Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

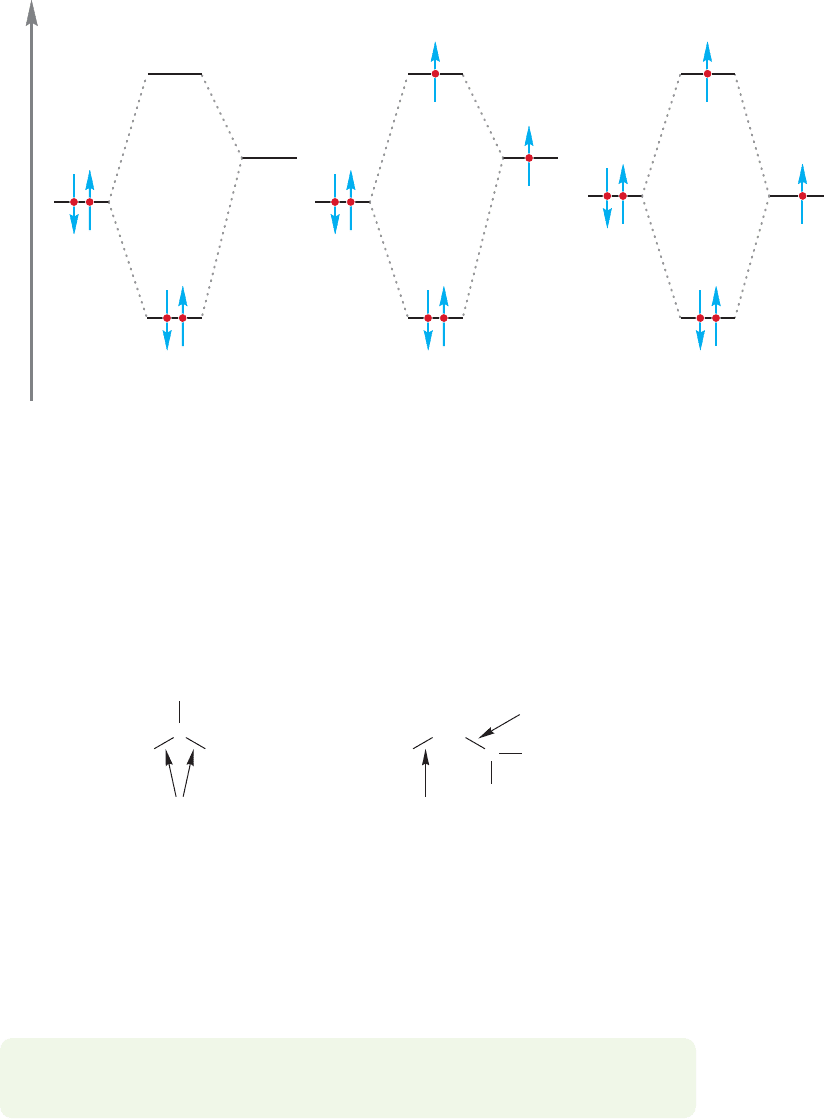
11.3 Structure and Stability of Radicals 479
Energy
σ
Antibonding
molecular orbital
Bonding
molecular orbital
2p
2p
σ
Antibonding
molecular orbital
Bonding
molecular orbital
1s
1s
Antibonding
molecular orbital
Bonding
molecular orbital
He
He
+
Cationic, two-electron
system
Radical, neutral,
three-electron system
He
2
+
FIGURE 11.19 A comparison of the stabilizations of carbocations, radicals, and He
2
. Notice the electron in the
antibonding orbital of He
2
and the radical.
Recall He
2
in which a similar orbital system was encountered (p.41). Figure 11.19
compares hyperconjugation of a carbocation, hyperconjugation of a radical, and
the He
2
molecule. As we saw in Chapter 1, He
2
is held together (bound) by
over 60 kcal/mol.
H
H
H
3
C
CH
3
C
H
3
C
CH
CH
2
2-Propyl radical 1-Propyl radical
Both sp
2
/sp
3
sp
3
/sp
3
sp
2
/sp
3
Weaker than the others
.
.
FIGURE 11.20 The 2-propyl radical
contains two relatively strong sp
2
/sp
3
bonds, whereas the 1-propyl radical
has one strong sp
2
/sp
3
bond and one
less strong sp
3
/sp
3
bond.
Consider the bond strengths in the carbon radicals, as we did earlier in discussing
alkenes and carbocations (p. 378, Problem 9.7). In the 2-propyl radical we have two
sp
2
/sp
3
carbon–carbon bonds, whereas in the less stable 1-propyl radical there is one
sp
2
/sp
3
bond and one less strong sp
3
/sp
3
carbon–carbon bond (Fig. 11.20).
Of course, we should analyze the carbon–hydrogen bonds as well. If we do this,
we find that the differences in the strengths of the bonds should favor the
1-propyl radical. As with carbocations, it is the bonds that dominate and
determine the issue. As a practical matter, it appears that we can “get away with” an
analysis of the bonds alone.C
O
C
C
O
C
C
O
H
PROBLEM 11.15 Analyze the difference in bond strength between the
bonds in the 2-propyl and 1-propyl radical.
