Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

WEB 3D
C
CH
2
H
Styrene
(
vin
y
lbenzene
)
11.6 Radical-Initiated Addition of HBr to Alkynes 489
PROBLEM 11.21 Write a mechanism for the formation of polystyrene, the product
of the polymerization of styrene (vinylbenzene).
PROBLEM 11.20 Styrene (vinylbenzene) is an example of a molecule in which the
addition of a radical is especially favorable. Explain why.
cis- and trans-1-Bromopropene 2-Bromopropene
H
H
initiation
CC
–70 ⬚C
+
CC
H
H
CH
3
not
CH
3
Br
..
..
..
CC
H
H
CH
3
Br
..
..
..
Br
..
..
..
Br
..
..
..
CC
CH
3
H
H
FIGURE 11.34 The radical-induced addition of HBr to propyne.
Propagation steps
H
3
C
.
.
Br
..
..
..
.
.
cis- and trans-1-
Bromopropene
2-Bromopropene
(not formed)
H
More stable
vinyl radical
Br
..
..
..
+
Br
..
..
..
.
Br
..
..
..
.
+
Br
..
..
..
.
+
CC
CH
3
.
H
Less stable vinyl
radical (not formed)
Br
..
..
..
C
H
H
HCC
CH
3
CC
H
Initiation steps
In2 In
In
H
Br
..
..
..
CC
H
H
CH
3
Br
..
..
..
..
..
..
CC
H
H
CH
3
Br
..
..
..
CC
H
Br
H
3
C
H
3
C
..
..
..
C
Br
Br
..
..
..
.
hν
.
InBr
+
H
Br
In
H
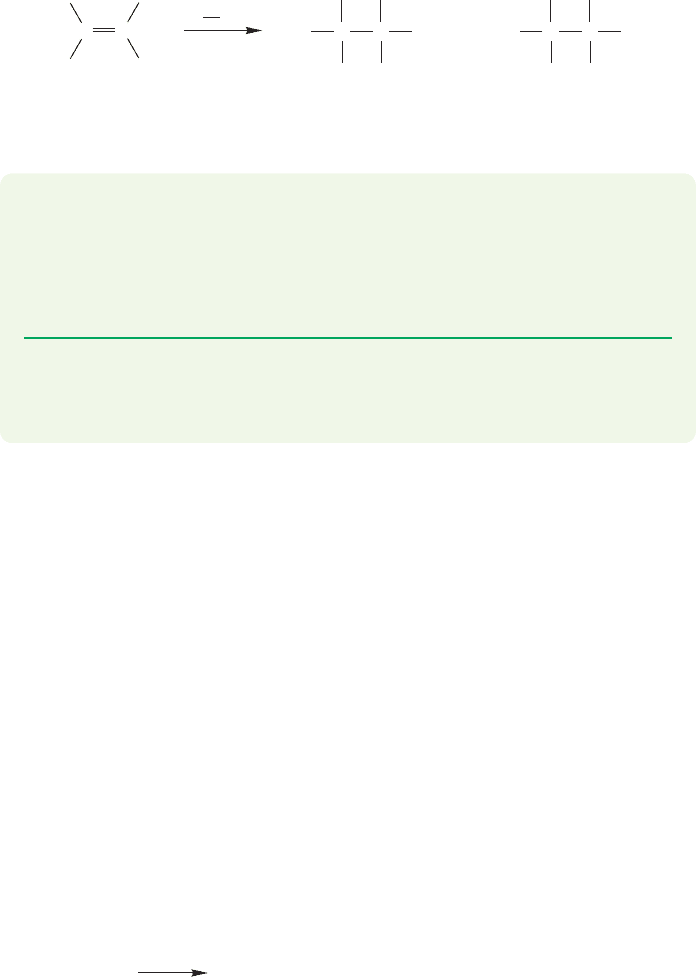
FIGURE 11.35 Addition of a bromine atom to 1-propyne gives the more stable vinyl radical, which then abstracts the
hydrogen atom from hydrogen bromide to give the anti-Markovnikov product.
The free-radical chain reaction for addition to alkynes is quite analogous to that
operating in additions to alkenes.The crucial step is the formation of the more sta-
ble vinyl radical rather than the less stable vinyl radical (Fig. 11.35).
11.6 Radical-Initiated Addition of HBr to Alkynes
Just as with alkenes, hydrogen bromide adds to alkynes in the anti-Markovnikov
sense when free radicals initiate the reaction.The light-initiated reaction of
with propyne gives cis- and trans-1-bromopropene,not 2-bromopropene (Fig. 11.34).
H
O
Br
490 CHAPTER 11 Radical Reactions
H
3
C
H
3
C
H
Vicinal dibromide
(>97%)
Geminal dibromide
(<3%)
C
–80 ⬚C
initiation
+
Br
H
CH
3
CH
3
HBr
Br
Br
H
C
H
3
C
H
C
CH
3
Br
Br
H
C
CC
FIGURE 11.36 A second radical
addition of hydrogen bromide to a
vinyl bromide generates the vicinal
dibromo compound as the major
product, not the geminal dibromo
compound. Problem 11.22 asks you
to explain why.
In principle, a second radical addition of hydrogen bromide could form either a
geminal or vicinal dihalide. In contrast to ionic reactions, it is the vicinal dibromide
that is preferred (Fig. 11.36).
CH
3
Cl CH
2
Cl
2
CHCl
3
CCl
4
Methyl
chloride
Methylene
chloride
Chloroform Carbon
tetrachlorid
e
hν
or Δ
++
+
+
CH
4
Cl
2
FIGURE 11.37 Photolysis or pyrolysis
of chlorine in methane gives a
mixture of chlorinated methanes.
