Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

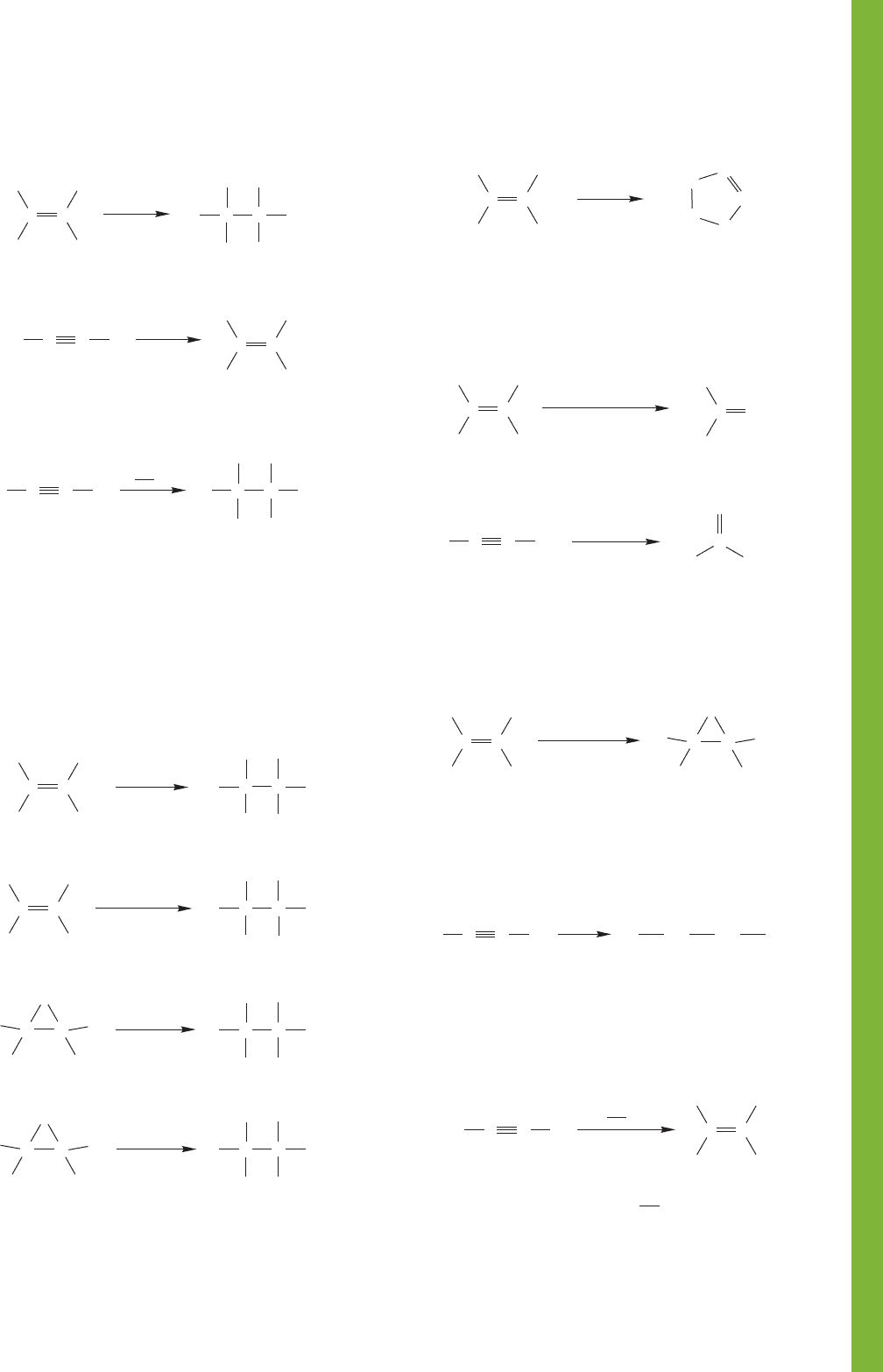
10.13 Summary 459
9. Dihalides
R
R
R
R
Vicinal dihalide, anti addition, X = Br or Cl
Vicinal dihalide, mostly anti addition,
X = Br or Cl, further reaction likely
Geminal dihalide, X = Br, Cl, or I,
double HX addition
CC
X
X
R
RX
C
C
X
2
X
2
RR
C
C
RR CC
R
X
R
H
X
X
H
excess
RR
CC
H
X
R
R
CC
10. Vicinal Diols
OH
HO
R
syn Addition
RR
RR
R
R
R
R
anti Addition
OH
HO
R
anti Addition
CC
C
O
C
–
–
+
1. KMnO
4
2. H
2
O/HO
H
3
O /H
2
O
R
R
R
R
C
O
C
H
2
O/HO
RR
RR
CC
HO OH
RR
RR
HO OH
CC
R
R
R
CC
R
RR
CC
RR
RR
CC
1. OsO
4
2. H
2
O
Na
2
SO
3
syn Addition
12. Ketones
RR
RR
This is only one example; there is a different
product for every 1,3-dipole; alkynes work too
C
C
RN
3
N
R
2
C
R
2
C
NR
N
11. Heterocyclic Five-Membered Rings
RR
RR
An ozonide is an intermediate; other reagents
will also decompose the ozonide
Forms methyl ketones, never the aldehyde
CC
1. O
3
2. Zn/CH
3
COOH
CH
3
C
HR
CC
+
H
3
O /H
2
O
O
R
2
R
R
OC
Hg(OAc)
2
13. Epoxides (Oxiranes)
syn Addition
CF
3
COOOH
R
R
RR
CC
C
O
C
R
RR
R
14. Tetrahalides
The intermediate vicinal dihalide can be isolated
RR
CC
CX
2
CX
2
X
2
excess
RR
15. Vinyl Halides
Markovnikov addition and it’s hard to stop here;
a second addition of H X occurs
RX
H
CC
H
RR
CC
X
R
X = Br or Cl

460 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
Common Errors
What psychopathology exists in this chapter comes not so
much from “something everyone always gets wrong” or from an
overwhelmingly difficult concept, but from the mass of detail.
There have been those who have succumbed to despair at sort-
ing out all the stereochemical nuances, at dealing with the many
new mechanisms and synthetic methods—at just making
rational sense of all the material in this chapter and Chapter 9.
There certainly is a lot of information, and you must be very
careful to keep your new synthetic methods properly cataloged.
There is a unifying principle that may help you to keep from
getting lost in the mechanism jungle: “Lewis acids (electrophiles)
react with Lewis bases (nucleophiles).”
