Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

10.2 Addition of H
2
and X
2
Reagents 419
Br
..
..
..
–
Br
..
..
..
..
+
H
H
Br
2
S
N
2
trans Br and OH
g
rou
p
s
H
H
2
O
..
..
..
..
H
H H H
OH
2
..
+
Br
..
..
..
..
O
+
..
H
H
H
Br
..
..
..
OH
H
Br
..
..
..
WEB 3D
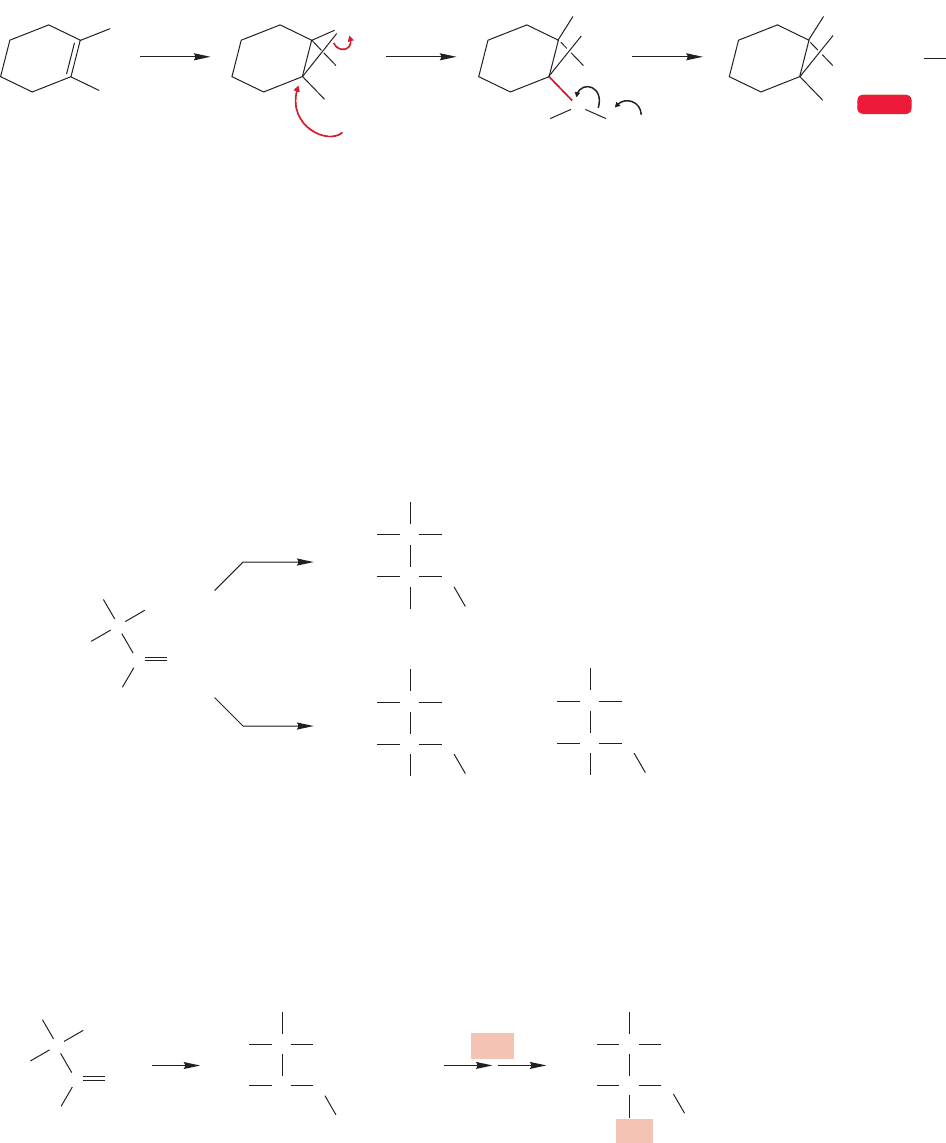
FIGURE 10.16 The bromonium ion mechanism demands that the products be formed by anti addition, as they are.
This stereochemical experiment uses cyclohexene to show that the bromine and solvent molecule become attached
from different sides of the ring through the anti addition enforced by the presence of the bromonium ion.
Addition of X
2
in nucleophilic solvents presents us with another opportunity.
We can measure the regioselectivity of the reaction. For example, in the bromina-
tion of 2,3,3-trimethyl-1-butene in carbon tetrachloride, there is no way to deter-
mine the regiochemistry of the bromide attack of the bromonium ion. Bromination
in water allows us to see where the hydroxyl group goes as it opens the bromonium
ion. There are two possibilities (Fig. 10.17). The hydroxyl group can attach to the
more substituted or to the less substituted carbon of the original double bond.
AB
2,3,3-Trimethyl-1-butene
C
CH
3
C
C
H
3
C
CH
3
CH
3
CH
3
Br
2
CH
2
CH
3
Br
Br
C
CH
2
H
3
C
CCl
4
C
CH
3
H
3
C
Br
2
Br
OH
C
CH
2
H
3
C
C
CH
3
H
3
C
OH
Br
or
C
CH
2
H
3
C
H
2
O
H
3
C
H
3
C
H
3
C
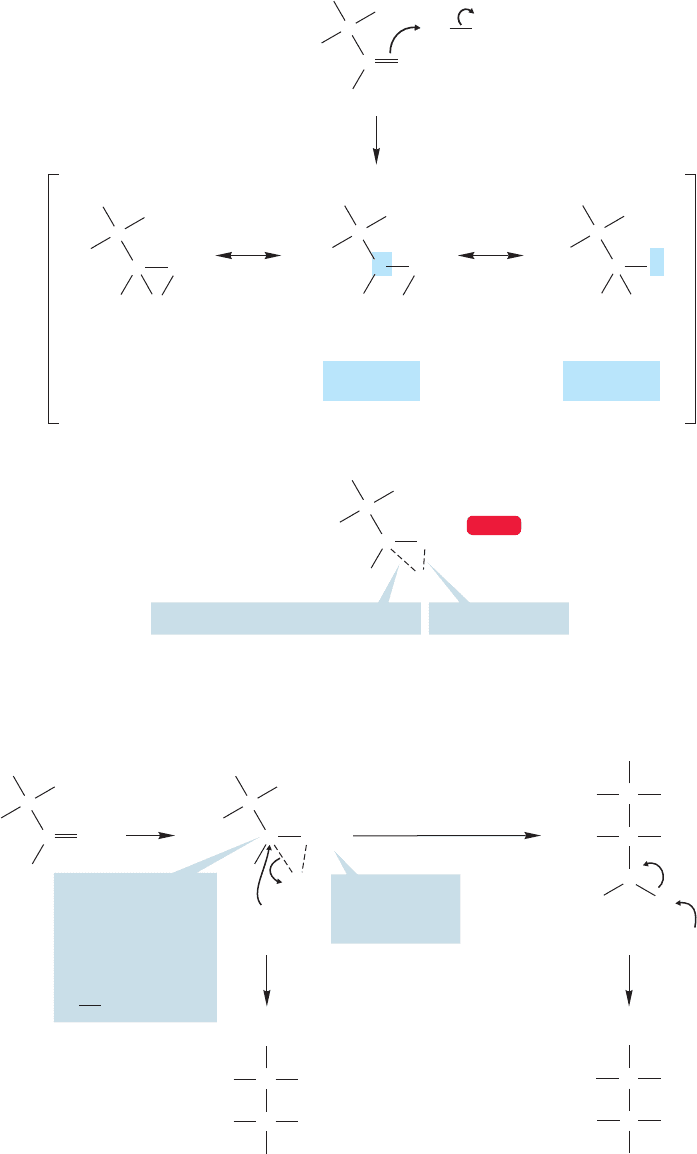
FIGURE 10.17 A test of the
regiochemistry of the addition of
Br
2
/H
2
O to alkenes. Halohydrin A
would result from water attacking
the more substituted carbon of the
bromonium ion and halohydrin B
would result from attack at the less
substituted carbon.
