Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

11.2 Formation and Simple Reactions of Radicals 469
1s
1
Hydrogen
Methane
Ethane
.
HH
H
1s
1
1s 1s
.
H
H
C
1s
1
.
H
(sp
3
)
1
.
H
H
H
C
(sp
3
)
1
(
s
p
3
)
1
.
.
H
H
H
H
C
H
H
H
H
H
H
CC
H
H
H
C
+
+
+
+
1ssp
3
+
sp
3
sp
3
+
H
H
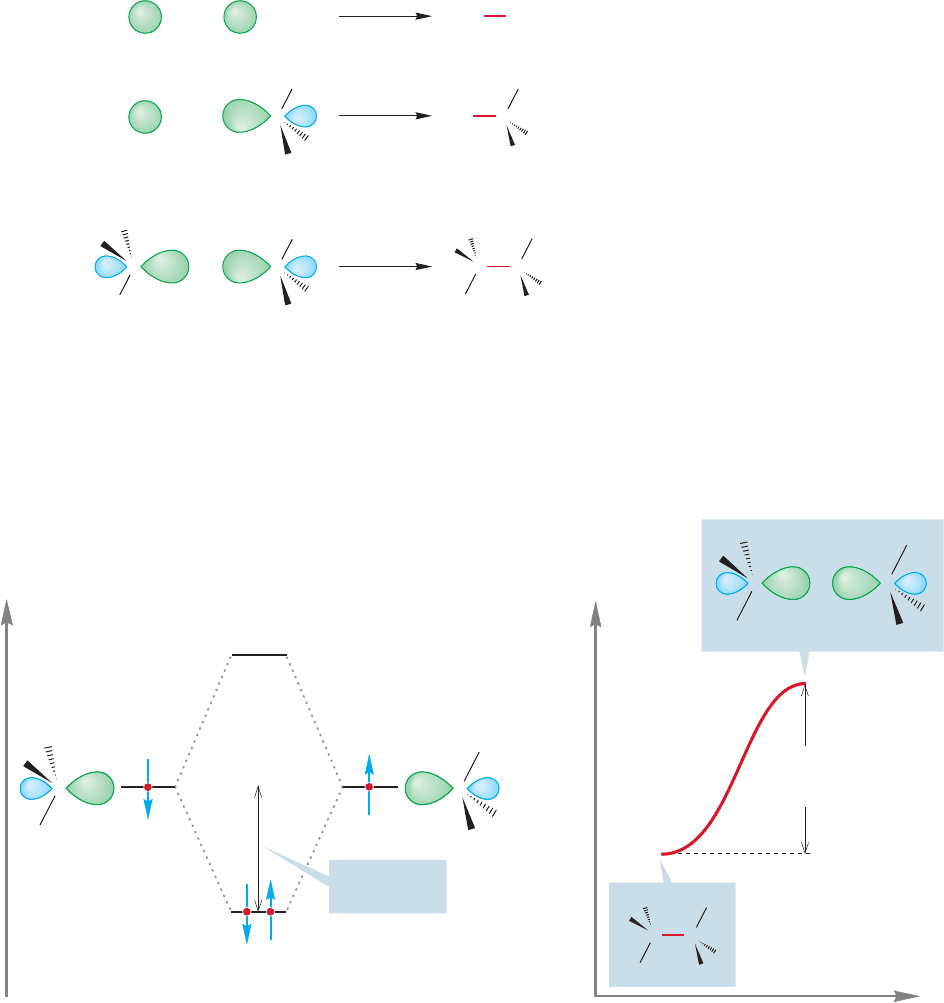
FIGURE 11.1 The formation of
hydrogen, methane, and ethane
through reactions of the radicals, H
and CH
3
.
.
.
Can we reverse the bond-forming process to produce radicals from these mol-
ecules? Energy will surely have to be added in the form of heat, because bond form-
ing is highly exothermic and bond breaking is highly endothermic. Figure 11.2a
Energy
Energy
Antibonding
molecular orbital σ*
Bonding
molecular orbital σ
H
H
H
H
H
H
CC
H
C
(sp
3
)
1
(sp
3
)
1
.
.
H
C
Stabilization
~90 kcal/mol
Reaction progress
H
C
(sp
3
)
1
(sp
3
)
1
.
.
H
C
90 kcal/mol
(bond dissociation
energy)
H
H
H
H
H
H
H
H
(a) (b)
FIGURE 11.2 (a) A schematic picture of the formation of a covalent bond through overlap of two half-filled sp
3
orbitals.
(b) To reverse the process, energy must be supplied. For example, methyl radicals can be formed by supplying enough
energy (90 kcal/mol) to cause homolytic cleavage of the carbon–carbon bond in ethane.
11.2 Formation and Simple Reactions of Radicals
We saw radicals in Chapters 1 and 2 when we considered the formation of molecules
such as hydrogen, methane, and ethane from their constituent parts: the hydrogen
atom, which is the smallest radical, and the methyl radical (see Sections 1.5, 2.4
and 2.5; Fig. 11.1).
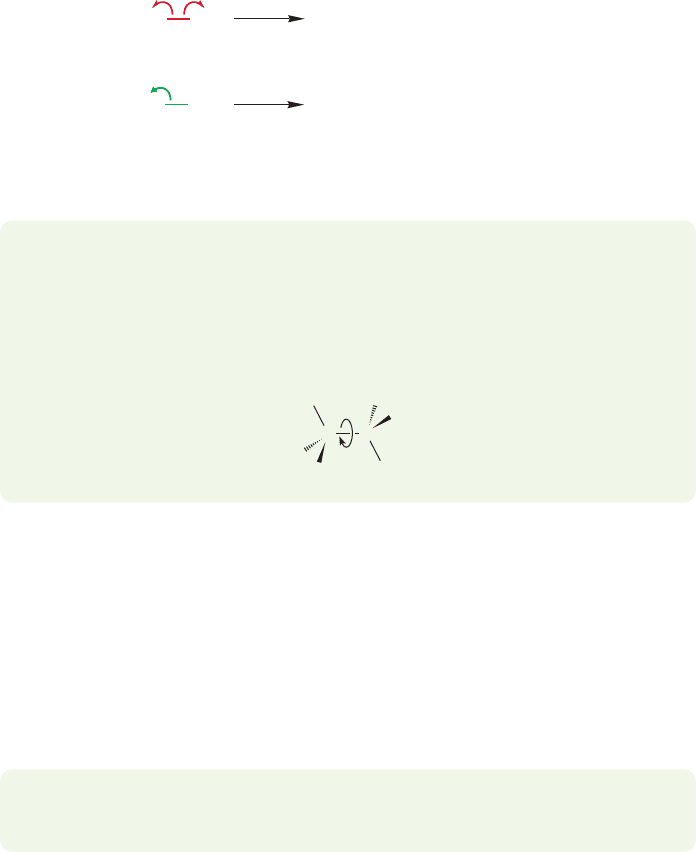
470 CHAPTER 11 Radical Reactions
–
..
