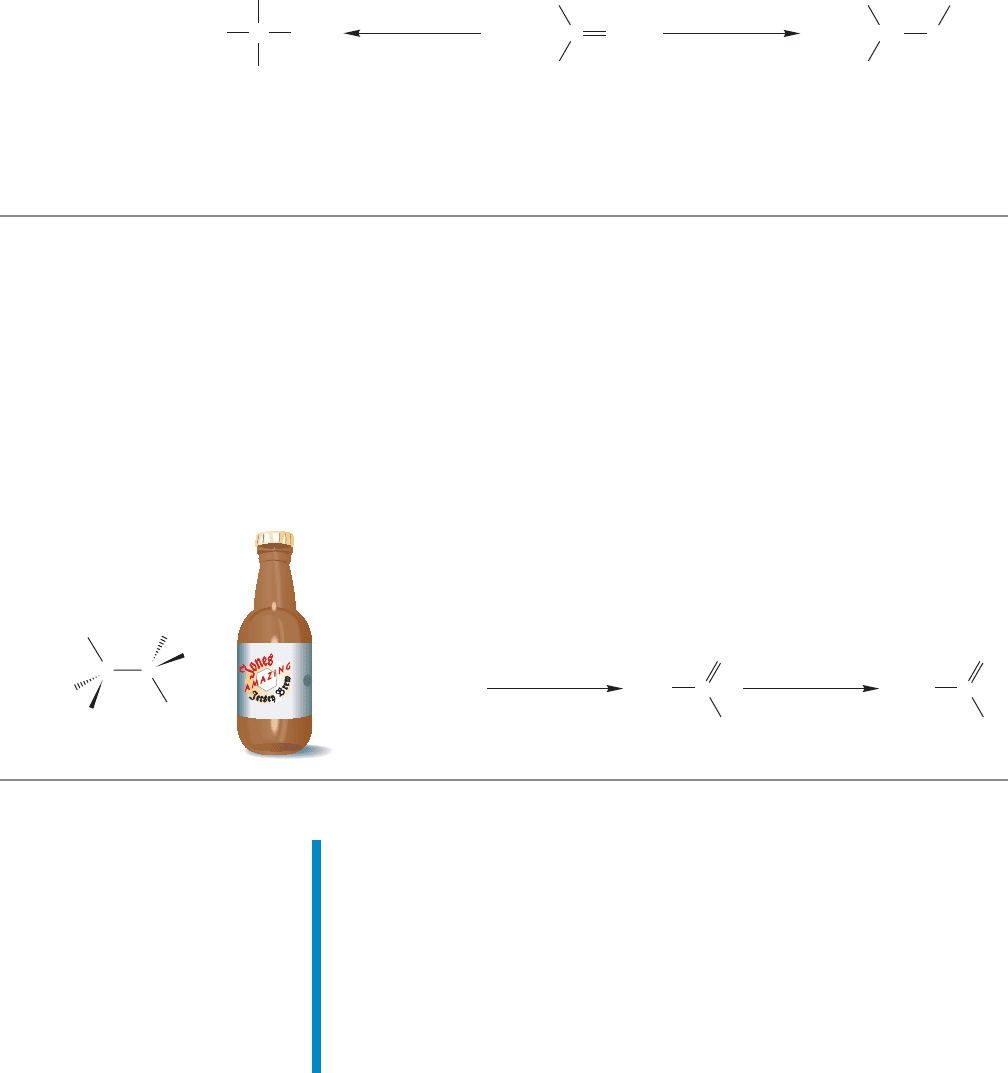
400 CHAPTER 9 Additions to Alkenes 1
Our previous method for alcohol synthesis, hydration of alkenes, necessarily
produced the more substituted alcohol. So now we have complementary synthetic
methods for producing both possible alcohols from a given alkene (Fig. 9.69).
Direct hydration gives the more substituted product (Markovnikov addition) and
the indirect hydroboration/oxidation method gives the less substituted alcohol (anti-
Markovnikov addition). Be sure to add these reactions to your file-card collection
of synthetically useful reactions.
ETHYL ALCOHOL
Ethyl alcohol, or ethanol, can be produced by several reac-
tions described in this chapter. Although it has other uses,
most ethyl alcohol is made for the production of alcoholic
beverages, a use deeply rooted in history. Indeed, the rise
of civilization has been attributed to the discovery of beer,
and the necessity to give up a nomadic life to attend to the
cultivation of the ingredients. In 2005, the average adult
American consumed slightly more than 31 gal of beer and
more than 4.5 gal of wine and spirits.
Ethyl alcohol
CC
H
OH
H
H
H
H
Ethyl alcohol acts as a depressant in humans, interfering
with neurotransmission. It works by binding to one side of
the synapse, changing its shape slightly and making it able
to bind γ-aminobutyric acid (GABA) more efficiently.
Bound GABA widens the synaptic channel, allowing chlo-
ride ions to migrate in, thus changing the voltage across the
synapse. The nerve cell is less able to fire, and neurotrans-
mission is inhibited. Your body cleanses itself of alcohol by
converting it into acetaldehyde with the enzyme alcohol
dehydrogenase. A second enzyme, aldehyde dehydrogenase,
completes the conversion into acetic acid. There are indi-
viduals, indeed whole races, who are short of aldehyde
dehydrogenase and thus overly susceptible to the deleterious
effects of alcohol. By contrast, alcoholics have been found to
contain elevated levels of this enzyme.
1. BH
3
2. HOOH / HO
–
Anti-Markovnikov addition
CCH
2
CH
3
CH
2
CH
3
CH
2
H
3
C
CH CH
2
H
3
C
H
3
C
OH
CH
3
C
Markovnikov addition
CH
3
CH
2
H
2
O
HOSO
2
OH
OH
FIGURE 9.69 Two addition reactions that can produce alcohols with different regiochemistry.
Summary
Hydroboration/oxidation is a process that yields an alcohol that is the prod-
uct of overall anti-Markovnikov addition. The mechanism of hydroboration
is complex, but several lines of evidence have led to the picture we have
of a concerted reaction with an unsymmetrical transition state in which
one of the alkene’s carbon atoms becomes partially positively charged
(Figs. 9.55–9.60). The synthetic utility of this reaction is not complex at all.