2. deprotonate
1.
C
CH
3
H
3
C
CH
3
Br
2
C
CH
2
H
3
C
C
CH
3
H
3
C
CH
3
OH
C
CH
2
H
3
C
H
2
O
Br
..
..
..
..
–
+
+
C
C
CH
2
CH
3
H
3
C
H
3
C
H
3
C
Br
..
..
..
Br
..
..
..
..
..
..
..
FIGURE 10.18 The bromine becomes
attached to the less substituted
carbon and the nucleophilic solvent
to the more substituted carbon of the
alkene. It is as if the reaction went
through a carbocationic intermediate.
In practice, the hydroxyl group becomes attached to the more substituted carbon. It
is as if the reaction were proceeding through a carbocation (Fig. 10.18).But we are quite
certain that it does not! The problem now is to reconcile the observed regioselectivity
with the intermediacy of a bromonium ion (recall trans addition and the lack of carbo-
cation rearrangements).
hydroxyl groups have a trans relationship. Such compounds are called halohydrins.
The bromonium ion mechanism fits all the data (Fig. 10.16).
The bromonium ion is symmetrical only when formed from an alkene in which
the two carbons of the double bond are the same. In all other cases, the two
carbon–bromine bonds must be of different strength. In resonance terms,we say that
the weighting factor (coefficient) for the more stable form is greater than that for the
420 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
This bond is longer and weaker…
Summary
structure
Tertiary
carbocation
(more important)
Primary
carbocation
(less important)
Br
..
..
..
Br
..
..
..
CH
2
CH
2
Br
+
CH
2
c
1
c
2
c
1
>>c
2
CH
2
…than this bond
..
..
Br
+
Br
+
..
..
..
Br
..
..
..
+
C
C
CH
2
CH
3
H
3
C
H
3
C
H
3
C
C
C
CH
3
H
3
C
H
3
C
H
3
C
C
C
CH
3
H
3
C
H
3
C
H
3
C
C
C
CH
3
H
3
C
H
3
C
H
3
C
C
C
CH
3
H
3
C
H
3
C
H
3
C
WEB 3D
FIGURE 10.19 A resonance
description of the bromonium ion.
HH
C
CH
3
CH
3
H
3
C
C
CH
2
H
3
C
C
CH
3
CH
3
H
3
C
HO
C
CH
2
H
3
C
Not much δ
+
on this primary
carbon
addition of water
breaks weaker
carbon–bromine bond
deprotonationaddition of
(60%)
(24%)
C
CH
3
CH
3
H
3
C
C
CH
2
H
3
C
Br
..
..
..
Br
..
..
..
..
–
..
..
..
..
..
Br
..
..
..
Br
..
..
..
Br
O
+
..
CH
2
Most δ
+
is on this
tertiary carbon;
the bond between
it and Br is longer
and weaker
than the other
C
Br bond
..
..
Br
+
H
2
O
..
..
H
2
O
..
..
Nu = H
2
O
..
(Nu = Br
–
)
..
C
C
CH
2
CH
3
H
3
C
H
3
C
H
3
C
C
C
CH
3
H
3
C
H
3
C
H
3
C
FIGURE 10.20 Addition of
nucleophiles breaks the longer,
weaker bond.C
O
Br
less stable form (c
1
c
2
, Fig. 10.19).The two carbons share the positive charge, but
not equally.The charge is more stable on the more substituted carbon.Therefore,the
more substituted carbon–bromine bond is longer and weaker than the less substituted
bond in this positively charged intermediate. For this reason, the transition state for
7
breaking the bond to the more substituted carbon will be lower in energy than that
for breaking the bond to the less substituted carbon. Therefore nucleophiles such as
methyl alcohol, water, and halide add at the more substituted position (Fig. 10.20).