PROBLEM 11.22 Explain in resonance and molecular orbital terms why the vicinal
dihalide is formed in Figure 11.36. Be careful! This question is more subtle than
it looks at first. Consider the stabilization of an adjacent radical (a half-filled
orbital) by an adjacent bromine atom. Why is this a stabilizing interaction?
Hint: Recall the molecule He
2
from p. 41, and see p. 479 in this chapter.
PROBLEM 11.23 Now predict whether will abstract hydrogen from the
methylene or methyl position of diethyl ether (CH
3
CH
2
OCH
2
CH
3
). Explain
your answer.
R
.
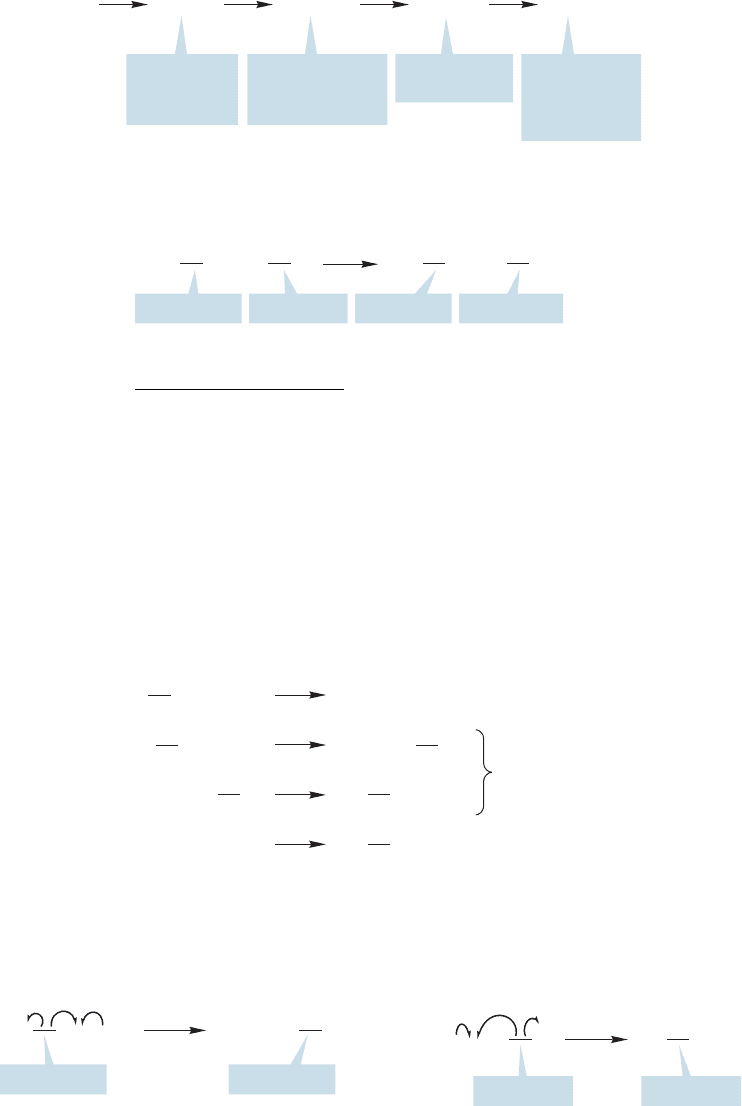
The composition of the product mixture depends on the amount of chlorine
available and the length of time the reaction is allowed to run. If we use a suffi-
cient amount of chlorine and allow the reaction to run long enough, all the methane
can be converted into carbon tetrachloride. However, measuring the product
11.7 Photohalogenation
So far, we have seen a variety of reactions of radicals with alkenes. Alkanes are much
less reactive than alkenes, and we have not seen ways to induce these notoriously
unreactive species into reaction in a specific way. We have seen pyrolysis, the ther-
mal cracking of saturated hydrocarbons (p. 470), but this method is anything but
useful in making specific molecules. Here we find a way to use alkanes in synthe-
sis. There still will be little specificity here, and these reactions will not find a
prominent place in your catalog of synthetically important processes. Yet they are
occasionally useful, and provide a nice framework on which to base a discussion of
selectivity.
11.7a Halogenation of Methane A mixture of chlorine and methane is sta-
ble indefinitely at room temperature in the dark,but reacts vigorously to give a mix-
ture of methyl chloride, methylene chloride, chloroform (trichloromethane), and
carbon tetrachloride when the mixture is either irradiated or heated (Fig. 11.37).
11.7 Photohalogenation 491
composition as a function of time shows that the initial product of the reaction is
methyl chloride. Methylene chloride and chloroform are formed on the way to
carbon tetrachloride (Fig. 11.38).
CH
3
Cl CH
2
Cl
2
CHCl
3
CCl
4
Methyl
chloride
(first formed)
Methylene
chloride
(second formed)
Chloroform
(third formed)
Carbon
tetrachloride
(the ultimate
product)
CH
4
hν
Cl
2
hν
Cl
2
hν
Cl
2
hν
Cl
2
FIGURE 11.38 The chlorinated
products are formed by sequential
chlorinations of methane.
+
105 kcal/mol 59 kcal/mol
187 kcal/molBonds made
164 kcal/mol
23 kcal/mol exothermic
Bonds broken
84 kcal/mol 103 kcal/mol
HH
3
CH
3
CCl Cl
+
H
ClCl
FIGURE 11.39 The formation of
methyl chloride is exothermic.
H
3
CH
3
C
Initiation
Termination
2
Propagation
.
HH
++
H
3
CH
3
C
.
++
H
3
CH
3
C
.
