Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


10.4 Other Addition Reactions Involving Three-Membered Rings 429
PROBLEM 10.12 If hydroxide is such a poor leaving group, one would expect RO
would also be a bad leaving group, and it is. In fact, simple ethers ( )
are not generally cleaved in strong base. Why, then, do oxiranes open so easily in
base (Fig. 10.32)? What is special about the three-membered ring that facilitates
the displacement?
R
O
O
O
R
We have seen that the apparently mysterious change in regiochemistry of the
opening in acid and base can be reconciled easily. The trick is to learn to see new
reactions in terms of what you already know. Books will always be organized in this
way. Questions are set up by recently discussed phenomena. Nature is not always so
cooperative and organized, however.
In Chapter 6,we met two organometallic reagents,the Grignard reagent, RMgX,
and organolithium reagents, RLi. These compounds are very strong bases (p. 227)
and are nucleophiles as well. Like other nucleophiles, organometallic reagents react
with oxiranes to open the strained ring.The initial product is an alkoxide, which is
protonated in a second step to give the alcohol.This reaction is quite useful for syn-
thesizing alcohols in which the initial carbon atom frame of the organometallic
reagent has been augmented by the two carbons originally in the oxirane (Fig.10.35).
O
dioxane
H
2
C CH
2
CH
3
CH
2
MgBr, 25 ⬚C
H
2
O
O
–
CH
3
CH
2
CH
2
CH
2
The intermediate alkoxide (82%)
OH
CH
3
CH
2
CH
2
CH
2
FIGURE 10.35 Opening of an epoxide by an organometallic reagent.The initial product is
an alkoxide, which is protonated in a second step to give the alcohol.
The strongly basic organometallic reagents inevitably attack at the less substi-
tuted, less sterically hindered carbon of the oxirane, as does any strong nucleophile
(Fig. 10.36).
..
..
..
O
..
..
C
H
CH
2
C
O
..
..
HCH
2
CH
3
PhMgBr
35 ⬚C, 20 h
Ph
CH
3
–
..
..
C
(60%)
OH
HCH
2
CH
3
H
2
O
Ph
FIGURE 10.36 The S
N
2 ring opening
occurs at the less sterically
demanding carbon of the epoxide.
The Grignard reagent and organolithium reagents are sources of “R
.” Because
they are not free carbanions, we call them carbanion equivalents. There are also
reagents that are hydride (H
) equivalents. We will see several in Chapter 16 and
beyond, but it is useful to introduce lithium aluminum hydride (LiAlH
4
,“LAH”)
now. Like the organometallic reagents, lithium aluminum hydride opens oxiranes by
nucleophilic addition, and this reaction provides you with another synthesis of alco-
hols. Addition of hydride is predominately from the less hindered side of the epoxide.
:
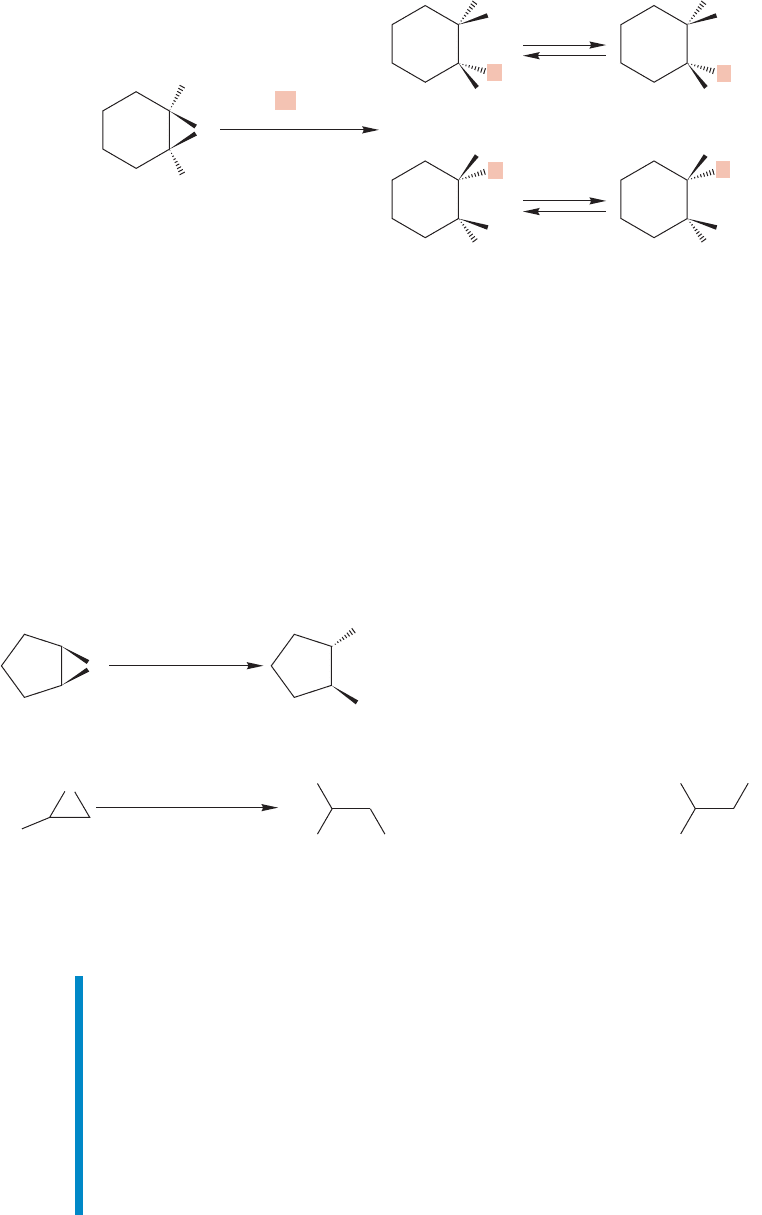
430 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
H
LiAlH
4
/ether
25 ⬚C, 3 h
H
2
O
H
2
O
O
Ph
H
O
–
Ph
H
Intermediate alkoxides
Ph
H
Ph
H
O
–
H
H
Ph
H
H
OH
(78%)
(8%)
OH
FIGURE 10.37 Lithium aluminum
hydride reduction of epoxides,
followed by hydrolysis, is yet another
pathway for alcohol synthesis.
