Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

10.5 Dipolar Addition Reactions 439
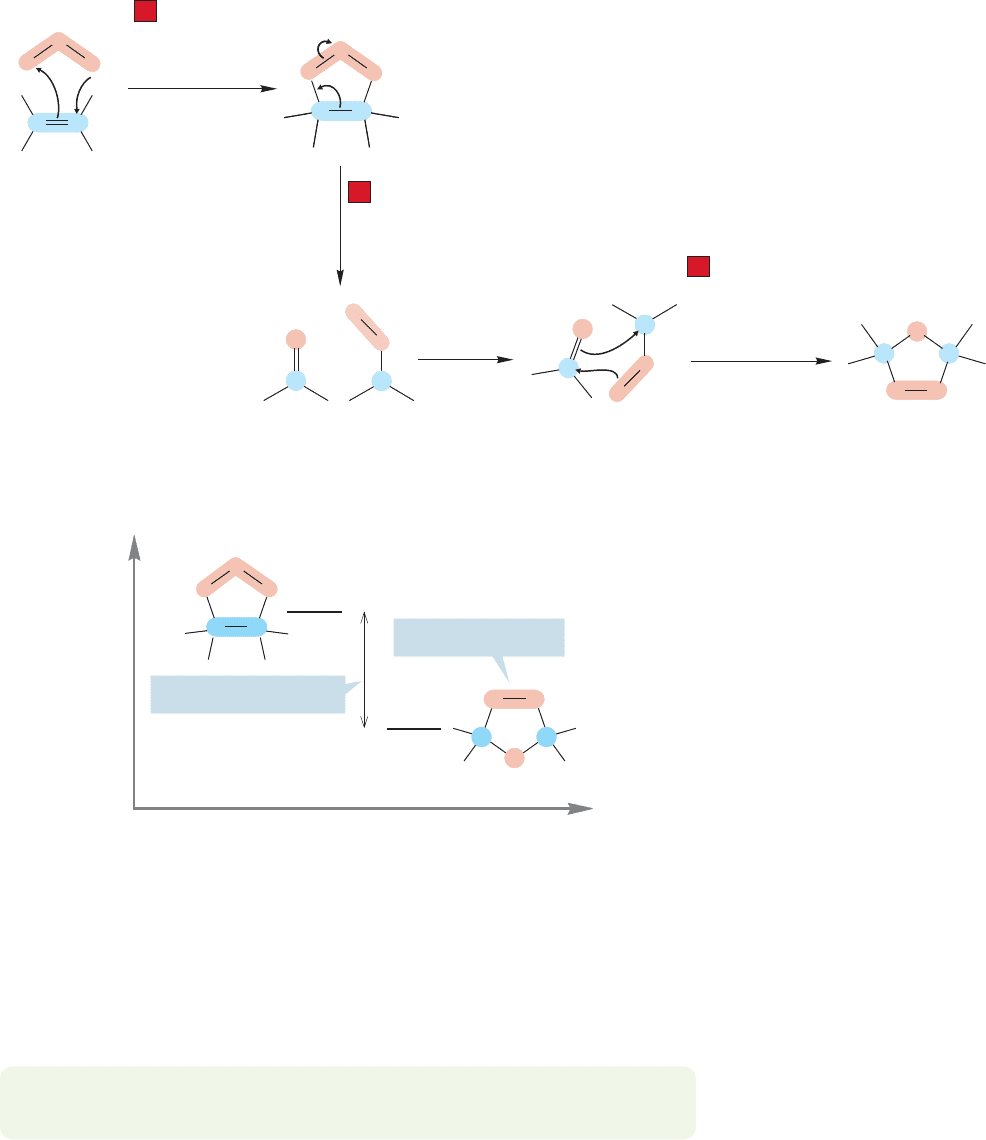
The overall reaction is a series of three 1,3-dipolar additions (Fig. 10.56). First,
ozone adds to the alkene to give the primary ozonide. Then the primary ozonide
undergoes a reverse 1,3-dipolar addition to give a carbonyl compound and a new
1,3-dipole, the carbonyl oxide. Finally, the carbonyl oxide turns over and re-adds to
the carbonyl compound in the opposite sense to give the new ozonide.The reaction
is a sequence of three reversible 1,3-dipolar additions driven by thermodynamics
toward the relatively stable ozonide.
Now that we have described the reaction, however, the tougher part is to explain
why it runs the way it does. Why does thermodynamics favor the final, more stable
ozonide over the primary ozonide (Fig. 10.57)?
A forward
1,3-dipolar
addition
reaction
1
A reverse
1,3-dipolar
addition
reaction
2
A second
forward
1,3-dipolar
addition
reaction
3
turn
carbonyl
oxide over
O
CC
O
O
..
..
O
O
O
O
O
C
C
+
+
–
–
..
..
C
..
..
..
..
..
..
..
..
..
..
C
O
..
..
O
..
..
+
CC
–
O
..
..
..
O
..
..
C
C
O
O
O
..
..
..
..
..
..
..
..
..
..
FIGURE 10.56 In the formation of
the final ozonide from ozone and an
alkene, Steps 1 and 3 are 1,3-dipolar
additions. Step 2 is the reversal of a
1,3-dipolar addition.
Energy
Reaction progress
This energy difference…
…favors this ozonide
O
CC
O
O
..
..
..
..
..
..
CC
O
..
..
O
O
..
..
..
..