For unsymmetrical alkenes, hydroboration/oxidation leads to the less substi-
tuted alcohol.
alcohol
dehydrogenase
aldehyde
dehydrogenase
CH
3
CH
2
OH H
3
CC
O
H
H
3
CC
O
OH
Acetic acidAcetaldehyde
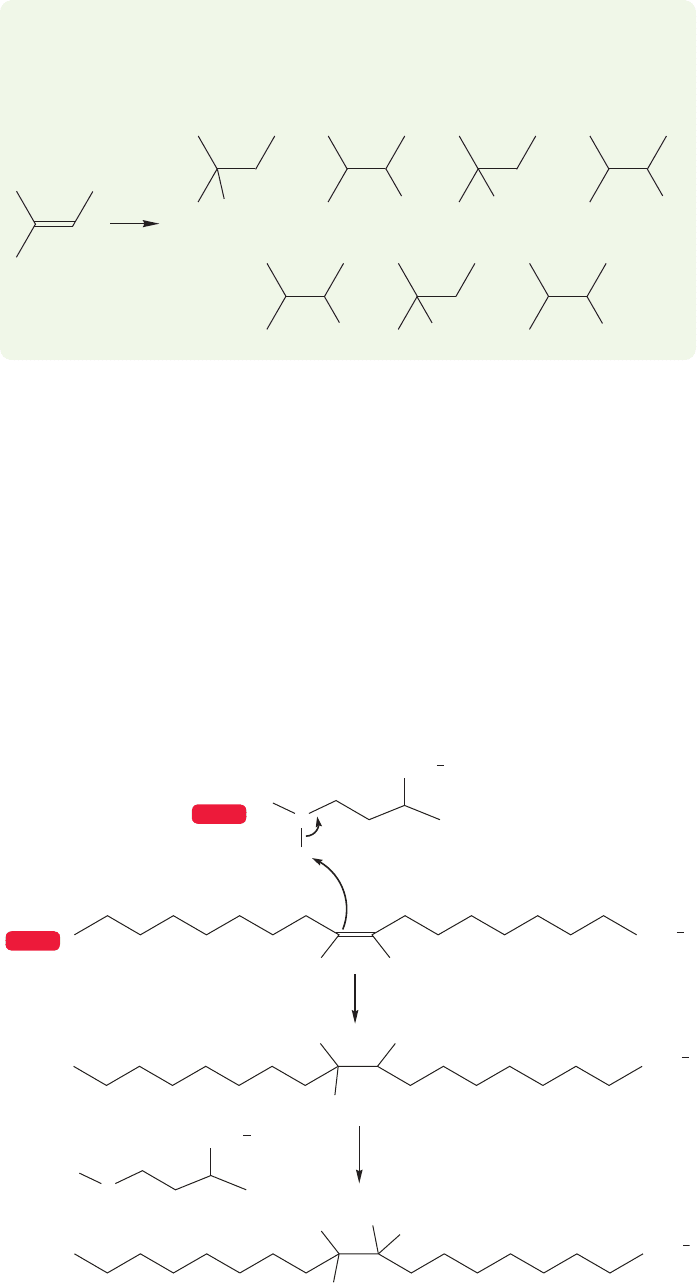
9.12 Special Topic: Rearrangements in Biological Processes 401
PROBLEM 9.22 Devise syntheses for the following molecules from 2-methyl-
2-butene. You may also use any inorganic reagent as well as organic compounds
containing no more than one carbon.
(a)
Cl
(b)
Cl
(c)
OH
(d)
OH
(e)
Br
(f)
Br
(g)
OCH
3
9.12 Special Topic: Rearrangements
in Biological Processes
The hydride shifts and alkyl migrations discussed in Section 9.9 are not arcane exam-
ples fit only for the nether regions of an organic text (and for especially vexing prob-
lems).They are going on all the time as part of many of the enzyme-driven reactions
in your body. So, as you read this, hydrides are migrating somewhere deep inside you.
Here is an example of a biological reaction in which an intramolecular hydride
shift must be occurring. Remember the section in Chapter 3 (p. 142) on methyl
transfer to an alkene? We are a bit further along now and can flesh out the
rather sketchy mechanistic description given earlier. In particular, Figure 9.70
shows the ultimate product of the reaction introduced in Figure 3.82. It looks as
HH
COO
R
S
H
3
C
COO
NH
3
H
3
C
H
H
S -Adenosylmethionine
COO
+
10-Methylstearate
COO
H
3
C
H
H
H
+
+
R
S
COO
NH
3
+
+
A
Oleate
WEB 3D
WEB 3D
FIGURE 9.70 The final product
of the methylation of oleate is
10-methylstearate.