The Lewis base or nucleophile in most of the reactions in
this chapter and Chapter 9 is the π bond of an alkene or an
alkyne. The Lewis acids or electrophiles are numerous, but most
add to give either an intermediate, or a stable three-membered
ring (not all, however; hydrogenation does not, and 1,3-dipolar
addition leads to a host of five-membered rings).
The three-membered rings are themselves often prone to
further reaction with nucleophiles, and the final products of
reaction may be quite far removed in structure from the start-
ing material! Moreover, not all the mechanistic details are
known about all these processes. There are reactions about
which we need much more information. The reactions of
alkynes with halogens and HX are examples, and even hydro-
genation has a complicated mechanism, still somewhat
obscure to organic chemists.
A good technique that helps one not to get too over-
whelmed or lost is to anchor oneself in one or two specific
reactions and then to generalize; to relate other reactions to
the anchor reaction. For example, the polar addition of
hydrogen chloride and hydrogen bromide to alkenes
(p. 365) is within anyone’s ability to master. Extensions to
hydration reactions of alkenes and alkynes and to hydrobora-
tion become easier if the analogy with the anchor is always
kept in mind. Similarly, use the reaction of alkenes with Br
2
as an anchor on which to hang the other ring-forming
addition reactions. It is useful to start a set of mechanism
cards to go along with your synthesis cards in order to keep
track of the detail.
10.14 Additional Problems
PROBLEM 10.29 Show the reaction you would use to synthe-
size bromoethene if your only source of carbon is acetylene.
PROBLEM 10.30 Show how you would make 2-azidobutane if
your only source of carbon is 1-butene.
PROBLEM 10.31 Show how you would make trans-2-methoxy-
cyclohexanol starting with cyclohexene.
PROBLEM 10.32 Show how you would make cis-1-bromo-2-
methoxycyclohexane from cyclohexene and any other reagents
you might need.
PROBLEM 10.33 Show the major organic product(s) expected
when 1-methylcyclohexene reacts with the following reagents.
Pay close attention to stereochemistry and regiochemistry
where appropriate.
(a) D
2
/Pd/C
(b) Br
2
/CCl
4
(c) Br
2
/CH
3
OH
(d) Hg(OAc)
2
,H
2
O, then NaBD
4
in base (no stereochemical
preference in this reaction)
(e) 1. B
2
H
6
(BH
3
)/THF; 2. H
2
O
2
/HO
(f) CF
3
COOOH
(g) CH
2
N
2
, hν
(h) 1. O
3
;2.(CH
3
)
2
S
(i) 1. OsO
4
; 2. NaHSO
3
/H
2
O
(j)
PROBLEM 10.34 Show in detail how both enantiomers of
product are formed in Problem 10.33a.
PROBLEM 10.35 Predict the major product(s), including
stereochemistry where relevant, for the bromination reaction
(Br
2
/CCl
4
) with each of the following alkenes:
(a) 1-pentene
(b) cis-2-pentene
(c) cyclopentene
(d) cis-3-hexene
(e) trans-3-hexene
PROBLEM 10.36 Which of the products in the previous prob-
lem are chiral and which are achiral?
PROBLEM 10.37 Predict the possible products in the reaction
between bromine in water with the following alkenes:
(a) cyclobutene
(b) trans-2-butene
(c) 2-methyl-2-pentene
(d) cis-2-hexene
HN
P
NH
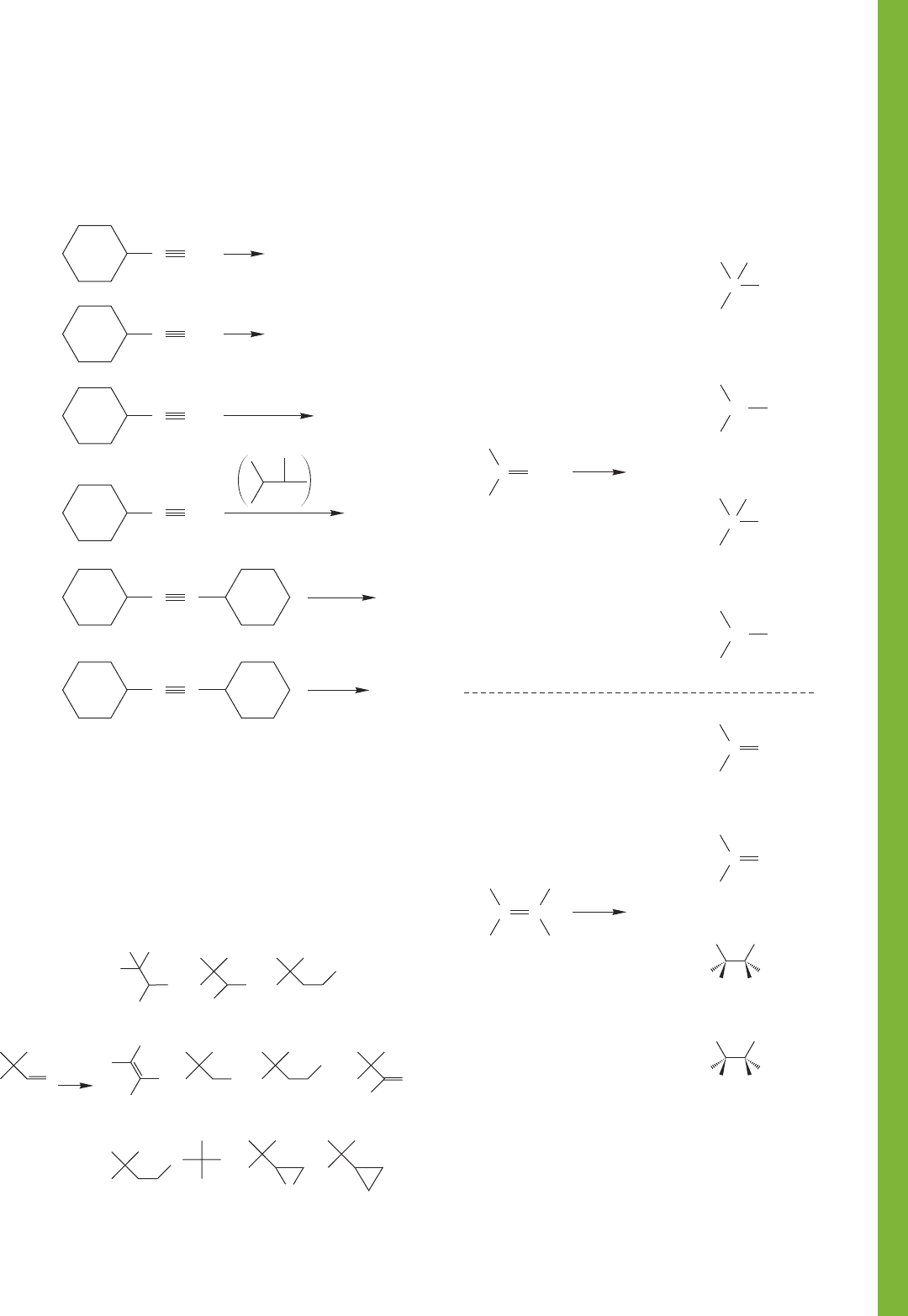
10.14 Additional Problems 461
(a)
CCH
HBr
excess
(b)
CCH
Cl
2
excess
(c)
CCH
Hg
2+
H
2
O/H
3
O
+
(d)
C
1.