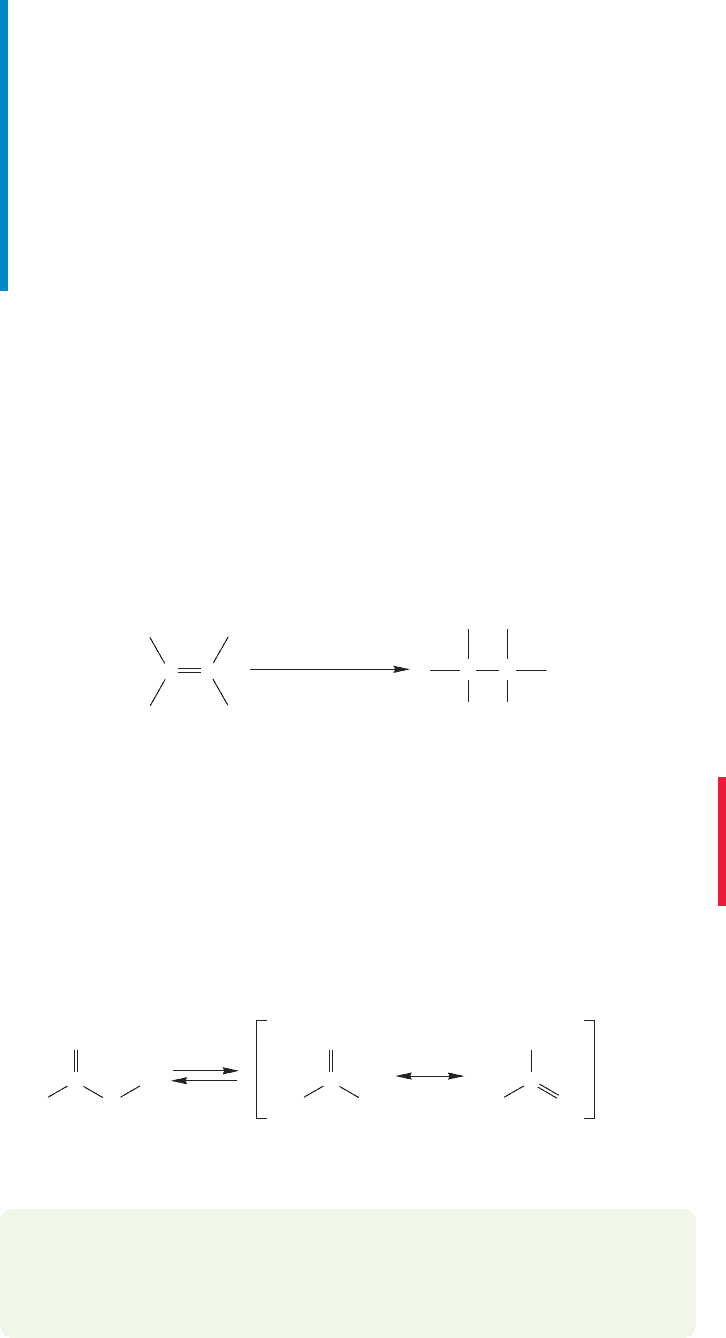
Summary
The critical difference between addition of halogen (X
2
) and the many HX addi-
tions we saw in Chapter 9 is the presence of the intermediate halonium (bromo-
nium or chloronium) ion. This intermediate is demanded by the observed
preference for anti addition. However, occasionally the cyclic halonium ion is less
stable than the corresponding open ion and the open ion will be favored. One
way to stabilize the open ion is through resonance. If the open ion is the inter-
mediate, the products of both cis and trans addition will be observed. When a
protic nucleophilic solvent is used in the reaction,the solvent as well as the halide
will add to the intermediate. In such cases halohydrins or halogenated ethers are
formed along with dihalides.
10.3 Hydration through Mercury Compounds: Oxymercuration 421
10.3 Hydration through Mercury Compounds:
Oxymercuration
For small-scale hydrations, it is convenient to use mercury salts, such as mercuric
acetate, Hg(OAc)
2
, to carry out the addition of across a double bond.
This process is called oxymercuration. In this two-step process, an alkene is first
treated with mercuric acetate, then the initial alkylmercury compound formed is
reduced with sodium borohydride (Na
BH
4
).
H
O
OH
1. Hg(OAc)
2
/H
2
O
2. Na
+
–
BH
4
H
C
OH
C
C
C
The abbreviation AcO
(or
OAc) represents the acetate ion (CH
3
COO
), the
conjugate base of acetic acid, CH
3
COOH (often abbreviated AcOH). Lewis struc-
tures are given in Figure 10.21, which shows the relationship between the acetate
ion and its conjugate acid (AcOH). Note the resonance stabilization of the acetate
ion—the two oxygen atoms are equivalent.
Acetic acid, the
conjugate acid of…
AcOH
…the acetate ion
H
3
C
CH
O
..
..
H
3
C
O
C
O
+
O
..
..
..
..
..
..
O
..
..
..
..
–
–
H
3
C
C
O
H
3
O
..
+
H
2
O
..
..
..
..
AcO
–
FIGURE 10.21 Note the resonance
stabilization of the acetate ion.
CONVENTION ALERT
PROBLEM 10.8 The pK
a
of acetic acid is 4.8. The corresponding two-carbon
alcohol, ethyl alcohol (CH
3
CH
2
OH), is a much weaker acid, as its pK
a
of
15.9 demonstrates. Explain why acetic acid is much more acidic than ethyl
alcohol.
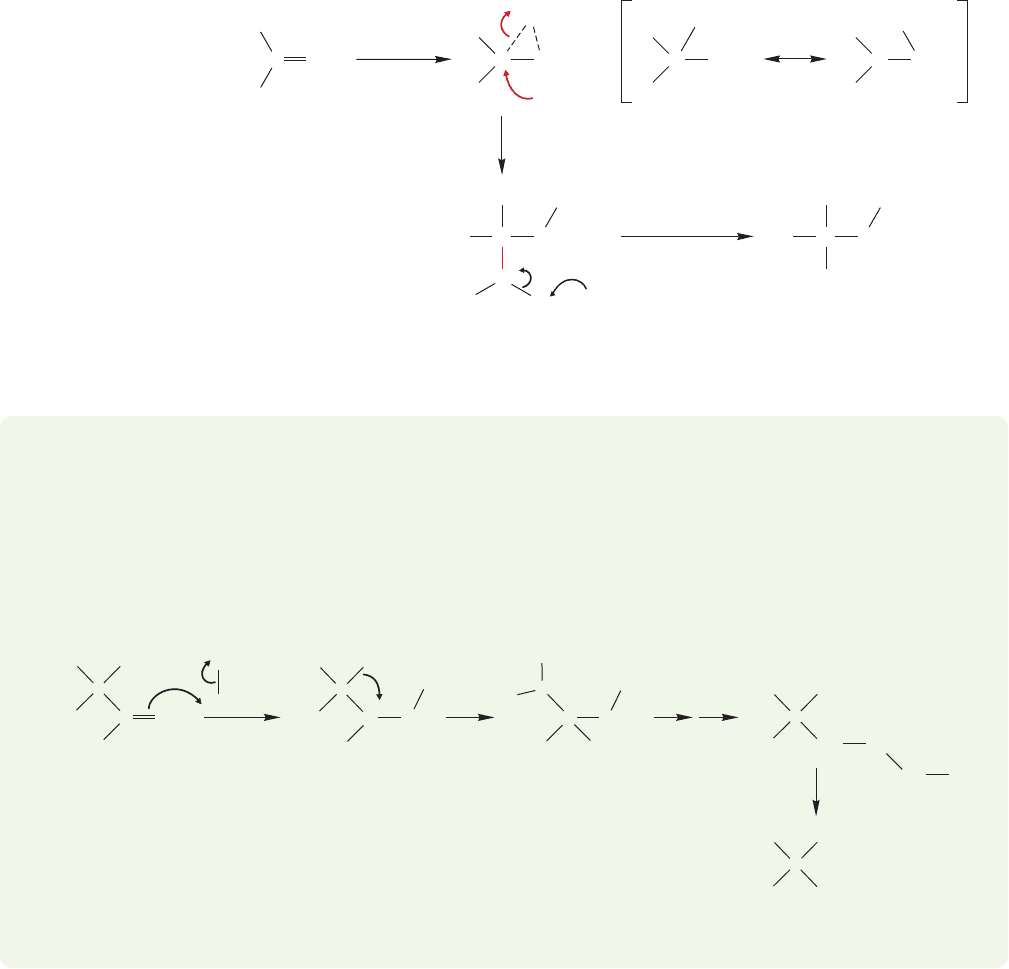
422 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
The first step in the oxymercuration reaction is the formation of a cyclic mer-
curinium ion (Fig. 10.22). Open carbocations are unlikely intermediates, as they
would surely undergo carbocationic rearrangements, and these are not seen even in
systems normally prone to rearrangement.
