Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


Additions to Alkenes 2
and Additions to Alkynes
409
10.1 Preview
10.2 Addition of H
2
and X
2
Reagents
10.3 Hydration through Mercury
Compounds: Oxymercuration
10.4 Other Addition Reactions
Involving Three-Membered
Rings: Oxiranes and
Cyclopropanes
10.5 Dipolar Addition Reactions:
Ozonolysis and the Synthesis
of Carbonyl ( )
Compounds
10.6 Addition Reactions of Alkynes:
Addition
10.7 Addition of X
2
Reagents to
Alkynes
10.8 Hydration of Alkynes
10.9 Hydroboration of Alkynes
10.10 Hydrogenation of Alkynes:
Alkene Synthesis through syn
Hydrogenation
10.11 Reduction by Sodium in
Ammonia: Alkene Synthesis
through anti Hydrogenation
10.12 Special Topic: Three-
Membered Rings in
Biochemistry
10.13 Summary
10.14 Additional Problems
HX
R
2
C
P
O
10
NATURAL DYES The red dragonfly may be one of several insects that produce
the red cochineal dye, a tricyclic compound with many multiple bonds.

410 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
1
Neal Stephenson (b. 1959) is the author of the brilliant Snow Crash, as well as The Diamond Age, Zodiac: The
Eco-Thriller, and, of course, Cryptonomicon.
A red dragonfly hovers above a backwater of the stream, its wings
moving so fast that the eye sees not wings in movement but a probability
distribution of where the wings might be, like electron orbitals: a quantum-
mechanical effect that maybe explains why the insect can apparently
teleport from one place to another, disappearing from one point and
reappearing a couple of meters away, without seeming to pass through the
space in between. There sure is a lot of bright stuff in the jungle. Randy
figures that, in the natural world, anything that is colored so brightly must
be some kind of serious evolutionary badass.
—NEAL STEPHENSON,
1
CRYPTONOMICON
10.1 Preview
We continue the discussion of additions to π systems in this chapter. Most reactions
we see here follow the pattern seen in Chapter 9, in which a Lewis acid (usually a
cation) is formed, and subsequently captured by a nucleophile,often an anion. Other
reactions follow a different path. We begin with one of these, hydrogenation, the
addition of H
2
. We then move on to reactions involving X
2
reagents, which often
proceed through the formation of an intermediate nonisolable, three-membered
ring. Next, we take up additions in which the three-membered ring is stable, and
finish with reactions forming different-sized rings. We will also discuss the related
additions to alkynes. Throughout this chapter, attention is paid to what all these
reactions allow us to do in the way of synthesis, which is rather a lot.
ESSENTIAL SKILLS AND DETAILS
1. Addition reactions have stereochemical consequences. In Chapters 3 and 9, where we first
encountered additions, we focused mainly on regiochemistry, the direction of addition.
Here,when we describe additions of X
2
and XY reagents, the stereochemistry, syn or anti, of
the reaction can also be followed easily, and it is essential that you keep this factor in mind.
2. Addition reactions can take place through either open or cyclic intermediates, and you
will learn to assess the factors that control which way the reaction goes. If an open
cation is well stabilized, usually by resonance, it is likely to be favored. If not, the cyclic
intermediate generally prevails.
3. Open cations permit addition of a nucleophile to each lobe of the empty orbital. Cyclic
ions require opening in S
N
2 fashion, leading to overall trans addition.
4. Epoxides open differently in acid and base. In base, the nucleophile adds to the less
hindered position, whereas in acid the nucleophile becomes attached to the more
substituted, more hindered side.
10.2 Addition of H
2
and X
2
Reagents
In this section, we see two very different reactions of diatomic reagents, the addition
of H
2
and of X
2
. In the first reaction, the concerted addition of hydrogen to the
double bond of an alkene, a catalyst is required to get things going. The second
reaction, halogen addition, fits better into the pattern seen in Chapter 9, in which
a two-step process is followed.
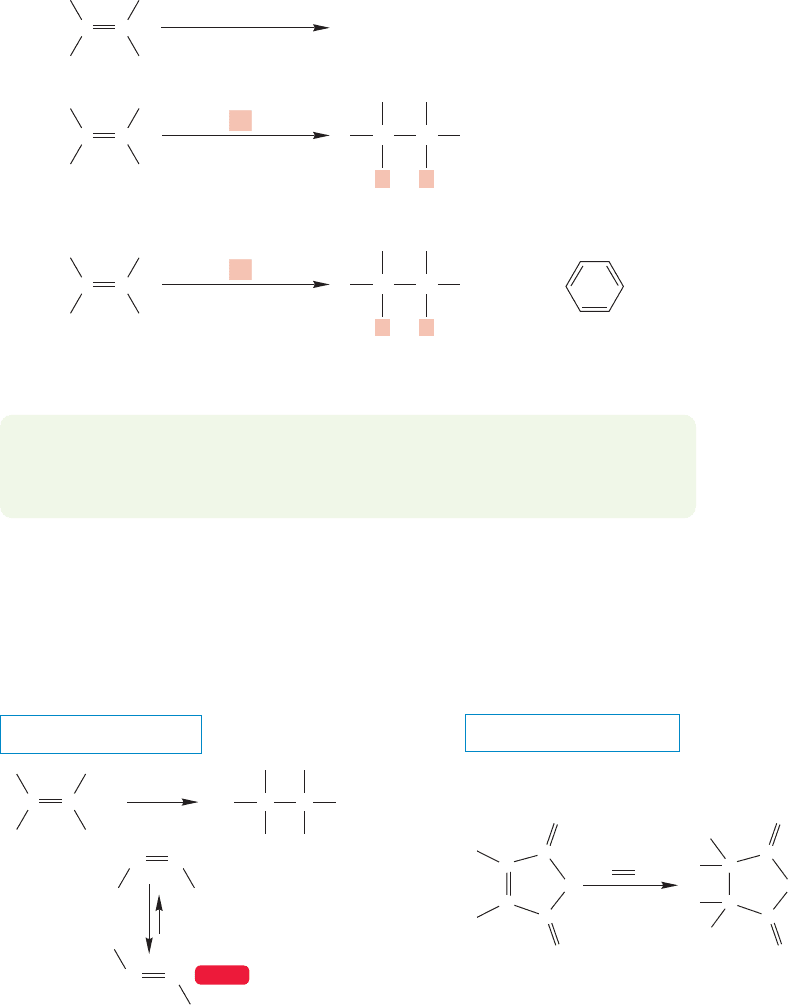
10.2 Addition of H
2
and X
2
Reagents 411
H
2
No reaction
H
2
H
2
Insoluble—
heterogeneous catalysis
H
C
H
Pd/C
Ph =
C
RhCl(PPh
3
)
3
Wilkinson’s catalyst
Soluable—
homogeneous catalysis
H
C
H
C
C
C
C
C
C
C
FIGURE 10.1 Hydrogen gas and an
alkene react very quickly in the
presence of a metal catalyst such as
palladium adsorbed on charcoal
(Pd/C).The rhodium-based
Wilkinson’s catalyst is typical of
soluble molecules that also catalyze
hydrogenation.