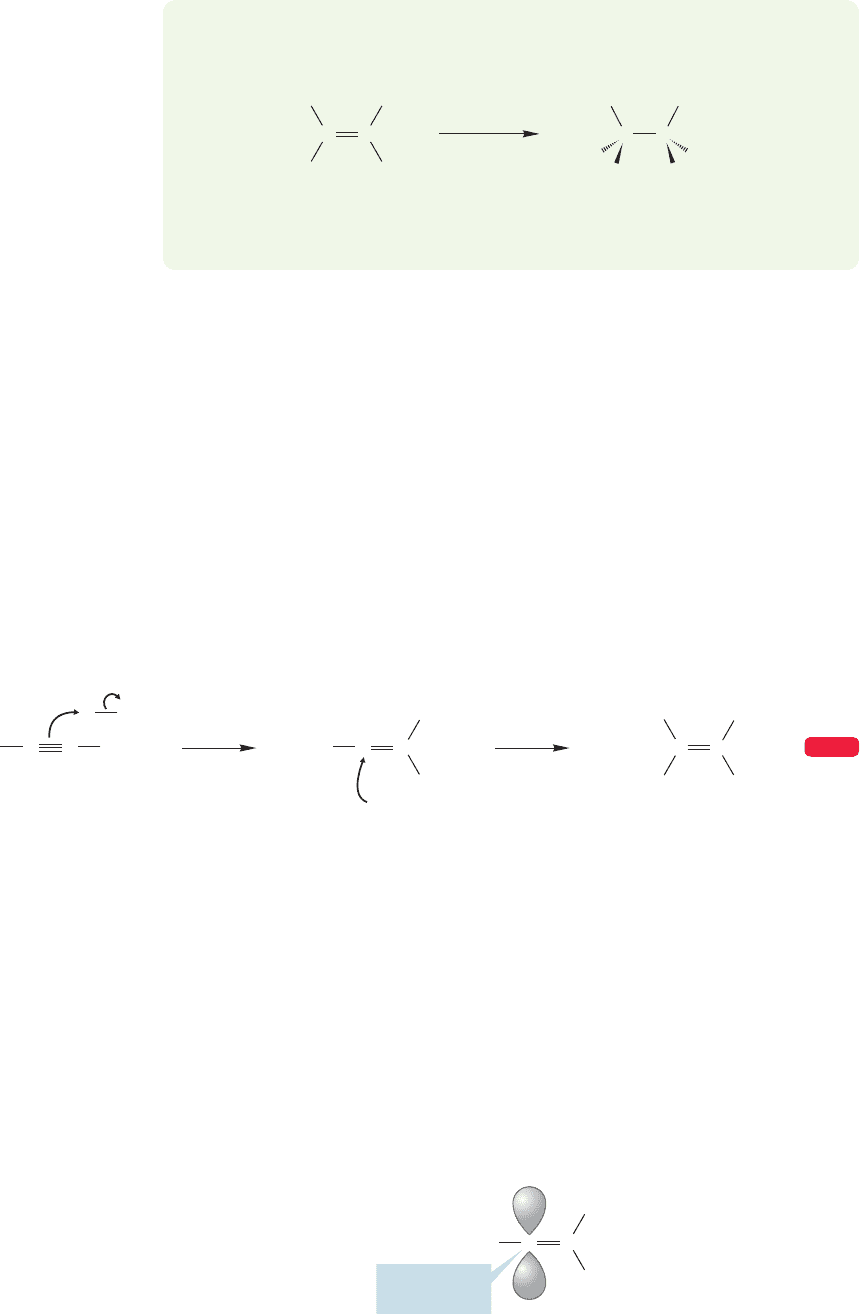
FIGURE 10.57 The ozonide contains
fewer weak oxygen–oxygen bonds
than the primary ozonide and is
therefore more stable.
The oxygen–oxygen bond is quite weak ( 40 kcal/mol). Much more stable,
though, is the carbon–oxygen bond (85–90 kcal/mol). The most important factor
that makes an ozonide more stable than a primary ozonide is the difference in the
numbers of weak oxygen–oxygen bonds and strong carbon–oxygen bonds. As long
as the kinetic barriers to the forward and reverse 1,3-dipolar additions are not too
high, thermodynamics favors formation of the stronger carbon–oxygen bonds and
the ultimate result is the final, more stable, ozonide.
'
PROBLEM 10.18 Use Table 8.2 (p. 337) to do a full calculation of the bond energy
difference between a primary ozonide and an ozonide.
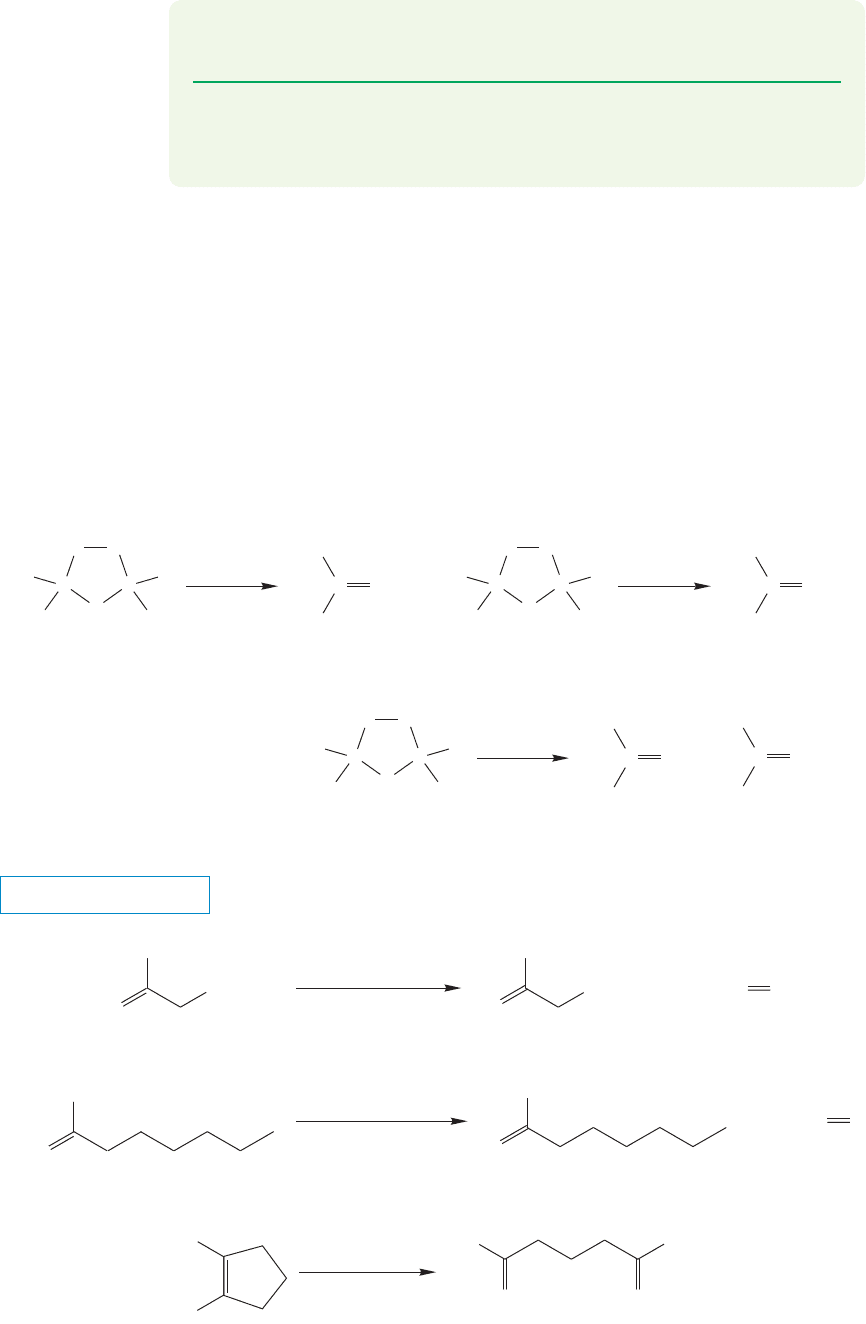
440 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
(75%)
1. O
3
, –30 C
2. (CH
3
)
2
S, ~0 C
(74%)
1. O
3
, –78 C
2. H
2
/ Pd
CC
O
RR
RR
R
R
O
O
Ketones Aldehydes
reductive
workup
C
2
O
CC
O
RH
RR
R
R
O
O
reductive
workup
C
O
reductive
workup
2
CC
O
HH
RR
R
H
O
O
C
O
One ketone
+
One aldehyde
R
H
C
O
H
O
H
2
C
1. O
3
, –78 C, 16 h
(93%)
2. Zn/H
2
O
CH
3
CH
3
O
C(CH
3
)
3
C(CH
3
)
3
H
2
C
H
2
CO
H
3
C
H
3
C
O O
H
3
C
CH
3
H
SPECIFIC EXAMPLES
+
H
2
CO
+
FIGURE 10.58 Reductive
decomposition of ozonides leads to
aldehydes and/or ketones.