Methyl radicals
Methyl
cation
Homolytic
cleavage
Heterolytic
cleavage
Methyl
anion
H
3
CH
3
CCH
3
CH
3
..
H
3
CH
3
CCH
3
CH
3
+
FIGURE 11.3 Two possible bond
breakings in ethane. Homolytic
cleavage requires less energy because
no charges are generated.
WORKED PROBLEM 11.3 In addition to bond vibration, what hard-to-see process
is going on in ethane as we heat it?
ANSWER Don’t forget rotation about the carbon–carbon bond! No new product
is formed, but this process is still occurring.
H
H
H
H
H
H
CC
Although adding heat is effective in producing methyl radicals from ethane, it
is not a generally useful method of making radicals.The carbon–carbon sigma bond
in hydrocarbons is strong, and therefore harsh conditions are required to break it,
even in a homolytic fashion. Other, more delicate functional groups will not survive
such treatment. In practice, this means that if we want to generate radicals in the
presence of other functional groups we should not attempt to do so by heating
simple hydrocarbons.
PROBLEM 11.4 When ethane is heated, why does the carbon–carbon bond break
rather than one of the carbon–hydrogen bonds?
shows schematically the stabilization gained when two singly occupied sp
3
orbitals
overlap to form a bond. In order to reverse the bond-making process and form two
radicals, energy must be provided up to the amount of the stabilization achieved
in the bond making. For example, we can apply heat to ethane in a process called
pyrolysis or thermolysis, eventually providing 90 kcal/mol and homolytically
breaking the carbon–carbon bond (Fig. 11.2b). Carbon–carbon bond cleavage is the
lowest energy process easily detectable in ethane.
Recall that the energy required to break the sigma bond homolytically is called
the bond dissociation energy (BDE, p. 337). Note that the bond breaks to give two
neutral species, two radicals, and not to give two polar species, a cation and an anion
(Fig. 11.3).
The pyrolysis of alkanes can be effective in decreasing the overall chain length
of the molecule and in introducing unsaturation. Let’s consider the pyrolysis of
butane. First, there are two possible homolytic cleavages of carbon–carbon bonds
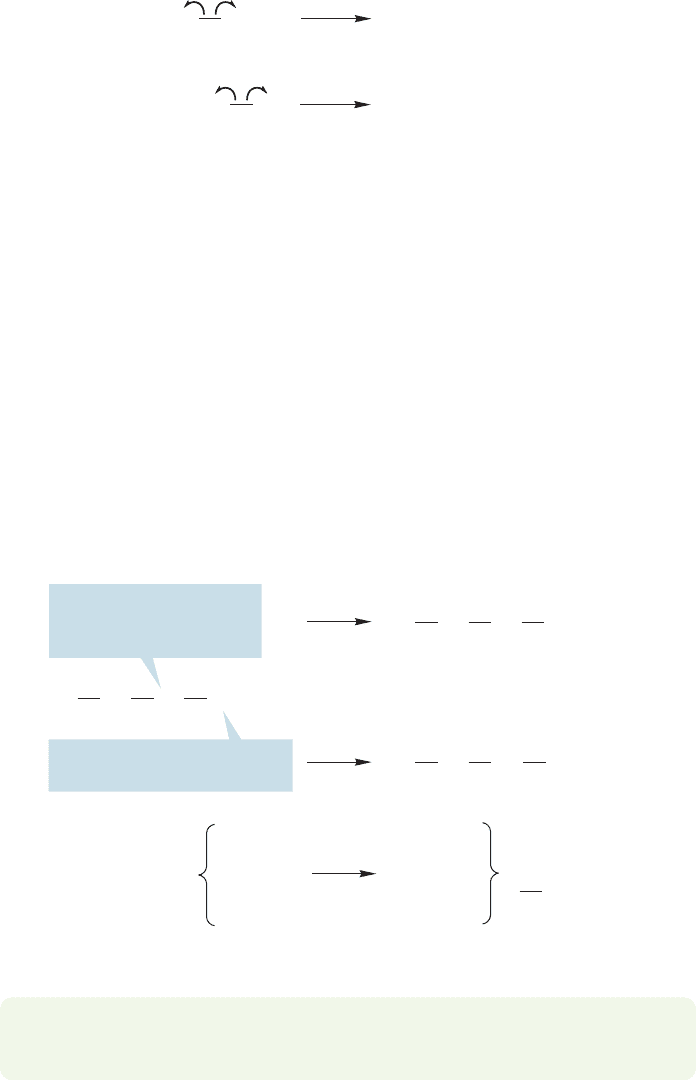
11.2 Formation and Simple Reactions of Radicals 471
Ethyl radicals
Propyl
radical
Methyl
radical
CH
3
CH
2
CH
3
CH
2
CH
2
CH
3
CH
2
CH
3
.
..
.
CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
CH
3
FIGURE 11.4 There are two possible
homolytic cleavages of
carbon–carbon bonds in butane.
Abstraction from the
secondary position gives
the sec-butyl radical
sec-Butyl radical
Abstraction from the primary
position gives the butyl radical
Abstracting radical
Products from
radical abstraction
.
CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
CH CH
3
+RH
+RH
R
H
Butyl radical
.
CH
3
CH
3
CH
2
CH
2
CH
2
.
CH
3
.
.
CH
3
CH
2
CH
2
.
CH
3
CH
3
CH
4
CH
3
CH
2
CH
3
CH
2
R
.
R
.
R
FIGURE 11.5 Hydrogen abstraction
from butane by a radical ultimately
leads to the formation of smaller
hydrocarbons and two possible butyl
radicals.
Now we need to evaluate the pathways available for these reactive intermedi-
ates. One reaction possible for these radicals is hydrogen abstraction. Abstraction
is used to describe the process of a radical species taking a hydrogen atom (not a
proton) from another molecule.In this case, one of the new radicals (methyl, ethyl,
and propyl) can abstract hydrogen from butane to make the smaller hydrocarbons
methane, ethane, and propane, along with the butyl radical. There are two posi-
tions from which hydrogen abstraction can take place in butane, the primary
of the methyl group or the secondary of the methylene group, to
give the primary radical or the secondary radical (Fig. 11.5). We will consider the
question of selectivity, of choosing between the two different positions,a little later
in this chapter.
