Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


10.8 Hydration of Alkynes 449
through ozonolysis of alkenes (p. 436). Here, ketones are the final products of the
hydration reactions of alkynes (Fig. 10.72).The mechanism of their formation from
enols is simple: The alkene part is protonated on carbon and the oxygen of the
enol is deprotonated to generate the compound containing the carbon–oxygen
double bond. Note that this process gives you another synthetic pathway to car-
bonyl compounds. We will see this process many times in the future.
H
2
O
..
OH
2
..
O
H
3
O
..
..
..
..
..
+
C
+
OH
2
..
H
H
H
H
Deprotonate on oxygenProtonate on carbon
Enol Ketone
C
..
..
HO
H
C
C
H
OH
2
..
C
C
H
R
R
R
R
R
R
+
+
+
O
..
..
H
FIGURE 10.72 Protonation of carbon,
followed by deprotonation at oxygen,
generates the carbonyl compound.
This sequence is a general reaction
of enols.
Nearly all ketones and aldehydes containing a hydrogen on the carbon adjacent
to the carbonyl carbon are in equilibrium with the related enol forms. How much
enol is present at equilibrium is a function of the detailed structure of the molecule,
but there is almost always some enol present.In simple compounds, the ketone form
is greatly favored. This interconversion is called keto-enol tautomerization.
PROBLEM 10.23 Draw a mechanism for the acid-catalyzed hydration of an
alkyne.
WORKED PROBLEM 10.24 Why were we so sure the enol in Figure 10.72 would
protonate to give the cation shown? There is another cation possible. Why is it
not formed?
ANSWER Protonation as shown in Figure 10.72 leads to a resonance-stabilized
carbocation. The adjacent oxygen shares the positive charge. Protonation on the
other carbon of the double bond leads to a carbocation that is not resonance
stabilized, which is therefore of much higher energy.
+
..
..
..
..
..
Not resonance stabilized!
Resonance stabilized!
H
H
R
C
CC
R
R
HO
..
C
R
H
C
R
H
+
H
H
R
HH
C
H
R
C
C
R
O
+
+
O
H
2
OH
H
O
..
..
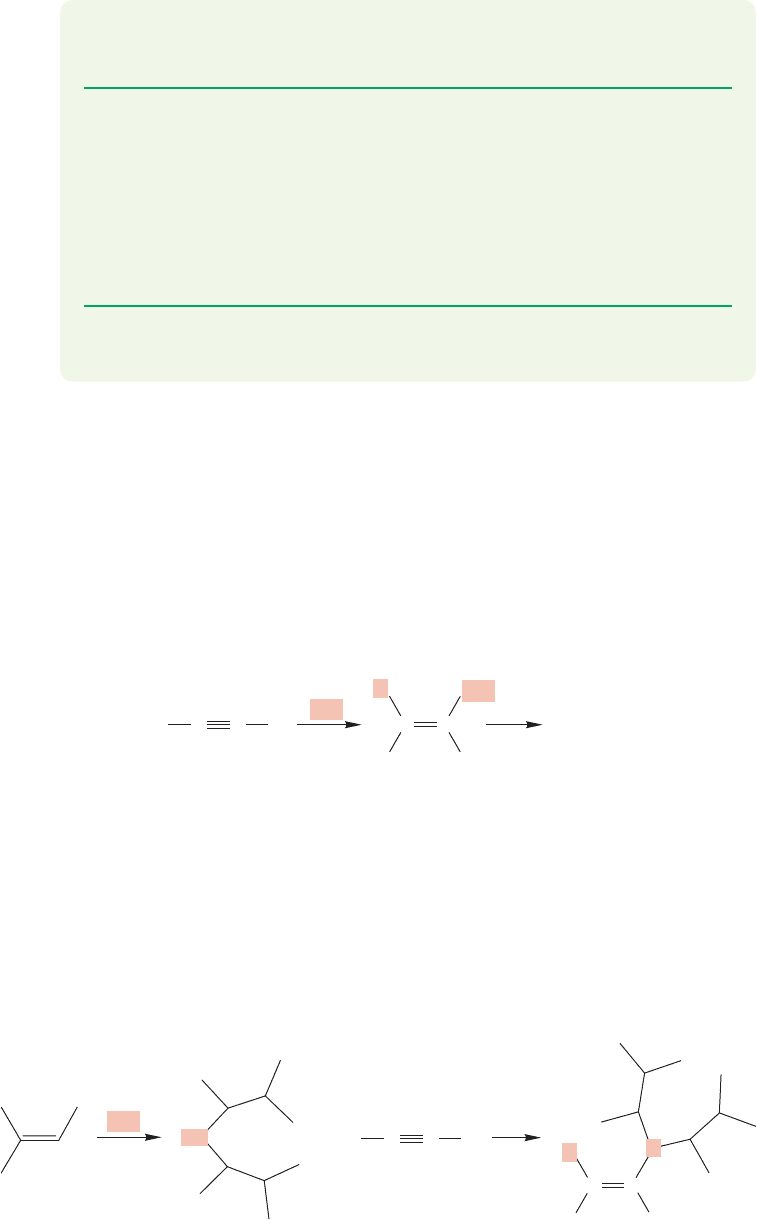
450 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
PROBLEM 10.25 In general, hydration of unsymmetrical alkynes such as 2-pentyne
is not a practical source of ketones. Why not?