10.5b The Synthetic Potential of Ozonolysis: Carbonyl Compounds
Why do we spend so much time with this complicated reaction? We have already
mentioned generality. A vast number of 1,3-dipolar agents has been made, and
many of them will add to π bonds to give five-membered rings.
Second, and more important, the ozonides are very useful compounds. Their
further transformations give us entry into new classes of compounds. Ozonides can
be reduced (Fig. 10.58) and, depending on the structure of the starting alkene, the
products can be ketones ( ), which are compounds that have two R groups
attached to the carbonyl carbon; aldehydes ( ), which are compounds
that have an R group and a H attached to the carbonyl; or a mixture of the two. Many
reducing agents are known, among them H
2
/Pd,Zn, and (CH
3
)
2
S (dimethyl sulfide).
RCH
P
O
R
2
C
P
O
PROBLEM 10.19 Why are oxygen–oxygen sigma bonds so weak? Hint: It is not just
electron–electron repulsion. Do a quick molecular orbital analysis.
PROBLEM 10.20 Complete the Energy versus Reaction progress diagram of
Figure 10.57 to show the conversion of the primary ozonide into the ozonide.
Assign the intermediates to the appropriate points in the reaction pathway.
10.5 Dipolar Addition Reactions 441
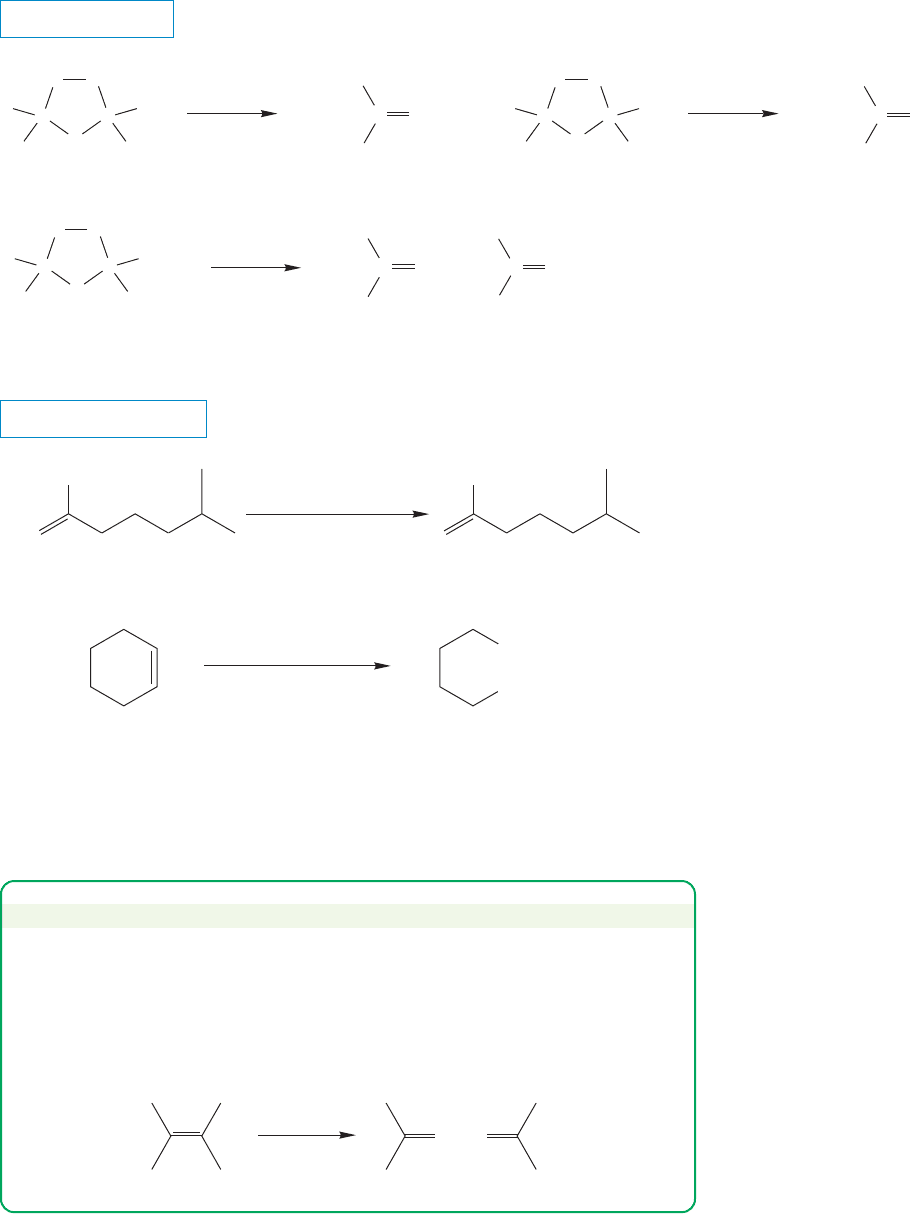
Oxidation of the ozonides, usually with hydrogen peroxide (H
2
O
2
), leads either
to ketones or carboxylic acids (see Fig. 10.24). The structure of the product again
depends on the structure of the ozonide, which itself depends on the structure of
the original alkene (Fig. 10.59).
(67%)
1. O
3
, –78 C
2. H
2
O
2
/CH
3
COOH
OHH
(85%)
1. O
3
, –70 C
2. H
2
O
2
/HCOOH
COOH
COOH
H
2
C
O
R
R
R
R
Ketones
oxidative
workup
C
O
2
One acid One ketone
+
C
O
oxidative
workup
R
2
Carboxylic acids
C
O
HO
oxidative
workup
CC
O
RR
RR
R
HO
O
O
C
O
CC
O
H
R
RR
O
O
CC
O
HH
RR
O
O
GENERAL CASES
SPECIFIC EXAMPLES
+
HCOOH
FIGURE 10.59 Oxidative
decomposition of ozonides leads to
ketones and/or carboxylic acids.