C
O
HC
O
H
PROBLEM 11.5 Draw the transition states for the two possible abstractions of
hydrogen from butane by a methyl radical.
in butane. The methyl, ethyl, and propyl radicals can all be formed from breaking
carbon–carbon bonds (Fig. 11.4).
It is also possible for one radical to abstract a hydrogen from the vicinal (adjacent
or β) carbon of another radical in a process called disproportionation,which produces
an alkane and an alkene. In the propyl radical, for example, a carbon–hydrogen bond
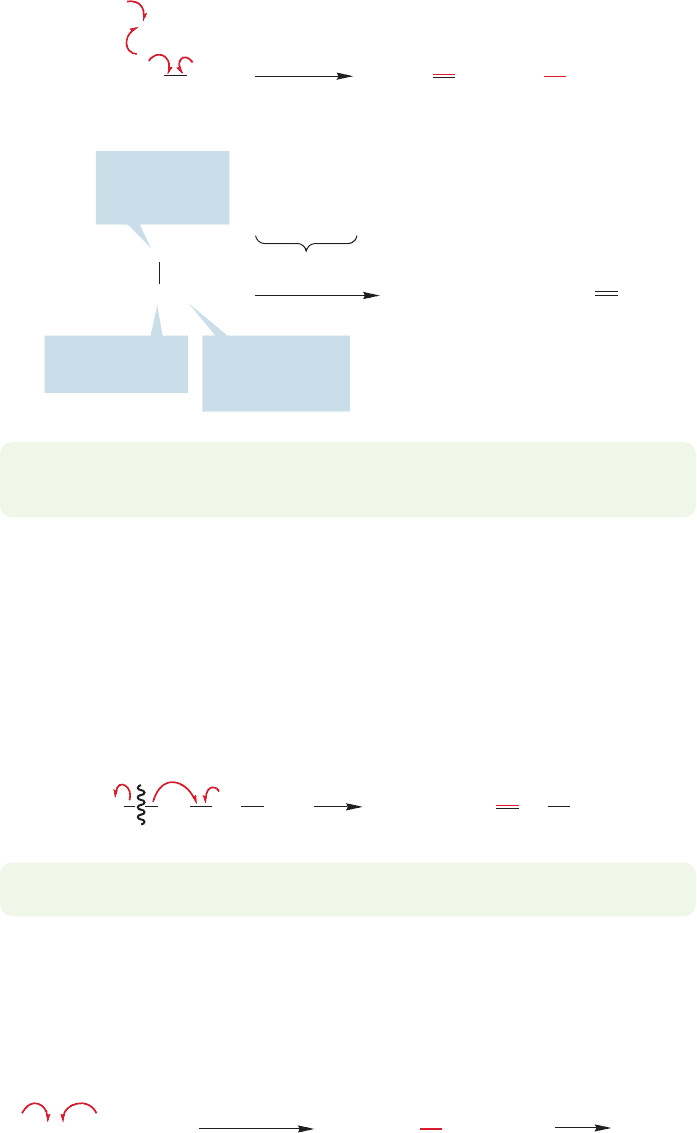
472 CHAPTER 11 Radical Reactions
could be broken by a methyl radical to give propene and methane (Fig. 11.6). In the
butane pyrolysis example, any of the radicals can do the abstracting so an is used
in Figure 11.6.
R
.
+
+
This carbon is in
the
α position
Hydrogen to be
removed is in
the
β position
available R
radicals
Reference point
is this carbon
(the radical itself)
.
.
.
CH
3
CH
CH
2
CH
2
HR
H
H
R
CH
3
CH
CH
2
CH
3
CH
Propyl radical Propene Alkane
CH
3
CH
2
.
CH
3
.
CH
3
CH
2
CH
2
CH
3
CHCH
2
.
.
CH
3
CH
3
CH
4
CH
3
CH
2
CH
3
..
FIGURE 11.6 The disproportionation
reaction produces an alkane and
an alkene from a pair of radicals.
A hydrogen atom of one radical is
abstracted by another radical.
.
.
H
3
C
CH
2
CH
3
CH CH
CH
3
H
3
CCH
2
βα
.
.
.
.
.
.
+
FIGURE 11.7 β Cleavage of the sec-
butyl radical.
..
CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
CH
2
CH
2
CH
3
dimerization
Two propyl radicals Hexane
Δ
New
products
FIGURE 11.8 Two radicals can form a bond in a process called dimerization.
PROBLEM 11.6 Draw the transition state for hydrogen abstraction in a dispropor-
tionation reaction.
PROBLEM 11.7 Why will β cleavage be favored at high temperature?
To make the issue even more complex, two radicals can occasionally react with
each other (dimerize) in a very exothermic formation of new hydrocarbons (dimers)
that can reenter the reaction sequence and provide new species. For example, two
propyl radicals might combine to form hexane (Fig. 11.8). Further pyrolysis of
hexane would lead to many other products.
Another reaction possible for many radicals is  cleavage (Fig. 11.7). In the
terminology of radical chemistry, the point of reference is the radical itself; the adja-
cent atom is α and the next atom is β. If an α–β carbon–carbon bond is present, it
can break to give a new radical and an alkene.This cleavage is simply the reverse of
the addition of a radical to an alkene (discussed later in this chapter).In the sec-butyl
radical, for example, cleavage between the methylene and methyl groups produces
a methyl radical and propene. The β cleavage reaction is favored at high tempera-
ture and can be quite important in high-temperature reactions of alkanes.