PROBLEM 10.26 Terminal alkynes are certainly unsymmetrical, yet in these
cases hydration is a useful process. In principle, either ketones or aldehydes
could be formed, but in practice only ketones are produced. Write mechanisms
for both ketone and aldehyde formation and explain why the product is always
the ketone. You may write open vinyl carbocations in your answer for simplic-
ity, but be aware that the real intermediates may be more complex cyclic
species.
PROBLEM 10.27 Write a mechanism for the acid-catalyzed conversion of diethyl
ketone into its enol form.
10.9 Hydroboration of Alkynes
Like alkenes, alkynes can be hydroborated by boranes. The mechanism is very sim-
ilar to that for alkenes (p. 390); the stereochemistry of addition of HBH
2
is syn, and
it is the boron that becomes attached to the less substituted end of a terminal alkyne
(Fig. 10.73).
BH
2
BH
3
CCH
R
R
Further
hydroborations
Vinylborane
H
H
C
C
FIGURE 10.73 Hydroboration of
alkynes gives vinylboranes that can
react further (R alkyl).=
Dialkylborane
BH
3
CR
R
C
2
H
H
(100%)
C
C
+
HB
B
H
FIGURE 10.74 This sterically hindered dialkylborane reacts only once with alkynes to give an
isolable vinylborane (R alkyl).=
The initial products of hydroboration are prone to further hydroboration
reactions.This complication can be avoided by using a sterically hindered borane
so that further reaction is slowed. A favorite suitably hindered reagent is the
borane formed by reaction of two equivalents of 2-methyl-2-butene with BH
3
(Fig. 10.74).
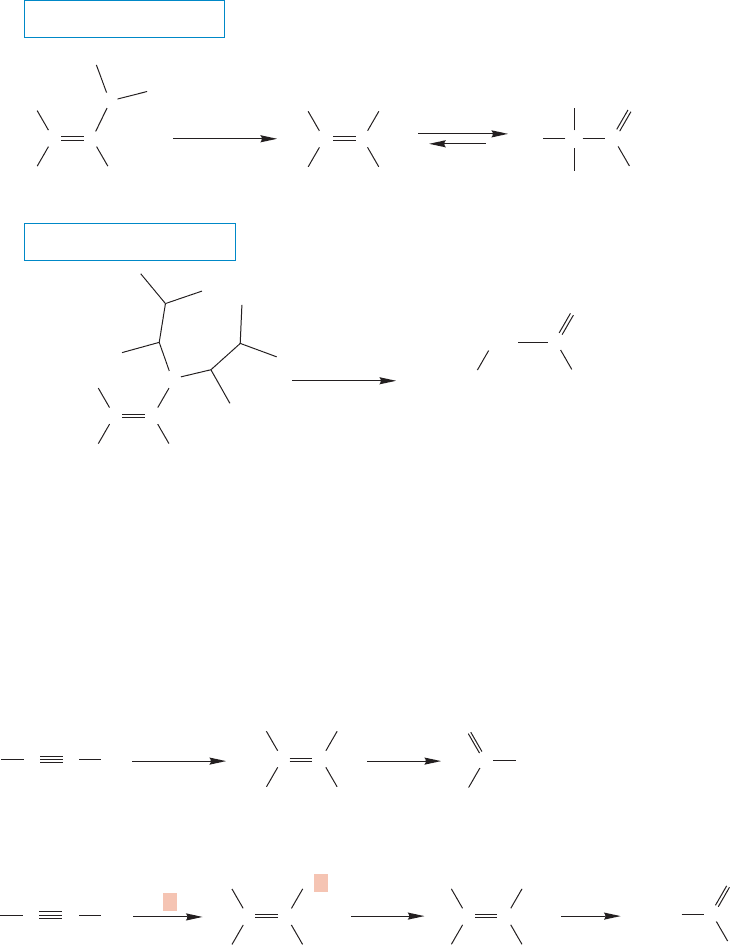
10.9 Hydroboration of Alkynes 451
Like alkylboranes, vinylboranes can be converted into alcohols by treatment with
basic peroxide (Fig. 10.75).The products are enols in this case,and they are in equi-
librium with the corresponding carbonyl compounds. Here’s an important point:
The regiochemistry of this reaction is the opposite of the regiochemistry of the
hydration reaction of alkynes.This situation is exactly the same as the one that exists
with the alkenes: Hydration of an alkene gives overall Markovnikov addition,
whereas the hydroboration/oxidation sequence gives the anti-Markovnikov product.