In addition to providing a synthetic pathway for numerous carbonyl compounds,
ozonolysis has another use for us: The structures of the products of an ozonolysis
reaction can be used to reason out the composition of an unknown alkene. To see
ozonolysis used this way, try Problem 10.21.
1. O
3
2. workup
O
R
1
R
2
O
R
3
R
4
R
1
R
2
R
3
R
4
PROBLEM SOLVING
Whenever you see ozone (O
3
) in a problem, you can bet the farm that a double
bond is going to be converted into a pair of carbonyl compounds. The question
may be mechanistic or synthetic in nature, but that processs is going to be
involved. It is not a bad idea at this early point to jot down the general scheme
shown below whenever you see ozone.
442 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
ANSWER (c) Ozonolysis converts a carbon–carbon double bond into two carbon–
oxygen double bonds.
[from two starting materials,
(1) C
6
H
10
(2) C
12
H
20
]
OH
HO
O
O
O
H
O
O
OH
OH
HO
O
O
H
O
(a)
*(c)
(d)
(b)
+
+
2
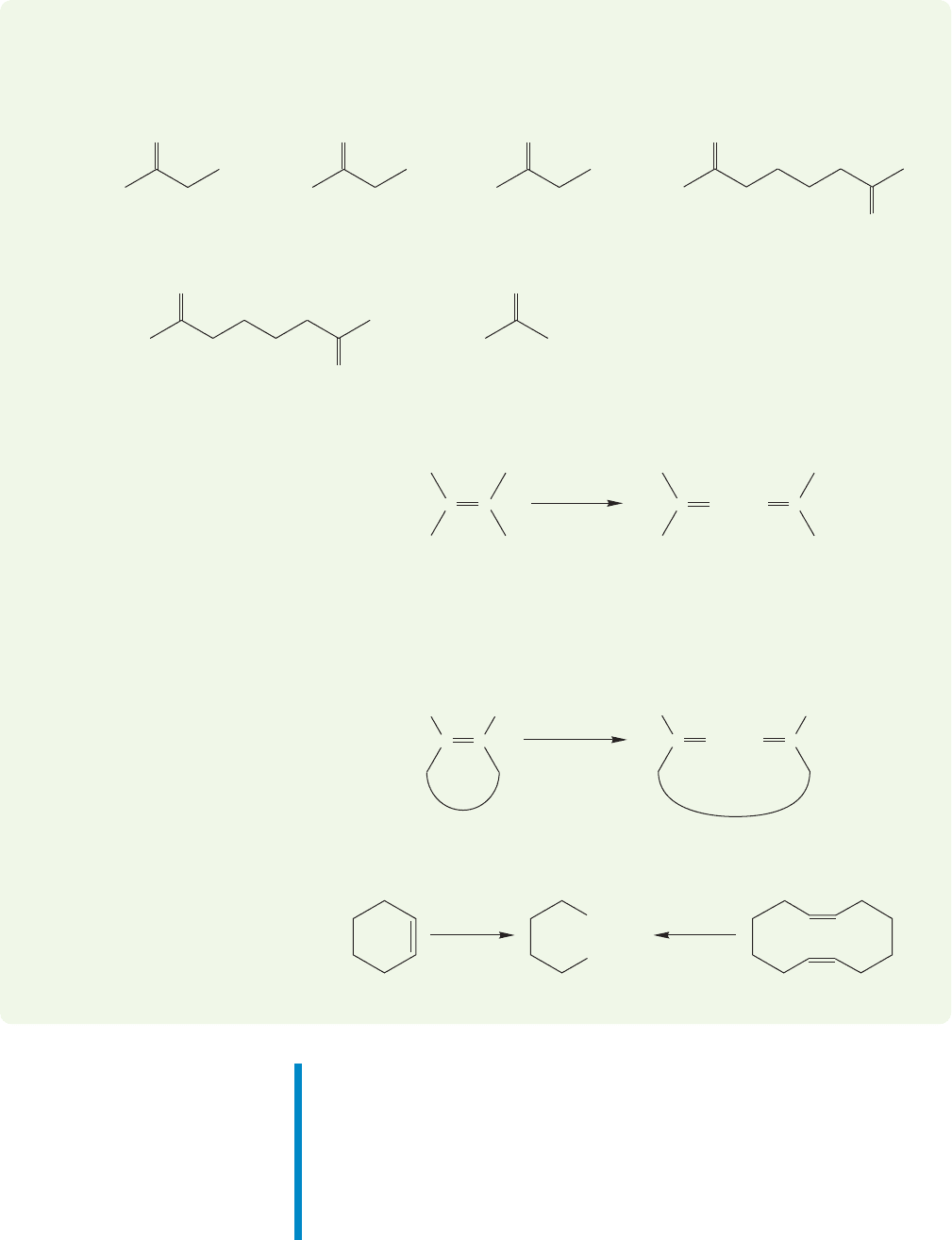
WORKED PROBLEM 10.21 Ozonolysis of alkenes can give the carbonyl products
shown below. Supply an alkene structure that can produce the carbonyl compounds
shown.Specify the nature of the workup step in the process (oxidation or reduction).
In this case, both carbon–oxygen double bonds are in the same product molecule,
which means that the starting material must be a cyclic alkene.The product con-
tains carboxylic acids, so we know that an oxidative workup is required. A reduc-
tive workup would give an aldehyde.
1. O
3
2. oxidative
or reductive
workup
O
+
C C
C
O
C
1. O
3
2. oxidative
workup
C
C
C
HOHHOH
OO
+
C
CC
There are several ways to construct an appropriate starting material. Two
possibilities are
1. O
3
2. HOOH
COOH
COOH
1. O
3
(C
6
H
10
)(C
12
H
20
)
2. HOOH
Summary
Ozonolysis of alkenes breaks both bonds in the carbon–carbon double bond and
generates a pair of carbonyl groups. A double bond carbon bearing two alkyl
groups is transformed into a ketone ( ) regardless of workup conditions.
When a double bond carbon bears one alkyl group and one hydrogen it is trans-
formed into an aldehyde ( ) if the workup is done under reducing con-
ditions and a carboxylic acid (RCOOH) if oxidizing conditions are used.