CH
BH
2. H
2
O
2
/NaOH
H
2
NH
3
(e)
CC
Lindlar
catalyst
Na
(f)
CC
2
HO
HO
OH
(a) (b) (c)
O
(h) (i)
O
(j)
O
(d)
Br
(e) (f) (g)1
HO
PROBLEM 10.38 Show the major organic products expected
when the following acetylenes react with the reagents shown.
Pay attention to stereochemistry and regiochemistry where
appropriate.
PROBLEM 10.40 What reagents would you use to convert the
following starting materials into the products shown? There are
no restrictions on reagents, but you must start from the mol-
ecule shown.
H
3
C
C
??
CH
2
H
3
C
H
3
C
CH
3
C
C
HH
??
H
3
C
C
O
H
(e)
H
3
C
C
O
HO
(f)
H
3
C
H
3
C
CH
(b)
CH
2
Br
H
3
C
H
3
C
C
(a)
Br
CH
3
H
3
C
H
3
C
C
CH
3
OH
(c)
CH
CH
2
OH
H
3
C
H
3
C
(d)
HO OH
(g)
HH
H
3
C CH
3
(h)
HO OH
H
H
H
3
C
CH
3
PROBLEM 10.39 Devise syntheses for the following molecules
starting from 3,3-dimethyl-1-butene (1). You may use any inor-
ganic reagent (no carbons), and the following “special” organic
reagents: diazomethane, carbon tetrachloride, tert-butyl alcohol,
tert-butyl iodide, Hg(OAc)
2
, carbon, (CH
3
)
2
S, trifluoroperacetic
acid, strychnine, and Igor Likhotvorik’s famous boiled goose-in-
a-kettle. Your answers may be very short, and no mechanisms
are required.
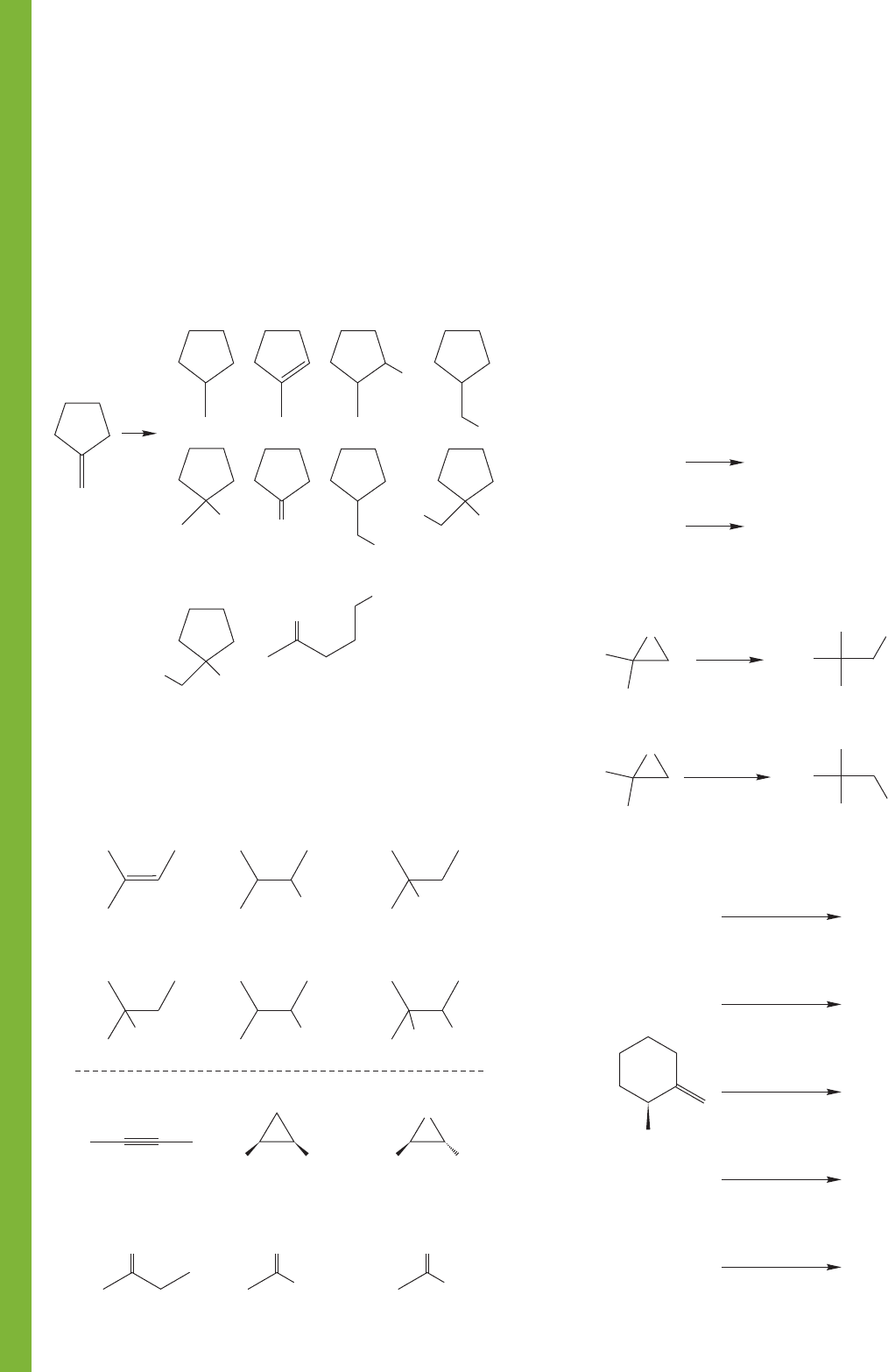
462 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
(a)
(f) (g) (h)
(
i
) (j)
(b) (c) (d)
(e)
OH
OH
Br
O
OH
OH
HO
OCH
3
Br
O
COOH
1
??