H
3
CCH
3
.
+
Cl
..
..
..
Cl
..
.
..
..
Cl
..
.
..
..
Cl
..
..
..
.
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
FIGURE 11.40 The mechanism of
methyl chloride formation.
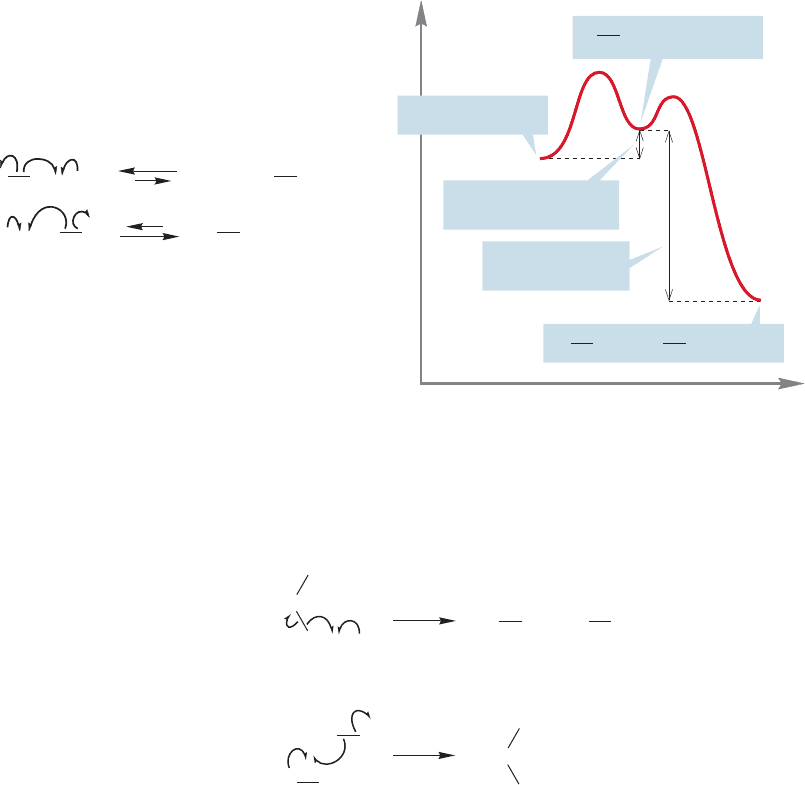
Let’s first examine the formation of the initial product,methyl chloride.The reac-
tion is exothermic by about 23 kcal/mol (Fig. 11.39).
The first step is the thermal or photochemical breaking of the chlorine–chlorine
bond. This initiation reaction is followed by the first propagation step (Fig. 11.40),
the abstraction of a hydrogen atom from methane by a chlorine atom to produce a
methyl radical and hydrogen chloride. In the second propagation step, the methyl
radical abstracts a chlorine atom from a chlorine molecule to give methyl chloride
and another chlorine atom that can carry the chain reaction forward.There are many
possible termination reactions; Figure 11.40, which shows the overall mechanism,
includes only one.
Now let’s look at the thermochemistry of the propagation steps. The second
propagation step is exothermic by 25 kcal/mol and thus is very favorable (Fig.11.41).
.
+
H
3
CH
3
C
H
First propagation step
This reaction is endothermic by 2 kcal/mol
H
105 kcal/mol 103 kcal/mol
Cl
..
.
..
..
Cl
..
..
..
Second propagation step
This reaction is exothermic
by 84 – 59 = 25 kcal/mol
+
H
3
CH
3
C
.
59 kcal/mol 84 kcal/mol
Cl
..
.
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
FIGURE 11.41 The thermochemistry of the two propagation steps for methyl chloride formation.
492 CHAPTER 11 Radical Reactions
The first propagation step is endothermic by about 2 kcal/mol. Will this endothermic
step be enough to stop the reaction? No.Although an energy difference of 2 kcal/mol
produces an unfavorable equilibrium mixture, the second highly exothermic step
results in the overall reaction being favored. The few methyl radicals formed react
rapidly to give methyl chloride,thus disrupting the slightly unfavorable equilibrium
Reestablishment of this equilibrium generates more methyl radicals, which go on
to make more methyl chloride and chlorine radicals (Fig. 11.42), which continue
the chain reaction. Note that a chlorine radical is just a chlorine atom.
Cl
.
+ CH
4
U
Z——
Cl
O
H +
.
CH
3
.
+
H
3
CH
3
C
H H
+
H
3
CH
3
C
.
Cl
..
.
..
..
Cl
..
.
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Energy
Reaction pro
g
ress
++
CH
3
Cl ClH
CH
4
+
Cl
2
+
Cl
.
+2 kcal/mol
(slightly endothermic)
–25 kcal/mol
(very exothermic)
++
H
CH
3
.
Cl
2
Cl
.
Cl
FIGURE 11.42 In the formation of
methyl chloride from methane and a
chlorine radical, the high exothermicity
of the second step overcomes the slight
endothermicity of the first step.
Methylene
chloride
Methyl chloride
.
+
H
2
C
H
2
C
H
H
H
2
C
H
2
C
..
.
Cl
..
..
Cl
..
..
..
+
Cl
..
.
..
..
Cl
..
.
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
FIGURE 11.43 The formation of
methylene chloride (H
2
CCl
2
) from
methyl chloride and a chlorine atom.
Moreover, the carbon–hydrogen bond in methyl chloride is weaker than the one
in methane, and thus easier to break.So methyl chloride competes favorably with
methane and cannot build up in the reaction unless there is a vast excess of
methane.
As methyl chloride builds up in the reaction chamber, it, too,begins to react with
chlorine atoms in another chain reaction (Fig. 11.43).