+
O
1. Ph
2
Cu(CN)Li
2
2. H
2
O
THF, 0 ⬚C
1. (C
4
H
9
)
2
Cu(CN)Li
2
2. H
2
O
THF, 0 ⬚C
O
Ph
OH
(98%)
(85%) (8%)
Ph
HO
Ph
CH
2
CH
2
CH
2
CH
3
OH
Ph
CH
3
CH
2
CH
2
CH
2
FIGURE 10.38 Organocuprates also open epoxides.
Organometallic reagents have drawbacks, however. Reduction of the oxirane by
hydride transfer from the organometallic reagent sometimes occurs and rearrange-
ments can be induced, especially by organolithium reagents.Moreover, Grignard and
organolithium reagents inevitably add to any carbonyl groups present in the molecule
(Chapter 16).By contrast, R
2
Cu(CN)Li
2
,the organocuprates which are formed from
RLi and CuCN,open epoxides smoothly without rearrangement (Fig.10.38) and are
not reactive enough to attack a carbon–oxygen double bond.As in other S
N
2 displace-
ments, opening of the three-membered ring is from the rear and occurs predominately
at the less hindered position.
Once again, an initially produced alkoxide is protonated by water in a second step
(Fig. 10.37).
Summary
Epoxides are typically synthesized through peracid-mediated additions to alkenes.
Opening of epoxides in either acid or base is stereospecifically trans (here is the
S
N
2 reaction at work), but the regiochemistry of opening depends on the condi-
tions. In base, steric factors lead to addition of the nucleophile at the less hin-
dered position. In acid, the nucleophile adds predominantly to the protonated
epoxide at the more sterically hindered carbon.
Openings of oxiranes by organometallic reagents or hydride reagents such as
lithium aluminum hydride are valuable synthetic tools for making alcohols.
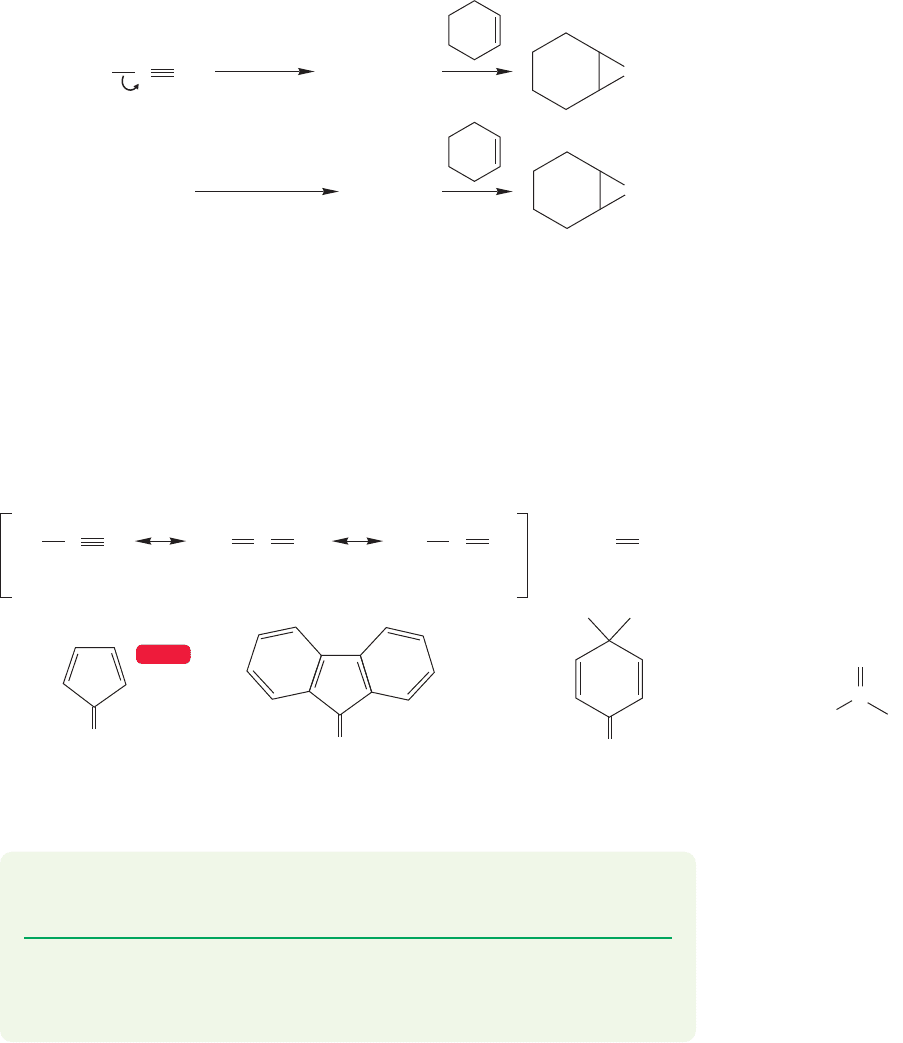
10.4 Other Addition Reactions Involving Three-Membered Rings 431
10.4d Carbenes Additions to alkenes lead to cyclopropanes through interme-
diates called carbenes. Carbenes are neutral compounds containing divalent car-
bon, and they are often formed from nitrogen-containing molecules called diazo
compounds (Fig. 10.39). Heat (Δ) or light (
hν) liberates carbenes from their par-
ent diazo compounds through loss of nitrogen, and they react rapidly with alkenes
(which are generally used as solvent for the reaction) to give cyclopropanes.
Another common source of carbenes is halocarbons. Treatment of chloroform
(CHCl
3
) or bromoform (CHBr
3
) with strong bases such as hydroxide or potassium
tert-butoxide gives dichlorocarbene or dibromocarbene (Fig. 10.39).
(a)
(b)
CH
2
..
CHX
3
N
2
OC(CH
3
)
3
..
..
..
..
–
+
h ν
(or )
Diazomethane
N
H
2
C
..