10.2a Hydrogenation When an alkene is mixed with hydrogen gas, nothing
happens (unless one is careless with oxygen and a match). If the two reagents are
mixed in the presence of any of a large number of metallic catalysts, however, an
efficient addition of H
2
converts the alkene into an alkane. This reaction is called
hydrogenation, and it can be achieved by either of two catalytic processes. First,
there is heterogeneous catalysis, in which an insoluble catalyst, often palladium
(Pd) on charcoal, is used. Many other insoluble metallic catalysts work as well in
heterogeneous catalysis. A second process is homogeneous catalysis, in which a
soluble catalyst, often the rhodium-based Wilkinson’s catalyst, after Geoffrey
Wilkinson (1921–1996), is employed (Fig. 10.1).
PROBLEM 10.1 Use bond dissociation energies (BDE) (Table 8.2, p. 337) to
calculate the exothermicity or endothermicity of the hydrogenation of ethylene
to ethane.
Nonmetallic reducing agents can be used in alkene hydrogenation as well.
Diimide ( ) is a good example. It is not stable under normal conditions
and is made only as it is needed. Diimide exists in two forms, cis and trans, but only
the less stable cis form is active in hydrogenation (Fig. 10.2).
HN
P
NH
N
2
C
HH
C
(95%)
+
HN NH
O
O
O
C
C
C
C
H
H
O
O
O
C
C
CC
WEB 3D
..
..
NN
trans Form
cis Form
....
NN
HH
H
H
Diimide
A SPECIFIC EXAMPLE
THE GENERAL CASE
H
H C
H
H
C
FIGURE 10.2 An effective nonmetallic
reducing agent for alkenes is diimide
(HNNH).
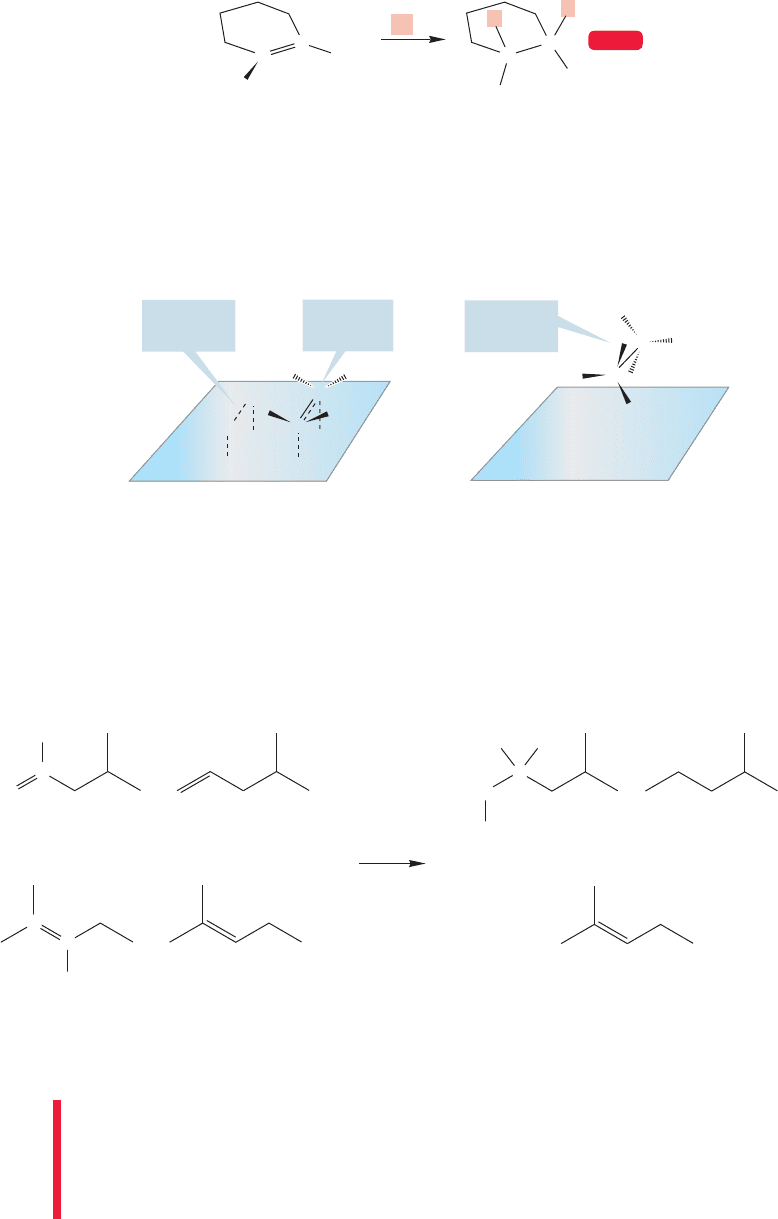
412 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
CH
3
H
3
C
H
3
C
CH
3
94.5% cis
PtO
2
C
C
D
2
D
D
C
C
WEB 3D
FIGURE 10.3 Metal-catalyzed
addition to a cycloalkene shows that
deuterium is added in syn fashion.
Detached
alkane
Metal surface
Adsorbed
hydrogen
Adsorbed
alkene
H
H
H
H
C
C
C
C
Metal surface
FIGURE 10.4 Partial bonding to the
metal surface is followed by hydrogen
transfer to the alkene.