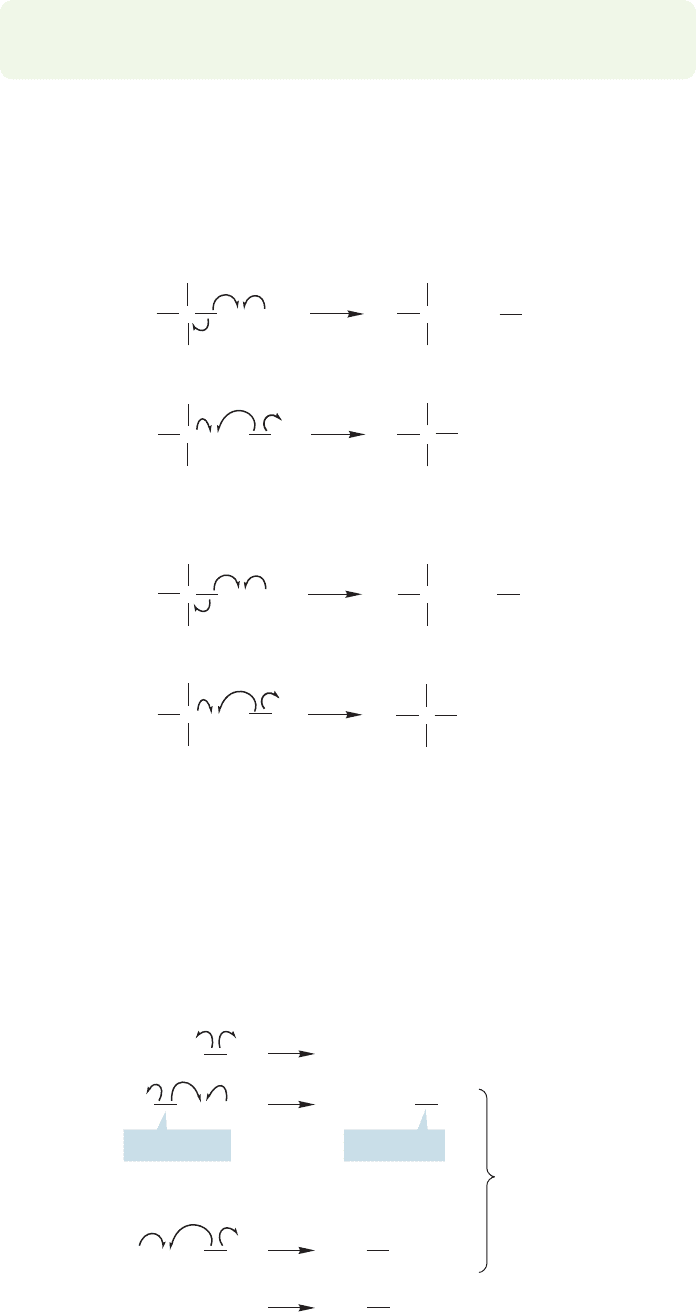
11.7 Photohalogenation 493
Methylene chloride is more reactive than either methyl chloride or methane,and
so it reacts to form chloroform as it builds up. Chloroform is more reactive still,
and rapidly produces carbon tetrachloride through abstraction of its single hydro-
gen (Fig. 11.44).
Chloroform
Carbon
tetrachloride
+
C
C
HH
H
C
H
H
..
.
Cl
..
..
Cl
..
..
..
+
Cl
..
.
..
..
Cl
..
.
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
C
H
.
Cl
..
..
..
Cl
..
..
..
.
+
C
C
C
HH
C
..
.
Cl
..
..
..
Cl
..
..
..
Cl
..
..
Cl
..
..
..
+
Cl
..
.
..
..
Cl
..
.
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
FIGURE 11.44 The formation of
chloroform from methylene chloride
and of carbon tetrachloride from
chloroform.
Methane can also be photohalogenated with bromine, but the overall reaction
is much less exothermic than is chlorination. Moreover, the first propagation step is
endothermic by the substantial amount of 17 kcal/mol (Fig. 11.45). As we will see,
this strongly endothermic step will have important consequences for the selectivity
of the reaction when a choice of carbon–hydrogen bonds exists.
H
3
CH
3
C
Initiation
105 kcal/mol
This first propagation step is
105 – 88 = 17 kcal/mol endothermic!
88 kcal/mol
Termination
2
.
..
Propagation
.
HH
+
H
3
CH
3
C
.
H
3
CH
3
C
.
H
3
CCH
3
.
+
Br
..
..
Br
..
..
..
..
Br
..
..
Br
..
..
..
..
+
Br
..
.
..
..
Br
..
.
..
..
Br
..
..
Br
..
..
..
Br
..
..
..
FIGURE 11.45 The formation of
methyl bromide from bromine and
methane.
PROBLEM 11.24 Explain why the carbon–hydrogen bond in methyl chloride is
easier to break than the carbon–hydrogen bond in methane itself.

494 CHAPTER 11 Radical Reactions
carbons of butane are more reactive than the hydrogens on the primary carbons.
Abstraction by a chlorine atom occurs more easily at the secondary position than at
the primary position. The percentages of products shown in Figure 11.46 indicate
that the preference is 72.2:27.8, or a factor of 2.6. However, this simple analysis
ignores the fact that in butane there are more primary hydrogens (6) than second-
ary hydrogens (4). In order to correct this value for the statistical advantage of the
primary bonds, we must multiply it by 6/4 to produce the true factor of 3.9. On a
per hydrogen basis, secondary hydrogens are abstracted more easily than primary
hydrogens by a factor of 3.9.
sec-Butyl chloride
(72.2%)
Butyl chloride
(27.8%)
CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
CH
2
ClCH
3
CH
2
CHCH
3
+
hν, 35 ⬚C
Cl
2
Cl
FIGURE 11.46 On a per hydrogen
basis, abstraction of a secondary
hydrogen is favored over abstraction
of a primary hydrogen by a factor of
(72.2/27.8) 6/4.