N
..
CH
2
CX
2
K
+
CX
2
+
–
(X = Cl, Br)
FIGURE 10.39 (a) Loss of nitrogen
from a diazo compound liberates a
carbene that can add to an alkene to
give a cyclopropane. (b) Dihalocarbenes
can be made by treatment of
halogenated alkanes with strong base.
Dihalocarbenes also add to alkenes.
Diazo compounds have the attractive feature (aside from being sources of
carbenes) of often being quite handsomely colored. Diazomethane (CH
2
N
2
) is
a bright yellow gas, diazocyclopentadiene an iridescent orange liquid, and
4,4-dimethyldiazocyclohexa-2,5-diene an almost blue liquid (Fig.10.40). However,
diazo compounds have a downside: They are certainly poisonous, demonstrably
explosive, and probably carcinogenic. So one must be careful (and brave, and
perhaps a little crazy) to do carbene chemistry! But this care is worth it because
carbenes are fascinating species.
+
N
Diazomethane (a yellow gas)
Diazocyclopentadiene
(an orange liquid)
4,4-Dimethyldiazocyclohexa-
2,5-diene (a purple liquid)
Diazofluorene
(an orange solid)
Methyl diazoacetate
(a yellow liquid)
N
..
..
–
++
..
..
H
2
C
..
N
..
N
H
2
C N
2
N
2
N
2
N
2
N
....
..
–
N
H
2
C H
2
C=
CHN
2
H
3
CO
..
..
–
O
C
WEB 3D
FIGURE 10.40 Some diazo
compounds. Resonance forms are
drawn only for diazomethane.
PROBLEM 10.13 Write resonance forms for 4,4-dimethyldiazocyclohexa-2,5-
diene shown in Figure 10.40.
PROBLEM 10.14 Propose a mechanism for the conversion of chloroform (CHCl
3
,
pK
a
29) into dichlorocarbene. Hint: When the reaction is carried out in
D
2
O/DO
, recovered chloroform is partially deuterated (CDCl
3
).
=
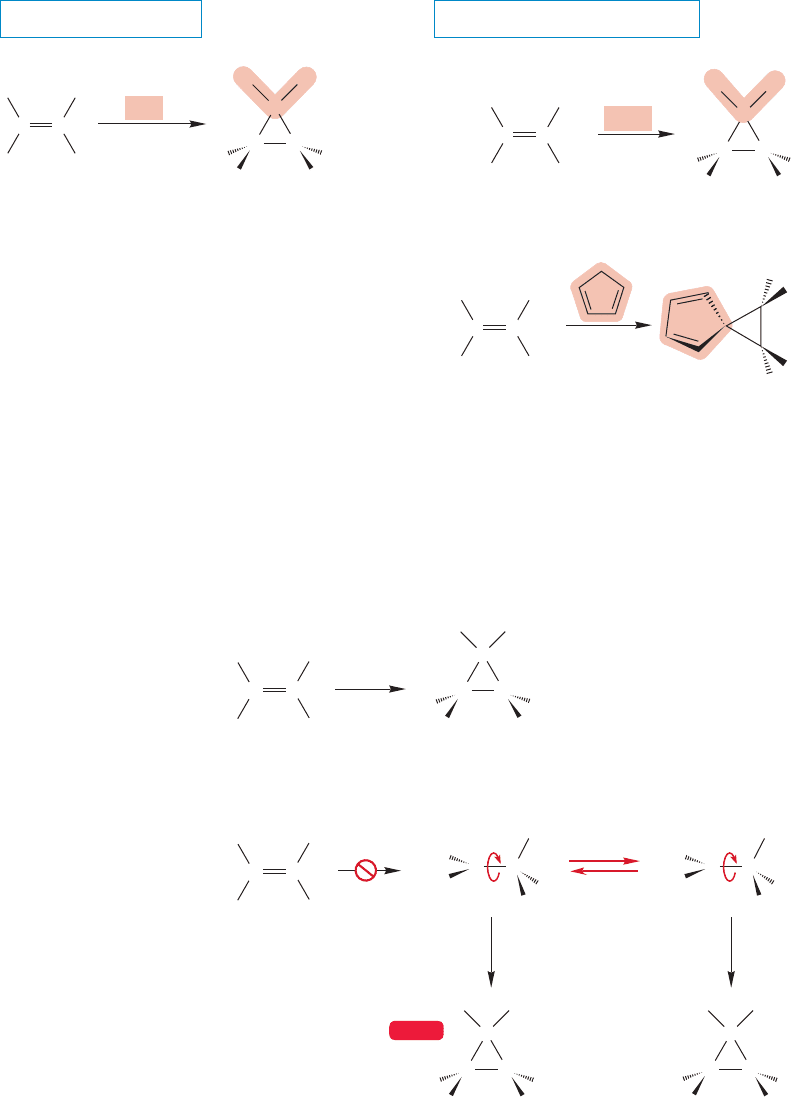
432 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
X
concerted,
syn addition
H
R
H
R
RR
HH
CX
2
CC
C
..
H
H
(70–80%)
(70%)
H
H
H
H
Br
Br
CBr
2
CH
3
CH
3
CH(CH
3
)
2
C
C
C
..
H
3
C
H
3
C
CH
3
X
HH
CH(CH
3
)
2
H
3
C
C
C
C
C
C
C
..
THE GENERAL CASE SOME SPECIFIC EXAMPLES
FIGURE 10.41 Addition of carbenes
to alkenes is a syn process with
retention of stereochemistry.
WEB 3D
Concerted reaction
H
H
H
H
Br
Br
CBr
2
CH
3
CH
3
CC CC
C
..
H
3
C
H
3
C
Br
Br
CC
C
Br
Br
CC
C
H
3
C
CH
3
H
H
CC
Nonconcerted, two-step reaction
close close
rotate
cis-1,1-Dibromo-2,3-
dimethylcyclopropane
cis-1,1-Dibromo-2,3-
dimethylcyclopropane
trans-1,1-Dibromo-2,3-
dimethylcyclopropane
H
CH
3
CBr
2
CC
..