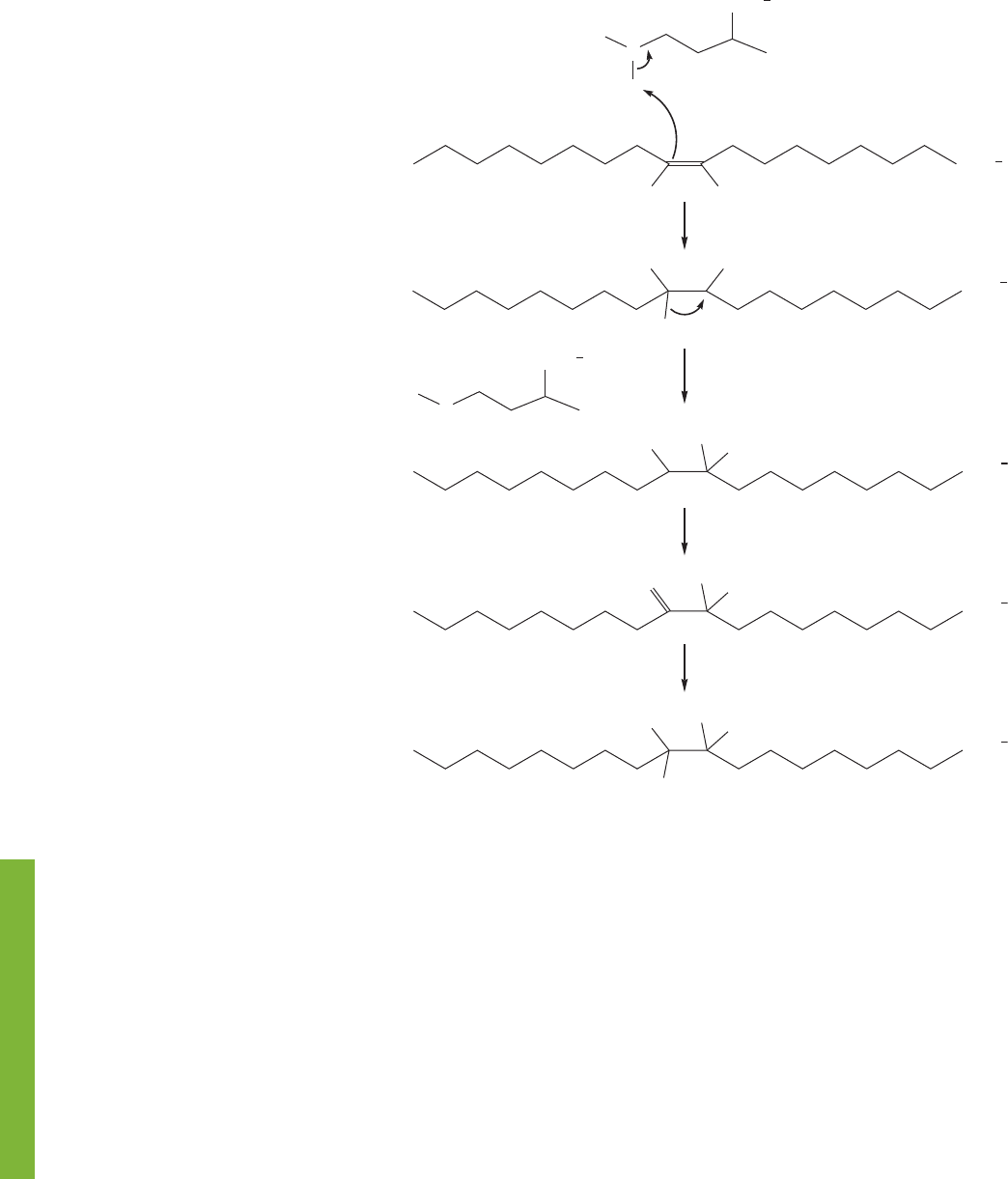
402 CHAPTER 9 Additions to Alkenes 1
9.13 Summary
New Concepts
DD
deuteride shift
elimination
enzymatic reduction
COO
R
S
H
3
C
COO
NH
3
H
3
C
D
D
S -Adenosylmethionine
COO
COO
+
+
H
3
C
D
D
COO
H
2
C
D
D
10-Methylstearate
COO
H
3
C
D
D
H
+
+
R
S
COO
NH
3
+
+
A'
B
FIGURE 9.71 This deuterium labeling
experiment shows that there is a
“hydride” shift at the center of this
reaction.
The central topic of this chapter is the addition of mol-
ecules to alkenes. These reactions begin by the addition of the
positive end of the dipole (the electrophile) to the alkene (the
nucleophile) in such a way as to form the more stable carbocation.
The anion (X
) then adds to the cation to form the final
addition product. The direction of addition—the regiochemistry
HX of the reaction—is determined by the relative stabilities of the
possible intermediate carbocations. The electrophile will always
add to give the more stable carbocation. Resonance and induc-
tive effects are important factors that influence stability.
Alkyl groups stabilize carbocations by a resonance effect in
which a filled σ orbital of the alkyl group overlaps with the
if intermediate A has somehow been reduced to give the final product.But a clever
labeling experiment (Fig. 9.71) shows that the process is far more complex and
that a hydride shift is involved. It should be no surprise to you that the secondary
carbocationic intermediate A′ undergoes an intramolecular hydride shift to give
the more stable tertiary carbocation B.A proton is then lost from the methyl group
(E1 reaction, p. 298) to give an alkene. It is this alkene that is reduced to give the
final product.

9.13 Summary 403
Key Terms
alkene hydrohalogenation (p. 365)
anti-Markovnikov addition (p. 399)
cationic polymerization (p. 386)
concerted reaction (p. 389)
hydration (p. 381)
hydride shift (p. 387)
hydroboration (p. 390)
hyperconjugation (p. 377)
inductive effects (p. 379)
Markovnikov’s rule (p. 375)
rearrangement (p. 387)
syn addition (p. 394)
Wagner–Meerwein rearrangement
(p. 387)
Reactions, Mechanisms, and Tools
Many acids ( , generally, ) will
add directly to a π bond. The first step is addition of a proton to
give the more stable carbocation. In the second step of the reac-
tion, the negative end of the original dipole adds to com-
plete the addition process. The regiochemistry of the addition is
determined by the formation of the more stable carbocation in
the original addition (Fig. 9.20).
Other molecules are not strong enough acids to proto-
nate alkenes. Water ( ) is an excellent example. However,
such molecules will add across the double bond if the reaction is
acid catalyzed. Enough acid catalyst is added to give the proto-
nated alkene, which is then attacked by water. The catalyst is
regenerated in the last step and recycles to carry the reaction
further (Fig. 9.34).
If the concentration of alkene is high enough, the nu-
cleophilic alkene can compete with other nucleophiles in the
HOH
HX
HX
HXHBr,
HCl, HI, HOSO
2
OH
system (for example, X
), and dimerization or even polymeriza-
tion of the alkene can take place. This reaction is not mysteri-
ous; the alkene is merely acting as a nucleophile toward the
electrophilic carbocation (Fig. 9.43).
In hydroboration, the boron atom of “BH
3
” adds to an alkene
to give an alkylborane. The mechanism involves a single step in
which the new carbon–hydrogen and carbon–boron bonds are
both made. Subsequent further hydroborations can give dialkyl-
and trialkylboranes. Treatment of these boranes with basic
hydrogen peroxide leads to alcohols (Figs. 9.66 and 9.67).
Hydroboration is especially important because it gives access to
the less substituted alcohol (anti-Markovnikov addition).
Additions that go through carbocationic intermediates can
be complicated by rearrangements of hydride (H
) or alkyl
groups (R
) to produce more stable carbocations from less
stable ones (Figs. 9.46 and 9.47).
:
:
empty 2p orbital on carbon. This effect is known by the curious
name of “hyperconjugation” (Figs. 9.24–9.26).