Vinylborane
H
C
H
R
H
O
C
H
2
O
NaOH/H
2
O
H
AldehydeEnol
H
R
R
R
C
C
H
(88%)
H
C
C
H
H
R
HOOH
NaOH/H
2
O
HOOH
CH
3
(CH
2
)
3
CH
2
CH
3
(CH
2
)
3
C
C
OH
B
B
C
H
O
THE GENERAL CASE
A SPECIFIC EXAMPLE
FIGURE 10.75 Oxidation of a
vinylborane leads to an enol that can
be converted into the aldehyde.
The ultimate formation of a carbonyl compound disguises the matter, but if we look
back one step to the enols, we can see that in alkyne reactions the same regioselec-
tivity holds (Fig. 10.76).Hydration goes through Markovnikov addition to the alkyne
and,ultimately,gives the ketone.The hydroboration/oxidation reaction goes through
anti-Markovnikov addition to the alkyne and, ultimately, gives the aldehyde.
CH
3
Hydration
H
2
O
H
2
O
H
3
O /H
2
O
Hg
2+
CR
R
R
R
R
R
C
C
C
H
3
O
Ketone
Aldehyde
H
H
H
H
HO
C
O
RCH
2
Hydroboration/oxidation
HOOH
HBR
2
BR
2
CC
C
C
HO
–
HO
–
H
H
C
C
H
OHH
C
O
H
+
+
FIGURE 10.76 It is possible to make
two different carbonyl compounds
from a terminal alkyne. Mercuric
ion-catalyzed hydration gives the
ketone, and hydroboration/oxidation
gives the aldehyde.
Be sure you see the similarities here as well as the mechanistic underpinnings of
the different paths followed. There is too much to memorize. Be sure to annotate
your file cards as you update your catalog of synthetic methods.
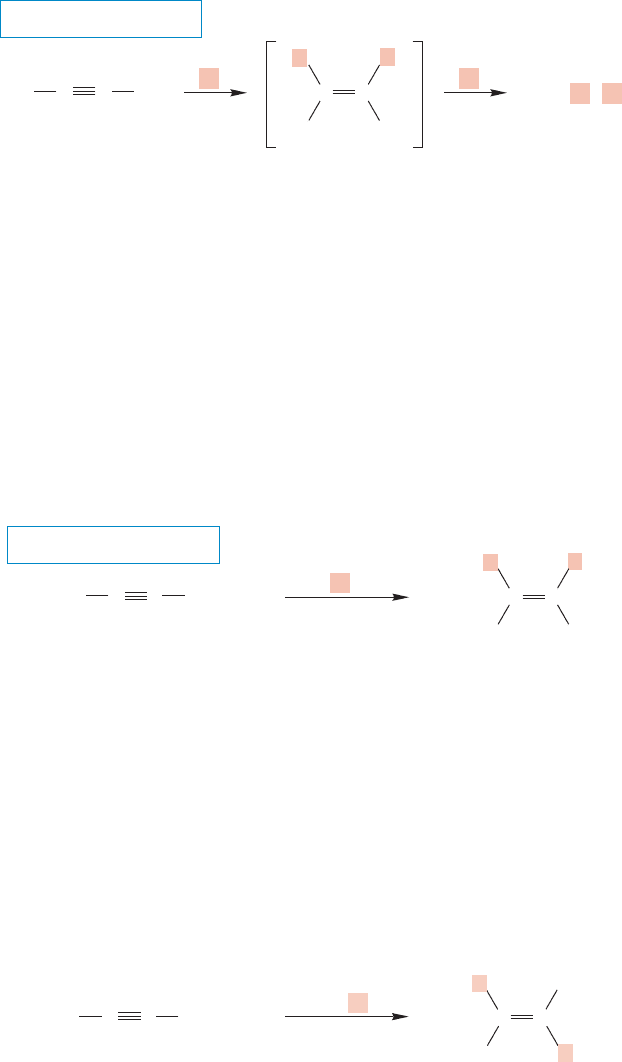
452 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
10.10 Hydrogenation of Alkynes: Alkene Synthesis
through syn Hydrogenation
Like alkenes,alkynes can be hydrogenated in the presence of many catalysts.Alkenes
are the first products, but they are not stable under the reaction conditions and are
usually hydrogenated themselves to give alkanes (Fig. 10.77). This reaction is your
second synthetic pathway to alkanes.
THE GENERAL CASE
CH
3
CH
2
CH
2
CH
3
Pd/C
CH
3
CH
3
H
2
H
3
C
H
3
C
CC
C
C
Pd/C
H
2
H
H
FIGURE 10.77 Hydrogenation of an
alkyne proceeds all the way to the
alkane stage.The intermediate alkene
cannot usually be isolated.
It would be extremely convenient to be able to stop the alkyne hydrogenation
at the alkene stage,and special,“poisoned”catalysts have been developed to do just
this. A favorite is the Lindlar catalyst (Fig. 10.78), which is Pd on calcium car-
bonate that has been treated with lead acetate in the presence of certain amines
(H. H. M. Lindlar, b. 1909). For many years it was thought that the lead treat-
ment poisoned the palladium catalyst through some sort of alloy formation,
rendering it less active and the reaction more selective. It now appears that the
treatment forms no alloys, but does modify the surface of the palladium.