OH
From
From
(a)
(b)
Br
(d)
OH
OH
(e)
(f)
OH
H
O
(c)
(h)
Br
H
3
C
CH
3
(g)
H
3
C
CH
3
O
(i)
O
OH
(j)
O
PROBLEM 10.41 Devise syntheses for the following molecules
starting from methylenecyclopentane (1). You may use any
inorganic reagent (no carbons), and the following “special”
organic reagents: diazomethane, carbon tetrachloride, tert-butyl
alcohol, methyl alcohol, ethyl alcohol, tert-butyl iodide,
Hg(OAc)
2
, carbon, (CH
3
)
2
S, trifluoroperacetic acid, and the
secret contents of Zhou Enlai’s favorite veggie dumplings. Your
answers may be very short, and no mechanisms are required.
PROBLEM 10.42 Provide syntheses for the following molecules
starting from the indicated compound. You may use any appro-
priate reagents you need, including Mrs. Tao’s incredible baby
eels in white pepper sauce.
PROBLEM 10.43 Draw four possible products for the reaction of
(R)-3-methylcyclopentene with bromine in methanol. Show the
reaction pathway for the compound you think would be the major
product and explain why you think it would be the major product.
PROBLEM 10.44 Which of the products in the previous prob-
lem are chiral and which are achiral?
PROBLEM 10.45 In Section 10.2b (p. 414), we considered two
possible mechanisms for the addition of Br
2
to alkenes. Ultimately,
a stereochemical experiment that used a ring compound was
used to decide the issue in favor of a mechanism in which addi-
tion proceeded through a bromonium ion rather than an open
carbocation. Use a detailed stereochemical analysis to show how
the experimental results shown below are accommodated by an
intermediate bromonium ion but not by an open carbocation.
PROBLEM 10.46 Explain the following regiochemical results
mechanistically.
PROBLEM 10.47 Predict the major product for the following
reactions:
CCl
4
cis-2-Butene Racemic dibromide
Br
2
CCl
4
trans-2-Butene meso Dibromide
Br
2
HOR
Major product
Ma
j
or
p
roduct
H
2
OR
O
CH
3
CH
3
OH
OR
H
3
C
H
3
C
HOR
–
ORNa
+
+
O
CH
3
CH
3
OR
H
3
C
H
3
C
OH
2) NaBH
4
1) Hg(OAc)
2
/H
2
O
C(CH
3
)
3
Pd/C
H
2
H
2
O
H
2
SO
4
2) NaOH/H
2
O
2
1) BH
3
/THF
2) Na
2
SO
3
/H
2
O
1) OsO
4
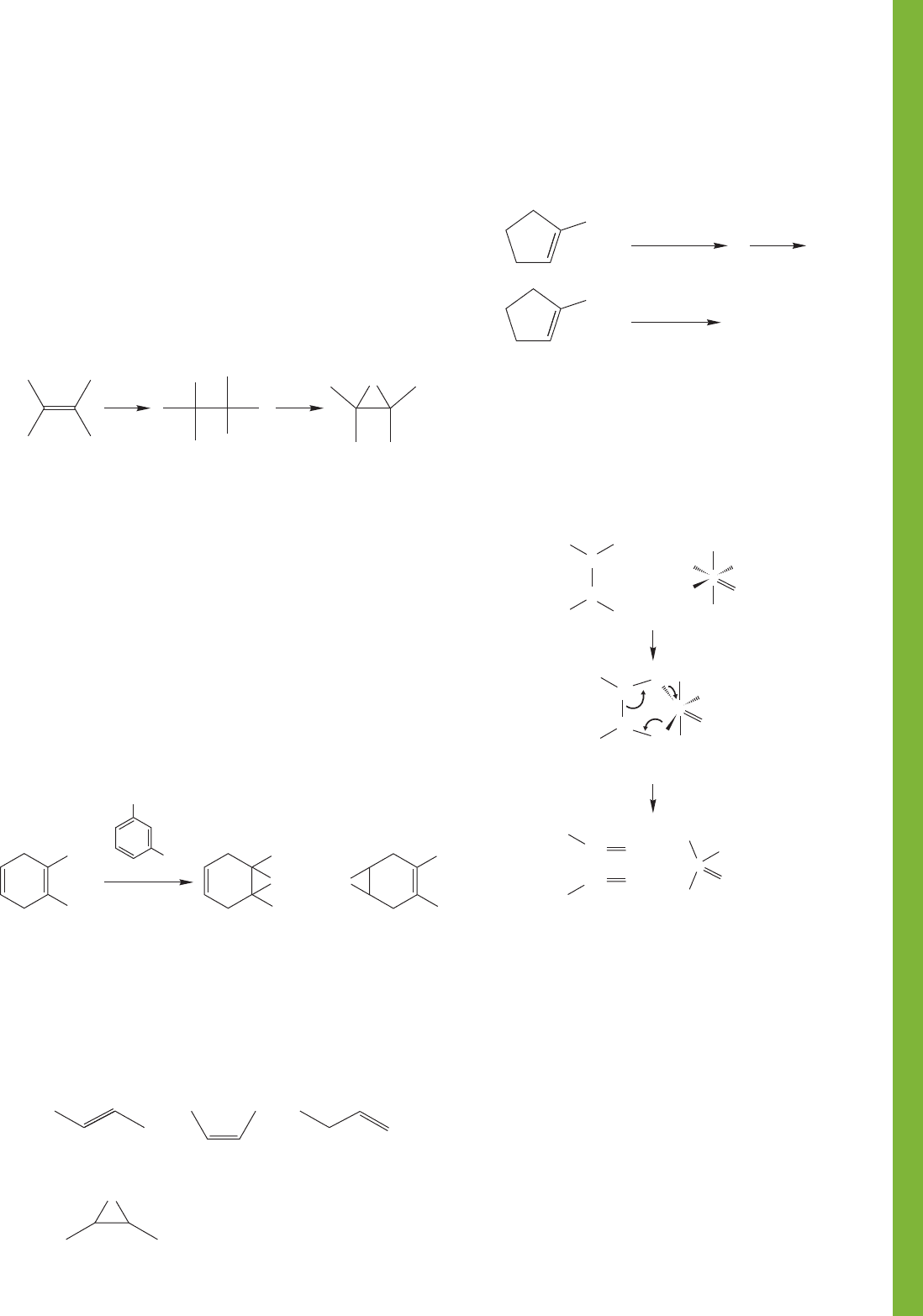
10.14 Additional Problems 463
Br
2
H
2
O
H
2
O
HO
–
O
Br
OH
A bromoh
y
drin
100 ⬚C, 3 h
(73%)
CH
3
(Not observed)
CH
3
Cl
COOOH
CH
3
CH
3
CH
3
CH
3
O
..