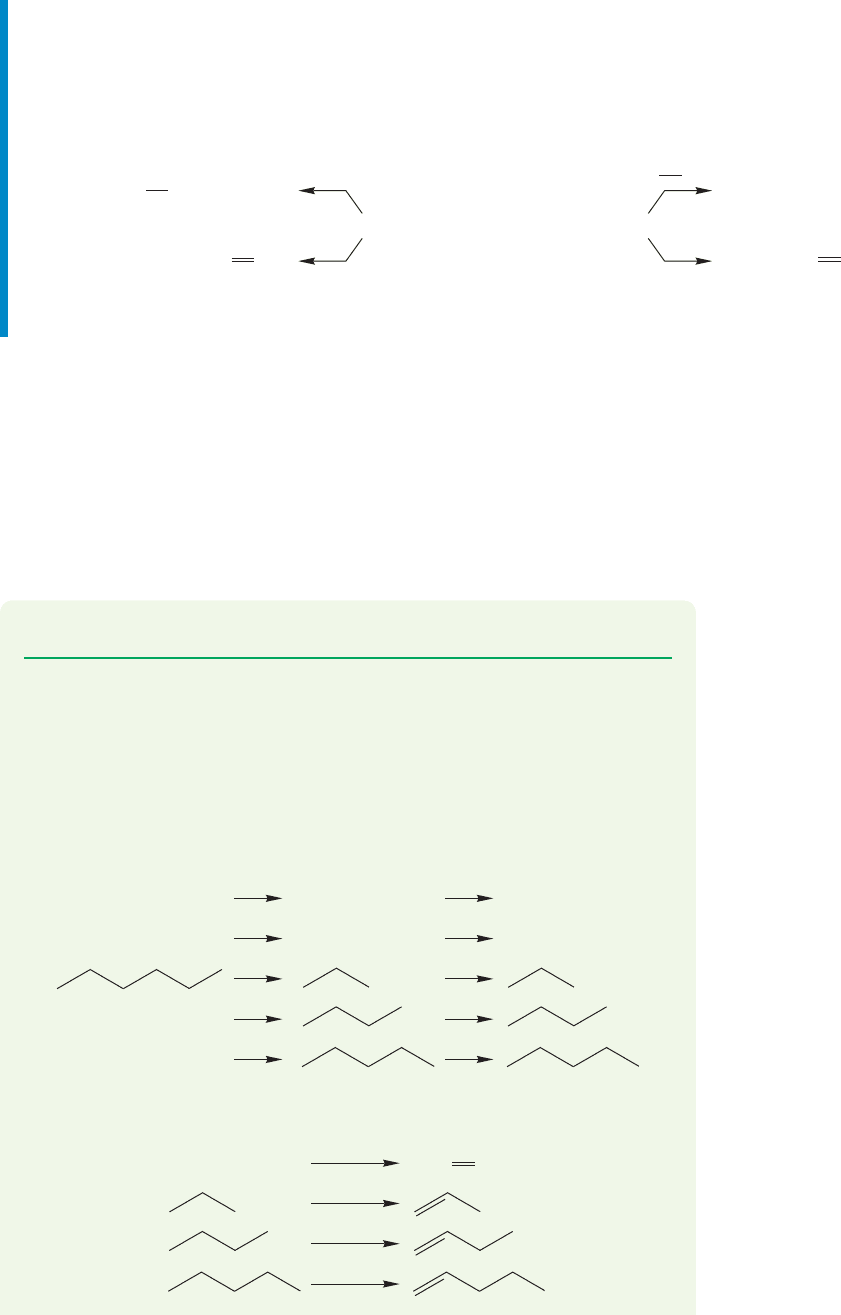
11.2 Formation and Simple Reactions of Radicals 473
Summary
The reactions available to an alkyl radical include hydrogen abstraction to make a
saturated compound and a new radical, disproportionation (hydrogen abstraction
from another radical to give an alkene and an alkane), β cleavage (the fragmenta-
tion of a radical into a new radical and an alkene), and dimerization. (Fig. 11.9).
.
.
CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
+
2 CH
3
CH
2
CH
2
CH
3
Hydrogen abstraction
Disproportionation
CH
2
CH
3
CH
2
CH
CH
3
CH
2
CH
2
CH
3
+
H
R
β Cleavage
.
CH
2
CH
2
CH
3
CH
2
+
Dimerization
CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
FIGURE 11.9 Simple reactions of radicals.
Clearly, little specificity is possible in hydrocarbon pyrolysis, and one might
at first expect that there would be little utility in such a series of reactions.That’s
not quite right. Using heat to form smaller hydrocarbons from larger ones is called
hydrocarbon cracking, and the petroleum industry relies on this process to con-
vert the high molecular weight hydrocarbons making up much of crude oil into
the much more valuable lower molecular weight gasoline fractions. It is also
possible to do the pyrolysis in the presence of hydrogen to help produce smaller
alkanes from the radicals that are produced initially.
CH
3
Δ
.
CH
2
CH
3
CH
4
CH
3
CH
3
....
Disproportionation gives the alkanes already mentioned, in addition to
ethylene, propene, 1-butene, and 1-pentene:
CH
2
CH
3
CH
2
CH
2
....
PROBLEM 11.8 What function does the hydrogen perform in hydrocarbon cracking?
WORKED PROBLEM 11.9 What products do you expect from the thermal cracking
of hexane?
ANSWER The point of this problem is to show you how incredibly complicated
this “simple” process becomes even in a relatively small alkane such as hexane.
First of all, carbon–carbon bond breakings give the methyl,ethyl, propyl, butyl,
and pentyl radicals,which can abstract hydrogen to give methane, ethane,propane,
butane, and pentane:
(continued)
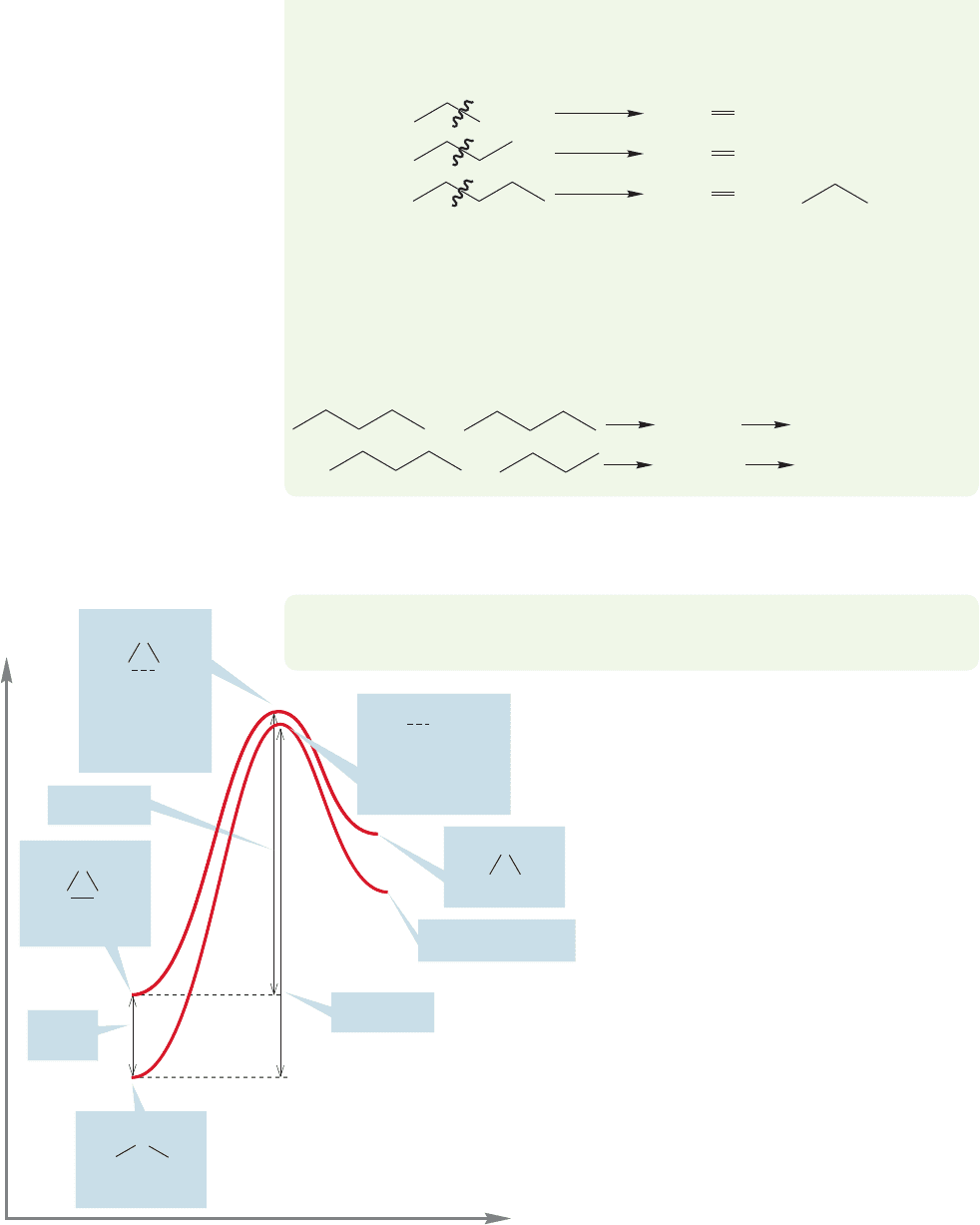
474 CHAPTER 11 Radical Reactions
β Cleavage of these radicals gives more ethylene and the methyl, ethyl, and
propyl radicals:
CH
3
.