=
H
H
2
C
H
2
Ru/C
=
HH
H
H
2
C
Less substituted (less sterically hindered,
faster to react)
More substituted (more hindered, slower to react)
C
=
H
+
+
C
C
C
FIGURE 10.5 It is possible to hydrogenate less-substituted double bonds in the presence of more-hindered,
more-substituted double bonds.The catalyst in this example is a ruthenium–carbon complex.
There are many kinds of selectivity in the hydrogenation reaction. Not all
carbon–carbon double bonds are hydrogenated at the same rate, and the differences
in rate often allow hydrogenation of one double bond in the presence of another.
Generally, the more sterically hindered a double bond, the more slowly it is hydro-
genated. This rate change is probably related to ease of adsorption on the catalyst
surface. Large groups hinder adsorption and therefore slow the rate of hydrogena-
tion. One can often hydrogenate a less substituted double bond in the presence of
more substituted double bonds, as shown in Figure 10.5.
We have to pay attention to the drawing conventions (codes) here. We do not
always draw out all of the carbon and hydrogen atoms—we are beginning to adopt
the convention most used by chemists—the “stick”drawings that show only the car-
bon framework of the molecule. Figure 10.5 shows two levels of code, but we will
often lapse into the most schematic representations of molecules.
If hydrogenation is carried out with a cycloalkene,transfer of hydrogen takes place
in predominately syn fashion. It is a syn addition because both new hydrogens are
delivered to the same side of the alkene. In Figure 10.3, D
2
is used so we can see
the stereochemical outcome of the reaction.
The mechanistic details of hydrogenation remain vague. Presumably, the metal
surface binds both hydrogen and alkene, weakening the π bond of the alkene and
the σ bond of hydrogen. The alkane is formed by addition of a weakened hydrogen
molecule to the coordinated alkene. As the transfer is completed, the product
alkane detaches from the metal surface (Fig. 10.4).
CONVENTION ALERT
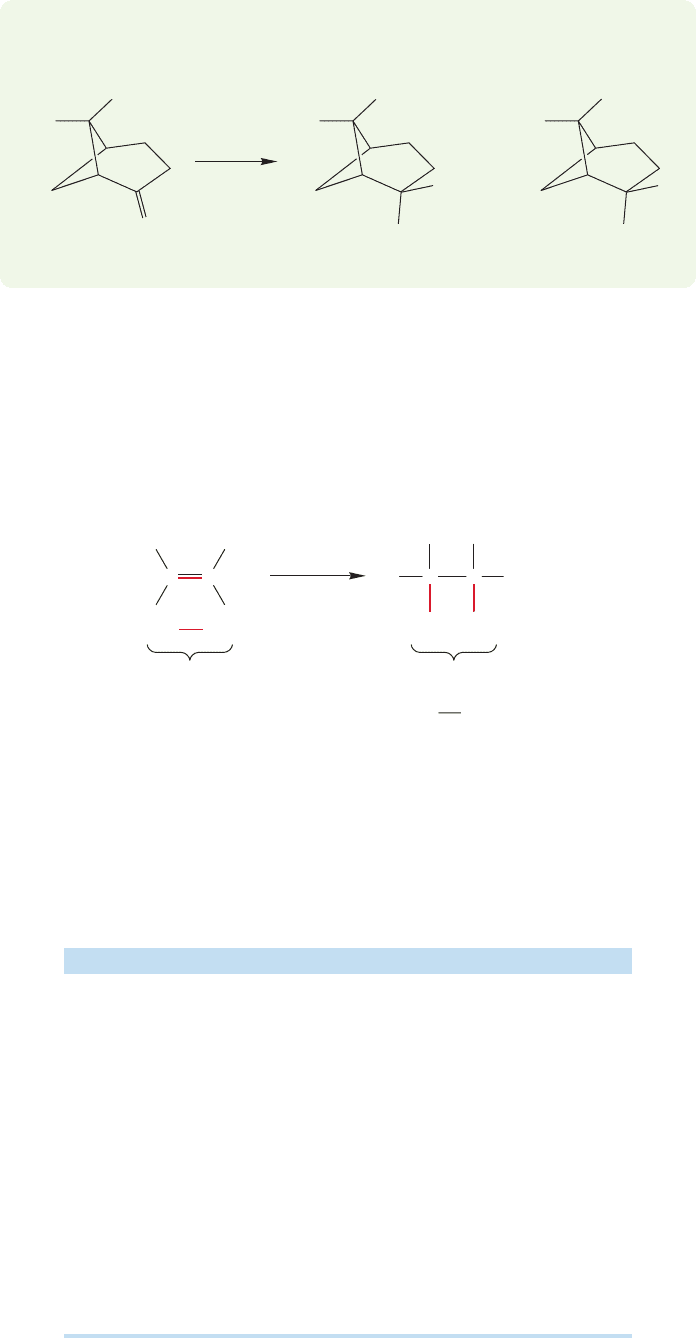
10.2 Addition of H
2
and X
2
Reagents 413
H
2
(Not observed)
catalyst
H
3
C
CH
3
H
CH
3
H
3
C
CH
3
H
3
C
CH
3
CH
3
H
CH
2
Now let’s consider the energetics of hydrogenation. Is it likely to be exothermic
or endothermic? As shown in Problem 10.1, a simple and common technique for
making such estimates uses bond energies.In the hydrogenation reaction,two bonds
are breaking: the hydrogen–hydrogen σ bond and the π bond of the alkene. Two
bonds are also being made in this reaction: the two new σ bonds in the alkane prod-
uct.If we know bond energies (Table 8.2,p.337), we can estimate ΔH ° for this reac-
tion.We made this estimate in Problem 10.1, and Figure 10.6 repeats the calculation.
The process is exothermic by about 30 kcal/mol.
Two C
H σ bonds =
~200 kcal/mol
π bond = 66 kcal/mol
σ bond = 104 kcal/mol
Bonds broken Bonds made
200 – 170 = 30 kcal/mol exothermic
H = –30 kcal/mol
H
C
H
C
HH
C
C
FIGURE 10.6 In a typical
hydrogenation, the π bond of the
alkene and the σ bond of hydrogen
are broken.Two new carbon–
hydrogen bonds are made.The
hydrogenation of an alkene is
exothermic by approximately
30 kcal/mol.