RHC
P
O
R
2
C
P
O
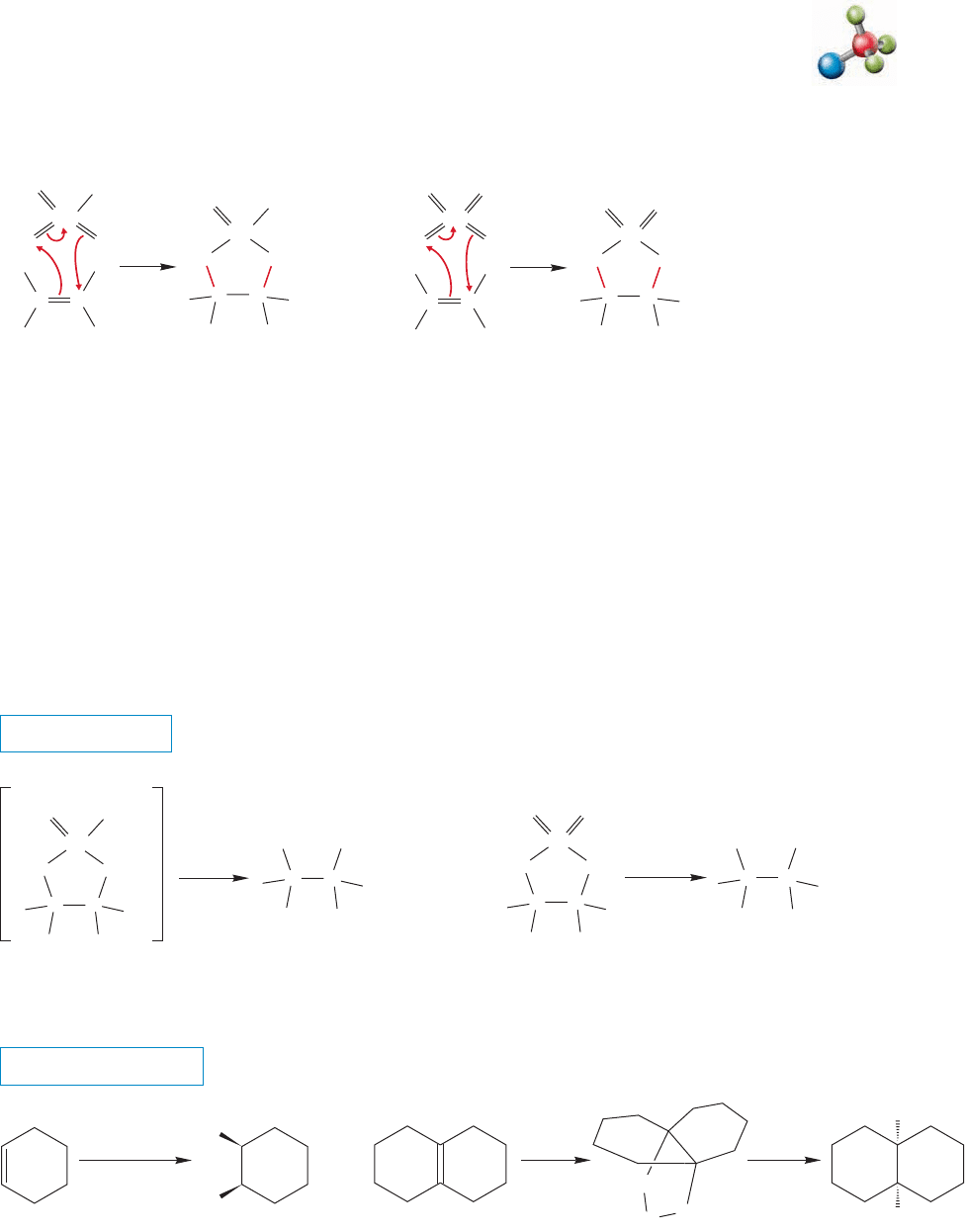
10.5 Dipolar Addition Reactions 443
10.5c Oxidation with Permanganate or Osmium Tetroxide Although
they are not 1,3-dipolar reagents, both potassium permanganate (KMnO
4
) and
osmium tetroxide (OsO
4
) add to alkenes to form five-membered rings in
reactions that are related to the additions of ozone and other 1,3-dipoles
(Fig. 10.60).
K
+
K
+
–
–
..
..
..
..
..
..
Osmate
ester
Mn
Mn
O
O
..
..
O
O
..
..
..
O
..
..
CC
C
C
O
O
..
..
..
..
..
..
..
..
..
..
..
..
..
Os
Os
O
O
O
O
O
O
..
..
..
..
..
..
..
..
O
..
..
CC
O
O
C
C
FIGURE 10.60 Addition of potassium
permanganate or osmium tetraoxide
to alkenes gives five-membered rings.
The cyclic intermediate in the permanganate reaction cannot be isolated and
is generally decomposed as it is formed to give vicinal diols (Fig. 10.61a). Good
yields of cis 1,2-diols can be isolated from the treatment of alkenes with basic
permanganate. The cyclic osmate ester can be isolated, but it, too, is generally
transformed directly into a diol product by treatment with aqueous sodium sul-
fite (Na
2
SO
3
) as shown in Figure 10.61b. In both of these reactions it is the
metal–oxygen bonds, not the carbon–oxygen bonds, that are broken. There are
many variations of these reactions, which are among the best ways of synthesiz-
ing vicinal diols.
(a) (b)
(85%)
CH
3
OH/H
2
O
NaOH, 20 ⬚C
(96%) (81%)
H
2
O
MnO
2
..
..
HO
..
..
–
OH
..
..
..
OH
..
..
OH
..
..
Not isolated Can be isolated,
but often is not
Vicinal diol
+
K
+
–
..
Mn
..
..
O
..
..
..
O
CC
O
O
CC
..
..
..
..
..
Os
..
..
..
..
O
O
CC
O
O
..
..
..
..
..
H
2
O
Na
2
SO
3
H
2
OsO
4
KMnO
4
..
HO
HO
HO
HO
HO
..
..
Vicinal diol
+
C
C
OsO
4
H
2
O
Na
2
SO
3
O
O
O
2
Os
GENERAL CASES
SPECIFIC EXAMPLES
FIGURE 10.61 A five-membered ring containing either (a) Mn or (b) Os can react further to generate vicinal diols.
Dihydroxylation of alkenes
444 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
cis-2-Butene meso-2,3-Butanediol
(shown in eclipsed
conformation for clarity)
H
3
C
H
3
C
CH
3
CH
3
H
H
CC
H
H
HO
OH
CC
1. OsO
4
2. H
2
O
Na
2
SO
3
PROBLEM 10.22 What mechanistic conclusions can be drawn from the observa-
tion that reaction of OsO
4
with cis-2-butene gives this single product?
WEB 3D
–
Cl
..
..
..
..
Cl
..
..
..
Cl
..
..
..