In this chapter, we continue to use stereochemical experi-
ments to determine reaction mechanisms. Generally, one-step
(concerted) reactions preserve the stereochemical relationships
of groups in the reacting molecules. Multistep processes intro-
duce the possibility of rotation around carbon–carbon bonds
and loss of the initial stereochemistry.
Remember: No experiment can prove a mechanism. We are
always at the mercy of the next experiment, the results of which
might contradict our current mechanistic ideas. Experiments can
certainly disprove a mechanism, but they can never prove one.
Syntheses
In this chapter, we see the synthesis of alkyl halides and alco-
hols from alkenes through addition reactions. Hydroboration
allows us to do addition reactions in the anti-Markovnikov
sense. The S
N
2 and S
N
1 reactions from earlier chapters allow us
to do further transformations of the alcohols and halides.The
new synthetic methods are summarized below.
1. Alcohols
H
3
O
+
H
2
O
2
HO
–
HO
H
OHBH
2
More highly substituted alcohol is formed
(Markovnikov addition)
Less highly substituted alcohol is formed
(anti-Markovnikov addition)
2. Alkylboranes
BH
3
BH
2
H
B
repeat
The number of hydroborations depends on the bulk of the
alkene; boron becomes attached to the less substituted
end of the alkene

404 CHAPTER 9 Additions to Alkenes 1
9.14 Additional Problems
Common Errors
Keeping clear the difference between resonance and equilibrium
is a constant difficulty for many students. Resonance forms are
simply different electronic descriptions for a single molecule.
The key word is “electronic.” In resonance, only electrons are
allowed to move to produce the various representations of the
molecule. Atoms may not change their positions. If they do, we
are not talking about resonance, but about equilibrium. Be care-
ful! This point is trickier than it sounds.
Remember also that the two-dimensional paper surface can
fool us. Molecules are three-dimensional.To have delocalization
of electrons, orbitals must overlap. Sometimes it looks in two
dimensions as though resonance forms exist when, in fact, they do
not in the real world of three dimensions.Two excellent examples
appear in Figure 9.18 (p. 373) and in Problem 9.4 (p. 373).
The grammar of chemistry—our use of arbitrary
conventions—is important because we need to be precise in
communicating with each other. The double-headed resonance
arrow is reserved for resonance and never used for anything else.
There are several schemes for representing resonance forms,
ranging from drawing them all out in full, to the summary
structures of Figure 9.14 (p. 370).
Both mechanistic analysis and synthesis are gaining in
complexity. There is much stereochemical detail to keep track of
in many mechanistic analyses, for example. Perhaps the most
complex mechanism considered so far is that for hydroboration.
If you understand why it was necessary to modify the standard
mechanism for the addition of Lewis acids to alkenes to
accommodate the experimental observations, you are in fine
shape so far.
There are no really complex synthetic procedures yet.
However, even very simple steps taken in sequence can lead to
difficulty. This area will rapidly proliferate and become more
difficult, so do not be lulled by the deceptive simplicity of the
reactions so far.
This chapter and Chapter 10 continue our cataloging of the
standard reactions of organic chemistry. To the S
N
1, S
N
2, E1,
and E2 reactions we now add a variety of alkene addition
reactions. Although there are several different mechanisms
for additions, many take place through a three-step sequence
of protonation, addition, and deprotonation. The following
new problems allow you to practice the basics of addition
reactions and to extend yourself to some more complex mat-
ters. Even simple additions become complicated when they
occur in intramolecular fashion, for example. These problems
also allow you to explore the influence of resonance and
inductive effects, and to use the regiochemistry and stereo-
chemistry of addition to help work out the probable mecha-
nisms of reactions.
Your sophistication in synthesis is also growing, and the
variety of addition reactions encountered in this chapter adds
to the ways you have available to make differently substituted
molecules. You are still not quite ready to undertake multistep
syntheses, but you are now very close. In anticipation of these
tougher problems, be sure to start working synthetic questions
backward. Always ask the question, What molecule is
the immediate precursor of the target? Don’t start, even with
simple problems, thinking of how an ultimate starting
material might be transformed into product. That approach
will work in one- or two-step synthesis, but becomes almost
impossible to do efficiently when we come to longer multistep
syntheses.
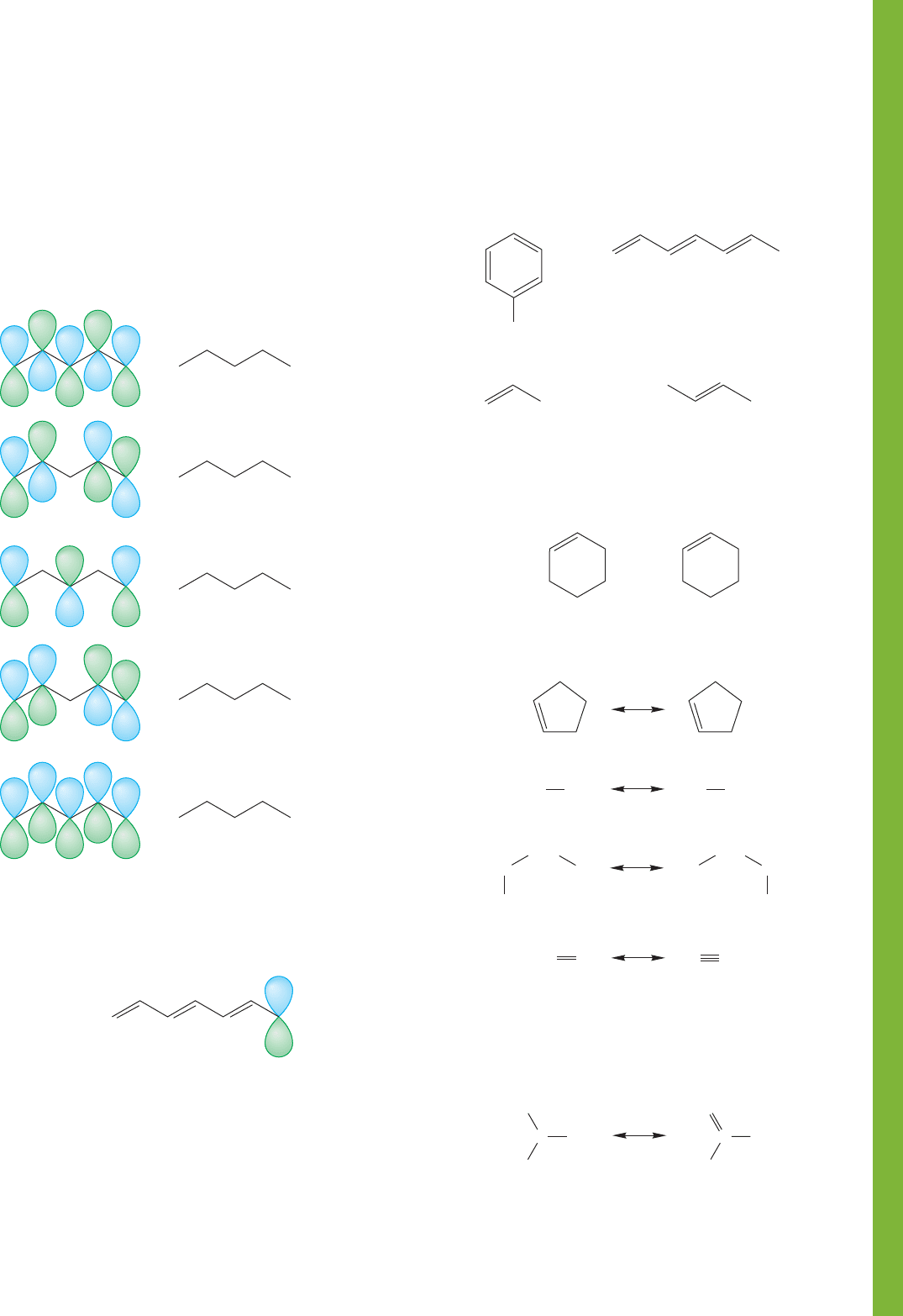
PROBLEM 9.23 See if you can write the π molecular
orbitals of allyl from memory. Show the electronic configura-
tion (orbital occupancy) for the allyl cation, radical, and
anion.