β cleavage
H
2
C
CH
2
.
.
..
+
CH
2
CH
3
.
+
+
H
2
C
CH
2
H
2
C
CH
2
Of course, the alkanes and alkenes formed in these reactions can react further.
Propane, butane, and pentane, as well as the alkenes formed in disproportionation
and β cleavage, can participate in similar radical reactions. Moreover, dimerization
reactions have the potential of producing larger hydrocarbons, only two of which
are shown here. These dimers can go on to produce other molecules as they, too,
participate in the radical reactions. It’s an incredible mess.
.
Decane
Nonane
Many more products
.
.
+
.
+
Now comes a big question. How can an alkane be modified in order to make
the carbon–carbon bond weaker and thus more easily broken?
Energy
CH
3
CH
2
H
3
C
.
δ
.
δ
Transition state
for diradical
formation
CH
2
CH
3
H
3
C
65 kcal/mol
89 kcal/mol
Propane
Cyclopropane
Reaction progress
.
.
Strain
energy
+
CH
2
CH
2
H
2
C
.
.
CH
2
CH
2
H
2
C
CH
2
CH
2
H
2
C
CH
2
CH
3
H
3
C
.
δ
.
δ
Transition state
for formation of
radical pair
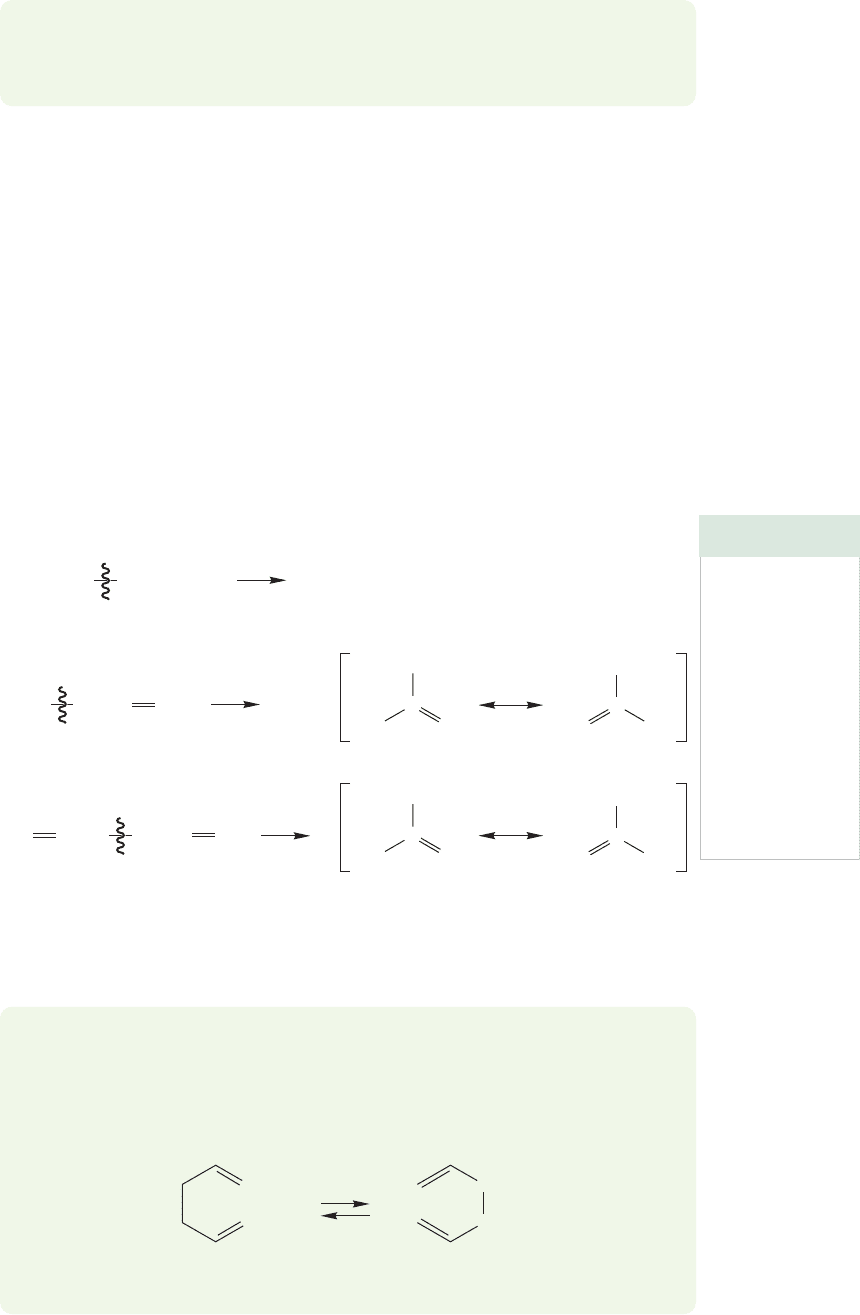
FIGURE 11.10 Strain can raise the energy of a hydrocarbon, making it easier
to break a carbon–carbon bond.