Table 10.1 collects some measured heats of hydrogenation. We can see that the
crude bond energy calculation of Figure 10.6 has actually done quite well; the heats
of hydrogenation of simple alkenes are quite close to our calculation of 30 kcal/mol.
PROBLEM 10.2 Hydrogenation of β-pinene gives only one of the possible
diastereomeric products, as shown below. Explain why.
TABLE 10.1 Some Heats of Hydrogenation
Alkene (kcal/mol)
32.7
30.0
30.3
28.6
27.6
28.4
26.9
29.6
27.3
26.4
28.0
26.6(CH
3
)
2
C
P
C(CH
3
)
2
CH
3
H
2
C
P
C
ƒ
O
CH
2
CH
2
CH
3
trans CH
3
CH
2
O
CH
P
CH
O
CH
2
CH
3
cis CH
3
CH
2
O
CH
P
CH
O
CH
2
CH
3
H
2
C
P
CH
O
CH
2
CH
2
CH
2
CH
3
(CH
3
)
2
C
P
CHCH
3
(CH
3
)
2
C
P
CH
2
trans CH
3
O
CH
P
CH
O
CH
3
cis CH
3
O
CH
P
CH
O
CH
3
H
2
C
P
CH
O
CH
2
CH
3
H
2
C
P
CH
O
CH
3
H
2
C
P
CH
2
≤H
°
H
2
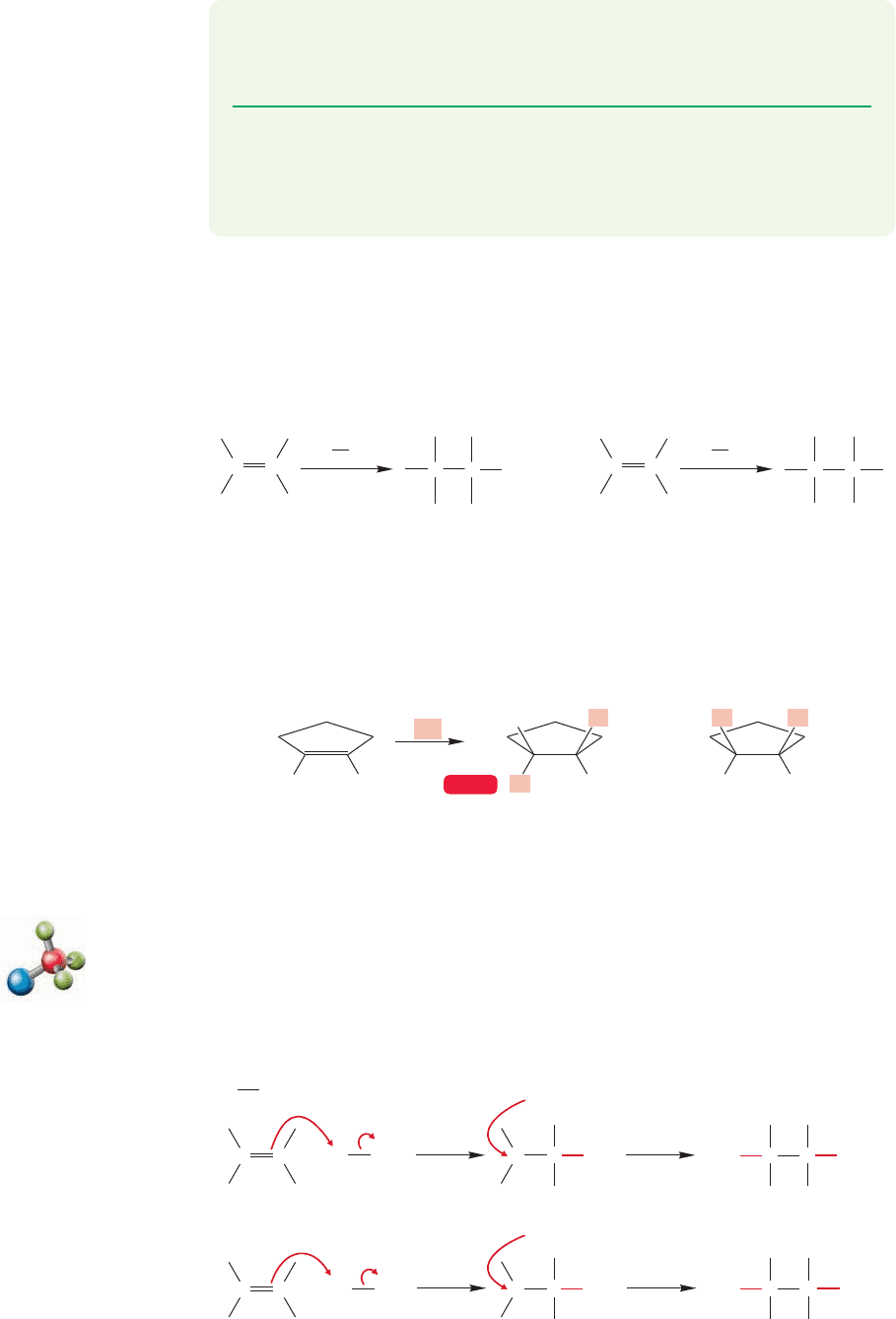
414 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
10.2b Additions of Halogens Alkene halogenation is the reaction between
X
2
and an alkene and it results in addition of a halogen atom to each of the carbons
of the double bond. Molecular bromine and chlorine (Br
2
and Cl
2
) do not need a
catalyst to add to alkenes to give these vicinal dihalides (Fig. 10.7). Groups are
described as vicinal when they are on adjacent atoms.
PROBLEM 10.3 Use the values in Table 10.1 to estimate the relative energies of the
hexenes. Compare your estimates with those generated from heats of formation
in Chapter 3 (p. 115).
PROBLEM 10.4 A mixture of an alkene and hydrogen gas has an energy that is
some 30 kcal/mol above the alkane product.Why doesn’t the reaction occur spon-
taneously? Use an Energy versus Reaction progress diagram to help explain in
general the role of the metal catalyst (Hint: See p. 381).