CH
2
CH
2
H
Ring opening by breaking the
weaker carbon–mercury bond
A mercurinium ion
deprotonation
addition
Initial isolable
product
C
CH
2
OH
..
..
C
H
R
+
HgOAc
Hg(OAc)
2
=C
CH
2
HgOAc
HgOAc
C
CH
2
HgOAc
+
+
C
H
H
H
C
CH
2
..
O
+
HgOAc
H
3
O
..
+
OH
2
..
..
OH
2
..
..
H
2
O
..
..
H
R
H
R
H
R
RR
+
FIGURE 10.22 Oxymercuration
begins by attack of the alkene on the
Lewis acid mercuric acetate to form a
cyclic mercurinium ion. The
nucleophilic solvent, water, then adds
in S
N
2 fashion to give the first
product, a mercury-containing
alcohol, where R is a simple alkyl
group.
CH
2
+
H
2
O
HgOAc
HgOAc
HgOAc
Hg
OAc
OAc
C
Secondary
carbocation
3-Methyl-1-butene
Rearranged alcohol
not formed!
H
2
O
+–
BH
4
Na
C
CH
2
CH
2
H
3
C
CH
3
H
Tertiary
carbocation
CH
3
HO
H
CH
2
C
CH
2
H
3
C
H
H
H
3
C
H
3
C
H
H
H
3
C
H
3
C
CH
3
HO
CH
2
CH
3
H
3
C
CC
C
C
C
+
With unsymmetrically substituted alkenes, the two bonds to mercury will not
be equally strong. (Recall our discussion of asymmetrical bromonium ions just a
few pages ago.) The bond between mercury and the more substituted carbon will be
longer and weaker than the bond to the less substituted carbon of the mercurinium
ion. It is at the more substituted carbon that water opens the ring. So, the first
product of this reaction is the mercury-containing alcohol shown in Figure 10.22.
WORKED PROBLEM 10.9 Design an experiment to test for carbocationic
rearrangements in the oxymercuration reaction.
ANSWER It’s simple. Just use any alkene in which migration of hydride (H
) or
migration of R
would give a more thermodynamically stable cation. 3-Methyl-
1-butene would work well. If addition gave an open carbocation rather than the
cyclic mercurinium ion, rearrangements would surely occur.That they are not observed
provides strong evidence that open cations are not involved in oxymercuration.
:
:
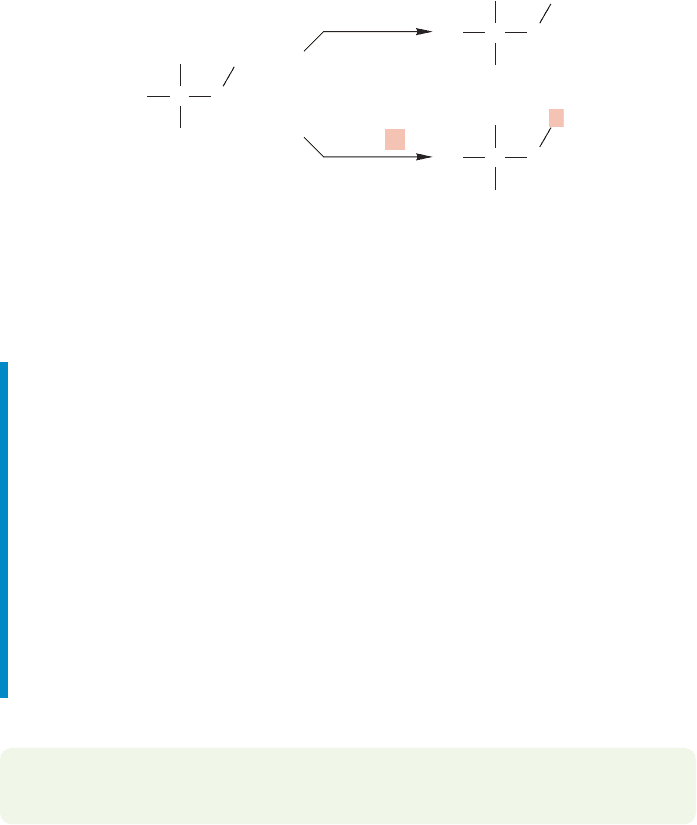
10.4 Other Addition Reactions Involving Three-Membered Rings 423
H
R
R
R
C
CH
2
OH
HgOAc
H
C
CH
2
OH
H
C
CH
2
OH
D
H
Na
+
–
BH
4
–
BD
4
Na
+
FIGURE 10.23 Reduction of the
mercury-containing products gives
alcohols.
Oxymercuration is synthetically useful because the reagent sodium borohydride
(Na
BH
4
) efficiently replaces the mercury with hydrogen. Figure 10.23 shows
the reaction with sodium borohydride and sodium borodeuteride (Na
BD
4
).
The use of the deuterated molecule allows us to see with certainty the position of
the entering deuterium.
So we now have a new hydration reaction, one that proceeds through a cyclic
mercurinium ion but ultimately gives the product of Markovnikov addition,the more
substituted alcohol.
Summary
Here are our three methods of hydration:
1. Direct hydration (p. 380) proceeds through an intermediate carbocation that is
captured by water to give the product of Markovnikov addition. The reaction is
limited in utility because rearrangements of the initially formed cation to more
stable species can lead to undesired, rearranged products.
2. Hydroboration/oxidation (p. 390) first generates an alkylborane that subse-
quently reacts with peroxide in base to give the product of anti-Markovnikov
addition.