C
O
H
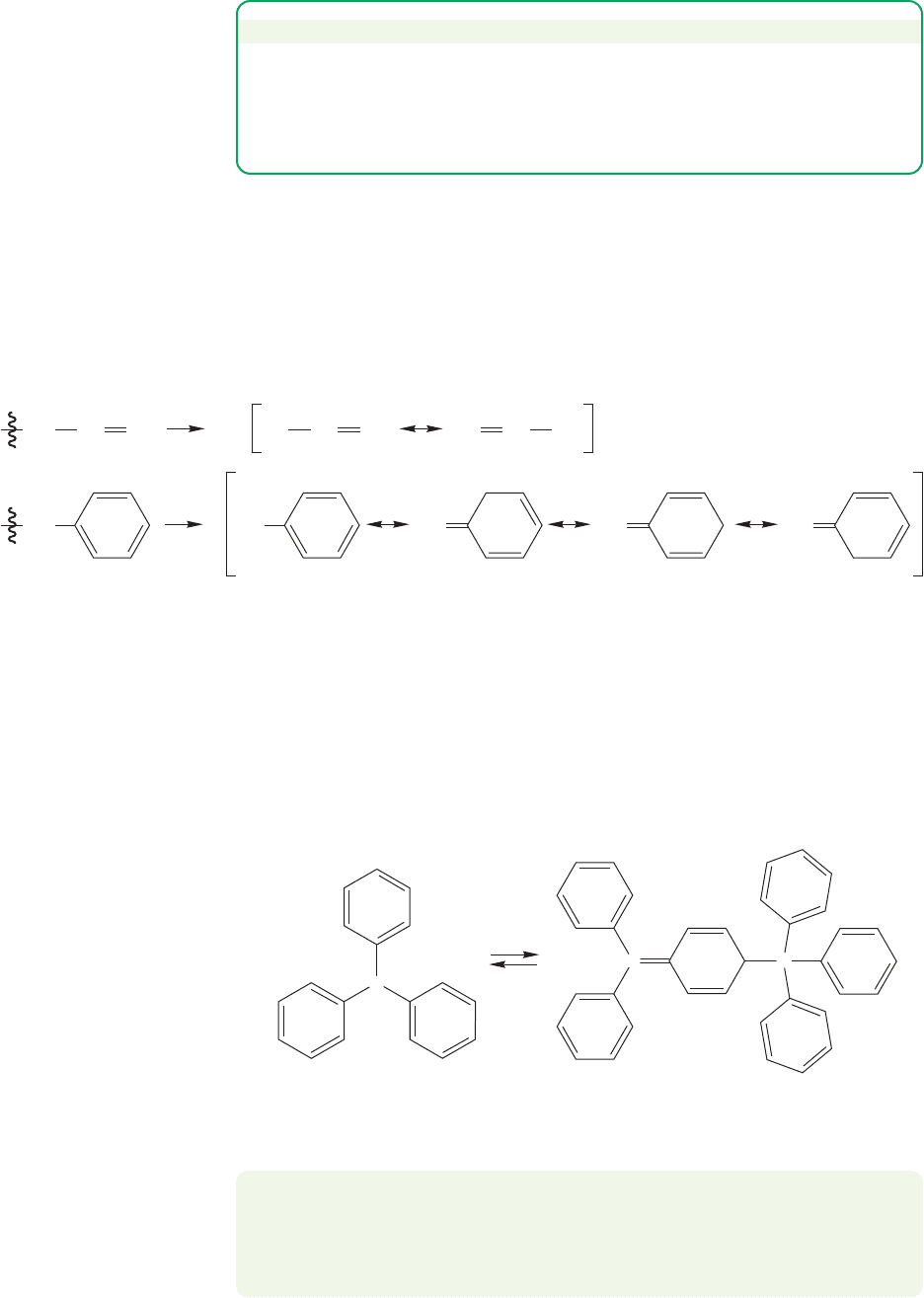
Radicals substituted with electron-delocalizing groups, such as the vinyl or
phenyl groups, are even more stable. As Table 11.1 shows, breaking a carbon-
hydrogen bond to give an allyl radical or benzyl radical is relatively easy.
The allyl and benzyl radicals are even more stable than a tertiary radical
(Fig. 11.21).
480 CHAPTER 11 Radical Reactions
.
.
CH HCH
2
CH
2
CH CH
2
CH
2
.
CH CH
2
H
2
CH
CH
2
H
2
CH
.
.
H
2
C
.
H
2
C H
2
C
.
FIGURE 11.21 Resonance stabilization in the allyl and benzyl radicals.
.
C
C
Dimer
Triphenylmethyl radical
2
C
FIGURE 11.22 The triphenylmethyl
radical exists in equilibrium with its
dimer.
PROBLEM 11.16 As you can see from Figure 11.22, the dimer is not the “obvious”
one, hexaphenylethane, but a much more complicated molecule. Can you see why
hexaphenylethane is a most unstable molecule, and draw an arrow formalism
mechanism for the formation of the real dimer?
PROBLEM SOLVING
Although the order of radical stability is the same as that for cation stability, the
absence of a charge makes a big difference. Primary and methyl radicals are not
“mechanistic stop signs,” as are the related carbocations. Their energies are not so
high as to make them unknown.
If they are sufficiently stabilized, radicals can be made and studied
spectroscopically at low temperature. A few highly delocalized radicals are even
stable under normal, room temperature conditions. For example, the triphenyl-
methyl radical happily exists in solution as an equilibrium mixture with its dimer
(Fig. 11.22).