Regardless, the reaction can be stopped at the alkene stage, and the alkenes formed
by hydrogenation with Lindlar catalyst have cis stereochemistry because the usual
syn addition of hydrogen has occurred.
A SPECIFIC EXAMPLE
Pd/CaCO
3
/Pb
Lindlar catalyst
H
2
CH
3
(CH
2
)
2
CH
3
(CH
2
)
2
(CH
2
)
2
CH
3
(CH
2
)
2
CH
3
CC
C
96% cis
C
H
H
FIGURE 10.78 If the Lindlar catalyst
is used in hydrogenation of a alkyne,
the cis alkene can be obtained.
10.11 Reduction by Sodium in Ammonia:
Alkene Synthesis through anti Hydrogenation
In a reaction that must at first seem mysterious, an alkyne dissolved in a solution of
sodium in liquid ammonia forms the trans alkene (Fig. 10.79). The practical con-
sequences of this are that we now have a way of synthesizing either a cis (Fig.10.78)
or trans (Fig. 10.79) alkene from a given alkyne. So, update your file cards.
Na/NH
3
CH
3
CH
2
CH
3
CH
2
(CH
2
)
3
CH
3
(CH
2
)
3
CH
3
CC
C
98% trans
C
H
H
FIGURE 10.79 Reduction of an
alkyne with sodium in ammonia gives
the trans alkene.
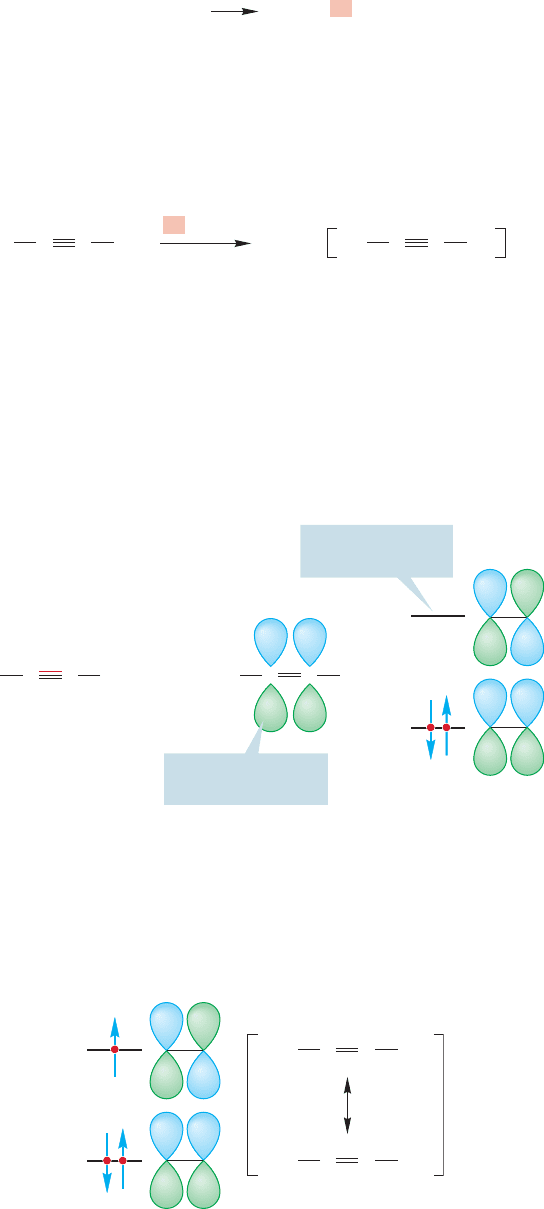
10.11 Reduction by Sodium in Ammonia: Alkene Synthesis through anti Hydrogenation 453
When sodium is dissolved in ammonia, the sodium atom loses its odd electron
to give a sodium ion and a solvated electron, which is an electron surrounded by
solvent molecules:
.
++
e
–
[NH
3
]
n
NH
3
Na Na
+
Solvated
electron
This complexed electron can add to an alkyne to give an intermediate called a
radical anion (Fig. 10.80). A radical anion is a negatively charged molecule that has
an unpaired electron.
–
.
+
CH
3
e
–
[NH
3
]
n
NH
3
CC
H
3
C
H
3
C
CH
3
CC
Radical anion
FIGURE 10.80 A solvated electron
can add to an alkyne to generate a
radical anion.
I (MJ) vividly recall being quite uncertain about the structure of the radical anion
when I was first studying organic chemistry. I suspect I solved the problem by mem-
orizing this reaction and hoping that no one would ever ask me to explain what was
happening.But it’s not so hard and you should not repeat my error. First of all, where
is the “extra”electron in the radical anion? It is most certainly not in one of the bond-
ing π orbitals of the alkyne, because they are each filled with two electrons already.
If it is not there, it must be in one of the antibonding π* orbitals (Fig. 10.81).
An added electron
must go here
One of the π bonds
of the alkyne
.
.
.
.
.
.