PROBLEM 11.10 Draw a molecule with a carbon–carbon bond weaker than the one
in a typical alkane.
There are many possible answers to
Problem 11.10. For example, we could
induce some strain in the molecule,raising
its energy and making bond breaking
easier. Cyclopropane is a nice example.
This small ring compound contains both
severe angle strain and substantial tor-
sional strain (Section 5.3).The strain ener-
gy of cyclopropane results in a low bond
dissociation energy of only 65 kcal/mol
(Fig. 11.10).
Breaking a carbon–carbon bond in
cyclopropane doesn’t yield two new separat-
ed radicals, but a 1,3-diradical, in which
two nonbonding electrons are present in
one molecule. We haven’t really done exactly
what we wanted—produce two new radi-
cals from a single molecule—so let’s try
another way to modify an alkane to make
it easier to cleave. We just tried making the
starting material less stable by introducing
strain. How about taking the opposite
approach by making the products of the
reaction, the two radicals, more stable?
11.2 Formation and Simple Reactions of Radicals 475
The question now becomes: How can we make a radical more stable than the
methyl radical? We know that delocalization of electrons is stabilizing, so one
approach would be to use a starting material that will yield resonance-stabilized rad-
icals.That change should lower the bond strength of the crucial carbon–carbon bond.
For example, introduction of a single vinyl group (as in 1-butene) lowers the bond
dissociation energy by about 13 kcal/mol to a value of 76 kcal/mol. As shown in
Figure 11.11, propane breaks a carbon–carbon bond to give a methyl radical and an
ethyl radical and 1-butene breaks a carbon–carbon bond to give a methyl radical and
an allyl radical.The difference in the energy required for the two bond breakings is
reasonably attributed mostly to the stabilization of the allyl radical, and, of course,
of the transition state leading to it. Introduction of a second vinyl group, as in 1,5-
hexadiene,reduces the BDE by approximately an additional 13 kcal/mol (Fig. 11.11).
The key to lowering the bond dissociation energy is the formation of allyl radicals
with their stabilizing delocalization of the nonbonding electrons.
Bond Dissociation
Energy (kcal/mol)
Propane
89
H
3
CCH
2
CH
3
CH
2
CH
3
.
.
+
H
3
C
An allyl radical
76
CH
2
H
1-Butene
+
H
3
CCH
2
CH CH
2
C
.
H
3
C
.
H
2
C
CH
2
H
C
.
H
2
C
63
CH
2
H
C
.
H
2
C
CH
2
H
1,5-Hexadiene
Two all
y
l radicals
CHCH
2
H
2
C CH
2
CH CH
2
2
C
.
H
2
C
FIGURE 11.11 Stabilization of the products of bond breaking through delocalization can make the bond
cleavage process easier.
PROBLEM 11.11 Construct an Energy versus Reaction progress diagram that
illustrates this second general approach of making the product of the reaction
more stable.
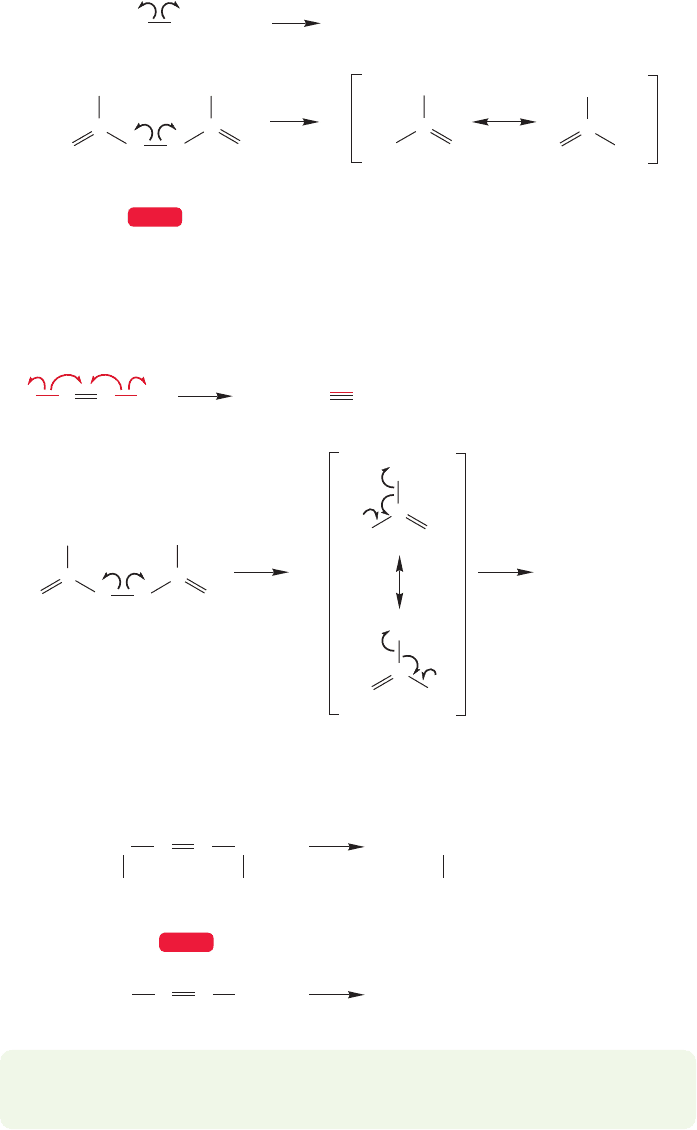
1,1,6,6-Tetradeuterio-
1,5-hexadiene
3,3,4,4-Tetradeuterio-
1,5-hexadiene
Δ
CD
2
CD
2
CD
2
CD
2
PROBLEM 11.12 There is an even lower energy process occurring in 1,5-hexadiene,
although no new molecule appears! Can you draw an arrow formalism for the
conversion of one molecule of 1,5-hexadiene into another?
Hint: 1,1,6,6-Tetradeuterio-1,5-hexadiene equilibrates on heating with 3,3,4,4-
tetradeuterio-1,5-hexadiene:
476 CHAPTER 11 Radical Reactions
WEB 3D
OR
..
..
..
..
RO
..
..
2 RO
..
..
R
.
R
C
R
Two carboxyl radicals
2
C
O
O
..
..
OO
C
RR
C
O
..
.
..
..
..
O
..
..
O
..
..
.
..
..
O
..
..