3. Oxymercuration proceeds through a cyclic mercury-containing ion and also gives
the product of Markovnikov addition. No rearrangements take place, which is
sometimes a great advantage.
PROBLEM 10.10 Work out the consequences of applying the three procedures out-
lined above to 3-methyl-1-butene.
10.4 Other Addition Reactions Involving
Three-Membered Rings: Oxiranes and
Cyclopropanes
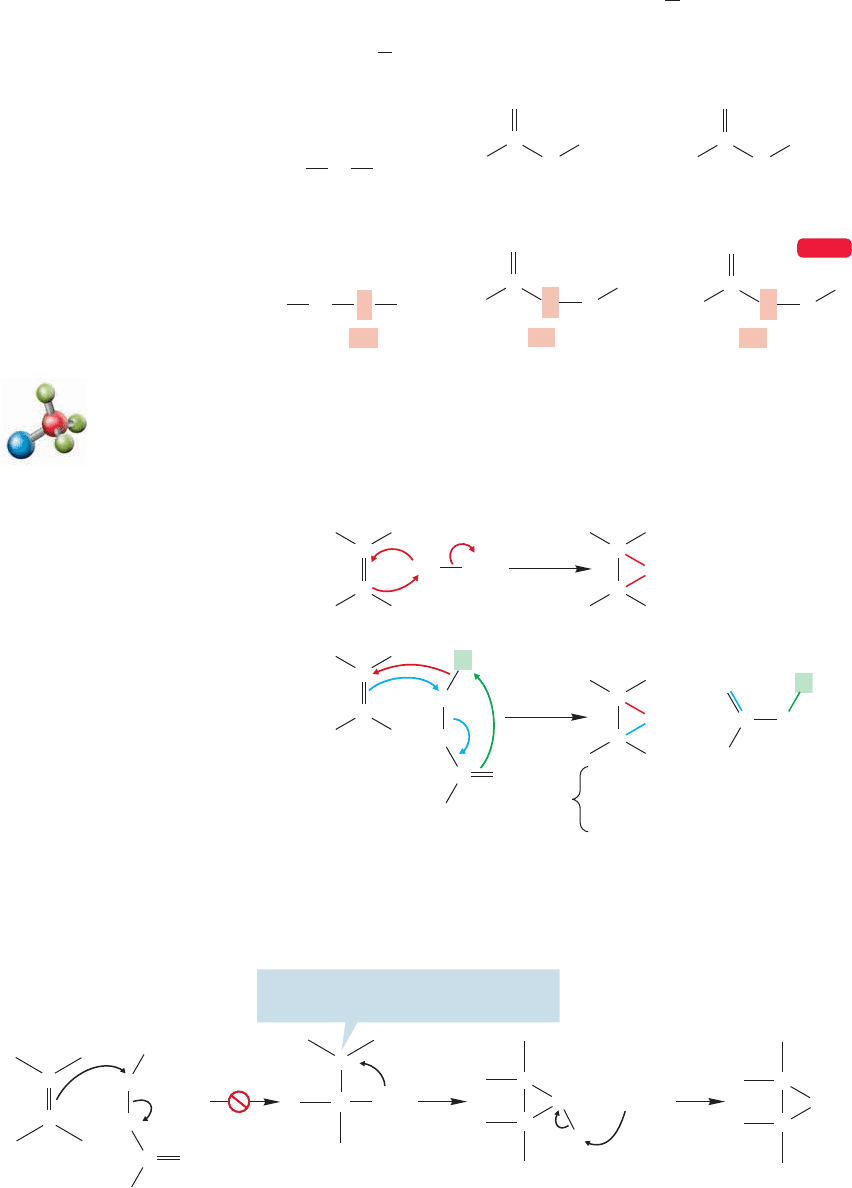
10.4a Oxiranes We have seen that many addition reactions begin with for-
mation of a three-membered ring. These intermediates have almost always been
rapidly converted into the final products through addition of a nucleophile. This
state of affairs will now change dramatically, as you learn about the addition reac-
tions that are the best routes to two types of stable three-membered rings: the
oxiranes (epoxides, p. 317) and cyclopropanes.
424 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
Hydrogen oxide
(water)
A carboxylic acid
A peracid
Hydrogen peroxide
CH
HH
R
R
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
O
..
..
HH
O
..
..
O
..
..
CH
O
..
..
O
..
..
H
Trifluoroacetic acid
Trifluoroperacetic acid
CH
O
..
..
C
F
3
C
F
3
C
WEB 3D
FIGURE 10.24 The prefix per- means
extra. So a “peroxide” and a “peracid”
each contain an extra oxygen atom,
one more than the oxide or acid.
An organic acid has the formula and is called a carboxylic acid.
Its structure is shown in Figure 10.24. We will study this functional group in detail
in Chapter 18. A peracid has the formula . Figure 10.24 shows the
difference in more detail.The “per”indicates an “extra”oxygen is present.Thus hydro-
gen peroxide is , whereas the normal oxide of hydrogen is water, HOH.HOO
H
R
O
COOOH
R
O
COOH
This open cation must lead to
rearrangements that are not observed
C
O
O
F
3
C
C
H
H
C
C
+
+
OH
..
..
..
..
H
3
O
..
O
..
C
+
C
C
C
C
O
O
OH
2
..
..
..
..
..
..
..
..
+
+
FIGURE 10.26 A stepwise (nonconcerted) mechanism for epoxidation does not happen.
(a)
(b)
epoxide
oxirane
oxacyclopropane
An
O
..
..
..
..
CF
3
Br
..
..
O
O
..
..
..
..
..
..
..
..
–
C
H
H
C
C
+
Br
..
..
Br
..
..
..
..
..
..
..
Br
+
F
3
C
O
C
+
O
..
O
C
C
C
C
C
C
FIGURE 10.25 (a) Bromination of
an alkene. (b) Epoxidation of an
alkene.There is an additional transfer
of hydrogen to give the isolable,
stable epoxide.
The epoxidation reaction resembles the formation of a bromonium ion. In both
reactions, a leaving group is displaced by the alkene acting as nucleophile in an S
N
2
reaction (Fig. 10.25). Simultaneous transfer of hydrogen to the carbonyl ( )
oxygen gives a stable three-membered ring, an epoxide.