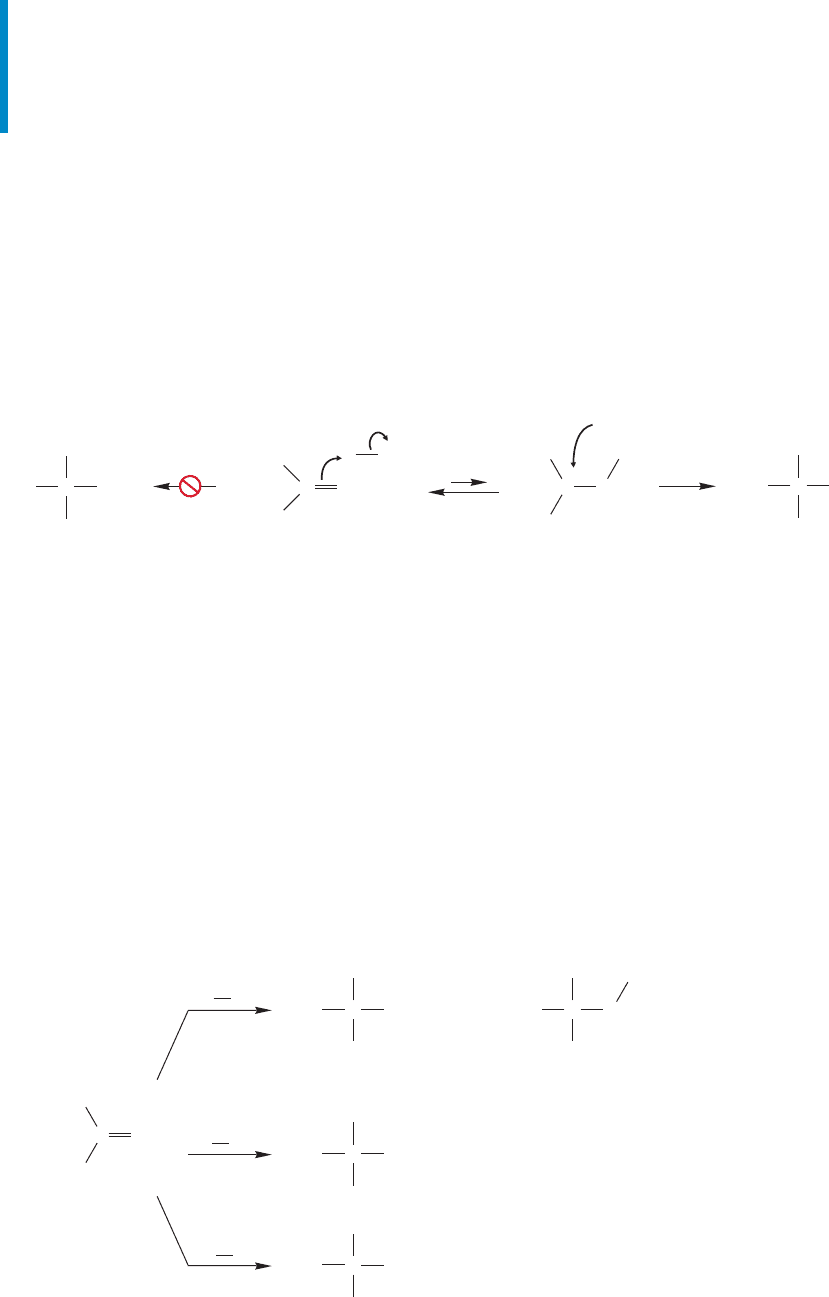
11.4 Radical Addition to Alkenes 481
+
+
Less stable,
primary carbocation
(
not formed
)
Much more stable
tertiary carbocation
is formed
H
–
Br
..
..
..
..
Br
..
..
..
Br
..
..
..
C
H
3
C
H
3
C
CH
2
H
CH
3
CH
3
C
H
3
C
CH
3
CH
3
C
H
C
H
3
C
H
3
C
CH
2
CH
2
FIGURE 11.23 The addition of hydrogen bromide to an unsymmetrical alkene follows the Markovnikov
rule because protonation of the alkene must give the more substituted (more stable) carbocation.
11.4 Radical Addition to Alkenes
In Section 9.3 (p. 366), the addition of hydrogen chloride to alkenes was presented
with the reaction always leading to Markovnikov addition. Chapter 9 stressed the
initial formation of the more stable carbocation followed by addition of the resid-
ual halide ion.The product of Markovnikov addition must be produced in this reac-
tion (Fig. 11.23). Of course, HBr reacts the same way as HCl.
The historical reality was far from that simple. The regiochemistry of the
reaction appeared strangely dependent on the source of the starting materials and
even on the place in which the reaction was carried out. In some worker’s hands, the
reaction did follow the Markovnikov path; in other places, with other investigators,
anti-Markovnikov addition was dominant. Confusion and argument were the rule
of the day in the late 1800s. Oddly enough, the problem was restricted to hydrogen
bromide addition. Both hydrogen chloride and hydrogen iodide addition remained
securely Markovnikov in everyone’s hands (Fig. 11.24).
Sometimes Markovnikov, sometimes not
Always Markovnikov
H
3
C
CH
3
CH
3
and/or
C
H
Br
H
3
C
CH
3
CH
2
C
H
B
r
Br
H
3
C
CH
3
CH
3
C
H
Cl
Cl
Always Markovnikov
H
3
C
CH
3
CH
3
C
H
I
I
C
H
3
C
H
3
C
CH
2
FIGURE 11.24 Both hydrogen
chloride and hydrogen iodide
addition always follow the
Markovnikov rule, but hydrogen
bromide addition is strangely
capricious.
Summary
Radical stability parallels carbocation stability: Benzylic and allylic are more
stable than tertiary, tertiary more stable than secondary, secondary more stable
than primary, and primary more stable than methyl.The determining factors are
resonance and hyperconjugative stabilization.