An alkyl peroxide
An acyl peroxide
FIGURE 11.12 In alkyl peroxides, the
weak oxygen–oxygen bond is easily
cleaved. In acyl peroxides, the product
radicals are stabilized and as a result
the oxygen–oxygen bond is even
weaker.
..
..
..
..
..
NRRRNN
N
Δ
..
Δ
Δ
.
.
R
.
.
An acyl peroxide
An azo compound
2
2 R 2 CO
2
(g)
O
..
..
O
..
O
O
.
..
OO
C
RR
R
R
C
C
C
+
++
O
..
..
..
..
..
O
..
..
..
..
..
(g)
FIGURE 11.13 Some molecules give
free radicals irreversibly through the
formation of gases.
(CH
3
)
3
C C(CH
3
)
3
2 (CH
3
)
3
C
+
N
2
(g)
..
NN
Δ
ΔH
= 42.2 kcal/mol
..
.
(CH
3
)
2
C C(CH
3
)
2
2 (CH
3
)
2
C
+
N
2
(g)
..
N
CN
AIBN
N
Δ
ΔH
= 31.9 kcal/mol
..
CN
CN
.
°
°
WEB 3D
(a)
(b)
FIGURE 11.14 Azobisisobutyronitrile
is an azo compound that is especially
easy to cleave.
Another technique for producing radicals is to start with molecules containing
especially weak, and therefore easily broken bonds. Examples are peroxides and acyl
peroxides (Fig. 11.12).The oxygen–oxygen bond in simple peroxides is already quite
weak (~38 kcal/mol; Table 8.2, p. 337). The addition of the carbon–oxygen double
bond of the acyl group ( ), helps delocalize the nonbonding electron in
the carboxyl radical and lowers the BDE to about 29 kcal/mol.
R
O
C
P
O
Some molecules give stable gases (often nitrogen or carbon dioxide) on heating
and thus produce radicals irreversibly. Azo compounds, which have the structure
, and acyl peroxides are good examples (Fig. 11.13).R
O
N
P
N
O
R
PROBLEM 11.13 Why does AIBN decompose much more easily than a simple azo
compound such as the molecule shown in Figure 11.14b?
A particularly useful source of radicals is azobisisobutyronitrile (AIBN), a mol-
ecule that decomposes much more easily than simpler azo compounds (Fig.11.14a).
11.3 Structure and Stability of Radicals 477
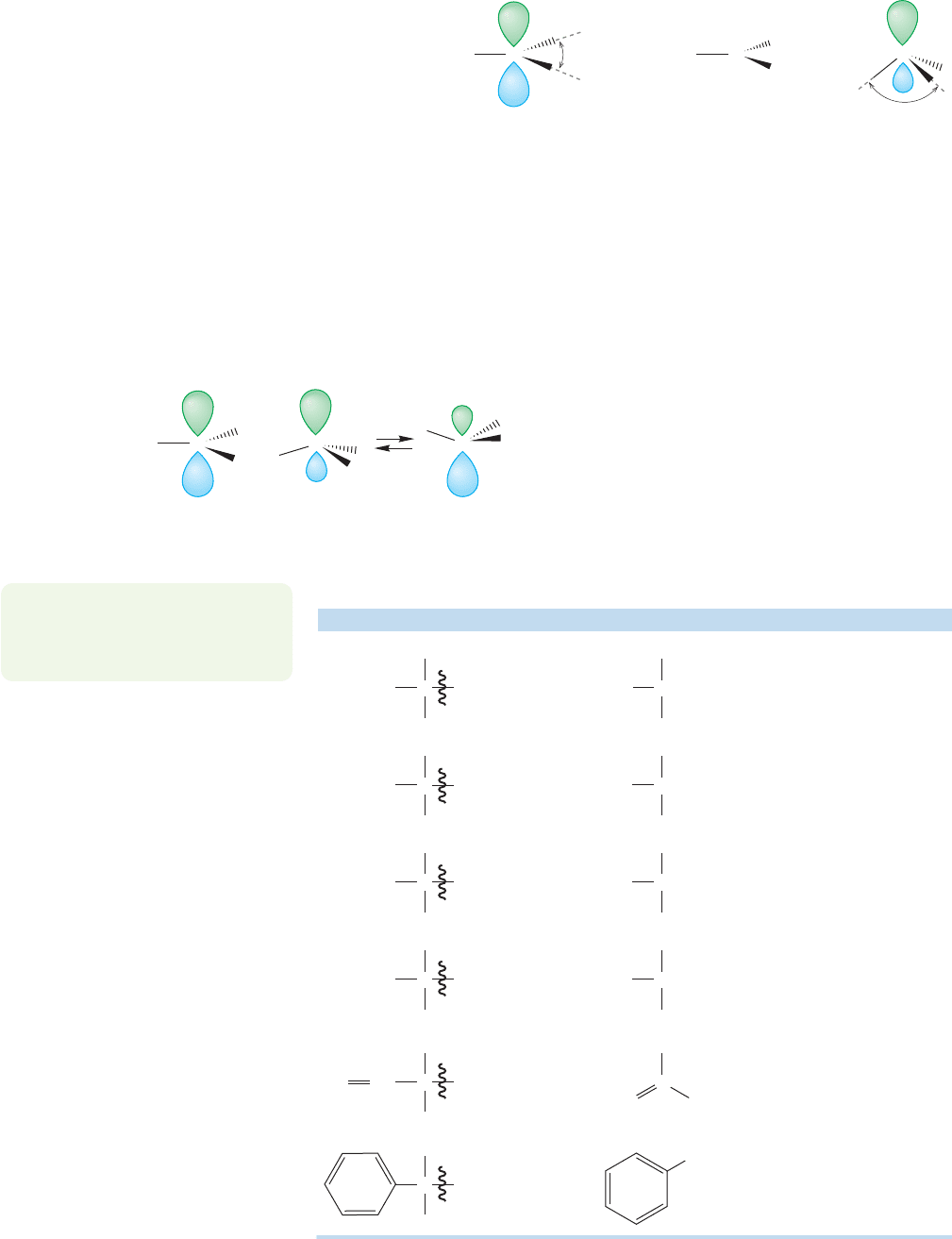
11.3 Structure and Stability of Radicals
We have already encountered both carbocations and car-
banions, and spent some time discussing their structures
(Section 2.4, p. 62).The methyl cation, and carbocations in
general,are flat,sp
2
-hybridized molecules with an empty 2p
orbital extending above and below the plane of the three
substituents on carbon (Fig.11.15).By contrast,the methyl
anion is pyramidal. One might well guess that the methyl
radical, with a single nonbonding electron, would have a
structure intermediate between that of the two ions.