PROBLEM 9.24 The molecular orbitals for pentadienyl are
shown on the next page.
Pentadienyl
3. Alkyl Halides
HX
X
H
More highly substituted halide is formed
(Markovnikov addition)
(X = Br, Cl, or I)
4. Allyl Halides
HX
X
H
X
H
1,2- and 1,4-Addition compete
(1,2) (1,4)
9.14 Additional Problems 405
Compare these molecular orbitals with those of allyl. What
rules can you develop to predict the molecular orbitals of any
odd-carbon, fully conjugated (2p orbital on each carbon) chain?
It is probably easiest to work from the schematic top views at
the right of the figure.
PROBLEM 9.25 Apply your rules to the molecular orbitals of
heptatrienyl.
PROBLEM 9.26 Draw a third resonance form for the allyl
cation that involves a three-membered ring. Be careful not
to move any atoms! What is the relative importance of this
resonance form? Explain.
Heptatrienyl
––
+++
–
–
+
+
=
=
0
–
+
+
00
–
+
–
+
0
+
+
+
+
+
=
=
=
=
=
=
=
+ – 0 + –
= + – + – +
+ 0 – 0 +
+ + 0 – –
+ + + + +
Views from the top of pentadienyl;
beneath every (
+) is a (–) and
beneath every (
–) is a (+); nodes
are shown as (0)
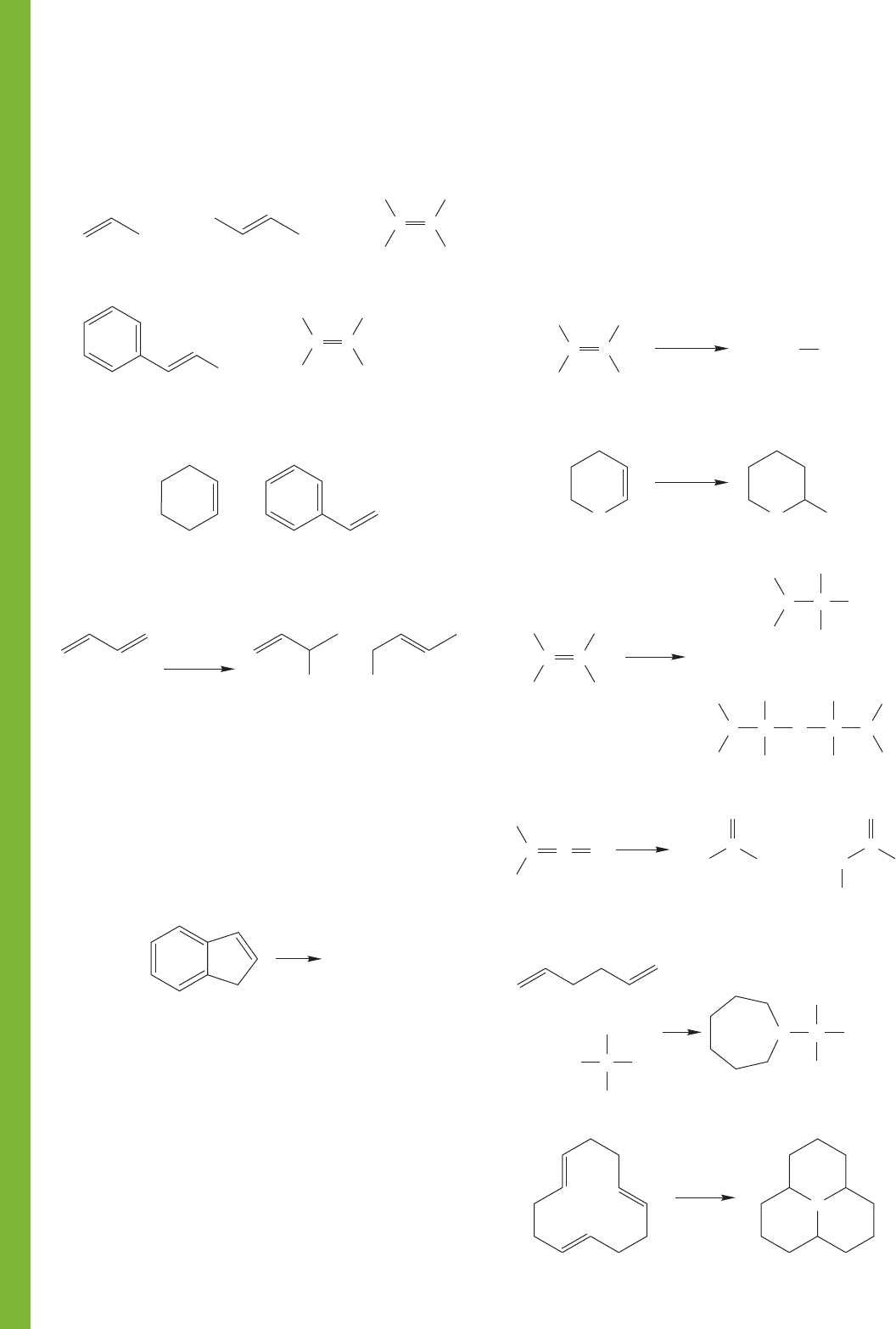
PROBLEM 9.27 Write resonance forms for the following
species. You will first have to write good Lewis structures, as the
lone pairs have been deliberately left out. You will also have to
write a full Lewis structure for the nitro (NO
2
) group.