A vinyl cation
CC
H
CH
3
CH
2
CH
2
CH
3
CH
2
CH
3
CH
2
CH
3
C
+
CCH
3
CH
2
CH
3
CH
2
H
H
(60%)
25 °C 25 °C
CC
(Z)-3-Chloro-3-hexene
(the ethyl groups are mostly
trans to each other
in the product)
FIGURE 10.62 Hydrogen halides add
to alkynes as well as to alkenes. Addition
is mostly anti.
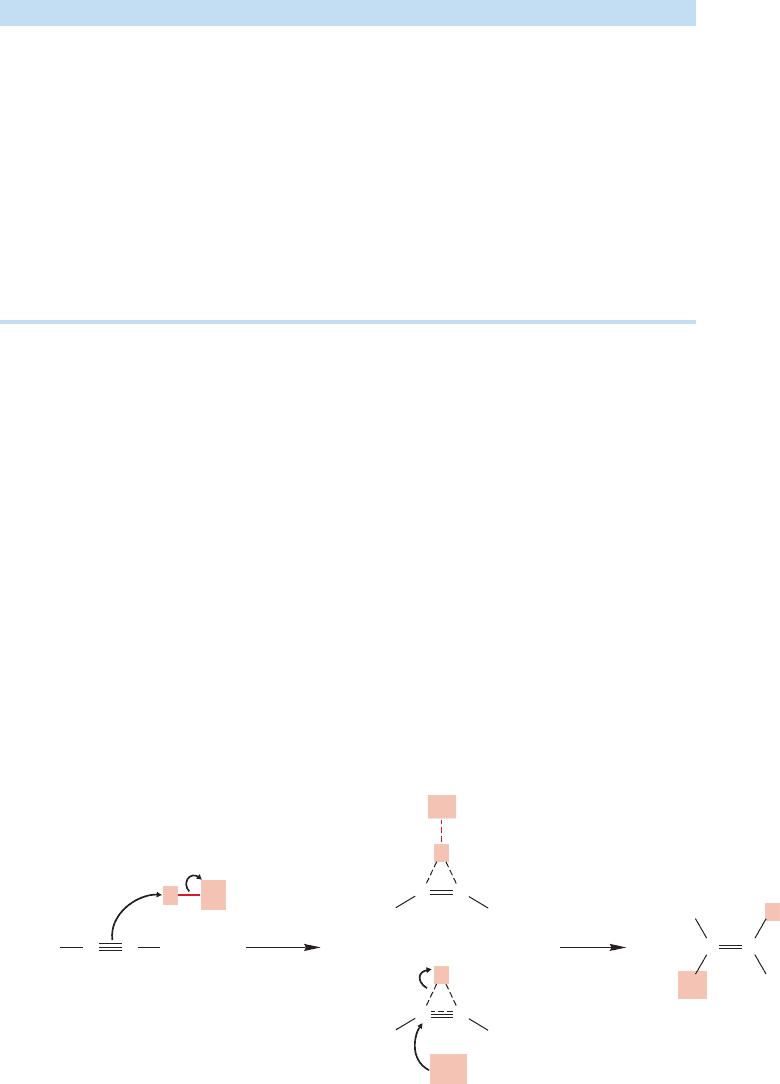
The stereochemistry of this addition is mixed, as would be expected from an open
carbocation, although the trans diethyl compound is favored (Fig. 10.62).
However, there are problems with this simple extension to alkynes of the mech-
anism for additions to alkenes. Vinyl cations are unusual species and we must stop
for a moment to consider their structures. The positive carbon in a vinyl cation is
attached to two groups: the other carbon in the original triple bond and an R group.
Accordingly, we would expect sp hybridization, and that leads to the structure in
Figure 10.63.
sp Hybridized
carbon
CH
2
CH
3
CC
CH
3
CH
2
H
+
FIGURE 10.63 A vinyl cation
contains an sp hybridized carbon.
10.6 Addition Reactions of Alkynes: Addition
Much of Chapters 9 and 10 has been devoted to the addition of various reagents to
carbon–carbon double bonds. Alkynes contain two π bonds and it would be quite
astonishing if they did not undergo similar addition reactions. If we keep in mind
what we have learned about alkene additions, it is reasonably easy to work through
alkyne additions.The presence of the second double bond will add mechanistic com-
plications, however, and some synthetic opportunities as well.
Addition of or to 3-hexyne gives the corresponding vinyl halides.
It is tempting to begin with a protonation of the π system to give a carbocation
and a halide ion as shown in Figure 10.62. In this case, the positive ion would be
a vinyl cation. Addition of the halide to this cation would give the vinyl halide.
HClHBr
HX
10.6 Addition Reactions of Alkynes: HX Addition 445
Measurements in the gas phase show that vinyl carbocations are very unstable.
Remember that the gas-phase heat of formation values do not include the solvent,
which is very important to any cationic species. Remember also that direct com-
parisons can be made only between isomeric species. Nonetheless, one can get an
approximate idea of the great instability of vinyl carbocations from Table 10.2.The
absolute values of the energy differences are not important, but the relative stability
order of the various carbocations is.
TABLE 10.2 Heats of Formation for Some Carbocations
Cation Substitution (kcal/mol)
Primary vinyl 285
+
CH
3
Methyl 261.3
Secondary vinyl 231
+
CH
2
CH
3
Primary 215.6
+
CH
2
CH
2
CH
3
Primary 211
+
CH
2
CH
2
CH
2
CH
3
Primary 203
Secondary 183
Secondary 190.9
Primary allyl 226
(CH
3
)
3
C
+
Tertiary 165.8
CH
2
P
CH
O
C
+
H
2
(CH
3
)
2
C
+
H
H
3
CC
+
HCH
2
CH
3
CH
3
O
C
+
P
CH
2
+
CH
P
CH
2
≤H°
f
Table 10.2 shows that,just as with alkyl carbocations,secondary vinyl cations are more
stable than primary vinyl cations.However,it also shows that even a secondary vinyl cation
is not even as stable as a primary alkyl carbocation. Primary carbocations serve as a kind
of mechanistic stop sign: They are generally too unstable to be viable intermediates in
most reactions. Presumably, we should treat unstabilized vinyl cations the same way.