CBr
2
.
CH
3
H
3
C
H
H
CC
CBr
2
.
.
.
H
3
C
H
H
H
3
C
H
3
C
CH
3
H
CH
3
H
H
FIGURE 10.42 The concerted
and nonconcerted mechanisms
contrasted.
Most carbenes add to the double bond of an alkene in stereospecifically syn
fashion, which means that cis alkenes react with carbenes to give only cis cyclo-
propanes and trans alkenes give the corresponding trans cyclopropanes (Fig. 10.41).
This stereospecificity tells us that there can be no intermediates capable of rota-
tion about carbon–carbon bonds in the ring-forming reaction. The mechanism
involves a single step; in the addition both new ring bonds are formed in a con-
certed fashion. A two-step addition would lead to formation of both cis and trans
cyclopropanes (Fig. 10.42).
Regardless of the mechanism, the reactions of carbenes with alkenes provide the
most widely used synthetic route to cyclopropanes.
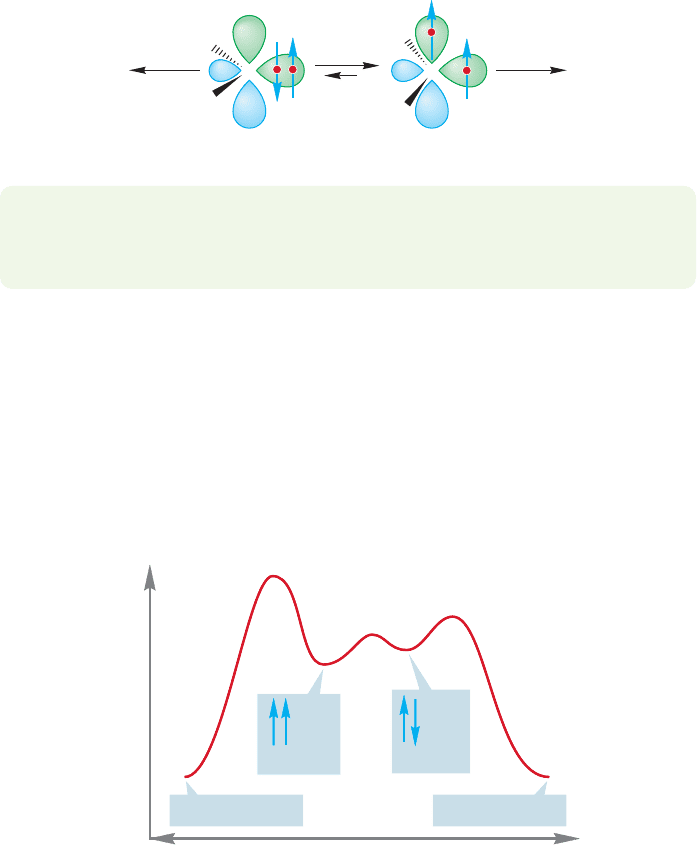
10.4 Other Addition Reactions Involving Three-Membered Rings 433
Sin
g
let carbene
Products Products
Triplet carbene
C
R
R
R
R
C
FIGURE 10.43 Both spin states of a
carbene may form products.
10.4e Triplet Carbenes One of the reasons carbenes are so interesting is that,
unlike almost all other reactive intermediates we know, they have two reactive
states, and these states are quite close to each other in energy.The reactions consid-
ered in Figure 10.41 were reactions of a carbene species in which two spin-paired
electrons occupied the relatively low-energy hybrid orbital and the higher energy 2p
orbital was empty, as shown in Figure 10.43. In fact, this species, called a singlet
carbene, generally lies a few kilocalories per mole higher in energy than another
form of divalent carbon called a triplet carbene. In the triplet species, one electron
occupies the hybrid orbital and the other has been promoted to the previously
empty 2p orbital. Of course, promotion incurs an energy cost—the approximately
sp
2
hybrid orbital has more s character than the 2p orbital, and thus an electron in
the sp
2
orbital is more stable than an electron in a pure 2p orbital. But there are
energetic compensations. For example, the two nonbonding electrons are farther
apart in the triplet and thus electron–electron repulsion is lowered. With some
exceptions (R halogen, for example), the triplets are somewhat more stable than
the corresponding singlets. For many carbenes the two species are in equilibrium,
and sorting out the chemistry can be complicated because both species can react.
=
PROBLEM 10.15 Singlet CF
2
is much more stable than the corresponding triplet.
Consider the structure of this intermediate and explain why the singlet is espe-
cially favored in this case.
:
Normally, even though the singlet is disfavored at equilibrium, reactions of the
singlet are the ones that are observed.The singlet is usually much more reactive than
the triplet. Even though there is more triplet present at equilibrium, its reactions
cannot compete with those of the small amount of the much more reactive singlet.
This point is important. A species does not have to be present in great quantity for
its chemical reactions to dominate those of a more thermodynamically stable (lower
energy) and relatively unreactive equilibrium partner. The activation barriers sur-
rounding the higher energy partner may be substantially lower than those surround-
ing the lower energy partner. Figure 10.44 shows this situation in an energy diagram.
Energy
Triplet products Singlet products
CR
2
..
Triplet
..
CR
2
Singlet
Reaction progress
FIGURE 10.44 Even though the
triplet carbene is usually lower in
energy and thus favored at
equilibrium, most of the chemistry
comes from the singlet.The triplet is
protected by quite high activation
barriers. As long as some of the
singlet is formed in equilibrium with
the triplet, the singlet accounts for
most or all of the chemistry.