PROBLEM 9.28 How many signals would appear in the
13
C
NMR spectra of the compounds in Problem 9.27 (a) and (b)?
PROBLEM 9.29 How many signals would appear in the
13
C
NMR spectra of the following compounds?
PROBLEM 9.30 Which of the following pairs represent
resonance forms and which do not? Explain your choices.
PROBLEM 9.31 Analyze the relative importance of the follow-
ing two resonance forms. What would you say to someone who
claimed that form B must be unimportant because the positive
charge is on the more electronegative atom? You will have to
add electron dots to make good Lewis structures first.
C
+
AB
H
H
CH
3
O
CH
3
O
+
CH
3
C
CH
3
+
OC
(a)
(b)
(c)
(d)
C
..
..
..
..
CH
2
..
–
CH
2
..
..
+
H
2
C
O
I
–
–
––
+
+
+
CH
2
H
2
C
CH
2
CH
2
I
H
2
C
CH
2
..
H
2
C
..
(a) (b)
+
+
(a) (b)
+
CH
2
CH
2
O
2
N
–
(d)(c)
N(CH
3
)
2
N(CH
3
)
2
406 CHAPTER 9 Additions to Alkenes 1
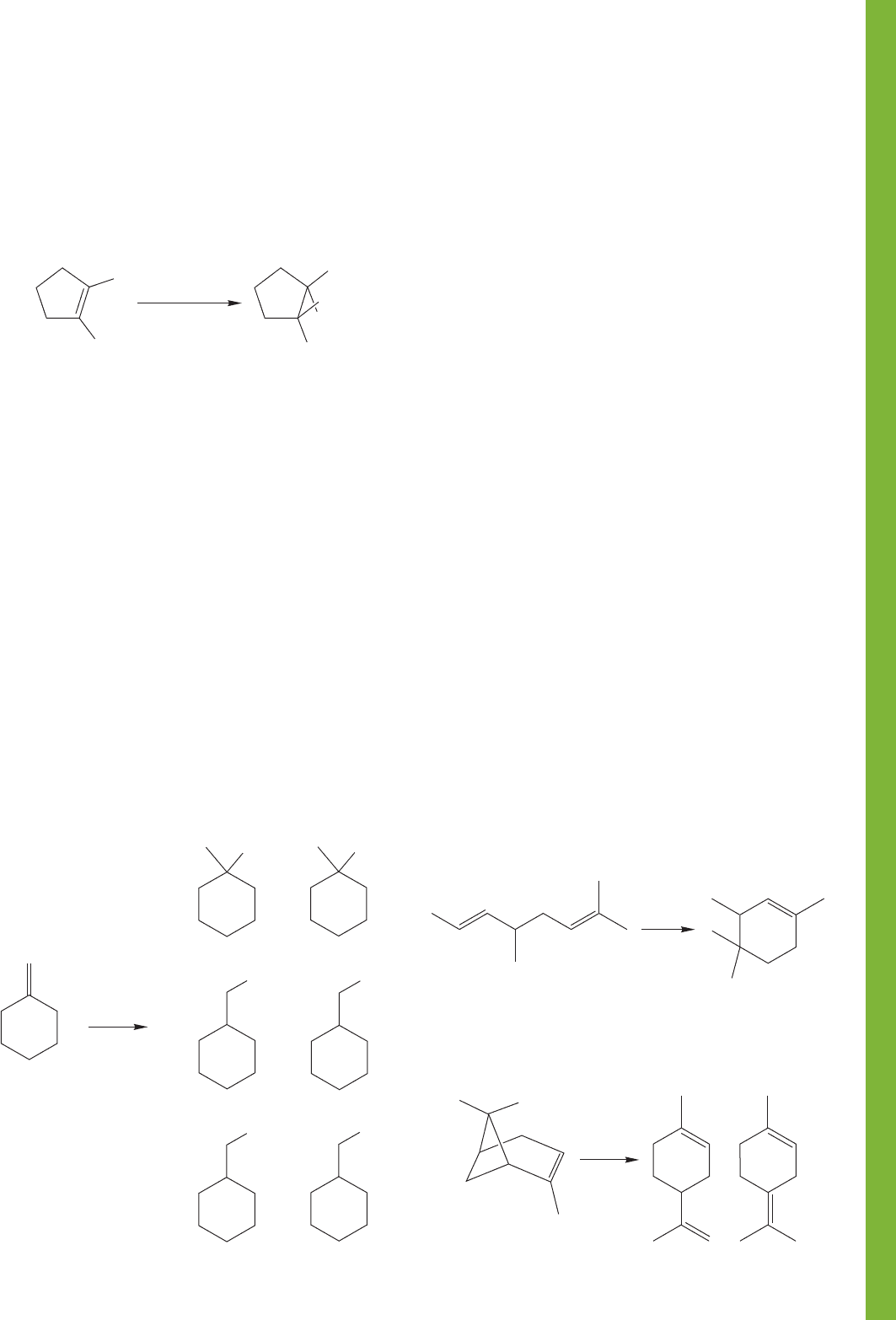
PROBLEM 9.32 Predict the regiochemistry of the following addi-
tion reactions of a generic acid, . Explain your predictions.
PROBLEM 9.33 What would be the structure of the polymer
produced from each of the following two monomers through
cationic polymerization?