..
O
..
..
(a) (b) (c)
(d)
O
(cis or
trans)
PROBLEM 10.49 Treatment of cis-2-butene with Br
2
/H
2
O
gives a product (C
4
H
9
OBr), that reacts with sodium hydride
to give a meso compound of the formula C
4
H
8
O. With the
same sequence of reagents, trans-2-butene gives a different
compound (a racemic mixture) of the same formula, C
4
H
8
O.
Explain mechanistically, paying close attention to stereochemi-
cal relationships.
PROBLEM 10.50 The difference in nucleophilicity between
differently substituted alkenes can lead to selectivity in the
epoxidation reaction. Rationalize the position of faster
epoxidation in the 1,4-cyclohexadiene below and explain your
reasoning.
PROBLEM 10.48 In Section 10.4a (p. 423), you saw that
epoxidation of an alkene with a peracid such as trifluoroper-
acetic acid results in addition of an oxygen atom to the alkene
in such a way as to preserve in the product epoxide the
stereochemical relationships originally present in the alkene.
That is, cis alkene leads to cis epoxide, and trans alkene leads
to trans epoxide. There is another route to epoxides that
involves cyclization of halohydrins. Use cis-2-butene as a sub-
strate to analyze carefully the stereochemical outcome of this
process. What happens to the original alkene stereochemistry
in the product?
PROBLEM 10.51 There is one alcohol that can be synthesized
in one or two easy steps from each of the following precursors.
What is the alcohol, and how would you make it from each
starting material?
H
2
O
NaOH
CF
3
COOOH
(a)
NaOH/H
2
O
KMnO
4
(b)
CH
3
CH
3
Periodate intermediate
O
HO
HO
OH
OH
OH
I
+
+
CH
OH
OH
R
R
CH
O
O
O
OH
OH
OH
I
CH
R
R
CH
O
O
O
HO
HO
OH
I
CH
R
R
CH
PROBLEM 10.52 Show the final products of the reactions
below. Pay attention to stereochemistry.
PROBLEM 10.53 Glycols react with periodic acid to form a
cyclic periodate intermediate.The periodate then decomposes
to a pair of carbonyl compounds.
If the product from (b) in Problem 10.52 is cleaved with
periodic acid, what would be the final product of the reaction
sequence?
From a synthetic point of view, this two-step sequence—the
treatment of an alkene with basic permanganate followed by
periodate cleavage—is equivalent to what other direct method
of cleaving carbon–carbon double bonds? What other way do
you know to convert an alkene into a pair of carbonyl
compounds?
PROBLEM 10.54 Why doesn’t the product of Problem 10.52a
react with periodate?
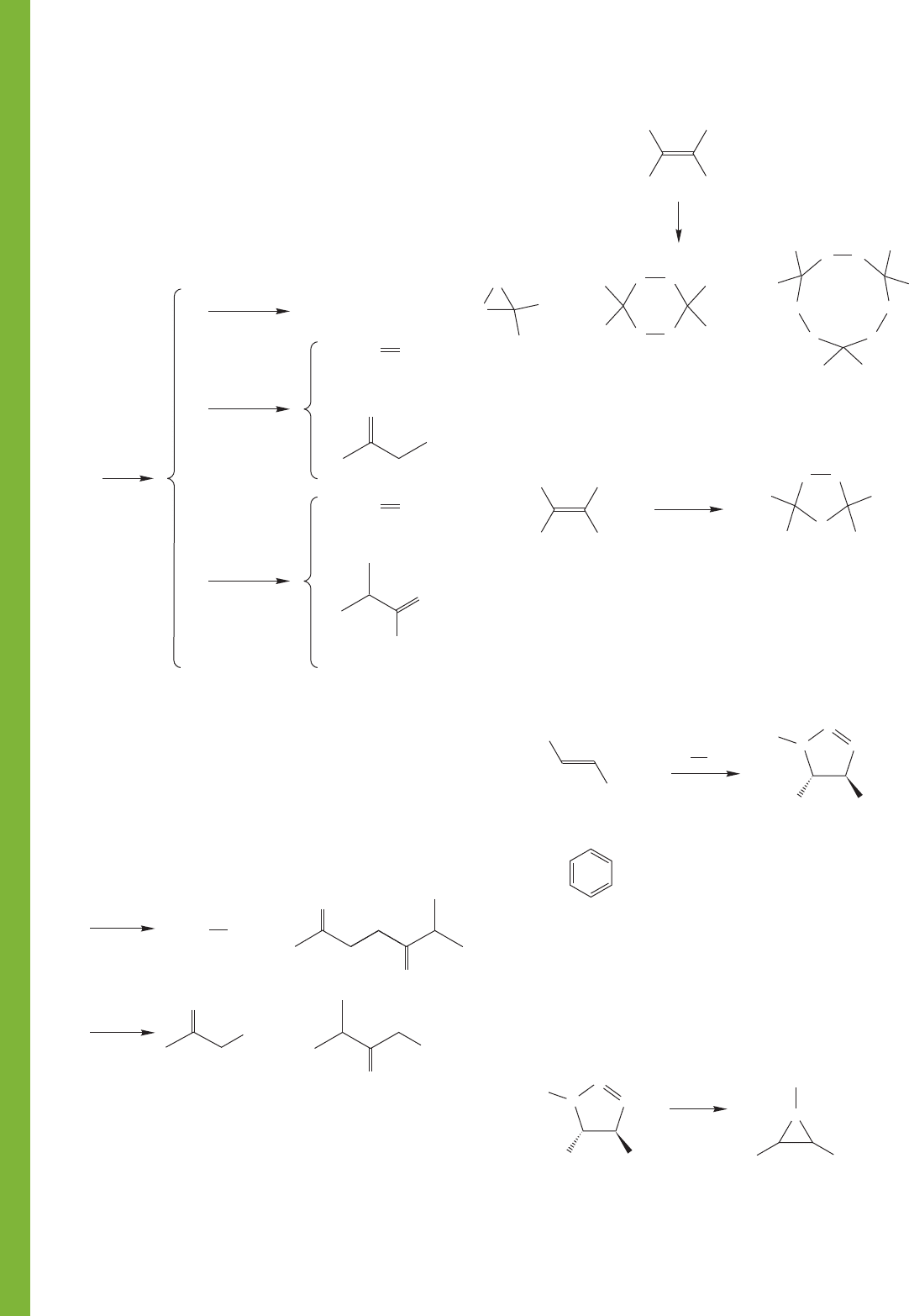
464 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
No reaction
Formaldehyde
2-Butanone
Isobutyraldehyde
+
+
1
2
3
4
H
2
O
3
Pd/C
1. O
3
2. (CH
3
)
2
S
1. O
3
2. (CH
3
)
2
S
H
2
CO
Formaldehyde
H
2
CO
O
O
H
+
+
1
2
1. O
3
2. H
2
O
2
HOOC COOH
O
O
COOH
COOH
O
O
1. O
3
2. H
2
O
2
++
O
3
R = CH
3
O
O
R
R
R
R
hexane
R
R
R
R
R
R
R
R
R
R
R
R
O
O
O
OO
O
O
O
O
O
PROBLEM 10.56 α-Terpinene (1) and γ-terpinene (2) are
isomeric compounds (C
10
H
16
) that are constituents of many
plants. Upon catalytic hydrogenation, they both afford
1-isopropyl-4-methylcyclohexane. However, on ozonolysis
followed by oxidative workup, each compound yields different
products. Provide structures for 1 and 2 and explain your
reasoning.