CH
3
CC =
H
3
C
CH
3
CC
H
3
C
π*
π
FIGURE 10.81 The electron must go
into an antibonding π* orbital of the
alkyne.
Why should the radical anion put up with this electron in an antibonding orbital?
The answer is twofold. First of all, the intermediate is held together by many elec-
trons occupying bonding orbitals, and a single electron in a high-energy orbital is
not destabilizing enough to overcome all the bonding interactions. Figure 10.82 gives
both molecular orbital and resonance pictures of the radical anion.
An alkyne radical anion
...
π*
π
CH
3
CC
H
3
C
.. .
CH
3
CC
H
3
C
–
–
FIGURE 10.82 The alkyne radical
anion.
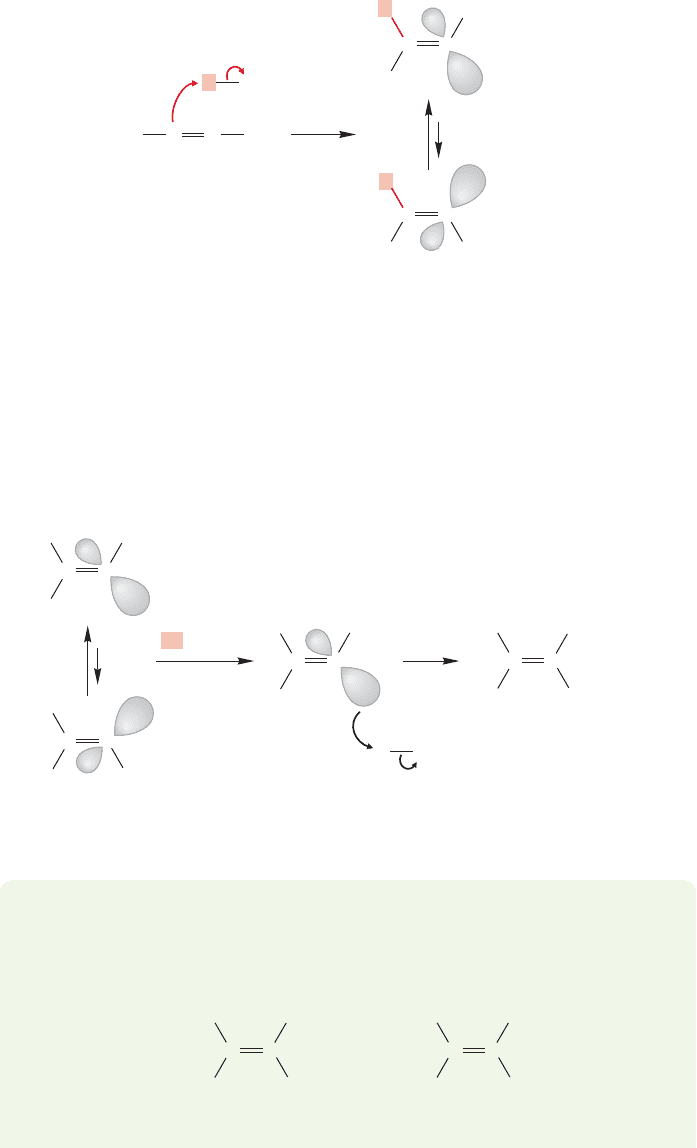
454 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
NH
2
..
..
+
Radical anion
Vinyl radical
.
.
.
.
.
..
..
CH
3
C
C
H
3
C
NH
2
Amide
ion
C
H
C
H
3
C
CH
3
C
H
C
H
3
C
CH
3
H
–
–
FIGURE 10.83 The radical anion can
abstract a proton from ammonia to
generate a vinyl radical and the amide
ion.The trans form is favored.
NH
2
..
..
..
+
Radical
.
.
.
.
..
H
NH
2
NH
3
Amide
ion
C
H
C
H
3
C
CH
3
C
Anion trans Alkene
C
–
C
C
CH
3
e
–
[NH
3
]
n
..
..
CC
H
H
H
3
C
H
H
3
C
H
H
3
C
CH
3
CH
3
–
FIGURE 10.84 Transfer of another
electron and protonation completes
the overall trans hydrogenation of
an alkyne.
Second, the radical anion is quite unstable and not much of it is likely formed
at equilibrium. Its very instability contributes to its great base strength,however, and
the small amount present at equilibrium is rapidly protonated by ammonia to give
the amide ion (
NH
2
) and the vinyl radical (Fig. 10.83).There are two forms of the
vinyl radical, and it will be the trans form, with the alkyl groups as far from each
other as possible, that is the more stable version. A solvated electron can be donated
to this radical forming the vinyl anion, which is rapidly protonated by ammonia to
give the final product, the trans alkene (Fig. 10.84).
PROBLEM 10.28 Devise a synthetic pathway for each of the following com-
pounds, starting in each case with 2-butyne:
(a) (b)
H
CH
3
C
C
HH
3
C
HH
C
C
CH
3
H
3
C
(continued)
10.12 Special Topic: Three-Membered Rings in Biochemistry 455
Summary
Normal hydrogenation of an alkyne adds two equivalents of H
2
to give the alkane.