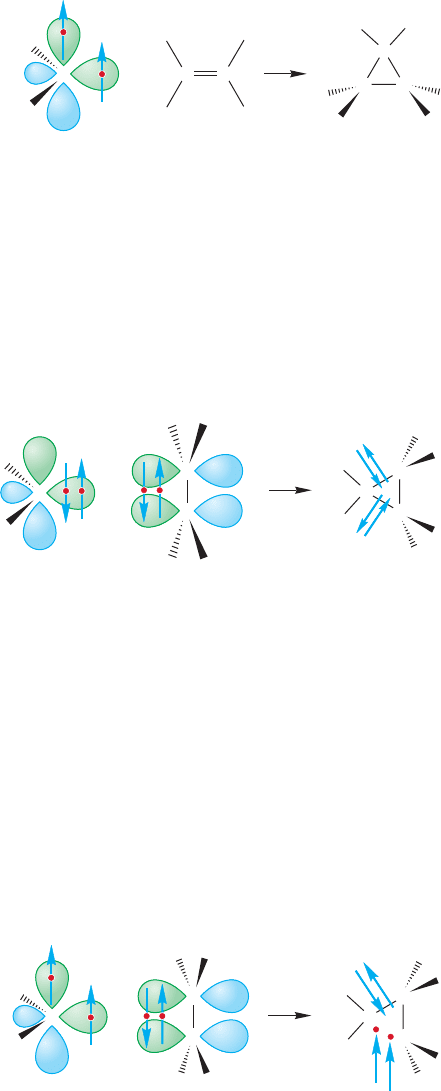
434 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
Triplet carbene
+
C
C
C
C
R
R
R
R
C
C
FIGURE 10.45 Triplet carbenes also
add to alkenes to give cyclopropanes.
C
R
R
CH
3
CH
3
CH
3
CH
3
C
C
Singlet carbene Spins in π bond
are
p
aired
All spins paired
H
H
H
H
C
R
R
C
C
FIGURE 10.46 In the addition of a
singlet carbene to an alkene, two
new bonds are formed in a concerted
fashion.
Now let’s look at electron spin. For a singlet carbene, the two nonbonding elec-
trons have paired (opposite) spins and can produce the two new cyclopropane
carbon–carbon bonds without any problem by combining with the two paired
electrons in the π bond of the alkene (Fig. 10.46). As we saw earlier (Fig. 10.41), the
reaction of a singlet carbene with an alkene takes place in a concerted fashion, pre-
serving the original stereochemical relationships of the groups on the alkene.
Sometimes the barriers to triplet reactions are not effectively higher than those
for singlets. In these cases, triplet carbene reactions can be observed. Like their singlet
counterparts, triplet carbenes add to alkenes to give cyclopropanes (Fig. 10.45).
What do we expect of the triplet? In particular, what will be the stereochemistry
of the addition reaction? The first step in the addition of a triplet carbene to an alkene
is shown in Figure 10.47. In contrast to the singlet, the triplet cannot form two new
bonds in a single step. A new species, called a diradical, is produced, with two
nonbonding electrons on different carbons. Now what can happen? Why not
simply close up to the three-membered ring and be done with it? Look closely in
Figure 10.47 at the spins of the electrons involved in the reaction.The second bond
cannot close! The two electrons that would make up the new bond have the same
spin and therefore cannot occupy the same orbital.
C
C
C
R
R
Triplet carbene
Triplet 1,3-diradical
(two electrons must
have the same spin).
A second bond
cannot be formed
Spins in
π bond
are paired
C
R
R
C
C
FIGURE 10.47 Only one bond
can be formed in the reaction of a
triplet carbene with an alkene. The
first step in triplet addition is reaction
with the π bond of the alkene to give
a 1,3-diradical.
C
O
C
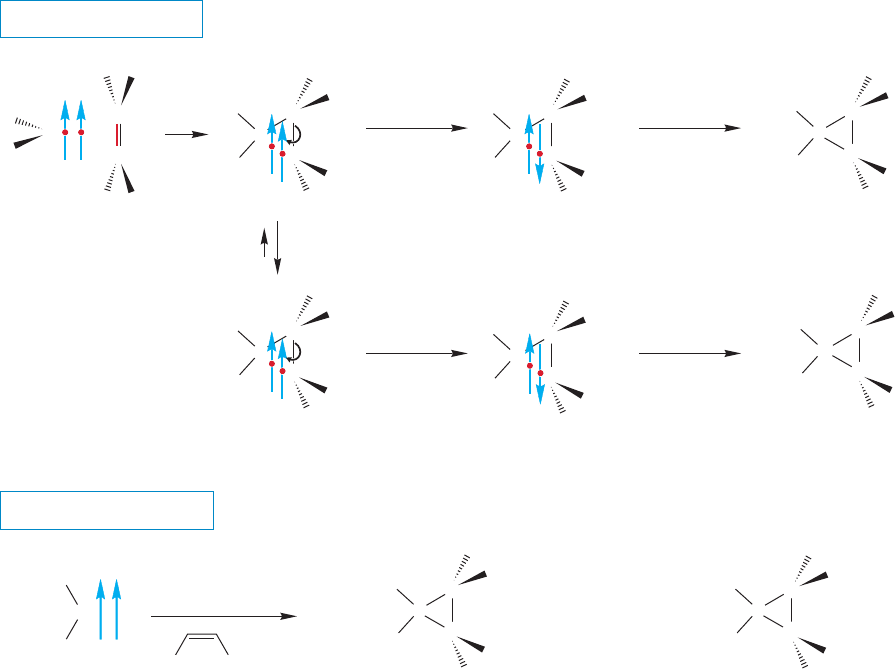
10.4 Other Addition Reactions Involving Three-Membered Rings 435
The triplet carbene, with its two nonbonding electrons of the same spin, must
produce an intermediate that cannot close to a cyclopropane until a spin is some-
how changed. Such spin “flips” are not impossible, but they are usually slow on the
time scale of molecular processes.In particular,they are slow compared with the very
rapid rotations about carbon–carbon single bonds.Therefore the stereochemistry of
the original alkene need not be maintained in the eventual product of the reaction,
the cyclopropane (Fig. 10.48).If rotation about the carbon–carbon bond is faster than
the rate at which the spin quantum number of one electron changes (spin flip), it
will not matter whether cis or trans alkene is used in the reaction with triplet meth-
ylene. The product will be a mixture of cis and trans 1,2-dialkylcyclopropanes in
either case.