PROBLEM 10.57 In practice, it is often very difficult to isolate
ozonides from ozonolysis of tetrasubstituted double bonds.The
product mixture depends strongly on reaction conditions, but
the products shown in the next column can all be isolated.
Construct reasonable arrow formalisms for all of them.
PROBLEM 10.58 Ozonolysis of 1 in solvent acetone
(dimethyl ketone) leads to 2 as the major product. Explain.
PROBLEM 10.59 trans-β-Methylstyrene reacts with phenyl azide
to give a single product, triazoline (1). What other stereoisomeric
products might have been produced? Draw an arrow formalism
for this reaction and explain what mechanistic conclusions can be
drawn from the formation of a single isomer of 1.
PROBLEM 10.60 Triazoline (1) (formed in Problem 10.59)
decomposes upon photolysis or heating to give aziridine (2).
Write two mechanisms for the conversion of 1 into 2; one a
concerted, one-step reaction, and the other a nonconcerted,
two-step process. How would you use the stereochemically
labeled triazoline (1) to tell which mechanism is correct?
O
3
12
acetone
BrH
2
C
H
3
CCH
2
Br
CH
3
O
O
O
CH
3
CH
3
H
3
C
BrH
2
C
N
3
Ph
trans -β-Methylstyrene
Ph
Ph
1
Ph
CH
3
CH
3
N
N
N
Ph =
h ν
or
1
2
(no stereochemistry
implied in the drawing)
CH
3
Ph
Ph
CH
3
N
N
N
N
Ph
Ph
PROBLEM 10.55 Incomplete catalytic hydrogenation of a
hydrocarbon, 1 (C
5
H
8
), gives a mixture of three hydrocarbons,
2, 3, and 4. Ozonolysis of 3, followed by reductive workup,
gives formaldehyde and 2-butanone. When treated in the
same way, 4 gives formaldehyde and isobutyraldehyde.
Provide structures for compounds 1–4 and explain your
reasoning.
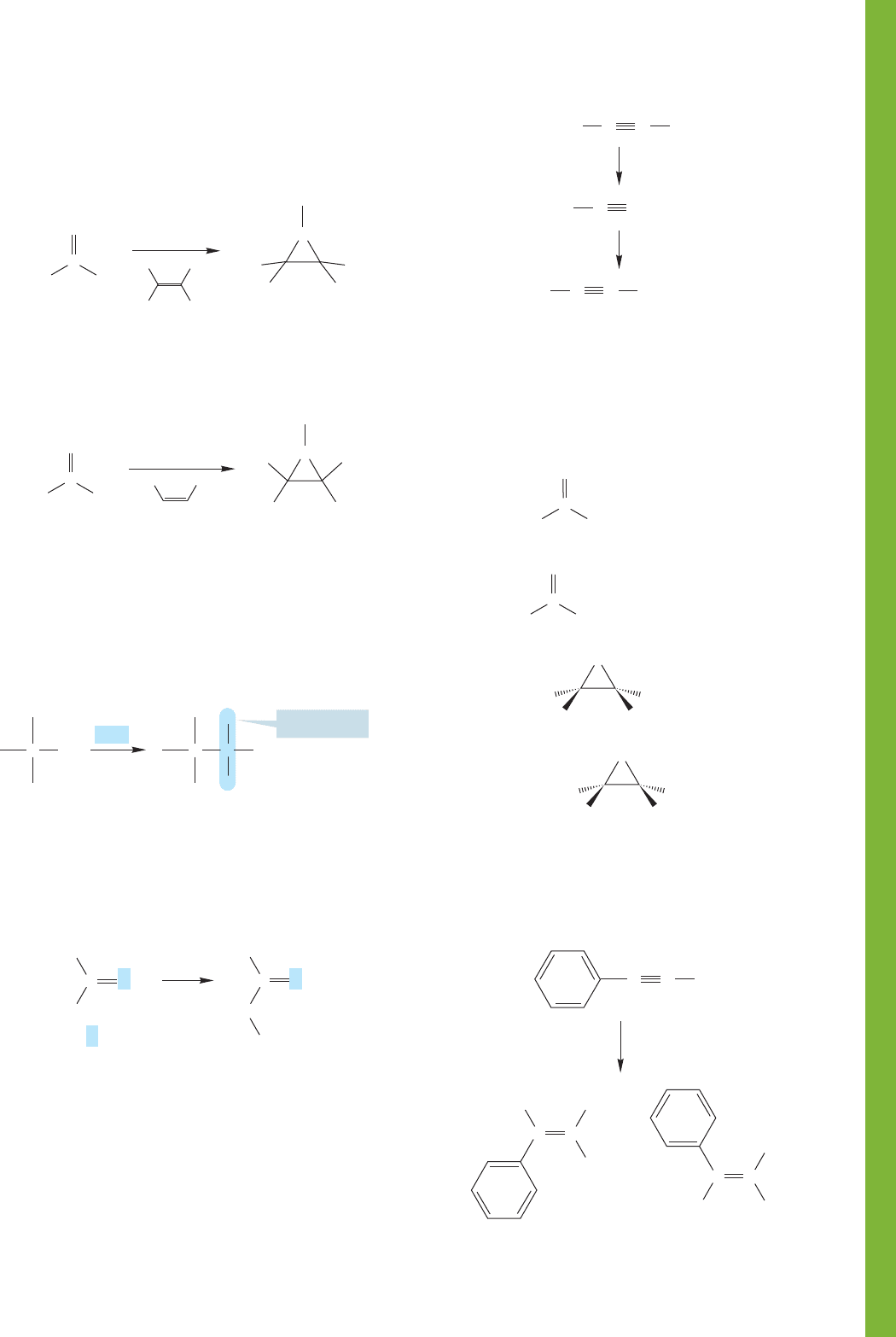
10.14 Additional Problems 465
hν
1
2
N
N
3
H
3
CO
H
3
C
H
H
C
O
COOCH
3
CH
3
CH
3
H
3
C
Old carbene
CR
2
H
C
C
R
R
H
C
..