Use of a “poisoned” Lindlar catalyst leads to absorption of one equivalent of H
2
and formation of the cis alkene. The trans alkene can be made by reducing the
alkyne with Na in ammonia.
10.12 Special Topic: Three-Membered
Rings in Biochemistry
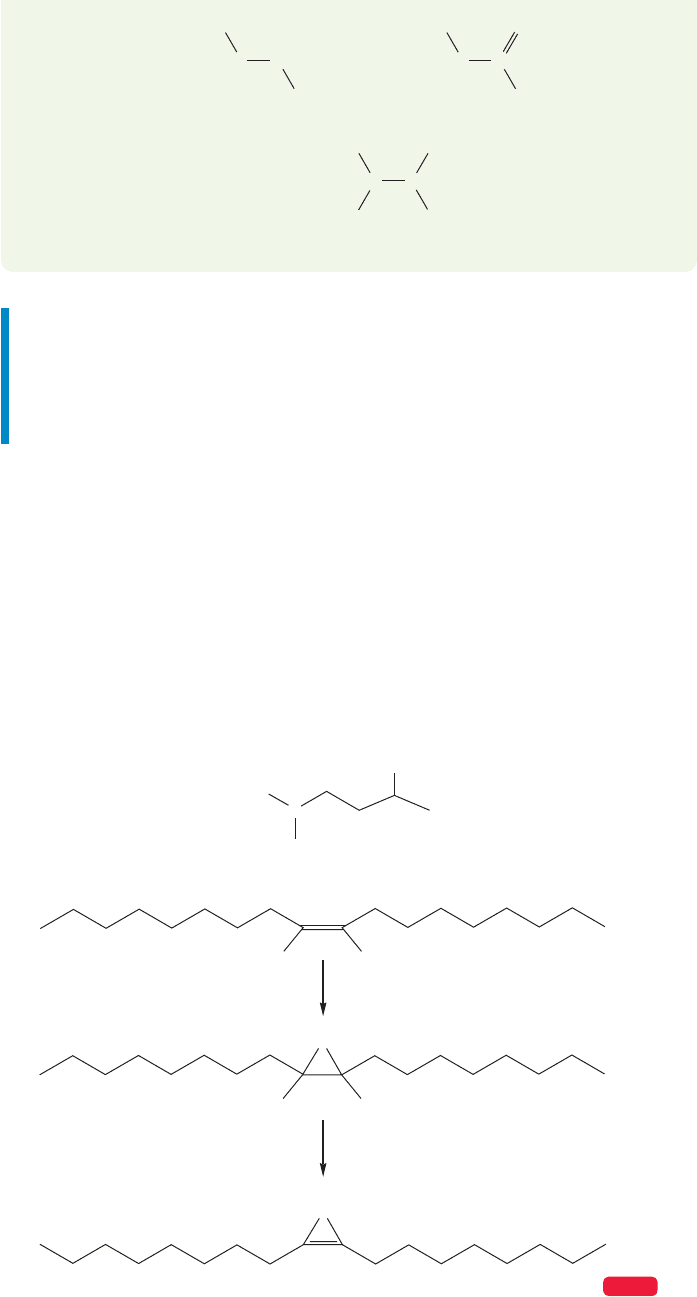
Despite the strain inherent in cyclopropanes, Nature finds ways to make them.
Bacteria, in particular, contain surprising amounts of cyclopropanated fatty acids.
Even more remarkable is the conversion of such molecules into cyclopropenes.
The source of the single “extra” carbon is S-adenosylmethionine, the same agent
involved in the methyl transfers discussed in Chapters 3 and 7 (pp. 142, 288;
Fig. 10.85). The mechanism of this intriguing change hasn’t been worked out yet.
R
S
H
3
C
COO
NH
3
S-Adenosylmethionine
A cyclopropanated fatty acid
cyclopropanation
dehydrogenation
A cyclopropene
+
+
+
HH
COOH
CH
2
CH
2
COOH
HH
COOH
WEB 3D
FIGURE 10.85 Cyclopropanation
of a fatty acid is followed by
dehydrogenation.
(c) (d)
(e)
meso
H
2
CCH
2
C
O
CH
3
H
3
C
H
2
C
CH
3
H
3
C
HO OH
HC
CH
CH
3
H
3
C

456 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
In Chapter 12 we will see other three-membered rings found in Nature.Epoxides
are used as triggers in the construction of the biologically active molecules called
steroids, and, amazingly enough, one of the most prominent molecules in interstel-
lar space is not only a cyclopropene, but a carbene as well.This molecule is known
as cyclopropenylidene:
O
Pyrethrin I
O
O
HH
Cyclopropenylidene
PYRETHRINS
Cyclopropanes are occasionally found in Nature, and one
class of naturally occurring cyclopropanes, the pyrethroids,
are useful insecticides. These compounds are highly toxic to
insects but not to mammals, and because they are rapidly
biodegraded, cyclopropanes do not persist in the environ-
ment. The naturally occurring pyrethroids are found in
members of the chrysanthemum family and are formally
derived from chrysanthemic acid. Many modified pyrethrins
not found in Nature have been made in the laboratory and
several are widely used as pesticides. The molecule known
as pyrethrin I has the systematic name 2,2-dimethyl-3-
(2-methyl-1-propenyl)cyclopropanecarboxylic acid
2-methyl-4-oxo-3-(Z-2,4-dipentadienyl)-2-cyclopenten-
1-yl ester. See why the shorthand is used?