C
P
O
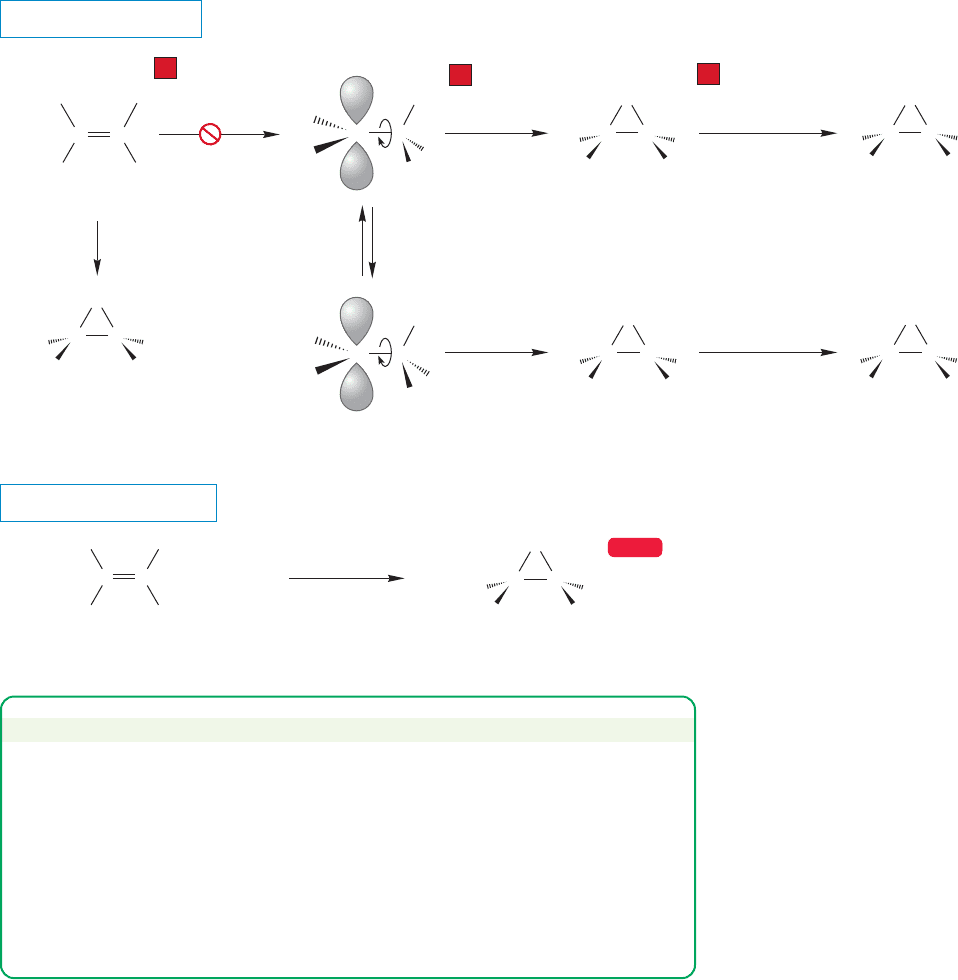
It is important to be clear why we are so sure that epoxidation occurs in a single
step.Why not just form the carbocation and then close up to the oxirane (Fig.10.26)?
Alkene epoxidation
10.4 Other Addition Reactions Involving Three-Membered Rings 425
If a free cation were involved, carbocation rearrangements would be expected, and
they are not found. Moreover it is easy to monitor the stereochemistry of this reac-
tion. Concerted (no intermediates) formation of the oxirane explains the observed
syn addition very well. A reaction in which the two new bonds are formed at the same
time cannot change the stereochemical relationships of the groups in the original
alkene. On the other hand, a mechanism involving an open carbocation would very
likely lead to a mixture of stereoisomeric oxiranes (Fig. 10.27). If only one bond were
made, there would be a carbocationic intermediate in which rapid rotation about
carbon–carbon single bonds would take place. These rotations would scramble the
original stereochemical relationship present in the alkene, and this result is not
observed. Instead, oxirane formation occurs with retention of the stereochemical
relationships present in the starting alkene.
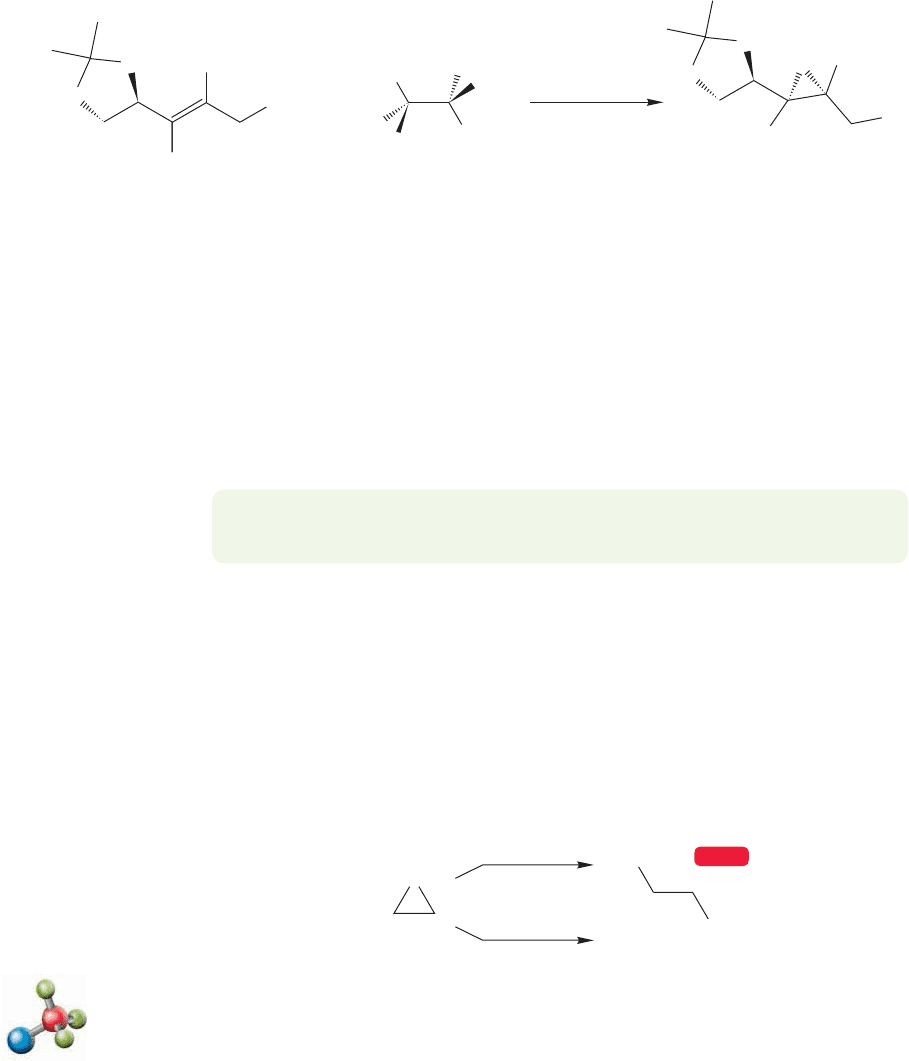
PROBLEM SOLVING
The mechanistic technique outlined in Figure 10.27 above is a general one,
often used to distinguish one-step from two-step mechanisms. Retention of
stereochemistry (cis cis and trans trans) implicates a one-step process,
whereas scrambling of stereochemistry (cis cis and trans, or trans cis and
trans) is indicative of a stepwise process proceding through an intermediate (or
intermediates) capable of rotation. We’ll see it again in a few pages. (Can you
spot it?) You should be alert for it, as it forms the basis for many mechanism-
based problems.