CH
3
CH
3
CH
3
CH
3
C
C
C
C
C
H
H
H
H
slow
spin flip
fast
closure
C
R
R
R
R
R
R
CH
3
CH
3
C
C
C
H
H
R
R
CH
3
CH
3
C
C
C
H
H
CH
3
CH
3
C
C
C
H
cis Cyclopropane
trans Cyclopropane
H
R
R
R
R
CH
3
CH
3
C
C
C
H
H
H
CH
3
C
C
C
CH
3
H
R
R
slow
spin flip
fast
closure
rotation
CH
3
OOC
CH
3
OOC
CH
3
OOC
CH
3
OOC
H
3
C
C
CH
3
C
C
C
H
H
cis Cyclopropane
(15%)
cis Alkene
CH(CH
3
)
2
CH(CH
3
)
2
CH
3
OOC
CH
3
OOC
CH
3
C
C
C
H
H
trans Cyclopropane
(85%)
CH(CH
3
)
2
+
..
..
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 10.48 Triplet carbene addition to alkenes.The formation of an intermediate 1,3-diradical induces
scrambling of stereochemistry. Unlike singlets, which add in a single step to generate cyclopropanes
stereospecifically, triplets give both cis and trans cyclopropanes.
It turns out that triplets do give mixtures of the cis and trans cyclopropanes,
and the examination of the stereochemistry of the addition reaction of carbenes
has become the method of choice for determining the spin state of the reacting
carbene.
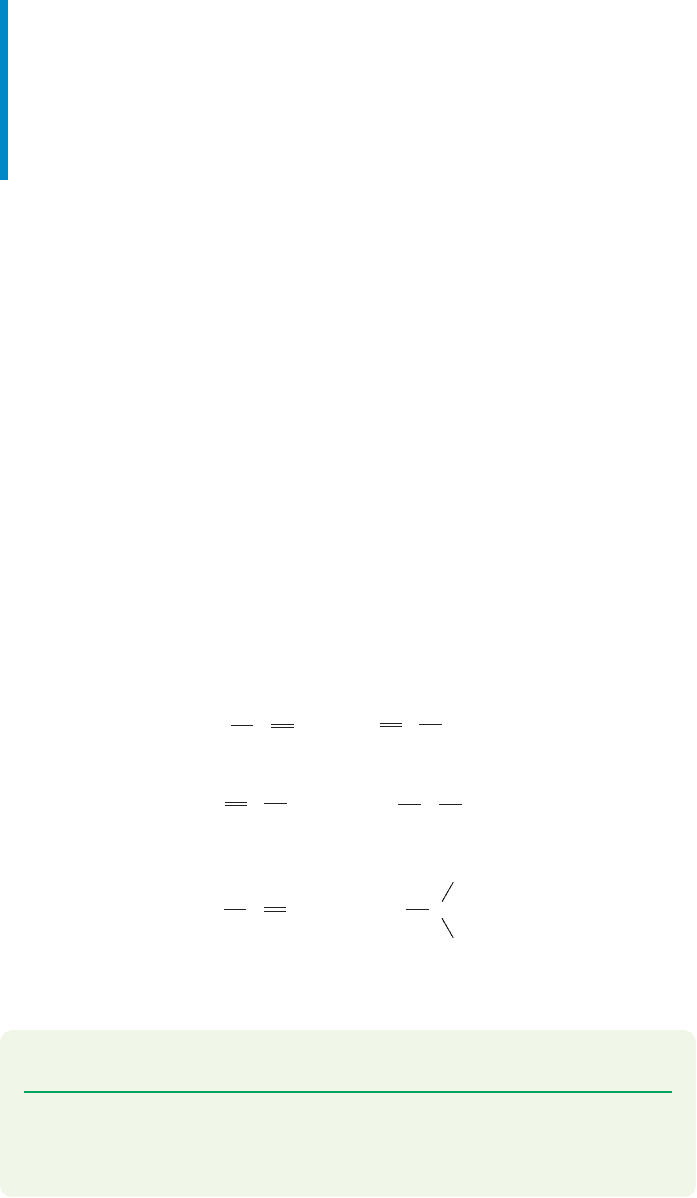
436 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
Summary
Carbenes come in two “flavors”—spin-paired singlets and spin-unpaired triplets.
Often the triplet is the more stable form, but the singlet state is usually more
reactive.Both singlet and triplet carbenes add to alkenes to give cyclopropanes.
In the concerted reaction of the singlets, any stereochemistry present in the
alkene is preserved. In the nonconcerted additions of triplets, stereochemistry
is lost.
PROBLEM 10.16 Draw more resonance forms for each 1,3-dipole in Figure 10.49.
PROBLEM 10.17 Use the arrow formalism to show the compounds formed from
reaction of the azides, nitrile oxides, and carbonyl oxides shown in Figure 10.49
with a typical alkene and alkyne.
+
+
NR
2
C
O
..
..
..
O
..
..
..
Diazo compounds
Nitrous oxide
Carbonyl oxides
Nitrile oxides
Nitrones
Azides
–
–
–
R
N
..
N
..
N
..
..
–
+
+
R
2
C
+
R
2
C
..
RC
O
..
..
O
..
..
..
–
O
..
..
..
–
N
..
RN
N
..
..
..
N
..
N
..
+
FIGURE 10.49 Some 1,3-dipolar
reagents.
10.5 Dipolar Addition Reactions: Ozonolysis
and the Synthesis of Carbonyl
( ) Compounds
So far,we have seen a number of addition reactions leading to three-membered rings,
either transient (bromonium ions, p. 414) or stable (oxiranes and cyclopropanes,
p. 423). Now we look at other additions leading to different-sized rings.These reac-
tions lead in new directions, and their products are not at all similar. Nonetheless
they are tied together and to earlier material by a mechanistic thread—they involve
an initial addition to an alkene to give a cyclic intermediate.