What intermediate could we use to replace the highly unstable vinyl cations in
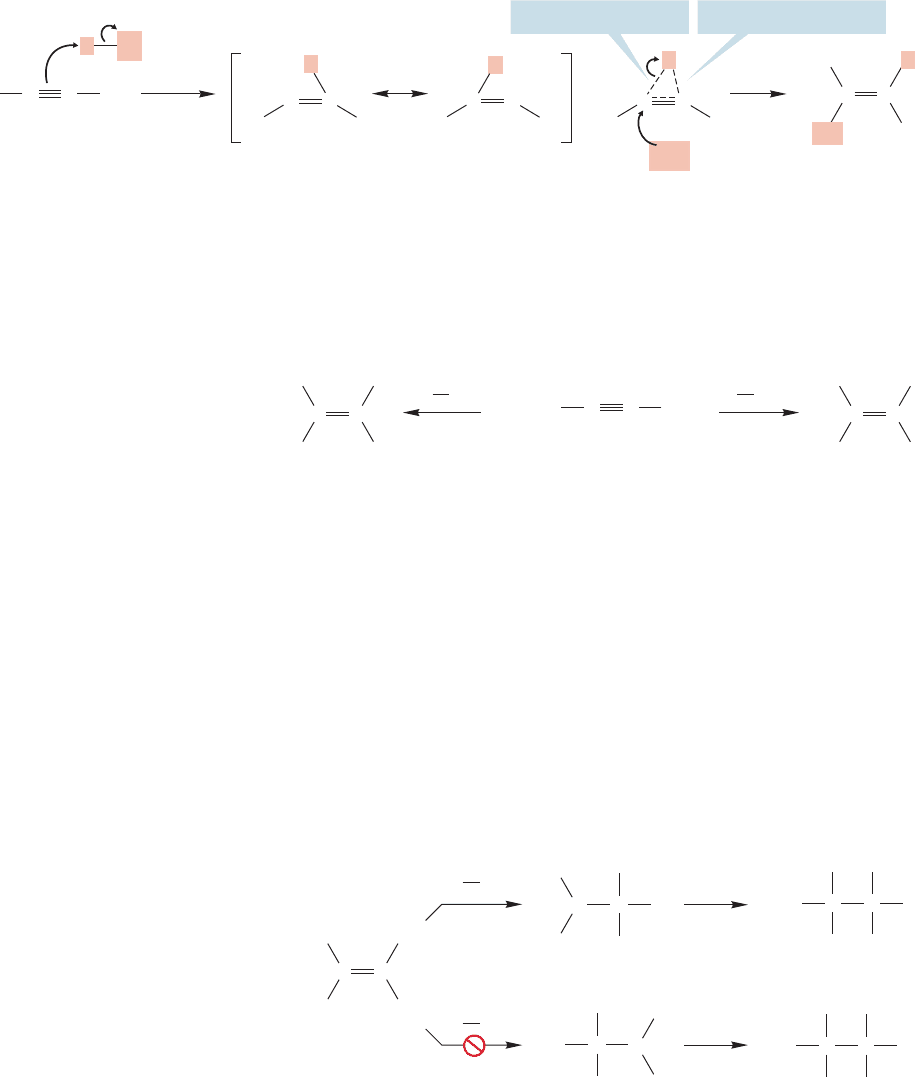
addition reactions involving alkynes? Perhaps cyclic intermediates are involved.
Moreover, alkynes are known to form complexes with HX acids. A cyclic protonium
ion or complex could accommodate the complicated kinetics, which show that more
than one molecule of halide is involved, and also account for the generally observed
predominance of trans addition (Fig. 10.64). We will write cyclic ions in the answers
to problems,but you should know that the intermediacy of vinyl cations in these reac-
tions is not a fully resolved issue. There is still lots to do in organic chemistry.
The modified mechanism shown in Figure 10.64 predicts that Markovnikov addi-
tion should be observed: that the more substituted secondary vinyl halide should be
δ
+
δ
+
δ
–
+
C
C
–
Cl
..
..
..
..
Cl
..
..
..
Cl
..
..
..
and/or
CC
H
CH
3
CH
2
CH
2
CH
3
CH
2
CH
3
CH
2
CH
3
+
CH
3
CH
2
CH
3
CH
2
H
H
CC
25 ⬚C
C
C
CH
2
CH
3
CH
3
CH
2
H
Cl
..
..
..
FIGURE 10.64 Possible cyclic
intermediates in the reaction of an
alkyne and hydrogen chloride.
446 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
–
Cl
..
..
..
..
Cl
..
..
..
CR
R
R
R
R
C
H
H
+
H
Markovnikov addition
p
redicted to be favored
C
C
C
C
H
=
H
Shorter, stronger bond
Longer, weaker bond
+
C
C
H
H
C
C
+
H
H
H
Cl
..
..
..
FIGURE 10.65 Addition of the
nucleophile to the cyclic ion takes
place at the more substituted position.
formed whenever there is a choice (Fig. 10.65). In the cyclic intermediate, there is a par-
tial positive charge on the carbons of the original triple bond.The partial bond to hydro-
gen from the more substituted carbon of an unsymmetrical alkyne is weaker than the bond
from the less substituted carbon because the partial positive charge is more stable at the
more substituted position. Accordingly, addition of halide would be expected to take
place more easily at the more substituted position, leading to Markovnikov addition.
H
3
C
H
3
C
CCH
H
H
(56%)
2-Chloropropene
Propyne
C
C
..
..
..
Cl
..
..
..
..
..
..
H
3
C
H
H
(35%)
2-Iodopropene
C
C
I
..
..
..
H I
HCl
FIGURE 10.66 Markovnikov addition
generally predominates in reactions
of HX with alkynes.
Let’s look at the experimental data.There is no difference in the two carbons of
the triple bond in a molecule such as 3-hexyne, so let’s examine the addition to a
terminal alkyne—a 1-alkyne—to see if Markovnikov addition occurs.Our mechanism
predicts that the more substituted halide should be the product, and it is.The prod-
ucts shown in Figure 10.66 are the only addition products isolated.
Additions of HX to alkynes are mechanistically complex. Also, they are usually not
practical sources of vinyl halides because the vinyl halides also contain π bonds and often
compete favorably with the starting alkyne in the addition reaction. The vinyl halide
products are not symmetrical and two products of further reaction are possible (p.367).
It makes a nice problem to figure out which way the second addition of
should go. As always in a mechanistic problem of this sort, the answer only appears
through an analysis of the possible pathways for further reaction.Draw out both mech-
anisms, compare them at every point, and search each step for differences.We will use
the reaction of propyne with hydrogen chloride as a prototypal example. The initial
product is 2-chloropropene,as shown in Figure 10.66.There are two possible ions from
the reaction of this vinyl halide with additional hydrogen chloride. Structure A in
Figure 10.67 has the positive charge adjacent to a chlorine, but structure B does not.
HX
HC
H
H
C
H
H
H
A
B
C
–
Cl
..
..
..
..
Cl
..
..
..
Cl
..
..
..
Two possible
carbocations
2,2-Dichloropropane
2-Chloropropene
H
3
C
H
3
C
HH C
H
C
1,2-Dichloropropane
CH
3
CH
3
C
+
Cl
..
..
..
H
H
H
C
–
Cl
..
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
+
C
H
3
C
H
H
C
C
Cl
..
..
..
..
..
..
..
..
..