PROBLEM 9.34 At 78 °C, hydrogen bromide adds to
1,3-butadiene to give the two products shown. Carefully write
a mechanism for this reaction, and rationalize the formation
of two products.
PROBLEM 9.35 Reaction of cis-2-butene with hydrogen
bromide gives racemic 2-bromobutane.
(a) Show carefully the origin of the two enantiomers of
2-bromobutane.
(b) In addition, small amounts of two alkenes isomeric with
cis-2-butene can be isolated. What are these two alkenes
and how are they formed?
PROBLEM 9.36 Indene can be hydrated to give a different
product (A or B) under the following two conditions.
Reagents for the first route: H
3
O
/H
2
O
Reagents for the second route: (1) BH
3
, (2) HOOH/HO
One of these two routes leads to a compound, A (C
9
H
10
O), which
has a
13
C NMR spectrum consisting of nine signals. The other
route leads to a different compound, B, which also has the formula
C
9
H
10
O, and has a
13
C NMR spectrum consisting of only five
signals. What are the structures of the two compounds A and B,
and which route leads to which compound? Explain your reason-
ing in mechanistic terms for each compound and make very clear
how you used the
13
C data in the structure determination. (You do
not have to sketch a mechanism for the oxidation step of route 2.)
C
9
H
10
O
Indene
–78 ⬚C
+
+
HBr
(81%)
Br
(18%)
Br
CC
CH
3
O
CH
3
O
CH
3
CH
3
Br
(b)
(a) (c)
CC
F
3
C
F
3
C
CH
3
CH
3
(e)(d)
CH
3
CH
3
CH
3
HX
In the following four problems, reactions appear that are quite
similar to those discussed in this chapter, but are not exactly the
same. Each raises one or more slightly different mechanistic points.
In this book, we aim to develop an ability to understand new obser-
vations in the light of old knowledge. In a sense, you are being put
in the place of the research worker presented with new experimen-
tal results. In each case, explain the products mechanistically.
PROBLEM 9.37
PROBLEM 9.38
PROBLEM 9.39
PROBLEM 9.40
(CH
3
)
2
CH
CH
3
CH
3
BH
2
(a)
C
CH
3
CH
3
CH(CH
3
)
2
C
+
B
CCO
H
H
C
O
OH
OH
not
H
3
O
+
H
2
O
H
3
C
C
O
H
H
2
C
HC
CH
3
CH
3
SH
C
CC
H
3
C
H
3
C
H
3
C
H
3
C
HC
CH
CH
3
CH
3
S
C
CH
3
CH
3
C
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
3
+
H
3
O
+
H
2
S
CC
H
H
3
O
+
H
CH
3
OH
OCH
3
(CH
3
)
3
C
CH
3
CH
3
(a)
(b)
O
H
3
O
+
CH
3
OH
OCH
3
O
(b)
BH
3
B
9.14 Additional Problems 407
PROBLEM 9.41
Given the following experimental observation
and the knowledge that the initial hydroboration step involves a
syn addition, what can you say about the overall stereochemistry
of the migration and oxidation steps of the organoborane (see
Fig. 9.67, p. 399). Is there overall retention or inversion of con-
figuration?
PROBLEM 9.42 Show the reactions you would use to synthe-
size 1-butanethiol from 1-butene.
PROBLEM 9.43 Show how you would make 1-azidobutane if
your only source of carbon is 1-butene.
PROBLEM 9.44 Show how you would make 1-isopropoxyhexane
starting with 1-hexene and using any other reagents you need.
PROBLEM 9.45 See the syntheses of 2-chloro-3-methylbutane
and 2-bromo-3-methylbutane suggested in the answer to
Problem 9.22 (b) and (e), given in the Study Guide. Carefully
consider the last step in the proposed syntheses, the reaction of
3-methyl-2-butanol with hydrogen bromide or hydrogen
chloride. Do you see any potential problems with this step?
What else might happen?
PROBLEM 9.46 Propose syntheses of the following molecules
starting from methylenecyclohexane, alcohols, iodides contain-
ing no more than four carbon atoms, and any inorganic materi-
als (no carbons) you want.
OH
Br
OH
Methylene-
cyclohexane
(a)
(b)
(c)
(d)
(e)
(f)
N
3
Br
OCH
3
?
H
CH
3
OH
H
1. BH
3
2. H
2
O
2
/HO
–
CH
3
H
PROBLEM 9.47 Predict the major product(s) for the hydro-
boration/oxidation reaction (1. BH
3
/THF; 2. H
2
O
2
/NaOH)
with each of the following alkenes:
(a) 1-pentene (b) 2-methyl-2-pentene
(c) cyclopentene (d) 3-hexene
PROBLEM 9.48 Which of the products in the previous
problem are chiral and which are achiral?
PROBLEM 9.49 Predict the possible products in the reaction
between HBr with the following alkenes:
(a) cyclopentene (b) trans-2-butene
(c) cis-2-butene (d) 3-methylcyclohexene
(e) 2-hexene
PROBLEM 9.50 Predict the major product(s) for the reaction
between (E)-2-pentene and the following reagents:
(a) H
3
O
/H
2
O (b) 1. BH
3
/THF; 2. H
2
O
2
/NaOH
(c) HCl (d) HBr
(e) H
3
O
/CH
3
CH
2
OH
PROBLEM 9.51 Predict the major product(s) for the
reaction between (E)-3-methyl-2-pentene and the following
reagents:
(a) H
3
O
/H
2
O (b) 1. BH
3
/THF; 2. H
2
O
2
/NaOH
(c) HCl (d) HBr
(e) H
3
O
/CH
3
CH
2
OH
In each of the following reactions, more than a simple addition
takes place. Keep your wits about you, think in simple stages,
and work out mechanisms.