C
C
C
Br
..
Br
..
..
..
..
..
Cl
..
..
..
Cl
..
..
..
..
..
..
..
..
..
C C
Vicinal (1,2-)
dihalides
Vicinal (1,2-)
dihalides
C
C
C
Br Br
..
..
..
..
..
..
Cl Cl
FIGURE 10.7 Addition of Br
2
and Cl
2
to yield vicinal dihalides.
trans -1,2-Dibromo-
cyclopentane
cis -1,2-Dibromo-
cyclopentane
(
Observed
)(
Not observed
)
HH
H
H
Br
Br
2
HH
Br Br Br
WEB 3D
FIGURE 10.8 Anti addition of Br
2
to cyclopentene to give the trans
product.
Halogen addition is stereospecifically anti; the groups add to opposite sides of
the alkene. For example, addition to cyclopentene gives only trans-1,2-dibromo-
cyclopentane (Fig. 10.8).
–
Br
..
..
..
..
CHH
Br
..
..
Br
..
..
Br
..
..
..
Br
..
..
..
..
..
Br
..
..
..
H
Br Addition
Possibly analogous
Br
2
addition (?)
Br
..
..
..
Br
..
..
..
C C
C
H
C
C
C
C
+
+
–
Br
..
..
..
..
+
C
C
+
C
C
FIGURE 10.9 A first-guess
mechanism for the reaction of an
alkene and bromine. For analogy, it
draws on the mechanism of hydrogen
bromide addition to an alkene.
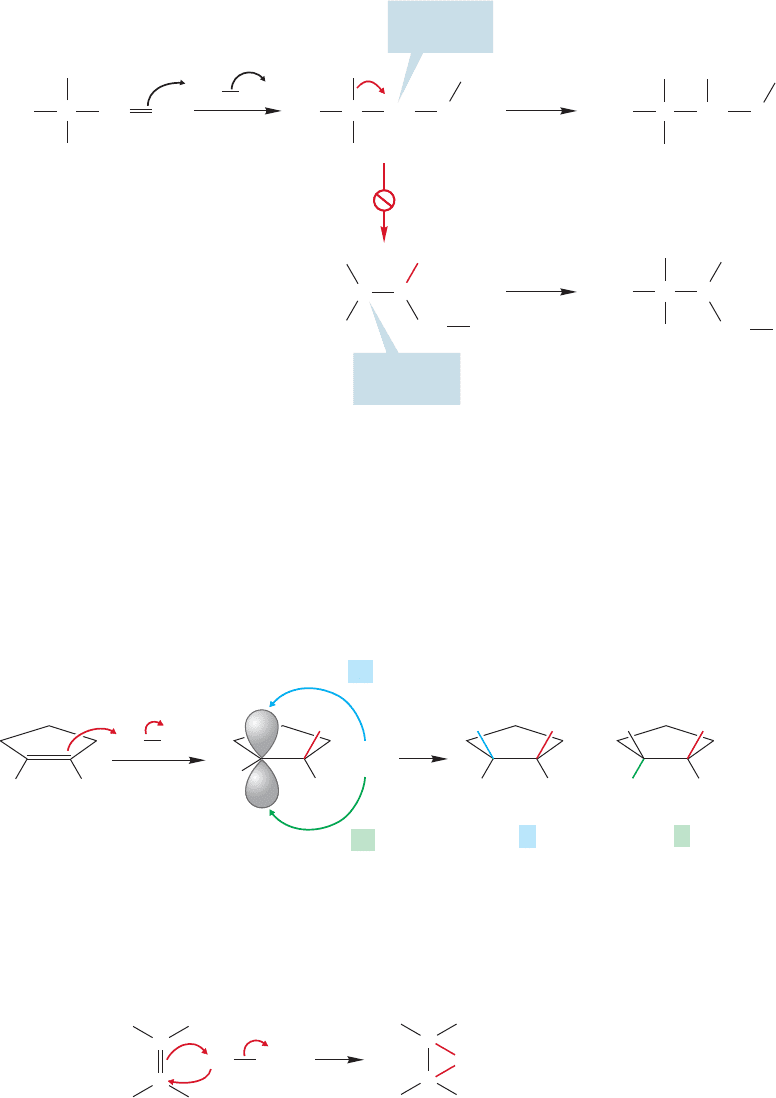
If we take the addition of hydrogen bromide to an alkene as a model for the
alkene halogenation reaction, we can build a mechanism quite quickly. Just as the
alkene reacts with hydrogen bromide to produce a carbocation and bromide (Br
),
so reaction with bromine–bromine might give a brominated cation and bromide
(Fig.10.9).The π bond of the alkene acts as a nucleophile,displacing Br
from bromine.
Addition of bromide to the carbocation would give the dibromide product.
Alkene halogenation
10.2 Addition of H
2
and X
2
Reagents 415
There are cases of bromination in which the mechanism shown in Figure 10.9 is
exactly correct, but for most alkenes it is not quite right. First, carbocation rearrange-
ments (p. 386) are not observed in most bromination and chlorination reactions. For
example, addition of bromine to 3,3-dimethyl-1-butene gives only 1,2-dibromo-3,3-
dimethylbutane, not 1,3-dibromo-2,3-dimethylbutane, as would be expected if an
open cation were involved (Fig.10.10).We already know that if a cycloalkene is used,
the product is exclusively the trans-1,2-dibromide. The cis compound cannot be
detected (Fig. 10.8).
+
+
Tertiary
carbocation
Secondary
carbocation
–
Br
..
..
..
..
..
..
..
–
CH
3
migration
This product is the only
observed dibromide
Not observed
..
C
CH
Br
Br
..
..
..
Br
..
..
..
Br
..
..
..
C
CH
C
Br
..
..
..
CH
C
..
..
..
Br
CH
–
Br
..
..
..
..
H
3
C
CH
3
CH
3
CCH
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
2
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
..
..
..
..
..
..
Br Br
FIGURE 10.10 Rearrangements of
carbocations are not observed in
most halogenation reactions.
If the addition involved an open cation, we would expect the bromide ion to add
from both sides to produce a mixture of products. The yields of cis and trans com-
pounds would not be equal, but both compounds should be formed (Fig. 10.11).