UU
UU
WEB 3D
THE GENERAL CASE
A SPECIFIC EXAMPLE
rapid
rotation
cis Alkene
cis Epoxide
+
trans Epoxide
(Not observed)
cis Epoxide
H
H
HH
R
R
R
R
R
R
H
RR
R
R
a peracid
a peracid
H
C
+
C
R
R
H
H
CC
CC
concerted,
syn addition
concerted,
syn addition
CH
3
COOOH
20 C, 3 h
Oleic acid
(CH
2
)
7
COOH
(CH
2
)
7
COOH
CH
3
(CH
2
)
7
CH
3
(CH
2
)
7
HH
H
RR
R
R
H
H
CC
(82%)
H
H
HH
CC
CC
H
CC
H
H
OH
..
..
C
+
C
OH
..
..
O
..
..
O
..
..
O
..
..
O
..
..
+
OH
..
CC
+
OH
..
Formation
of an open
carbocation
1
Closure
2
Deprotonation
3
C
C
FIGURE 10.27 A test of the mechanism
of epoxidation. A stepwise mechanism
predicts scrambling of stereochemistry,
whereas a concerted mechanism must
retain the stereochemistry of the
starting alkene. Because cis alkenes
give only cis epoxides, the reaction
must be concerted.
H
H
OH
HO
Ti[OCH(CH
3
)
2
]
4
(CH
3
)
3
COOH
CH
2
Cl
2
H
H
O
O
O
OH
The allylic alcohol
(S,S) Enantiomer
of diethyl tartrate
(78%)
+
COOEt
EtOOC
H
H
O
O
OH
FIGURE 10.28 Asymmetric (Sharpless) epoxidation of alkenes (Et CH
3
CH
2
).=
10.4b Asymmetric (Sharpless) Epoxidation In recent years, an enormously
useful synthetic technique for producing enantiomeric oxiranes (epoxides) has appeared.
Discovered by Barry Sharpless (b. 1941), now at the Scripps Research Institute, it uses
a witches’ brew of titanium isopropoxide, tert-butyl peroxide, and one enantiomer of a
tartaric ester to react with an allylic alcohol ( ). Part of the
great utility of this procedure comes from the observation that the two enantiomers of
tartaric ester lead to two different stereochemistries of product oxirane (Fig. 10.28).
Professor Sharpless shared the Nobel prize in chemistry in 2001 for his discovery of
chiral catalysts.
R
O
CH
P
CH
O
CH
2
OH
426 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
WEB 3D
OH
A 1,2-diol
A
n epoxide
..
..
H
2
O / H
3
O
..
..
–
..
..
..
..
H
2
O /
..
..
O
HO
HO
..
..
..
..
+
FIGURE 10.29 Epoxides (oxiranes)
open in either acid or base.
The mechanism of this complex reaction involves the titanium compound act-
ing as a clamp,holding the alkene,peroxide,and (S,S)-tartaric ester together.Because
the ester is asymmetric, the clamped combination of molecules is also asymmetric.
In one cluster, the oxirane oxygen is delivered from one side, whereas in the enan-
tiomeric cluster formed from (R,R)-tartaric ester, the oxygen comes from the other
side. These and other epoxides can then be transformed into all manner of com-
pounds, as we will now see.
PROBLEM 10.11 The (R,R) enantiomer of diethyl tartrate gives a diastereomer of
the product shown in Figure 10.28. Draw this diastereomer.
10.4c Further Reactions of Oxiranes Unlike the closely related bromonium
ions, oxiranes can be isolated under many reaction conditions.The bromonium ion
is doomed to bear a positive charge and is therefore a more powerful Lewis acid
than the neutral oxirane. Oxiranes will react, however, when treated in a second
step with either acids or bases. For example, reaction with either H
3
O
/H
2
O or
HO
/H
2
O leads to opening of the three-membered ring and formation of a
1,2-diol (Fig. 10.29).
The mechanisms of these ring openings are straightforward extensions of
reactions you already know. In base, the strongly nucleophilic hydroxide ion
attacks the oxirane and displaces the oxygen atom from one carbon in an S
N
2
Basic epoxide ring opening
10.4 Other Addition Reactions Involving Three-Membered Rings 427
OH
..
..
HO
..
..
..
+
+
OH
..
..
S
N
2
protonation
The mechanism of epoxide opening in base
protonation
H
HH
H
OH
..
HO
..
..
OH
2
..
..
deprotonation
The mechanism of epoxide opening in acid
O
+
+
..
H
H
2
O
OH
..
..
HO
..
..
O
+
–
–
OH
..
..
..
..
..
..
–
O
O
O
HO
..
..
H
2
O
..
..
H
2
O
..
..
H
3
O
..
+
..
..
..
..
..
S
N
2
FIGURE 10.30 Mechanisms for
epoxide opening in base and acid.
reaction (Fig. 10.30). In acid, there is no strong nucleophile, but protonation of
the oxygen atom of the oxirane creates a good Lewis acid. Water is a strong
enough nucleophile to open the protonated oxirane to give, after deprotonation,
the 1,2-diol (Fig. 10.30).
Acidic epoxide ring opening
–
+
(Not observed)
H
HH
OH
2
..
..
protonation deprotonation
O
+
..
S
N
2
OH
..
..
O
+
O
O
HO
..
..
..
..
..
O
..
..
..
..
H
2
O
..
..
H
2
O
..
..
H
2
O
..
..
H
3
O
..
+
H
3
O
..
+
+
HO
..
..
FIGURE 10.31 Water is not a strong
enough nucleophile to open the
unprotonated oxirane, because RO
is too poor a leaving group. Acid
catalysis changes the leaving group to
ROH, which is much easier to displace.