Both theory and experiment agree that the structure of simple radicals is hard
to determine! However, it is clear that these molecules are not very far from planar.
If the molecules are not planar, the pyramid is very shallow and the two possible
forms are rapidly inverting (Fig. 11.16).
Planar... or... ver
y
shallow p
y
ramid
Cor
C
.
C
.
.
FIGURE 11.16 Alkyl radicals are
either flat or very shallow, rapidly
interconverting pyramids.
PROBLEM 11.14 Draw the transi-
tion state for the inversion
process in Figure 11.16.
As with carbocations, more sub-
stituted radicals are more stable than
less substituted ones. The first four
entries of Table 11.1 show BDEs of
four hydrocarbons that lead to a
methyl, primary, secondary, and ter-
tiary radical. The more substituted
the radical, the easier it is to form,
and the easier it is to break the bond
that produces it.The data presented
in the table are slightly suspect,
because we are forming the radicals
from different starting materials.
Might not the different energies of
the different starting materials influ-
ence the BDEs? Yes they might, and
do. But this effect isn’t large, and the
data in the table can be used to show
that more substituted radicals are
more stable than less substituted
radicals.
Carbocation,
planar
Carbanion,
pyramidal
Radical—somewhere
in between the cation
and anion
+
C C
.
–
C
..
120⬚
99.8⬚
FIGURE 11.15 Radicals are intermediate in structure between
the planar carbocations and the pyramidal carbanions.
.
...
H
H
H
C
H
H
3
C
H
H
C
H
H
3
C
H
H
C
H
3
C
H
3
C
H
3
C
H
C
H
3
C
H
H
C
H
H
3
C
H
C
H
H
3
C
H
C
H
3
C
H
3
C
105.0
101.1
98.6
96.5
89.8
88.8
H
3
C
C
H
3
C
TABLE 11.1 Bond Dissociation Energies for Some Hydrocarbons
Hydrocarbon Radical BDE (kcal/mol)
H
2
C
C
H
C
H
2
.
.
CH
2
CHH
2
C
H
H
C
H
H
H
C
H
478 CHAPTER 11 Radical Reactions
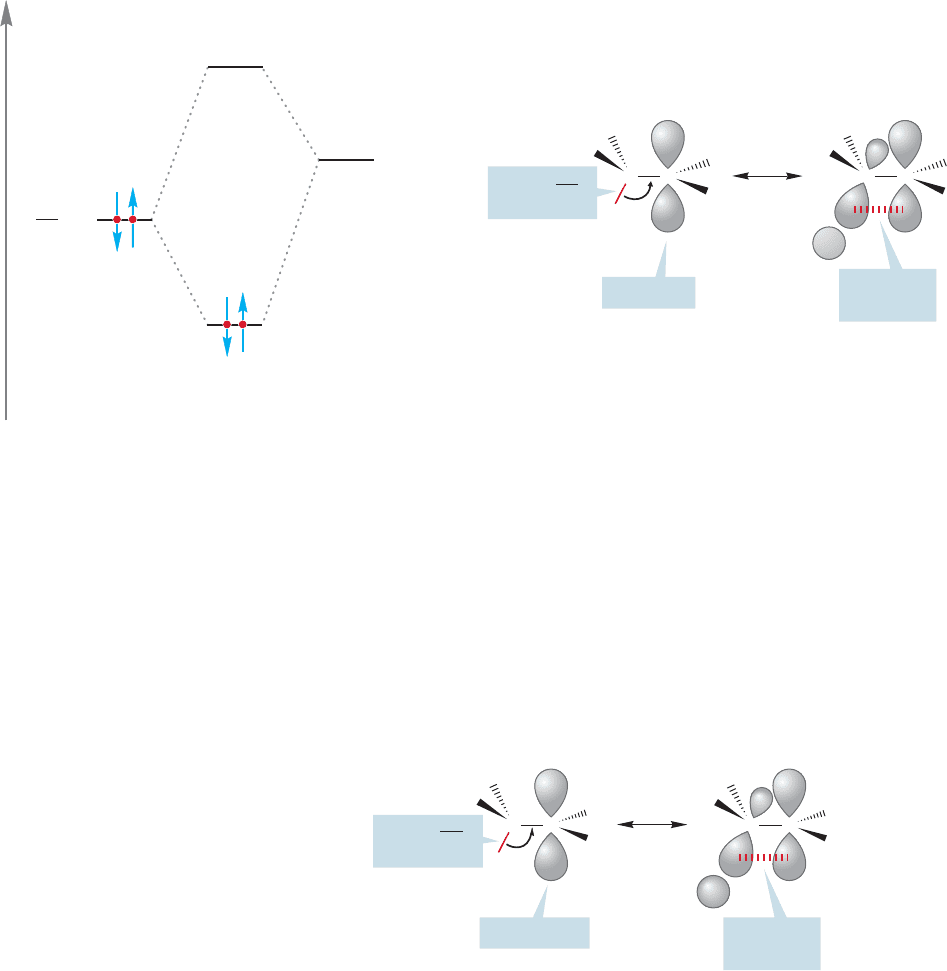
Why should more substituted radicals be more stable than less substituted
radicals? Let’s start with an analogy. In explaining the order of stability for
carbocations (tertiary > secondary > primary > methyl), we drew resonance forms
in which a filled carbon–hydrogen bond overlapped with the empty 2p orbital in
a process called hyperconjugation (Fig. 11.17).
Energy
CH σ
Antibonding
molecular orbital
Bonding
molecular orbital
2p
(empty)
Empty 2p
+
C
2p/σ bond
overlap
Filled C
H
σ bond
C CC
H
H
+
FIGURE 11.17 Resonance stabilization of a carbocation through hyperconjugation, shown both by an interaction energy
diagram and by an orbital overlap picture.
Half-filled 2p
2p/σ bond
overlap
Filled C
H
σ bond
CC CC
H
H
.
.
.
.
.
.
.
.
FIGURE 11.18 Resonance
stabilization of an alkyl free radical.
We can draw similar resonance forms for radicals. As before, the more
alkyl groups attached to the carbon bearing the nonbonding electron, the
more resonance forms are possible. But there is an important difference:
Stabilization of the carbocation involves overlap of a filled carbon–hydrogen
bond with an empty 2p orbital (Fig. 11.17), whereas in the radical, overlap
is between a filled carbon–hydrogen bond and a half-filled 2p orbital
(Fig. 11.18).
Why should this orbital mixing be stabilizing? The evident problem is that
this is a three-electron system. One electron must occupy the antibonding orbital,
and this is certainly destabilizing. Nevertheless, the overall system is still stabi-
lized because two electrons are able to occupy the low-energy bonding orbital.