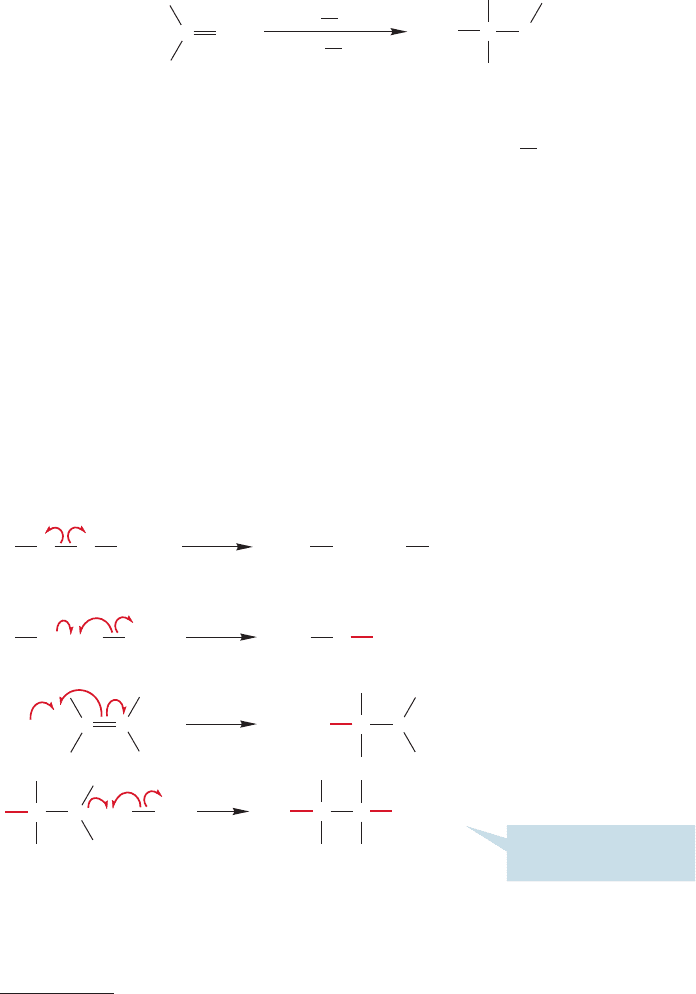
The product of
anti-Markovnikov
addition of H
B
r
H
H
3
C
CH
3
CH
2
C
H
B
r
Br
RO OR
(a peroxide)
C
H
3
C
H
3
C
CH
2
FIGURE 11.25 Addition of hydrogen
bromide to alkenes in the presence of
peroxide is anti-Markovnikov.
In the 1930s, largely through the efforts of M. S. Kharasch,
2
it was discovered
that the purity of the compounds used was vital.Pure materials led to Markovnikov
addition, and we can remain content with the mechanism for the polar reaction.
Impure reagents led to predominately anti-Markovnikov addition. A special culprit
was found to be peroxides (Fig. 11.25). Breaking the weak oxygen–oxygen bond in
peroxides provides a relatively easy route to free radicals. The mechanism of addi-
tion in the presence of peroxides is different from the familiar polar one and can be
triggered by these small amounts of radicals.
482 CHAPTER 11 Radical Reactions
C
C
Br
..
Br
..
Br
..
..
.
..
Br
..
..
..
..
..
..
C
C
or h
ν
Δ
OStep 1
.. ..
..
..
..
..
..
..
..
..
..
..
..
..
..
..
O
.
.
O O
+
OStep 2
Step 3
Step 4
H
.
.
.
.
.
.
CC
H
Recycle to Step 3 and
start all over again
Br
CC
Br
..
..
..
.
.
Br
..
..
..
H
ORR
RRRR
H
+
Br
..
..
..
.
.
+
FIGURE 11.26 The mechanism for the radical addition of HBr to an alkene.
11.4a The Mechanism of Radical HBr Addition As noted earlier (p. 476),
peroxides contain a very weak oxygen–oxygen bond and are easily cleaved by heat ( )
or light (
h ) to give two radicals (Fig. 11.26, Step 1). The radical can
react with by hydrogen abstraction to form and a new radical, a
bromine atom (Step 2). Addition of the bromine atom to an alkene gives still
another radical (Step 3), which can react with to produce an alkyl bromide
and another bromine atom (Step 4). The bromine atom can recycle to Step 3 and
start the reaction over again with another alkene molecule.The overall result is the
addition of hydrogen bromide to the alkene.
H
O
Br
R
O
OHH
O
Br
R
O
O
.
ν
¢
2
Kharasch, 1895–1957, is reported by one of his students, F. R. Mayo (b. 1908), to have suggested that the
direction of addition may have been affected by the phases of the moon.
11.4 Radical Addition to Alkenes 483
Br
..
.
.
CC
Br
..
..
CC
RRRR
Br
..
..
..
Br
..
..
..
..
..
.
.
.
.
Br
..
Br
..
..
..
..
..
..
Br
..
..
..
Br
..
..
..
FIGURE 11.27 Whenever two
radicals come together they can react
to form a bond. The bond formation
removes two radicals and terminates
this chain reaction.
Other products can be formed in this reaction.When two radicals meet they can
react in a very exothermic fashion to form a new bond and end the sequence, called
a chain reaction. There are many possibilities for this kind of termination reaction
using the radicals in Figure 11.26. A few ways are shown in Figure 11.27.
We have just outlined the radical-induced addition of hydrogen bromide to an
alkene. The steps of this chain reaction naturally divide into three kinds:
Initiation. The reaction cannot start without the presence of a radical. A radical
must be formed to start the process,in a reaction known as an initiation step.Peroxides
are good generators of radicals because of the weakness of their oxygen–oxygen bond
(Fig. 11.26, Step 1). Once a radical is created in the presence of hydrogen bromide, a
second initiation step, abstraction of hydrogen (Fig. 11.26,Step 2),takes place to form
a bromine atom, which is the radical that propagates the chain reaction.