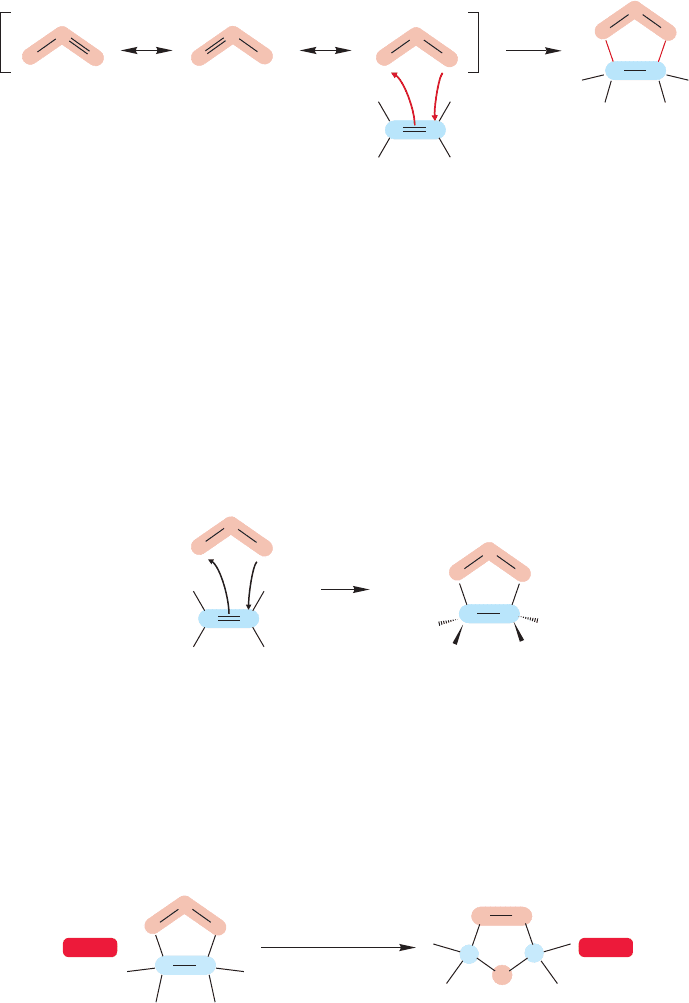
10.5a The Mechanism of Ozonolysis The precursors of carbenes, diazo
compounds (Fig. 10.39), are members of a class of molecules called 1,3-dipoles,or
1,3-dipolar reagents (Fig. 10.49). They are so called because good neutral reso-
nance forms cannot be written for them. An important characteristic of 1,3-dipoles
is that they add to alkenes to give five-membered rings.
R
2
C
P
O
This discussion of triplet carbenes and diradicals leads directly to Chapter 11,
which will concentrate on the chemistry of radicals: atoms with a single nonbond-
ing electron.
10.5 Dipolar Addition Reactions 437
Ozone (O
3
) A primary ozonide
O
..
..
..
..
..
..
..
..
O
+
O
..
O
O
–
+
CC
O
O
CC
..
..
O
..
..
..
O
..
O
O
–
+
..
..
..
–
..
..
..
..
..
O
..
..
FIGURE 10.50 A 1,3-dipolar addition
of ozone to an alkene gives a primary
ozonide, a five-membered ring
containing three oxygen atoms
in a row.
The arrow formalism lets us map out the formation of the ozonide. The
π bond of the alkene reacts with ozone to produce the five-membered ring.
These primary ozonides are extremely difficult to handle, and it took great experi-
mental skill on the part of Rudolf Criegee (1902–1975) and his co-workers at
the University of Karlsruhe in Germany to isolate the ozonide produced in
the reaction between ozone and trans-di-tert-butylethylene. A concerted
mechanism predicts that the stereochemical relationship of the alkyl groups in
the original alkene will be preserved in the ozonide, and this is what happens
(Fig. 10.51).
Ozone (O
3
) is a particularly important 1,3-dipole. The ozone molecule is often
in the news these days.There is too little of this gas in the upper atmosphere,where
it functions to screen the surface of the earth from much dangerous ultraviolet (UV)
radiation.Yet, in all too many places near the surface, there is too much of it. Ozone
is a principal component of photochemical smog.
The process called ozonolysis involves the reaction of ozone with an alkene
to give a five-membered ring containing three oxygen atoms adjacent to one
another, as shown in Figure 10.50. Such a ring is called a primary ozonide or a
molozonide.
H
C(CH
3
)
3
(CH
3
)
3
C
H
CC
C(CH
3
)
3
H
H
(CH
3
)
3
C
trans Alkene
Ozone
trans Primary ozonide
..
..
O
O
..
..
+
CC
–
O
..
..
..
..
O
..
O
O
..
..
..
..
FIGURE 10.51 The stereospecific
formation of a primary ozonide.
As implied previously, unless great care is taken, these initially formed primary
ozonides are not stable, but rearrange to another compound, known simply as an
ozonide (Fig. 10.52). The rearrangement of primary ozonides to ozonides involves a
WEB 3D WEB 3D
O
CC
C
C
Primar
y
ozonide Ozonide
–40 ⬚C to –100 ⬚C
O
O
O
O
..
..
O
..
..
..
..
..
..
..
..
..
..
FIGURE 10.52 The rearrangement of
a primary ozonide into an ozonide.