10.13 Summary
New Concepts
This chapter continues our discussion of addition reactions, and
many of the mechanistic ideas of Chapter 9 apply. For example,
many polar addition reactions start with the formation of
the more stable carbocation. The most important new concept
in this chapter is the requirement for overall anti addition
introduced by three-membered ring intermediates. This
concept appears most obviously in the trans
bromination and chlorination of alkenes.
The openings of the intermediate bromonium
and chloronium ions by nucleophiles are S
N
2
reactions in which inversion is always
required.This process insures anti addition
(Fig. 10.13).
Remember also the formation of epoxides,
stable three-membered rings containing an
oxygen atom, and cyclopropanes, formed by carbene additions
to alkenes (Fig. 10.86).
Alkenes and alkynes react in similar fashion in most
addition reactions, although additional complications and
opportunities are introduced by the second π bond in the
alkynes.
C
R
R
R
R
R
R
R
CF
3
COOOH H
2
CN
2
R
Oxirane Alkene Cyclopropane
O
C
C
CH
2
C
C
C
hν
R
R
R
R
FIGURE 10.86 Stable three-membered rings can be formed in some alkene
addition reactions.Two examples are epoxidation and carbene addition.
10.13 Summary 457
Reactions, Mechanisms, and Tools
Key Terms
aldehydes (p. 440)
alkene halogenation (p. 414)
bromonium ion (p. 415)
carbene (p. 431)
carbonyl compound (p. 438)
carboxylic acid (p. 424)
diazo compounds (p. 431)
1,3-dipolar reagents (p. 436)
1,3-dipoles (p. 436)
diradical (p. 434)
enol (p. 448)
epoxidation (p. 424)
geminal (p. 447)
halohydrin (p. 419)
heterogeneous catalysis (p. 411)
homogeneous catalysis (p. 411)
hydrogenation (p. 411)
ketone (p. 440)
molozonide (p. 437)
organocuprate (p. 430)
oxymercuration (p. 421)
ozonide (p. 437)
ozonolysis (p. 437)
primary ozonide (p. 437)
radical anion (p. 453)
singlet carbene (p. 433)
solvated electron (p. 453)
tautomerization (p. 449)
triplet carbene (p. 433)
vicinal (p. 414)
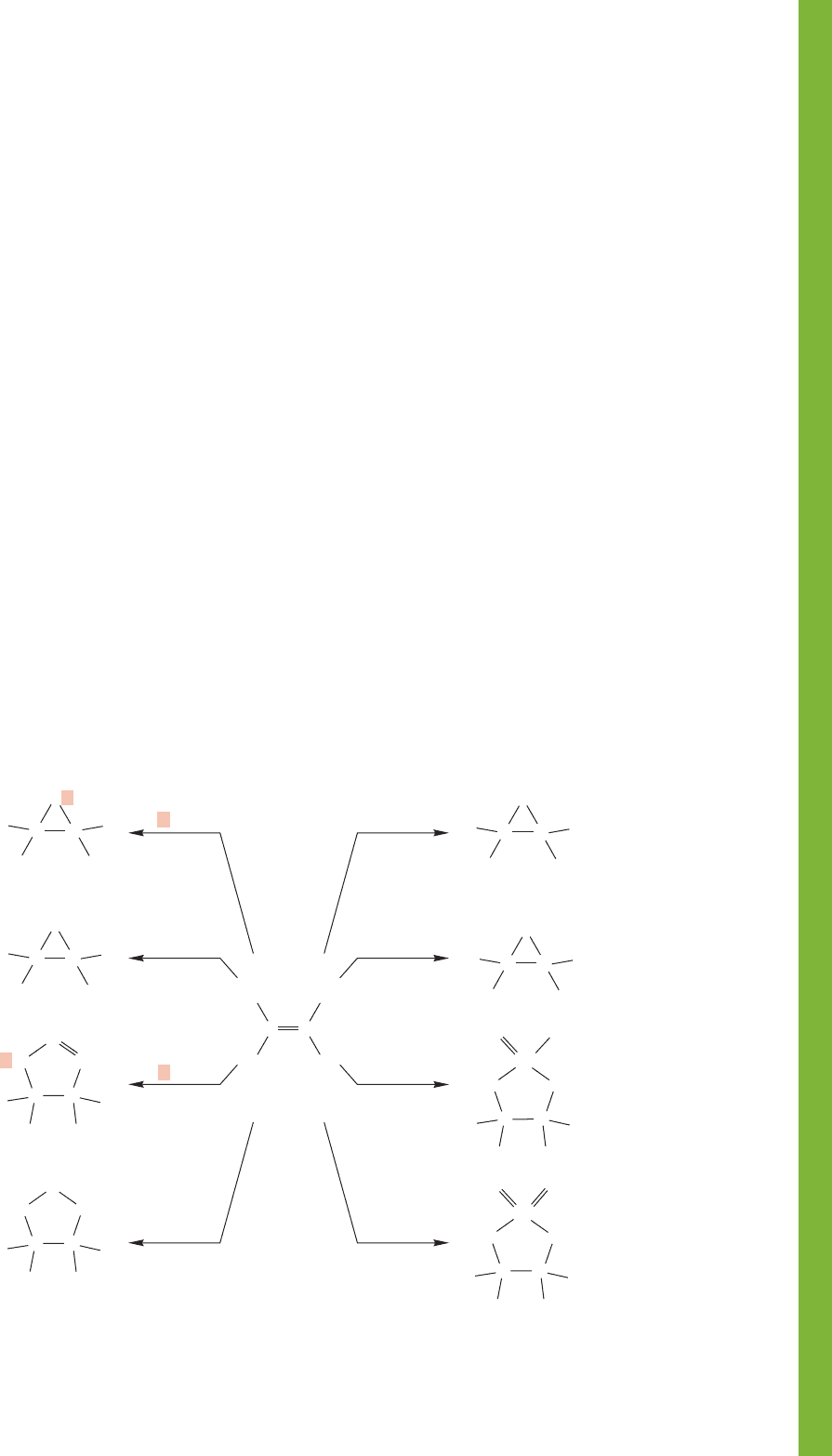
In this chapter, a series of ring formations is described: the
three-membered oxiranes, cyclopropanes, bromonium, chlor-
onium, and mercurinium ions, as well as the five-membered rings
formed through 1,3-dipolar additions, ozonolysis, and the addi-