Propagation. Once a bromine atom has been formed in the initiation steps, the
chain reaction can begin.The reactions making up the chain reaction are called prop-
agation steps. In this example, the two propagation steps are the addition of the
bromine atom to the alkene to give a new, carbon-centered alkyl radical and the sub-
sequent abstraction of hydrogen from hydrogen bromide by the alkyl radical
(Fig. 11.26, Steps 3 and 4).
The two radicals involved in the propagation steps, the bromine atom and the
alkyl radical, are called chain-carrying radicals.The chain reaction continues as the
newly formed bromine atom adds to another alkene to repeat the process over and
over again.
Termination.Any time one of the chain-carrying radicals (in this case a bromine
atom or an alkyl radical) is annihilated by combination with another radical, the chain
reaction is stopped (Fig. 11.27). If such a step does not happen, a single radical could
initiate a chain reaction that would continue until starting material was completely
used up. In practice, a competition is set up between the propagation steps carrying
the chain reaction and the steps ending it.Such reactions are called termination steps
and there are many possibilities.
For a chain reaction to produce large amounts of products effectively, the prop-
agation steps must be much more successful than the termination steps. Such is often
the case, because in order for a termination step to occur, two radicals must wander
through the solution until they find each other. Typically, the propagation steps
require reaction with a reagent present in relatively high concentration. So, even
though termination steps often can take place very easily once the reactants find each
other, propagation steps can compete successfully, and reaction cycles of many thou-
sands are common.Under such circumstances,the small amounts of product formed
by the occasional termination step are inconsequential.
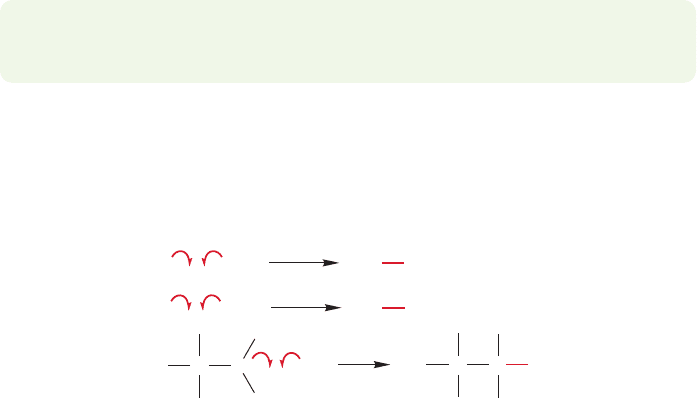
PROBLEM 11.17 Why is abstraction of H from preferred to abstraction of Br?
Hint: The bond dissociation energy of is 56 kcal/mol.
HO
O
Br
H
O
Br
11.4b Inhibitors Small amounts of substances that react with the chain-carrying
radicals can stop chain reactions by removing the chain-carrying radicals from the
process. These substances are called inhibitors. They act as artificial terminators of
the propagation steps (Fig. 11.28).
484 CHAPTER 11 Radical Reactions
CC
Br
..
..
.
..
Br
..
..
..
C
The first propagation step in a typical
radical chain addition reaction
The chain reaction can be stopped if the
chain-carrying radical reacts with an inhibitor, X
C
.
CC
X
Br
..
..
..
CC X
Br
..
..
..
.
.
.
FIGURE 11.28 An inhibitor of radical chain reactions can act by intercepting one of the
chain-carrying radicals.
WEB 3D
OH
OH
Hydroquinone BHT BHA
OH
(CH
3
)
3
C
C(CH
3
)
3
CH
3
C(CH
3
)
3
OCH
3
OH
(CH
3
)
3
C
OCH
3
OH
FIGURE 11.29 Some radical inhibitors.
.
.
More
substituted
radical
Less
substituted
radical
H
3
C
CH
3
CH
2
C
H
3
C
H
3
C
CH
2
.
.
H
3
C
H
3
C
CH
2
C
H
3
C
H
3
C
R
R
R
R
CH
2
C
C
FIGURE 11.30 When a radical adds
to an unsymmetrical alkene, there are
two possible radical products.
A few molecules can have an impact out of proportion to their numbers, because
stopping one chain reaction can mean stopping the formation of thousands of prod-
uct molecules. Oxygen (O
2
), itself a diradical, can act in this way, and many radical
reactions will not proceed until the last oxygen molecules are scavenged from the
system, thereby allowing the radical chains to carry on. Radical inhibitors are often
added to foods to retard radical reactions causing spoilage.Figure 11.29 shows a few
typical radical inhibitors. Both butylated hydroxy toluene (BHT) and butylated
hydroxy anisole (BHA) are widely used in the food industry. It is not important that
you memorize these structures. What is worth remembering is the general structur-
al features. You should also think a bit about how these molecules might function
in intercepting radicals.