CH
2
H
3
C
H
3
C
C
*
C =
14
C
*
CH
2
H
3
C
H
2
C
CH
3
C
*
CH
2
..
PROBLEM 10.61 Explain the formation of aziridine (2) in the
following reaction of azide (1). Hints: Draw a full Lewis struc-
ture for the azide, and see Section 10.4d (p. 431).
NaNH
2
NH
3
CH
3
I
S
N
2
H
3
C
CHC
H
3
CCH
3
CNaIC
H
3
C
C
+
NaC
–
..
+
PROBLEM 10.62 What does the formation of only cis aziri-
dine (2) from irradiation of azide (1) in cis-2-butene tell you
about the nature of the reacting species in Problem 10.61?
PROBLEM 10.63 A reaction of carbenes not mentioned in
the text is called “carbon–hydrogen insertion.” In this startling
reaction, a reactive carbene ultimately places itself between the
carbon and hydrogen of a carbon–hydrogen bond.
(a) Write two mechanisms for this reaction, one with a single-
step and the other having two steps.
PROBLEM 10.64 Contrast the results of hydroboration/oxida-
tion and mercury (Hg
2
)-catalyzed hydration for 2-pentyne
and 3-hexyne. Would any of these procedures be a practical
preparative method? Explain.
PROBLEM 10.65 Recall that terminal alkynes are among the
most acidic of the hydrocarbons (p. 129), and that the acetylide
ions can be used in S
N
2 alkylation reactions with an appropriate
alkyl halide. For example,
h ν
12
N
N
3
H
3
CO
R
R
R
R
R
R
R
R
C
O
COOCH
3
(b) In 1959, in a classic experiment in carbene chemistry,
Doering and Prinzbach used
14
C-labeled isobutene to dis-
tinguish the two mechanisms. Explain what their results
mean. The figure shows only one product of the many
formed in the reaction. Focus on this compound only.
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
H
3
C
C
H
H
O
C
O
H
(a)
(b)
(c)
(d)
O
CH
2
CH
2
CH
2
CH
3
CH
3
CH
2
HH
O
Br
2
acetic acid
HC
C
Br Br
H
(31%) (69%)
C
C
C
Br
B
r
H
C
+
Provide syntheses for the following molecules, free of other
isomers. You must use alkynes containing no more than four car-
bon atoms as starting materials. You may use inorganic reagents
of your choice, and other organic reagents containing no more
than two carbon atoms. Mechanisms are not required.
PROBLEM 10.66 Many additions of bromine to acetylenes give
only trans dibromide intermediates. However, there are excep-
tions. Here is one. Explain why phenylacetylene gives both cis
and trans dibromides on reaction with bromine.

466 CHAPTER 10 Additions to Alkenes 2 and Additions to Alkynes
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 10.67 The second page of reactions on the ORA
CD has several reactions that are discussed in Chapter 10.
Select the “Stabilized alkene halogenation” animation. This
animation shows the calculated pathway for the bromination
of acenaphthylene (see Problem 10.7). Notice the energy dia-
gram for the reaction. What does the energy diagram tell you
about the intermediate? Stop the animation at the intermedi-
ate and click on the LUMO track. Notice the interesting pat-
tern of LUMO (or cation) density. Draw all the resonance
structures that are consistent with this calculated representa-
tion of the intermediate. Based on the LUMO picture, which
of the resonance structures that you have drawn contribute
most to the overall species? Which contribute least? Can you
guess why?
PROBLEM 10.68 The “Halohydrin formation” animation was
calculated using a symmetrical alkene.The symmetry of the
alkene means that nucleophilic addition to the bromonium
intermediate will not be regioselective. Select play for this reac-
tion. Stop at the first intermediate and observe the calculated
LUMO. Based on this data, where is the positive charge locat-
ed? Show four products that would be formed through nucleo-
philic attack at each of the places of LUMO density.
PROBLEM 10.69 Epoxidation of alkenes is thought to be a
concerted process. Select the “Alkene epoxidation” animation.
Does the energy diagram indicate this reaction is concerted?
Why do the methyl groups on the cis-2-butene rotate during
the reaction? This reaction occurs because of the weak
oxygen–oxygen bond of a peracid. Hydrogen peroxide (H
2
O
2
)
also has an oxygen–oxygen bond. Would this reaction mecha-
nism work with hydrogen peroxide?
Radical Reactions
467
11.1 Preview
11.2 Formation and Simple
Reactions of Radicals
11.3 Structure and Stability
of Radicals
11.4 Radical Addition to Alkenes
11.5 Other Radical Addition
Reactions
11.6 Radical-Initiated Addition
of HBr to Alkynes
11.7 Photohalogenation
11.8 Allylic Halogenation:
Synthetically Useful Reactions
11.9 Special Topic: Rearrangements
(and Nonrearrangements)
of Radicals
11.10 Special Topic: Radicals in Our
Bodies; Do Free Radicals Age
Us?