H
3
C
CH
3
CH
3
Cl
CH
3
CH
2
Cl
C
hν, 35 ⬚C
H
3
C
CH
3
Cl
2
CH
+
H
3
C
CH
CH
3
1-Chloro-2-methylpropane
(64%)
2-Methylpropane
2-Chloro-2-methylpropane
(36%)
FIGURE 11.47 For a chlorine radical,
abstraction of a tertiary hydrogen is
favored over abstraction of a primary
hydrogen by a factor of about 5 on a
per hydrogen basis.
However, there are nine primary hydrogens and only a single tertiary hydrogen
in 2-methylpropane, so on a per hydrogen basis the primary:tertiary reactivity
ratio is 1.78 1/9 0.198. Put another way, the tertiary hydrogen is favored by
1:0.198 5.1.
Bromine is much more selective in the photohalogenation reaction than is chlo-
rine. That is, bromine is able to choose among the various reaction possibilities
with more discrimination and pick out the most favorable abstraction pathway by
a wider margin than chlorine. For example, a bromine atom prefers secondary to
11.7b Halogenation of Other Alkanes If we irradiate a mixture of chlorine
and a hydrocarbon more complicated than methane or ethane, there is usually more
than one possible initial product.For example, butane can give either 1-chlorobutane
or 2-chlorobutane. Figure 11.46 shows that the hydrogens on the secondary
PROBLEM 11.25 Write a reaction mechanism for the photochlorination of ethane.
PROBLEM 11.26 Analyze the thermochemistry of the two propagation steps for
the photochlorination of ethane. The bond dissociation energies of the
chlorine–chlorine and carbon–chlorine bonds of ethyl chloride and the
carbon–hydrogen bond of ethane are 59, 84.8, and 101.1 kcal/mol, respectively.
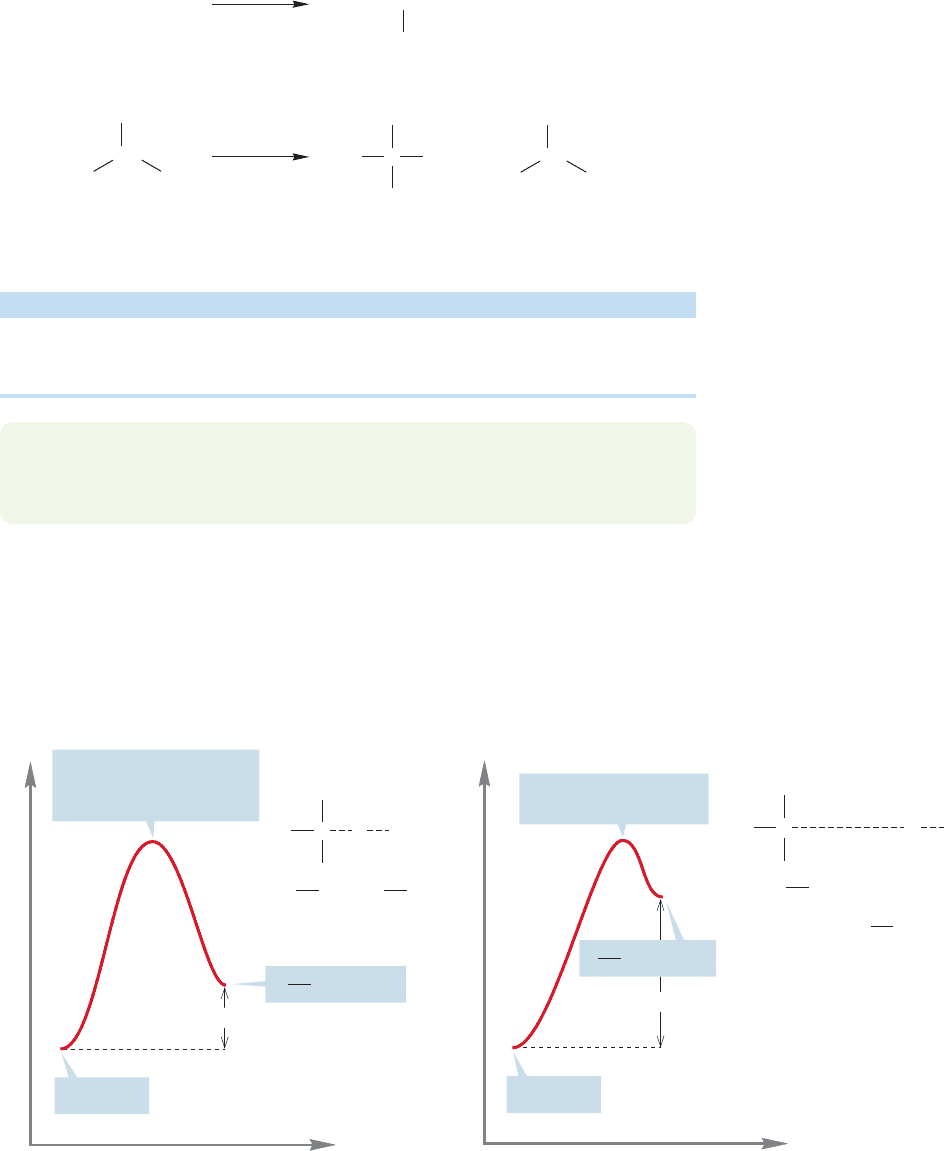
Selectivity is increased when the possibilities include the formation of a tertiary
radical. Photochlorination of 2-methylpropane, for example, gives a 64:36 mixture of
1-chloro-2-methylpropane (isobutyl chloride) and 2-chloro-2-methylpropane
(tert-butyl chloride). The primary:tertiary ratio is 64:36 1.78 (Fig. 11.47).