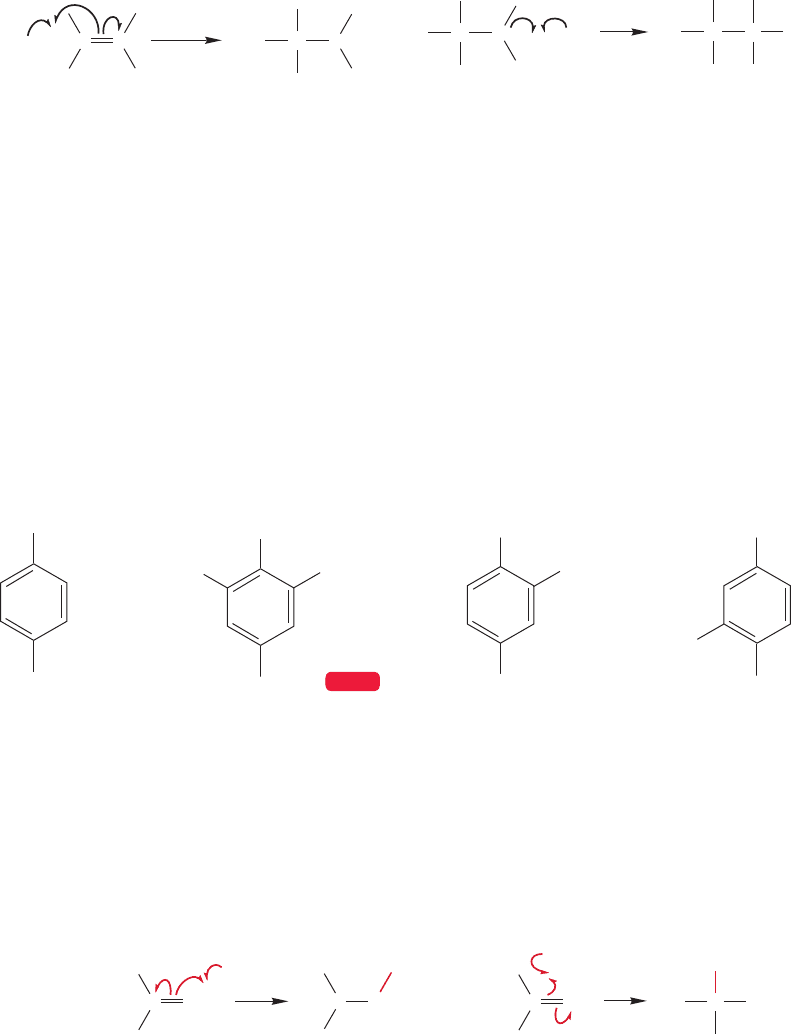
11.4c Regiospecificity of Radical Additions: Anti-Markovnikov Addition
When a radical adds to an unsymmetrical alkene, there are two possible out-
comes: formation of a more substituted or less substituted alkyl radical (Fig. 11.30).
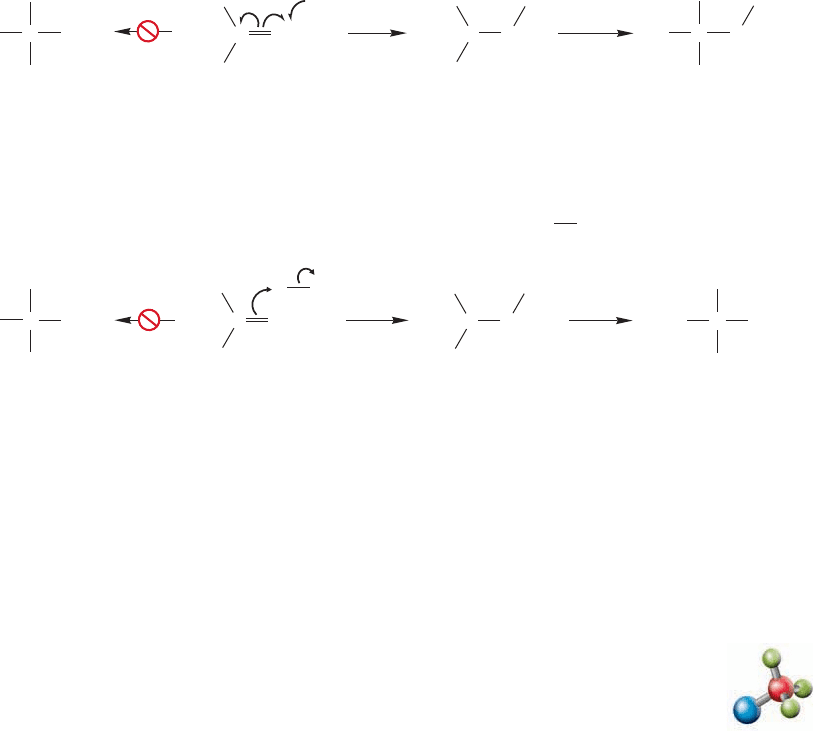
11.4 Radical Addition to Alkenes 485
So, the regiospecificity of radical addition to an unsymmetrical alkene such as
2-methylpropene (isobutene) is determined by the direction of radical attack on
the alkene, and thus by the stability of the alkyl radical formed. In the case of
alkene radical hydrohalogenation, the bromine atom adds to give the more sub-
stituted, more stable radical.The reaction continues when the newly formed alkyl
radical abstracts a hydrogen atom from hydrogen bromide to give, ultimately,
the compound in which the bromine is attached to the less substituted end of the
original alkene, the anti-Markovnikov product (Fig. 11.31a). This reaction is in
contrast to polar hydrohalogenation, in which the first step is protonation of
the alkene to give the more substituted carbocation, which is then attacked by
bromide ion to give the more substituted bromide, the Markovnikov product
(Fig. 11.31b).
(a)
(b)
+
–
.
+
This less stable primary
radical is not formed
Br
..
.
H
—
Br
..
C
CH
2
Br
CH
3
CH
3
C
CH
2
The more stable,
tertiary radical
is formed
This anti-Markovnikov
product is determined by
the initial addition of a
bromine atom to give the
more stable radical
.
.