Because only anti addition is observed, our initial proposed mechanism cannot be
completely correct. So our mechanism needs modification. How do we explain the
simultaneous preference for anti addition and absence of rearrangement? It seems
that an open carbocation, which would lead to cationic rearrangements and permit
both syn and anti addition, must be avoided in some way.
Br
..
..
..
..
..
..
..
..
..
..
+
+
–
Path a
(a)
Path b
(b)
HH
H
H
HH
H
H
Br
..
..
..
Br
..
..
..
Br
..
..
..
Br
Br
..
..
..
..
..
..
Br Br
FIGURE 10.11 An open carbocation
must lead to both syn and anti addition.
What if the initial displacement of Br
from Br
2
were to take place in a
symmetrical fashion (Fig. 10.12)? Once again, the filled π orbital of the alkene
acts as nucleophile in an S
N
2 reaction in which Br
is displaced from Br
2
.
As the figure shows,a bromine-containing three-membered ring, a bromonium ion
(brom bromine; onium positive), is formed. An open carbocation is avoided==
C
C
Br
..
..
Br
..
..
..
..
Br
..
..
Br
..
..
..
..
Symmetrical displacement A bromonium ion
+
+
–
C
C
FIGURE 10.12 A symmetrical attack
of the alkene on bromine yields a
cyclic bromonium ion along with
bromide, Br
.The π bond is the
nucleophile in this S
N
2 reaction.
416 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
and rearrangements cannot occur. But what of the preference for anti addition? Look
closely at the second step of the reaction, the opening of the bromonium ion by the
nucleophile Br
(Fig. 10.13). This reaction is an S
N
2 displacement, and therefore
must take place with inversion, through addition from the rear as we saw so often
in Chapter 7. Overall anti addition of the two bromine atoms is assured.
WEB 3DWEB 3D
trans -1,2-Dibromo-
c
y
clo
p
entane
HH
S
N
2
H
H
H
H
Br
..
..
Br
..
..
..
..
Br
..
..
..
..
..
..
..
..
..
..
–
Br
Br
..
..
Br
+
FIGURE 10.13 This anti addition
of the two bromine atoms requires
the formation of trans-
1,2-dibromocyclopentane.
HH
Mirror
Enantiomers!
(a)
(a)
(b)
(a)
(b)
(b)
H
H
Br
..
..
Br
..
..
..
..
Br
..
..
..
..
..
..
..
..
..
..
–
Br
Br
H
..
..
..
Br
+
H
H
..
..
Br
H
..
..
..
Br
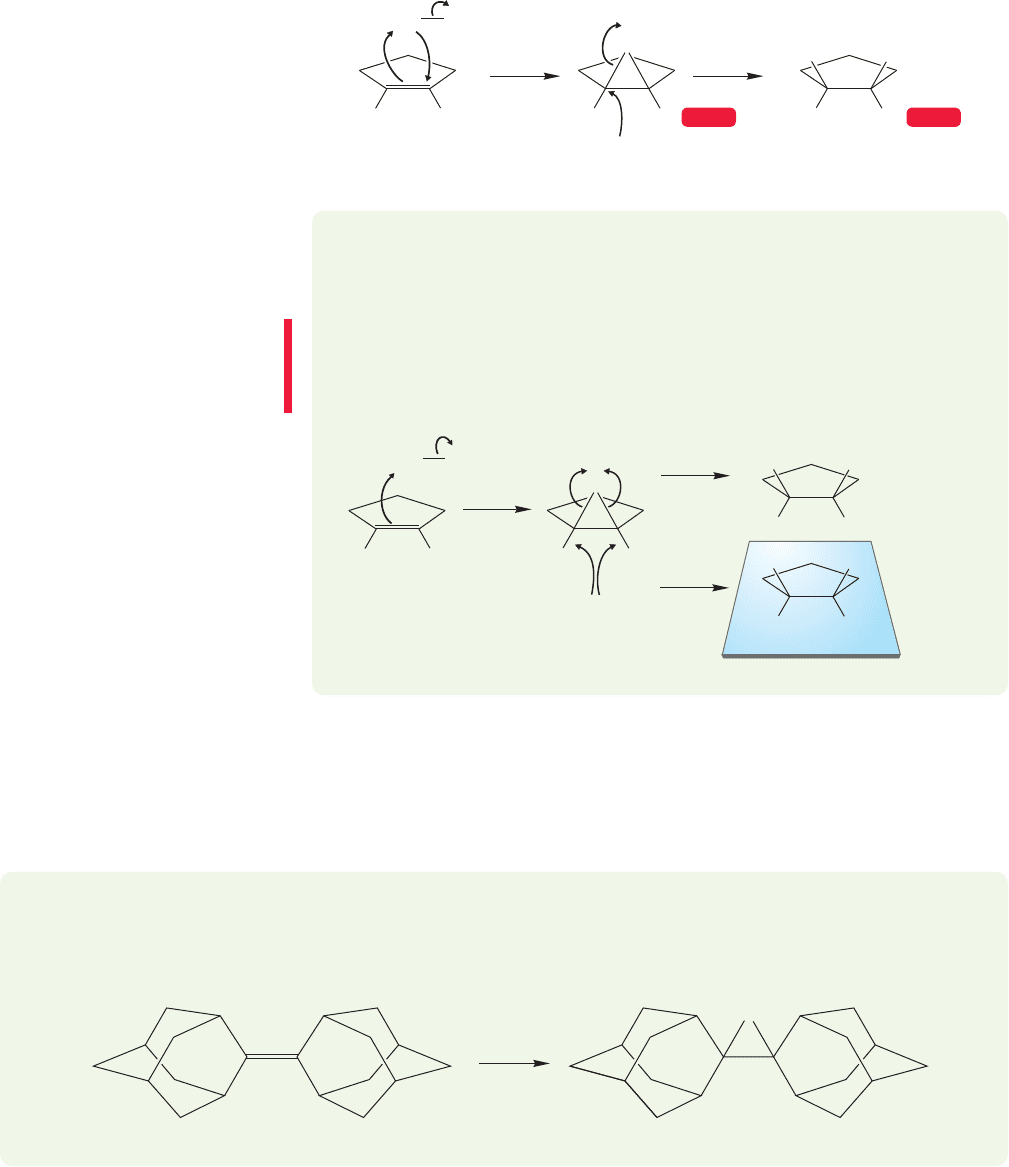
WORKED PROBLEM 10.5 In Figure 10.13, the reaction starts with achiral molecules.