tions of osmium tetroxide and potassium permanganate. Many
of these compounds react further to give the final products of
the reaction (Fig. 10.87).
Cyclopropanes and oxiranes can be isolated, but the other
rings react further under the reaction conditions to give the
final products. Oxiranes can be opened in either acid or base
to give new products. Additions to alkynes resemble those of
alkenes, but the second π bond adds complications, and
further addition reactions or rearrangements occur. Alkynes
can be reduced in either cis or trans fashion (Fig. 10.78 and
Fig. 10.79).
Both homogeneous and heterogeneous catalytic hydrogena-
tion are syn additions, because both added hydrogens are trans-
ferred from the metal surface to the same side of the alkene.
R
R
R
R
R
2
CN
2
Alkene
C
CR
2
C
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
R
C
C
KMnO
4
OsO
4
R
2
CN
2
O
3
CF
3
CO
3
H
X
2
Hg(OAc)
2
C
O
C
C
X
X = Br or Cl
C
C
HgOAc
C
–
+
+
+
Mn
OO
C
C
O
O
Os
O
O
R
K
R
R
R
R
R
R
R
C
C
O
O
O
R
R
R
R
C
C
O
O
N
R
2
C
R
R
R
R
C
C
N
hν
FIGURE 10.87 Ring formation through addition across the double bond.

458 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
Syntheses
This chapter provides many new synthetic methods.
R
R
H
H
Via ozonide intermediates
C
C
R
2
HO
O
C
1. O
3
2. H
2
O
2
2. Alcohols
R
R
R
R
Markovnikov addition, no rearrangements, anti addition
Organocopper reagents also work
C
C
1. Hg(OAc)
2
/H
2
O
2. NaBH
4
1. RMgX
2. H
2
O
ROH
R
R
R
CC
HR
OH
1. LiAlH
4
2. H
2
O
H
OH
O
O
3. Aldehydes
A vin
y
l borane and an enol are intermediates
R
H
CCH
2
HR
C
C
R
R
H
H
C
C
1. O
3
2. (CH
3
)
2
S
1. R
2
BH
2. H
2
O
2
/NaOH
R
2
H
O
O
C
4. Alkanes
R
R
R
RR
R
C
C
R
2
CH
CHR
2
H
2
/Pt
C
C
RCH
2
CH
2
R
H
2
/Pt
syn Addition, many catalysts possible
5. Alkenes
R
R
H
H
C
C
syn Addition
anti Addition
RR
C
C
H
2
Lindlar catalyst
R
H
H
R
C
C
RR
C
C
Na/NH
3
6. Halohydrins
R
R
R
CC
anti Addition, X = Br or Cl
X
HO
R
R
R
R
R
C
C
X
2
/H
2
O
7. Bromo Ethers and Chloro Ethers
anti Addition, X = Br or Cl
R
R
R
R
R
R
C
C
X
R
R
CC
X
2
/ROH
RO
8. Cyclopropanes
C
CH
2
C
R
RR
R
cis Addition for singlet carbene, but both
cis and trans addition for triplet carbene
R
R
R
R
C
C
CH
2
N
2
hν or Δ
1. Carboxylic Acids