There is a strong parallel here to reactions we have already seen. Hydroxide is a
poor leaving group. Treatment with acid or formation of sulfonate esters converts
hydroxide into water or another good leaving group, and many reactions require this
transformation (see p. 281 for examples).The opening of the oxirane in acid involves
exactly the same kind of transformation; protonation facilitates the opening by mak-
ing the leaving group ROH rather than RO
(Fig. 10.31).
Unsymmetrical oxiranes can open in two ways and the regiochemical result is gen-
erally different in acid and base (Fig. 10.32). How does the difference in regiochem-
istry arise? Why should the two openings be different? In base,the ring opening is an
In acid, the nucleophile
generally becomes
attached to the more
substituted carbon
In base, the nucleophile
generally becomes
attached to the less
substituted carbon
H
2
Nu
CH
2
OH
O
C
R
R
R
R
R
R
C
CH
2
OH
C
CH
2
HNu
HNu / Nu
Nu
Nu
..
..
+
–
FIGURE 10.32 The regiochemistry
of epoxide opening is different in acid
and base.
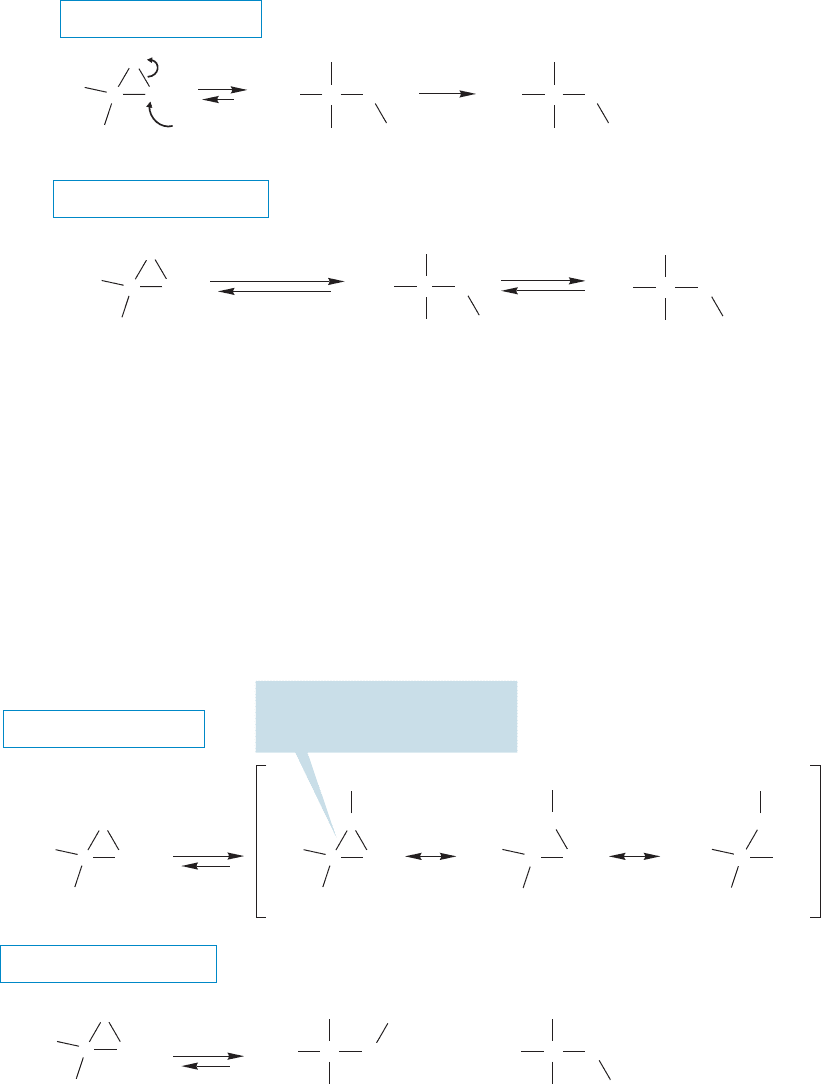
428 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
THE GENERAL CASE
A SPECIFIC EXAMPLE
Na
+
N
3
–
H
2
O
..
..
H
3
C
H
3
C
H
3
C
H
3
C
–
O
..
..
..
H
3
C
C
HNu
Nu
N
3
CH
2
(41%)
C
Nu
CH
2
C
H
3
C
OH
..
..
CH
2
+
H
3
C
H
3
C
H
3
CH
3
C
H
2
O/dioxane
80 ⬚C
–
–
O
..
..
..
C
CH
2
C
N
3
H
3
C
H
3
C
OH
..
..
..
CH
2
C
CH
2
+
..
..
HO
O
..
..
O
..
..
Nu
..
–
Nu
..
–
FIGURE 10.33 In base, the nucleophile adds at the sterically less encumbered carbon.
THE GENERAL CASE
A SPECIFIC EXAMPLE
HX
+
+
Cl
..
..
..
Cl
..
..
..
H
3
C
H
3
C
HCl
..
..
..
(55%) (45%)
..
H
H
3
C
CH
3
CH
2
C
H
CH
2
C
H
CH
2
C
H
3
C
H
3
C
OH
..
..
OH
..
..
CH
2
C
+
This weaker carbon–oxygen
bond will be the major bond
broken by an adding nucleophile
O
C
CH
2
H
3
C
O
C
CH
2
H
3
CH
3
C
H
3
C
H
3
C
O
C
CH
2
..
..
O
..
..
O
..
..
..
..
+
H
3
C
H
3
C
H
3
C
FIGURE 10.34 Both carbons help bear the positive charge in the protonated oxirane. Most of the positive
charge is on the more substituted carbon, and that is where the nucleophile adds.This reaction is much like
the opening of an unsymmetrical bromonium ion by water or alcohol.
S
N
2 displacement by hydroxide on the unprotonated oxirane. As in any S
N
2 reaction,
steric matters are important, and the ring opens at the sterically more accessible, less
substituted carbon (Fig. 10.33). In acid, the first step is the protonation of the oxygen
of the three-membered ring (Fig. 10.34). Recall the discussion of the regiochemistry
of the opening of unsymmetrical bromonium ions (p. 419) and mercurinium ions
(p. 422).The positive charge is borne not only by the oxygen but also by the two car-
bons of the oxirane. Most of the positive charge on carbon will reside at the more sub-
stituted position.The more substituted carbon–oxygen bond will be longer and weaker
than the other, less substituted carbon–oxygen bond (Fig. 10.34). As in unsymmetri-
cal bromonium ions, the transition state for addition at the more substituted position
will be lower in energy than that for addition at the less substituted position.