Yet the product, as drawn in the figure, is a single enantiomer. Clearly something is
awry. What is it? Hint: Convention Alert! (p. 157).
ANSWER You were warned about this! Of course there can be no optical activity
produced from a reaction of two achiral reagents. In this reaction, the bromonium
ion must open in two equivalent ways to produce a racemic pair of enantiomers. In
situations such as this one, it is the custom not to draw both enantiomers.
Br
3
Adamantylideneadamantane
A stable bromonium ion
+
..
..
Br
+
–
Br
2
PROBLEM 10.6 One example in which the bromonium ion is stable to further
addition of nucleophiles is the reaction of adamantylideneadamantane with
bromine (see below). Explain why this cyclic ion might be stable.
So, a modification of our earlier addition mechanism suffices to explain the reac-
tion.In contrast to the addition to alkenes we saw in Chapter 9 ( , carbocations,and
boranes),in alkene halogenation (bromination and chlorination) a three-membered ring
is produced as an intermediate.In some, very specialized cases the bromonium ion can
even be isolated, and it seems its intermediacy in most brominations is assured.
HX
CONVENTION ALERT
10.2 Addition of H
2
and X
2
Reagents 417
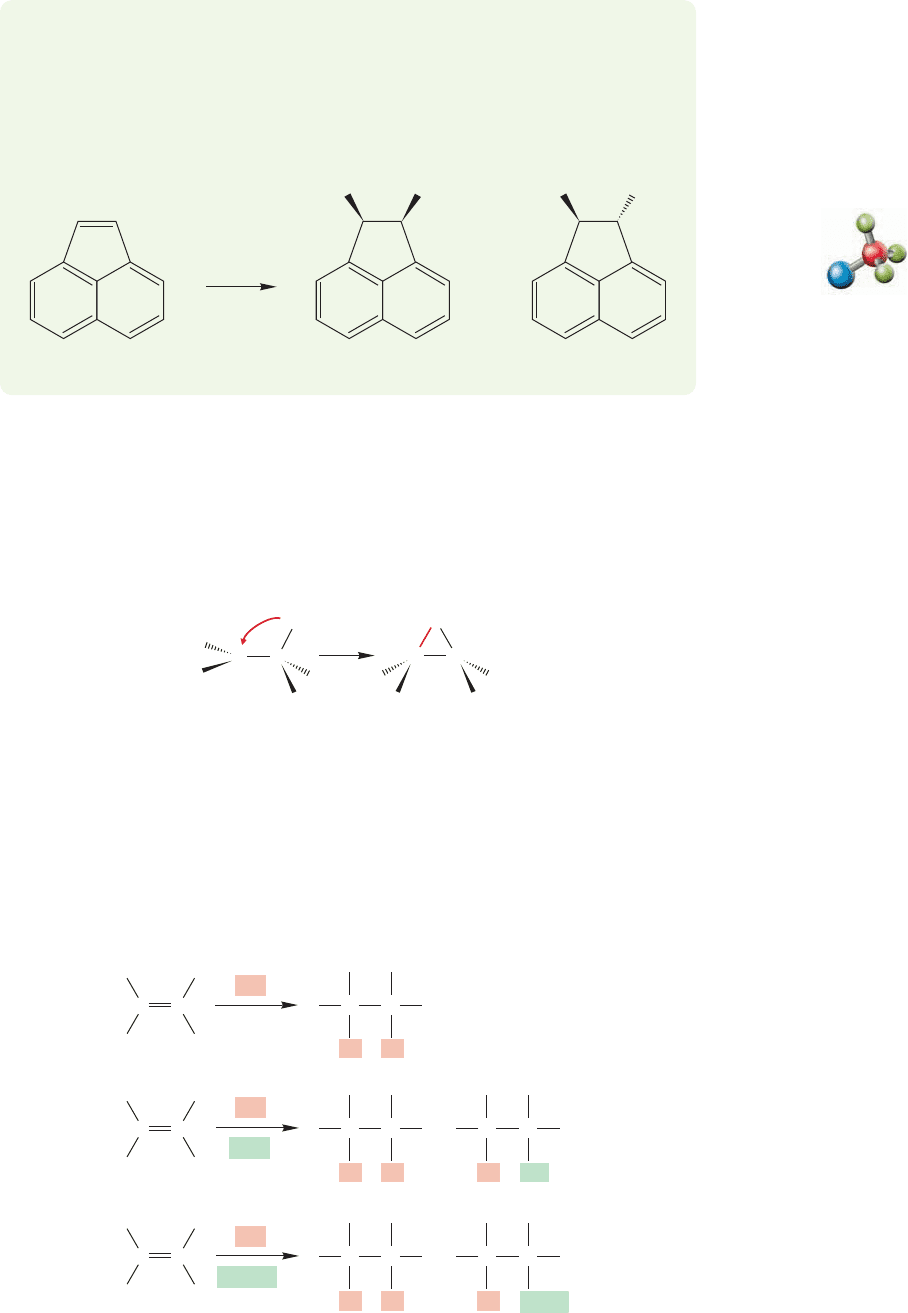
Br
2
CCl
4
Br
Br
Br
Br
trans DibromideAcenaphthylene cis Dibromide
+
PROBLEM 10.7 Addition of Br
2
to acenaphthylene gives large amounts of the
product of cis addition along with the usual trans dibromide. Give a mechanism
for this bromination and, more important, explain why the mechanism of the
reaction shown below is different from the typical selectivity for only trans addi-
tion. Why is cis product formed in this case?
Stabilized alkene halogenation
In the bromonium ion,there is the full complement of six electrons for the three
σ bonds binding the two carbons and the bromine. The bromonium ion is a nor-
mal compound even though it may look odd at first.To see this clearly, imagine form-
ing the bromonium ion from an open cation.