Alkane halogenation
11.7 Photohalogenation 495
PROBLEM 11.27 Use the bond dissociation energies of Table 8.2 (p.337) to estimate
the thermochemistry of the propagation steps for photochemical fluorination and
iodination of methane.
H
3
C
CH
3
CH
3
Br
CH
3
C
H
3
C
CH
3
CH
+
+
H
3
C
CH
CH
3
CH
2
B
r
(
~100%
)(
~0%
)
hν, 127 ⬚C
Br
2
hν, 127 ⬚C
Br
2
Br
(98.2%) (1.8%)
CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
CHCH
3
CH
3
CH
2
CH
2
CH
2
Br
FIGURE 11.48 Photobromination
is far more selective than
photochlorination.
TABLE 11.5 Selectivities of Halogen Atoms in Carbon–Hydrogen Bond Abstraction
Halogen Primary Secondary Tertiary
F 1 1.2 1.4
Cl 1 3.9 5.1
Br 1 82 1600
Energy
Reaction progress
+
CH
4
Br
.
Transition state—much
closer to product
17 kcal/mol
Energy
Reaction
p
ro
g
ress
+
CH
4
Cl
.
Transition state—nearly
at the midpoint for this
reaction
2 kcal/mol
C
H
H
The C
H bond is much more
fully broken than in the case
of chlorine; the H
Br bond
is nearly fully made, and the
free electron is nearly fully
developed on carbon
Cl CH
3
H
C
H and H
Cl
bonds approximately
equally formed
+
C
H
H
HCl
H
H
Br
.
Br CH
3
H
+
.
H
FIGURE 11.49 For the nearly thermoneutral abstraction of hydrogen by a chlorine atom, the transition state is almost
halfway between starting material and products. For the highly endothermic abstraction of hydrogen by the bromine
atom, the transition state will be quite product-like.
Neither fluorine nor iodine undergoes a useful photohalogenation reaction.The
reaction of fluorine with carbon–hydrogen bonds is overwhelmingly exothermic and
can even occur explosively. Almost no selectivity is possible (Table 11.5). In direct
contrast to the reaction of fluorine atoms, reaction of iodine atoms with hydrocar-
bons is highly endothermic. It is so slow as to be of no synthetic utility.
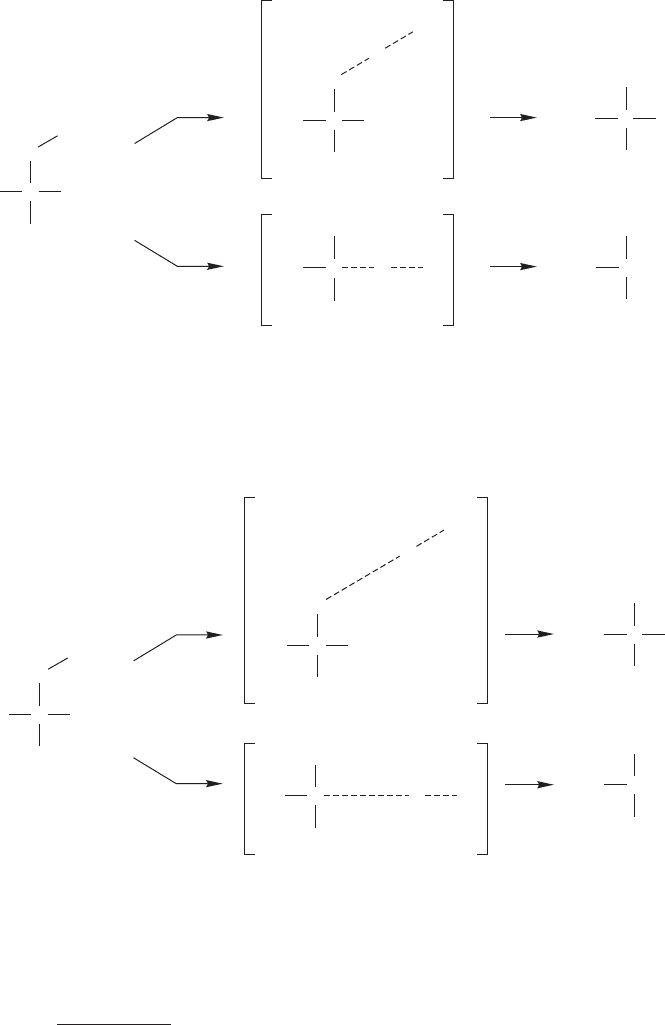
What is the reason for bromine being more selective than chlorine? Recall that
the abstraction reaction is close to thermoneutral for chlorine (Fig. 11.49), but very
primary hydrogens by a factor of 82, and tertiary hydrogens to primary hydrogens
by a factor of about 1600 (Fig. 11.48; Table 11.5).
496 CHAPTER 11 Radical Reactions
endothermic for bromine (p. 493, Fig. 11.45).
3
The Hammond postulate (the
transition state for an endothermic process resembles the product) tells us that the
transition state for the abstraction of hydrogen by chlorine is nearly halfway between
starting material and product (the radical intermediate) (Fig. 11.50a), but the
transition state is very product-like for bromine (Fig. 11.50b). Because the reaction
is more endothermic, the transition state for abstraction of hydrogen by bromine is
product-like. There is a strongly developed hydrogen–bromine bond and a large
degree of radical character on carbon in the transition state (Fig. 11.50b).