CH
3
CH
2
CH
3
C
Radical addition
Contrast radical addition with polar addition of H
Br
H
H
3
C
H
3
C
CH
2
This less stable primary
carbocation is not formed
H
C
CH
2
H
CH
3
CH
3
C
CH
2
The more stable,
tertiary carbocation
is formed
This Markovnikov product
is determined by the initial
protonation to give the
more stable carbocation
CH
3
CH
3
CH
3
C
C
H
H
3
C
H
3
C
CH
2
H
3
C
H
3
C
C
H
3
C
H
3
C
Br
Br
..
..
..
..
..
..
..
Br
..
..
..
Br
..
..
..
Br
..
..
..
Br
..
..
..
+
..
..
..
..
FIGURE 11.31 (a) The first step in a radical addition to an alkene gives the more
substituted, and therefore more stable radical. (b) The first step in the polar addition gives
the more stable intermediate, which leads to the Markovnikov product. Notice, however,
that in the polar reaction it is the hydrogen atom that adds first, and in the radical reaction
it is the bromine atom that adds first.
The historical difficulty in determining the regiospecificity of hydrogen bromide
addition had to do only with recognizing that radical initiators such as peroxides had
to be kept out of the reaction. If even a little peroxide were present, the chain nature
of the radical addition of hydrogen bromide could overwhelm the nonchain polar reac-
tion and produce large amounts of anti-Markovnikov product.This understanding of
the causes of the regiochemical outcome of these reactions presents an opportunity
for synthetic control.We now have ways to do Markovnikov addition of hydrogen chlo-
ride and hydrogen iodide, and either Markovnikov or anti-Markovnikov addition of
hydrogen bromide. Of course, the halides formed in these reactions can be used for
further synthetic work.
Radical alkene hydrohalogenation
486 CHAPTER 11 Radical Reactions
TABLE 11.2 The Thermochemistry of the First Propagation Step:
Addition of Halogen Radicals to Ethylene
Bond Strength Bond Strength
X(π bond) ( in ) (kcal/mol)
Cl 66 85 19
Br 66 72 6
I66 57 9
X
O
CH
2
CH
2
.
X
O
C
≤H
X
.
ⴙ H
2
C
P
CH
2
U
X
O
CH
2
CH
2
.
PROBLEM 11.18 Show how to carry out the following conversions. You need not
write mechanisms in such problems unless they help you to see the correct
synthetic pathways.
Br
Br
Devise two syntheses
for each
OH
OH
Neither hydrogen chloride nor hydrogen iodide will add in anti-Markovnikov
fashion, even if radical initiators are present. Now we have to see why.
11.4d Thermochemical Analysis of Radical HX Additions to Alkenes
Why does the chain reaction of Figure 11.26 succeed with but not for other
hydrogen halides? We can write identical radical chain mechanisms for and
, but these must fail for some reason.
We can understand the difference if we examine the thermochemistry of the two
propagation steps for the different reactions.
The energetics of the first propagation step are shown in Table 11.2. These val-
ues are estimated by noting that in this first reaction a π bond is broken and a
bond is made.The overall exothermicity or endothermicity of the reaction is a com-
bination of these two bond strengths.A π bond is worth 66 kcal/mol,and we approx-
imate the bond strength in the product by taking the values for ethyl halides.X
O
C
X
O
C
HI
HCl
HBr
We are making all sorts of assumptions, but the errors involved turn out to be rel-
atively small. For both bromine and chlorine this first step is exothermic—the
product is more stable than starting material and is favored at equilibrium. For
iodine, the reaction is substantially endothermic.The product is strongly disfavored
and the reaction proceeds very slowly. So now we know why the addition of
fails; the first propagation step of the reaction is highly endothermic and does not
compete with the termination steps. But both the bromine case, which we know
succeeds, and the chlorine case, which we know fails, have exothermic first steps.
HI

11.5 Other Radical Addition Reactions 487
TABLE 11.3 The Thermochemistry of the Second Propagation Step:
Abstraction of a Hydrogen Atom from
Bond Strength Bond Strength
X ( ) ( ) (kcal/mol)
Cl 103 98 5
Br 87 98 11
I71 98 27
H
O
C in H
O
CH
2
O
CH
2
O
XH
O
X
≤H
H
O
X ⴙ
.
CH
2
O
CH
2
X
U
.
X ⴙ H
O
CH
2
O
CH
2
O
X
H
O
X
The second step is exothermic for Br and I, but endothermic for Cl.So,we can
expect this reaction to succeed for Br and I, but fail for Cl. If we sum up the analy-
sis for the two propagation steps we see that it is only for addition that
both steps are exothermic. For and , one of the two propagation steps is
endothermic,and the radical addition reaction cannot successfully compete with the
termination steps. The polar addition of wins out.HX
HIHCl
HBr
ROORStep 1 RO OR+
CCl
4
CCl
3
+
.
ROStep 2
.
.
CCl
3
Step 3 CH
2
CH
2
CH
2
CH
2
CCl
3
CH
2
CH
2
CCl
3
Step 4
.
.
CCl
4
CCl
3
ClCH
2
CH
2
CCl
3
.
.
.
ROCl +
Propagation
Initiation
+
+
+
There are man
y
possible termination stepsStep 5
(recycle
to Step 3)
FIGURE 11.32 The mechanism for
the radical-induced addition of
carbon tetrachloride to an alkene.
11.5 Other Radical Addition Reactions
Hydrogen bromide addition to alkenes is only one example of a vast number of
radical chain reactions. Another example involves carbon tetrahalides, such as CCl
4
and CBr
4
, which add to alkenes in the presence of initiators. The steps in the
peroxide-initiated reaction are shown in Figure 11.32.
Let’s look at the second propagation step in Table 11.3.
Summary
The polar addition of HBr to alkenes leads to the more substituted bromide,
whereas the radical-initiated addition of HBr gives the less substituted bromide.