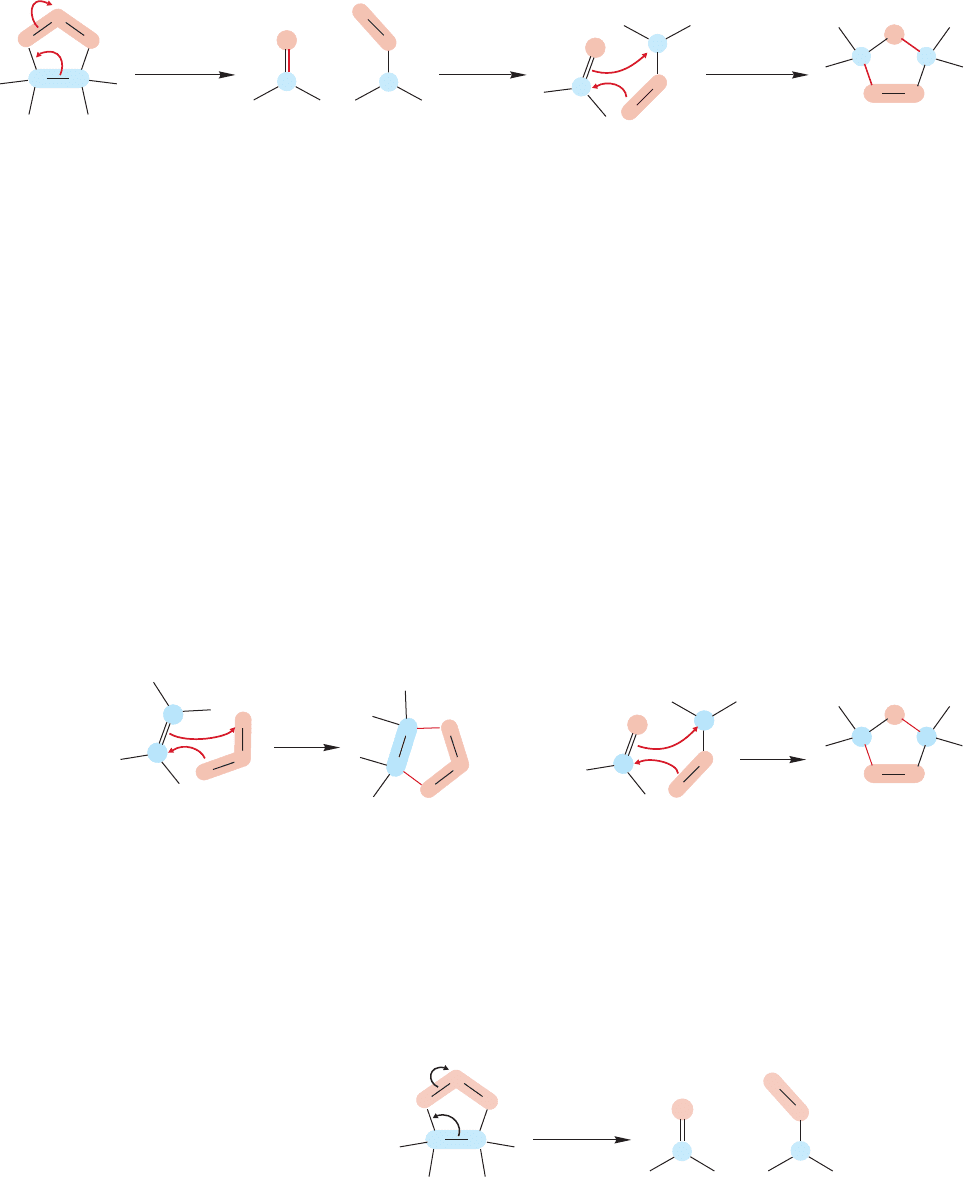
438 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
The mechanism of rearrangement from primary ozonide to ozonide may look a bit
bizarre, and there can be no hiding the fact that it’s not simple. It is not really far from
things we already know,however.More frustrating is our distance from being able to pre-
dict the course of complicated reactions.At this point, you have little choice but to learn
the course of the ozonolysis reaction, and that is not intellectually satisfying.It would be
a far better situation if you were able to predict what must happen in this multistep,multi-
intermediate process.Perhaps it is some comfort to know that you are not far behind the
current frontier.Organic chemists have only recently emerged from the “gee whiz”phase
to the point where advances in experimental skill (mostly in analytical techniques, if one
is to be honest) and theoretical methods have begun to allow reliable predictions to be
made in risky cases. All too often we are reduced to making explanations after the fact;
to rationalizing,as we are about to do with the rearrangement of one ozonide to the other.
We will take it one step at a time, working backward. In the final step,the addition
of the carbonyl oxide to a carbon–oxygen double bond is very little removed from the
addition of ozone to an alkene (Fig. 10.54a). Like ozone, the carbonyl oxide can add
to π bonds. In this case, such an addition leads to the observed ozonide (Fig. 10.54b).
(a) (b)
C
C
C
C
O
O
Primary ozonide Carbonyl
oxide
Ozone
The final ozonide
O
O
O
O
O
C
C
+
–
..
..
+
–
O
O
..
..
..
..
..
..
..
..
..
O
..
..
O
..
..
..
O
..
..
..
..
..
..
..
..
..
..
C
C
FIGURE 10.54 Like diazo compounds and ozone, a carbonyl oxide is a 1,3-dipole and adds to π systems to give
five-membered ring compounds.
Carbonyl
compound
Primary ozonide Carbonyl
oxide
reverse
1,3-dipolar
addition
O
CC
O
O
..
..
O
O
+
–
C
O
+
..
..
..
..
..
..
..
..
..
..
..
C
FIGURE 10.55 The first step in
the formation of an ozonide from
a primary ozonide is a reverse
1,3-dipolar addition to generate a
carbonyl compound and a carbonyl
oxide.
The first step in the conversion of the primary ozonide into the ozonide is anoth-
er 1,3-dipolar addition, but this time in reverse, and it is therefore a bit harder to
see (Fig. 10.55). The reaction is completed when the carbonyl oxide turns over and
re-adds to the carbonyl compound (Fig. 10.54b).
pair of reverse and forward 1,3-dipolar additions (Fig. 10.53). The first step in the
rearrangement is the opening of the primary ozonide to give a molecule containing a
carbon–oxygen double bond called a carbonyl compound,along with a carbonyl oxide,
a new 1,3-dipole (see Fig.10.49).These two species can recombine to give the ozonide.
O
CC
O
O
..
..
C
C
O
O
Primary ozonide Carbonyl
compound
Carbonyl
oxide
Ozonide
reverse
1,3-dipolar
addition
forward
1,3-dipolar
addition
O
O
O
turn
carbonyl
oxide over
O
C
+
+
–
–
C
O
C
..
..
O
C
+
..
..
..
..
..
O
..
....
..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
FIGURE 10.53 The mechanism of rearrangement by which a primary ozonide becomes an ozonide involves two steps.
In the first, a reverse 1,3-dipolar addition generates a carbonyl compound and a carbonyl oxide.The carbonyl oxide
then re-adds to the carbonyl in the opposite sense to give the new ozonide.