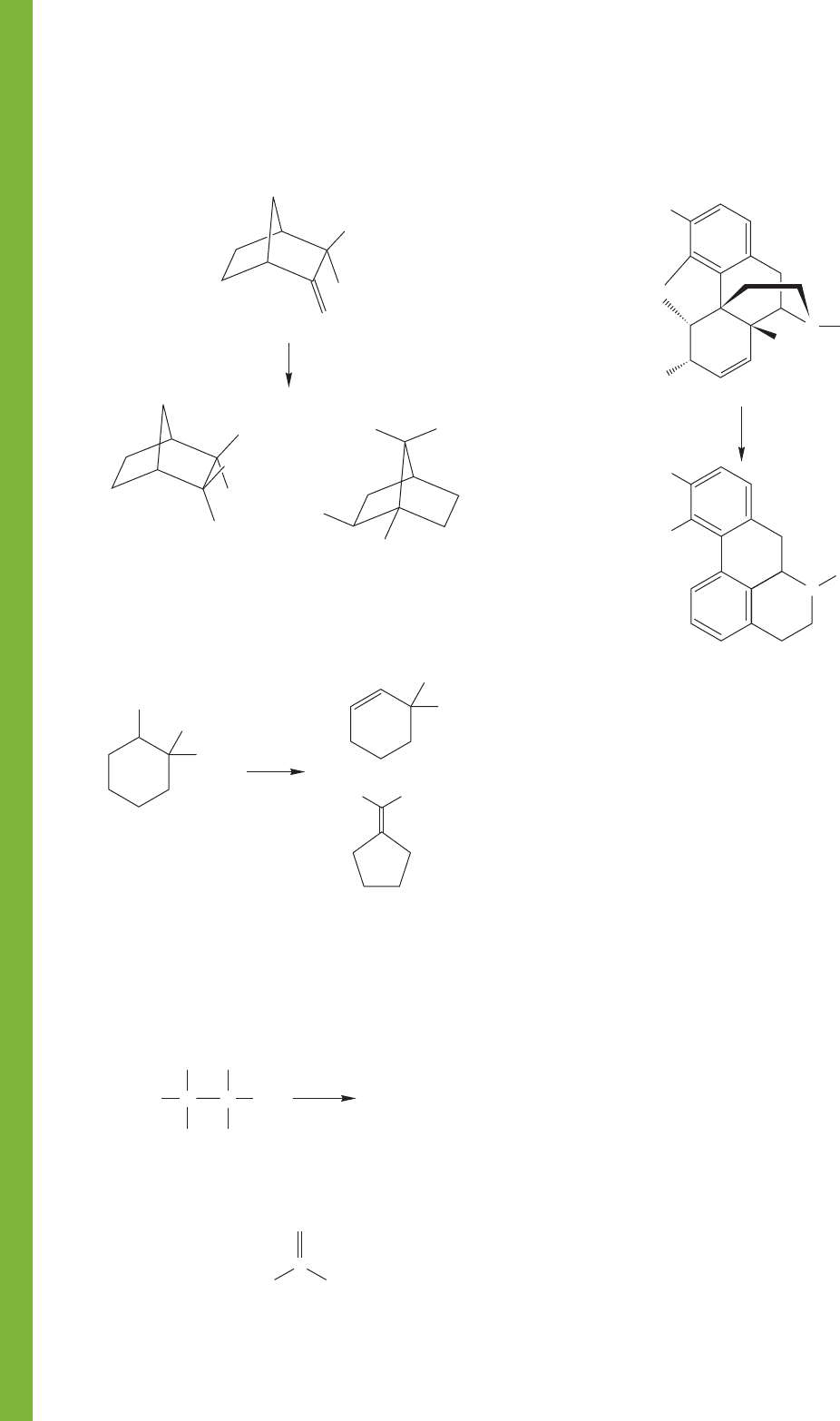
PROBLEM 9.52 Provide a mechanism for the following
change:
PROBLEM 9.53 Provide a mechanism for the following
change:
+
H
3
O
+
H
2
O
H
3
O
+
H
2
O
408 CHAPTER 9 Additions to Alkenes 1
PROBLEM 9.54 Provide a mechanism for the following
change: Hint for the hard part and for the next few problems: Think
Wagner–Meerwein, and use the CH
3
groups as markers (p. 387).
PROBLEM 9.55 Provide mechanisms for the following trans-
formations. Once again, use the CH
3
groups to track where the
atoms go.
PROBLEM 9.56 What two products do you anticipate for the
reaction shown below? Apply what you already know about the
chemistry of alcohols in acid.There are two reasonable products,
each with the formula C
6
H
12
O, but with very different structures.
In fact, these compounds are formed in only minor amounts. The
major product is pinacolone, shown in Problem 9.57.
PROBLEM 9.57 What is the mechanism of pinacolone
formation in the reaction in Problem 9.56?
Pinacolone
O
CH
3
(CH
3
)
3
C
C
CH
3
CCC
6
H
12
O
OH
CH
3
OH
Pinacolone
CH
3
H
3
C
H
3
O
H
2
O
+
+
H
3
O
+
H
2
O
CH
3
H
3
C
OH
CH
3
CH
3
CH
3
CH
3
+
H
3
C
Easy
HCl
Hard!!
CH
2
CH
3
CH
3
CH
3
CH
3
Cl
CH
3
CH
3
H
3
C
Cl
PROBLEM 9.58 Provide a mechanism for the following
transformation of morphine into apomorphine. Caution: This
problem is very hard.
Use Organic Reaction Animations (ORA) to answer the fol-
lowing questions:
PROBLEM 9.59 Select the animation titled “Alkene hydro-
halogenation” and observe the intermediate in the reaction.
What is the hybridization of the central carbon? Select the
LUMO representation of the intermediate. What do we learn
from this calculated information? What options are available
for the nucleophile in the second step of this reaction?
PROBLEM 9.60 Observe the “Alkene polymerization”
animation. What experimental conditions are necessary for
polymerization to be the major pathway? The animation shows
formation of the dimer and the trimer. How many alkene
molecules need to add before we can call the product a polymer?
PROBLEM 9.61 The “Alkene polymerization” animation does
not show a final product. Do you suppose an alkene polymer
would have a charge? Milk jugs are made from alkene poly-
mers. What do you know about the properties of a milk jug
that help you answer the question? What are possible final steps
in the formation of the polymer?
PROBLEM 9.62 The “Alkene hydroboration” reaction was a
challenge to calculate. Observe the first part of the hydrobora-
tion animation, the reaction of the alkene with the borane. Why
is it difficult to calculate this part of the reaction? Observe the
second part of the animation, the oxidation of the boron. Notice
that there is a 1,2-alkyl shift. What molecular orbital relation-
ship do you think is the key for that shift?
HO
HO
Morphine
O
H
N
CH
3
HO
HO
CH
3
Apomorphine
N
H
2
O/H
3
O
150 C
+