HCl
HCl
FIGURE 10.67 2-Chloropropene can
protonate in two ways.
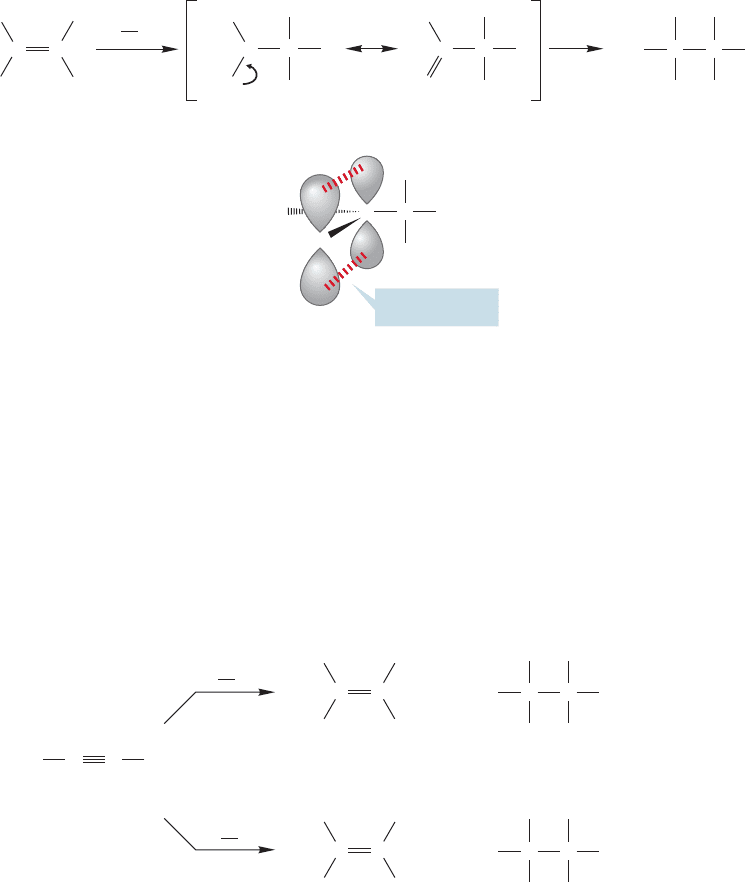
10.7 Addition of X
2
Reagents to Alkynes 447
There is a way for the chlorine in structure A to share the positive charge.The
charge is shared by the two atoms through 2p/3p overlap as shown in Figure 10.68.
In molecular orbital terms, we see that a filled 3p orbital on chlorine overlaps
with the empty 2p orbital on carbon, and this orbital overlap is stabilizing. The
primary carbocation B in Figure 10.67 is much less stable, and products from it
are not observed.
HCl
H
H
H
C
–
Cl
..
..
..
..
Cl
..
..
..
2,2-Dichloropropane
H
3
C
H
H
C
H
C
Resonance-stabilized cation
C
+
Cl
..
..
..
..
..
H
H
H
C
H
3
C
H
3
C
C
+
Cl
Cl
..
..
..
H
3
C
H
H
C
C
Cl
..
..
..
C
H
3
C
H
H
H
C
3p/2p Overlap
..
..
Cl
..
..
..
..
FIGURE 10.68 The carbocation
adjacent to chlorine is stabilized by
resonance.
H
excess
excess
I
HCl
HC
H
H
C
Cl
Cl
2,2-Dichloropropane2-Chloropropene
2-Iodopropene
Propyne
H
3
C
H
3
C
HC
H
C
2,2-Diiodopropane
I
H
I
H
3
C
H
CC
H
3
C
H
H
(56%) (44%)
(
65%
)
C
C
Cl
H
3
C
H
H
(
35%
)
C
C
I
+
+
FIGURE 10.69 Addition of excess
hydrogen halide to an alkyne gives
a mixture of products of mono- and
di-addition.
So, addition of a second halogen will preferentially result in formation of the
geminal dihalide (geminal means groups substituted on the same carbon) not
the vicinal (groups substituted on adjacent carbons) compound because the cation
with chlorine attached is lower in energy, and leads inevitably to the observed
geminal product. The reactions of Figure 10.66 are complicated by the for-
mation of compounds arising through double addition in the presence of
excess hydrogen halide. The double addition always gives the geminal dihalide
(Fig. 10.69).
10.7 Addition of X
2
Reagents to Alkynes
The pattern of alkene addition is also followed when X
2
reagents add to alkynes,
although the mechanism of the alkyne reaction is not well worked out. Both ionic
and neutral intermediates (i.e., radicals, which are discussed in Chapter 11) are
448 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
involved. Alkynes add Br
2
or Cl
2
to give vicinal dihalides. A second addition can
follow to give the tetrahalides (Fig. 10.70).
WEB 3D
H
3
C
H
3
C
H
3
C
(15–20%) (60–65%)
Cl
2
excess
Cl
Cl
C
Cl
Cl
C
+
CCH
H
H
C
C
Cl
Cl
65–70 ⬚C
Cl
2
excess
Cl
Cl
C
Cl
Cl
C
+
CRR
R
R
R
R
C
C
C
Cl
Cl
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 10.70 Addition of Cl
2
(or Br
2
) to alkynes gives both vicinal
dihalides and tetrahalides.
10.8 Hydration of Alkynes
Like alkenes, alkynes can be hydrated. The reaction is generally catalyzed by mer-
curic ions in an oxymercuration process (Fig. 10.71), although simple acid catalysis
is also known. In contrast to the oxymercuration of alkenes, no second, reduction
step is required in this alkyne hydration. By strict analogy to the oxymercuration of
alkenes, the product should be a hydroxy mercury compound, but the second dou-
ble bond exerts its influence and further reaction takes place. The double bond is
protonated and mercury is lost to generate a species called an enol. An enol is part
alkene
and part alcohol, hence the name.
Na
+
C
+
(+)
–
BH
4
..
..
+
OH
2
..
H
2
O /H
3
O
..
..
..
Hg(OAc)
2
HgOAc
HgOAc
H
2
O
HgOAc
An enol
C
Resonance
stabilized cation
RHC RHC
CHR CHR
OH
Oxymercuration of alkenes
An alcohol
Oxymercuration of alkynes
HO
RHC
CH
2
R
OH
HO
..
..
HO
Hg(OAc)
2
C
R
R
R
R
R
R
H
R
R
C
H
CC
C
C
H
+
..
..
FIGURE 10.71 The oxymercuration of alkynes resembles the oxymercuration of alkenes. In the alkyne case,
the product is an enol, which can react further.
Enols are extraordinarily important compounds, and more than one chapter
will mention their chemistry. Here their versatility is exemplified by their conver-
sion into ketones. We have already seen a synthesis of ketones in this chapter