δ
.
H
H
C
H
3
C
H
3
C
H
2
C
H
3
C
CH
3
C
H
3
C
H
3
C
CH
3
CH
3
HC
Cl
δ
.
+
In these transition states the
carbon–hydrogen bonds are
only about half-broken, so transition
states for abstraction of different
hydrogens don’t differ much
Cl
..
.
.
δ
.
H
H
3
C
H
2
C
C
H
3
C
H
H
2
C
CH
3
CH
3
HC
Cl
δ
.
HCl
+
HCl
+
.
..
..
δ
H
3
C
H
3
C
CH
3
C
H
H
C
H
3
C
H
2
C
CH
3
+
δ
.
H
3
C
H
3
C
CH
3
C
.
.
H
In these transition states, the carbon–hydrogen
bonds are largely broken and the radicals are
very well developed on the carbons; differences
in radical stability will be important in selection
of the hydrogen to be abstracted
Br
δ
.
HBr
+
δ
H
H
H
3
C
H
2
C
CH
3
C
δ
.
H
H
3
C
H
2
C
CH
3
C
Br
δ
.
HB
r
+
Br
..
.
..
..
(a)
(
b
)
FIGURE 11.50 (a) Because the
transition state for abstraction by
chlorine lies roughly half way
between starting material and
product there can be little selectivity.
(b) The product-like transition state
for abstraction by bromine means
that the radical on carbon will be well
developed and the substitution
pattern of the carbon atom (tertiary,
secondary, primary, or methyl) will be
relatively important in determining
the ultimate major product.
3
Watch out! One of the things chemistry professors laugh about is the number of people who call “thermoneu-
tral” reactions “thermonuclear” reactions. Believe it or not, this happens a lot!
11.8 Allylic Halogenation: Synthetically Useful Reactions 497
The transition states for abstraction of hydrogen from an alkane by chlorine and
bromine radicals are very different from each other. In the transition states for
abstractions by bromine, the radical on carbon is far more developed than it is in
the abstractions by chlorine, which means that differences in the stabilities of dif-
ferent radicals will be far more important in determining the major product of the
bromine abstractions than in the chlorine abstractions (Fig. 11.50).
Summary
When questions of selectivity arise, it is always important to consider what the
transition state looks like. Selective radicals will have more product-like transi-
tion states in any abstraction reaction, and therefore the factors influencing the
stability of those products will be important in the transition state as well.
11.8 Allylic Halogenation: Synthetically
Useful Reactions
One of the goals of synthetic organic chemists is to increase selectivity—to be able
to do chemical transformations at specific sites in a molecule without affecting the
rest of the molecule.Selectivity is useful—even necessary—when one is dealing with
a complicated molecule. We must have ways of inducing change at one place in a
molecule without affecting others if we are to be able to manipulate molecules ration-
ally. It’s quite clear that the photochlorination reaction of alkanes is not a good way
of doing this because this reaction is far too unselective. Chlorination takes place all
too easily at the various positions in the hydrocarbon.
Photobromination more closely approaches the goal of perfect selectivity because
the bromine atom abstracts tertiary hydrogens much more readily than secondary
or primary hydrogens. But even this reaction is not without its problems.It is a slow
process and the side products of di- and polybromination are formed.
There are some instances where the chlorination or bromination reaction can
be used to good effect, however. Alkenes that have allylic hydrogens can some-
times be halogenated specifically at an allylic position in a process called allylic
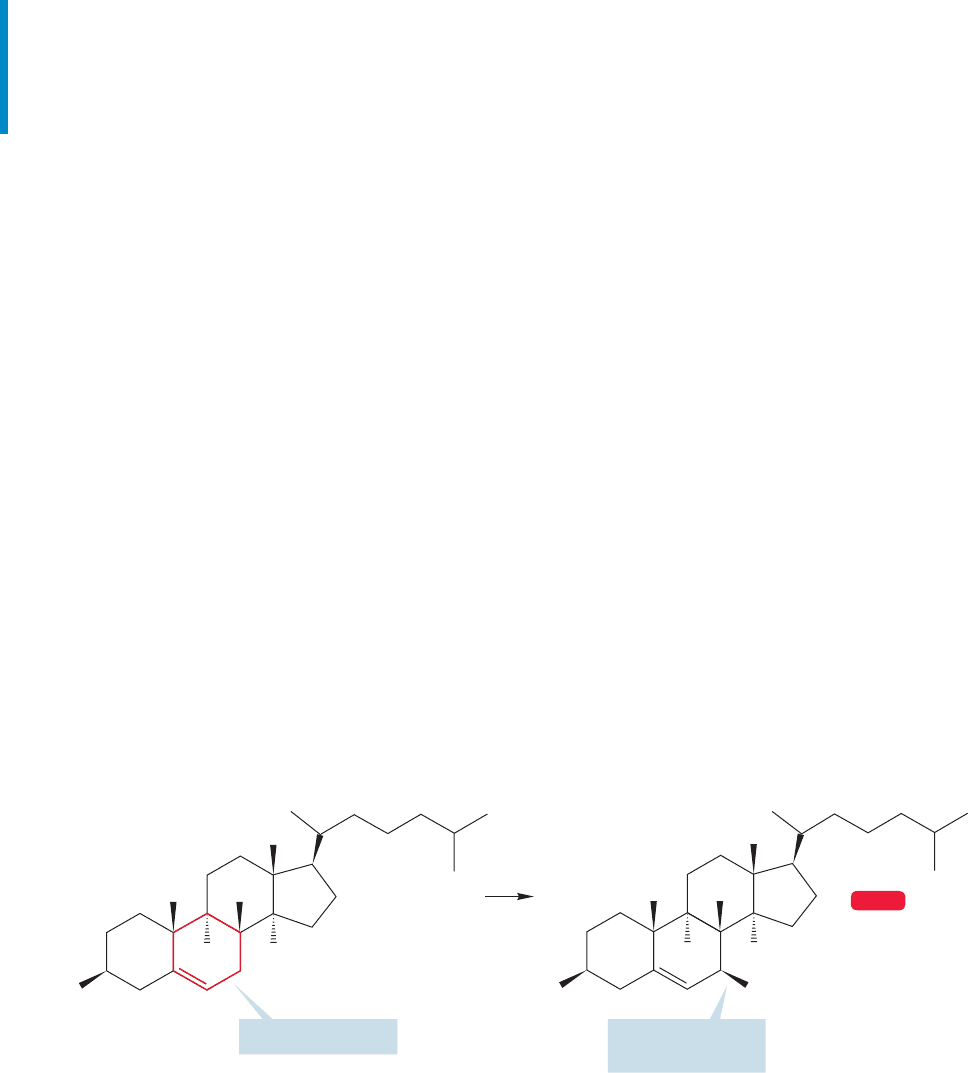
halogenation, another free radical chain reaction. For example, when low con-
centrations of bromine are photolyzed in the presence of the complicated cyclo-
hexene shown in Figure 11.51, the product is exclusively brominated in one allylic
position.