Thus, you have the ability to make either regioisomer when the alkene is unsym-
metrically substituted. The addition of HCl and HI always gives the more sub-
stituted halide.
PROBLEM 11.19 Write as many termination steps as you can imagine for the
reaction of Figure 11.32.
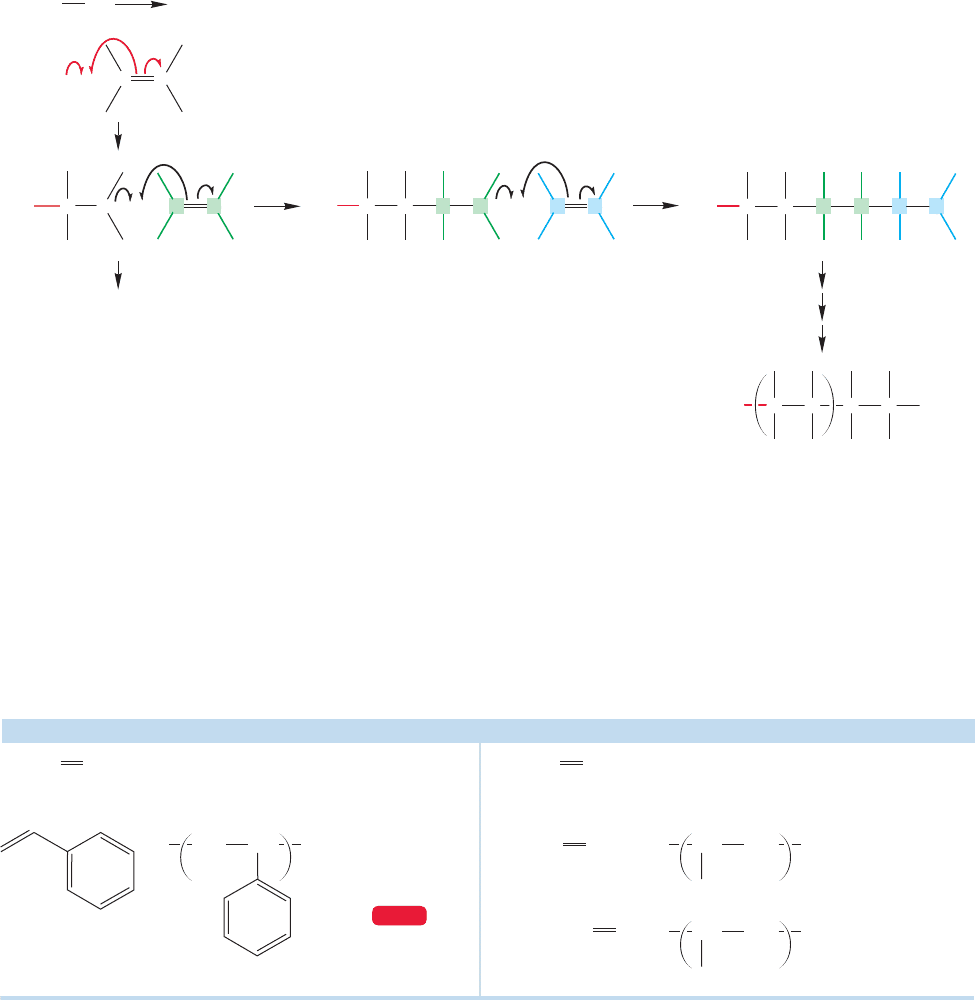
Under some conditions, alkenes can be polymerized by free radicals. In the
examples we have discussed, radical addition to an alkene produced an alkyl radi-
cal, which then abstracted hydrogen (or halogen in the case of CX
4
) in a second
propagation step to give the product of overall addition to the alkene. What if
Step 4 is not easy? If the concentration of alkene is very high, for example, or if
the addition step (Step 3) is especially favorable, the abstraction reaction (Step 4)
might not compete effectively with it. Under such conditions the alkyl radical can
undergo addition to the alkene to form a new alkyl radical (the dimer).The dimer
radical can react with alkene to form a trimer radical and so forth, until either a
termination step intercedes or the supply of alkene is exhausted (Fig. 11.33).
488 CHAPTER 11 Radical Reactions
CC
IN
IN IN 2 IN
Products of hydrogen
abstraction
Dimer radical Trimer radical
repeat many times
until a termination
step or hydrogen
abstraction stops
the chain reaction
Δ
.
.
n
CC
IN
C C H
IN
C C
.
C C
IN
IN
.
C
C
C
CC
CC
C
CC
C
.
C
FIGURE 11.33 In the radical-induced polymerization of alkenes, repeated
additions grow a long polymer chain. A high concentration of alkene favors this
process ( radical).IN
.
= initiator
TABLE 11.4 Some Monomers and Polymers
Monomer Polymer Formula NameMonomer Polymer Formula Name
Styrene
Ethylene
H
2
CCH
2
(CH
2
)
n
n
CH
2
CH
Polyethylene,
polymethylene
Polystyrene
(CF
2
)
n
n
CH
Cl
CH
2
n
CH
COOCH
3
CH
2
Polyacrylate
Poly(tetrafluoro-
ethylene), Teflon
Poly(vinyl chloride)
(PVC)
Tetrafluoroethylene
F
2
CCF
2
CH
2
ClCH
Vinyl chloride
CH
2
CH
3
OOCCH
Methyl acrylate
WEB 3D
Such polymerization reactions are common and the products often have very long
chain lengths. Very high molecular weight products can be formed. Table 11.4
shows a number of common polymers and the monomers from which they are
built up.