11.11 Summary
11.12 Additional Problems
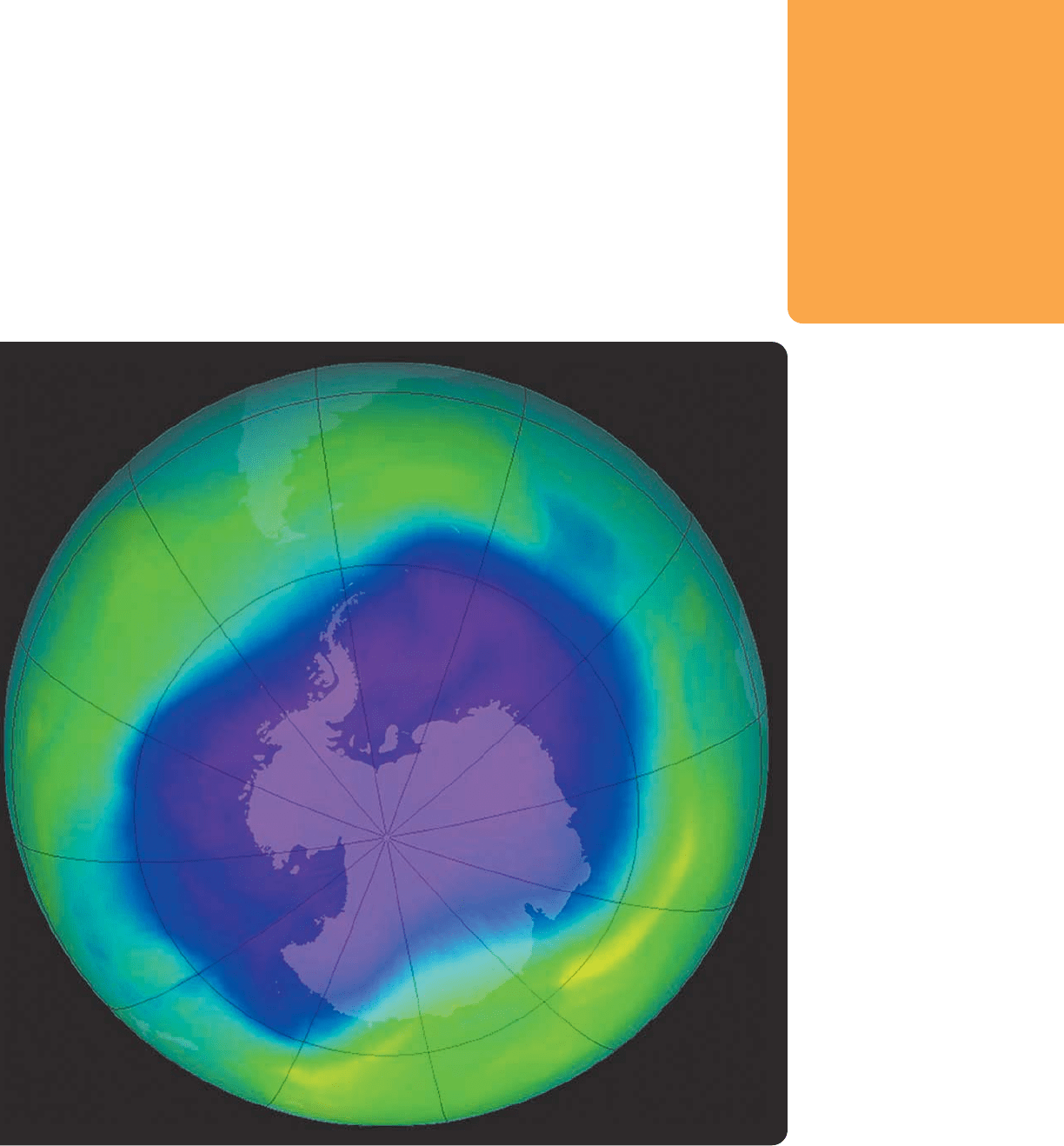
RADICALS IN THE AIR It is radical initiation and propagation steps like those
shown in Figure 11.40 that are thought to be the cause of ozone depletion.
This chart shows ozone concentrations over the Antarctic in 2006.
11
468 CHAPTER 11 Radical Reactions
1
Wystan Hugh Auden (1907–1973) was one of the best known and most influential British poets of his generation.
Knowledge may have its purposes,
But guessing is always
More fun than knowing.
—W. H. AUDEN
1
WORKED PROBLEM 11.1 Find an exception to the generalization stated in this chap-
ter’s first paragraph. What reaction have we studied that involves no polar inter-
mediates and doesn’t have a highly polar transition state? Hint: Think “recent.”
ANSWER One example is the reaction studied in Section 10.4e (p. 433), the addi-
tion of a triplet carbene to an alkene. Only neutral intermediates are involved in
this reaction. Another example is hydrogenation, the conversion of an alkene and
hydrogen into an alkane (p. 411).
PROBLEM 11.2 Name a reaction that involves no intermediates but certainly has a
highly polar transition state.
ESSENTIAL SKILLS AND DETAILS
1. You have to be able to sketch the steps of chain reactions.This chapter introduces
initiation, propagation, and termination steps, which are quite common in radical
reactions. In a chain reaction, a repeating process keeps the reaction going until starting
materials are used up or termination occurs. It is important to be able to write
initiation, propagation, and termination steps.
2. Another important skill is understanding the factors that influence selectivity. In
synthesis, selectivity is everything, because the synthetic chemist seeks a single product
over all others. In order to maximize the formation of that single product, we need to
understand the factors that control selectivity. In this chapter, selectivity is discussed
in the context of the photochemical halogenation of alkanes. This reaction is more
important for understanding selectivity than for its use in synthesis.
3. N-Bromosuccinimide (NBS) is a standard reagent for inducing bromine into the allylic
and benzylic positions.
11.1 Preview
Although there have been many variations in the details, with few exceptions all of the
reactions we have studied so far have been polar ones.The S
N
1 and S
N
2 substitutions,the
addition reactions of HX and X
2
reagents,and the E1 and E2 reactions all involve cation-
ic electrophiles and anionic nucleophiles,or at least have obviously polar transition states.
Now we come to a series of quite different processes involving the decidedly non-
polar, neutral intermediates called radicals or, sometimes, free radicals. We will also
encounter chain reactions, in which a small number of radicals can set a repeating reac-
tion in motion. In such a case, a few starter molecules can determine the course of an
entire chemical process.
The question of selectivity is prominent in this chapter.What factors influence the abil-
ity of a reactive species to pick and choose among various reaction pathways? In partic-
ular, what is the connection between the energy of a reactive molecule and its reactivity?
Don’t forget—throughout our discussion of these radical reactions, the motion
of single electrons is shown with single-barbed, “fishhook” arrows (see p. 38).
We begin with a section on the formation and simple reactions of radicals and
then move on to structure before considering the more complicated chain reactions.
CONVENTION ALERT