CC
CC
Bromonium ion
Br
Br
+
..
..
..
..
..
+
Normally, the nonnucleophilic solvent carbon tetrachloride (CCl
4
) is used for
the bromination or chlorination of alkenes. But the reaction will also work in pro-
tic solvents such as water and simple alcohols. In these reactions, new products
appear that incorporate molecules of the solvent (Fig. 10.14). How do the OH
or OR groups get into the product molecule? To see the answer, write out the
mechanism and look for an opportunity to make the new products.The first step
Br Br
Br Br Br OH
Cl Cl Cl OCH
3
CH
3
OH
Cl
2
H
2
O
Br
2
CCl
4
Br
2
C
C
C
C
C
C
C
C
C
C
+
+
C
C
C
C
C
C
FIGURE 10.14 In the presence of
nucleophilic solvents, addition
reactions of bromine and chlorine
give products incorporating solvent
molecules as well as dihalides.
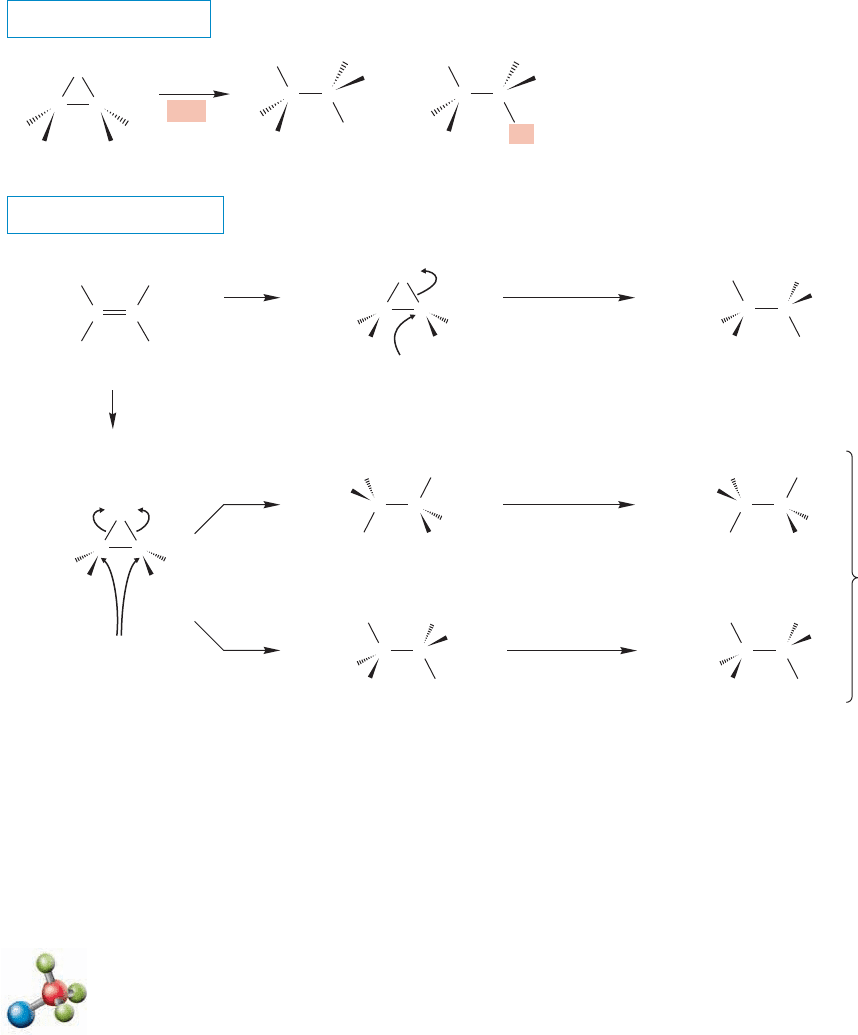
is the same as in Figure 10.12, in this case formation of the cyclic chloronium ion
(Fig. 10.15).Normally, the chloronium ion is opened in S
N
2 fashion by the nucleophilic
Cl
to give the dichloride.This opening must be the path for dichloride formation in
these examples. But in these reactions there are other nucleophiles present: molecules
of water or alcohol.Of course,they are weaker nucleophiles than the negatively charged
Cl
, but they are present as solvent and have an enormous numerical advantage over
the more powerfully nucleophilic chloride, which is present only in relatively low con-
centration (Fig. 10.15).
418 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
Cl
..
..
..
..
..
..
–
CC
(91%)
(8%)
H
H
H
H
H
H
H
H
CC
H
H
CC
CC
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
CH
3
CH
2
CH
3
OH
..
CH
3
O
CH
3
CH
2
S
N
2
CH
3
CH
3
CH
3
H
H
CC
CH
3
CH
2
CH
3
S
N
2
S
N
2
CH
3
Cl
2
Cl
2
/CH
3
OH
CH
3
CC
H
CH
3
CH
3
CH
2
H
OCH
3
..
..
CC
H
H
CH
3
CH
2
CH
3
deprotonation
deprotonation
..
HOCH
3
+
+
..
..
CH
3
OH
(a)
(a)
path a
(b)
(b)
path b
C
C
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
+
Cl
..
..
+
+
A SPECIFIC EXAMPLE
CH
3
OH
Nu
Cl
Cl
+
CC CC
CC
–
Cl
A chloronium ion
..
..
..
..
..
..
..
Cl
..
..
..
..
..
..
Cl
..
..
+
Nu
..
–
THE GENERAL CASE
FIGURE 10.15 Once the chloronium ion is formed, it is vulnerable to attack by any
nucleophile present. When a protic nucleophilic solvent is used in the reaction, it can
attack the cyclic ion to give the solvent-containing product. In this case, addition
of chloride accounts for 8% of the product, whereas the solvent methyl alcohol
produces 91%. Notice that methyl alcohol can produce two products through path a
and path b.
If this mechanism is correct, we can predict that the required inversion in the
opening of the cyclic ion must produce a trans stereochemistry in the products.
So, we have the opportunity to test the mechanism, and it turns out that addi-
tion of Br
2
(or Cl
2
) to cyclohexene in the solvent water exclusively produces the
compound resulting from anti addition in which the bromine (or chlorine) and
Halohydrin formation