(70%)
CH
3
CH
3
Cyclohexene in red
Brominated only in
this allylic position
Cholesterol benzoate
C
6
H
5
COO
Br
H
H
H
CH
3
CH
3
C
6
H
5
COO
H
H
H
hν
Br
2
WEB 3D
FIGURE 11.51 When low concentrations of bromine are photolyzed in the presence of the
substituted cyclohexene, the product is exclusively the allylic bromide shown.
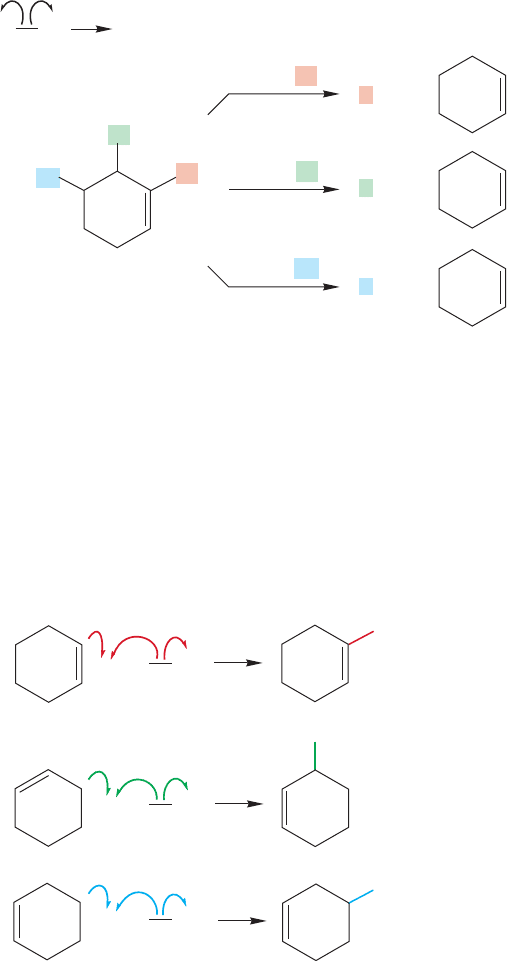
498 CHAPTER 11 Radical Reactions
The mechanism is a typical radical chain reaction in which a low concentration
of bromine atoms is first produced by breaking of the weak bromine–bromine bond
(initiation step). Abstraction of hydrogen from the cyclohexene is the first propa-
gation step, but there are three possible kinds of hydrogen that could be removed:
H
v
(vinylic), H
a
(allylic), and H
m
(methylene) (Fig. 11.52).
Br
..
..
..
Br
..
..
..
.
Br Initiation step
Three possible propagation steps
abstract H
v
..
.
..
..
Br
..
.
..
..
Br
..
..
..
H
a
H
m
H
v
abstract H
a
abstract H
m
+
+
HBr
..
.
.
.
..
..
+
HBr
..
..
..
+
HBr
..
..
..
+
FIGURE 11.52 In principle, a bromine
atom could abstract a vinylic (H
v
),
allylic (H
a
) or methylene (H
m
)
hydrogen.
Br
..
..
..
Br
..
..
..
Br
..
..
..
Br
..
..
..
Br
..
..
..
Br
..
..
..
Br
..
..
..
Br
..
..
..
Br
..
..
..
.
.
.
.
+
Br
..
..
..
.
Br
..
..
..
.
Br
..
..
..
+
+
FIGURE 11.53 In a propagation step,
any of the carbon radicals could
react with bromine to give a
bromocyclohexene and a chain-
carrying bromine atom.
As shown in Figure 11.53, each of these radicals could lead to a bromocyclo-
hexene through reaction with bromine in the second propagation step.The prop-
agation is then carried forward by the new bromine atom formed in this step.The
formation of only the allylic bromide tells us that only the allylic radical is pro-
duced originally.
Because there is only one product formed, the allylic bromide, the allylic radical
must be formed with high selectivity. Why? It is only this radical that is resonance sta-
bilized.There are two equivalent resonance forms for the allylic radical and this delo-
calization makes it far more stable than the other two possibilities (Table 11.1).
