Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

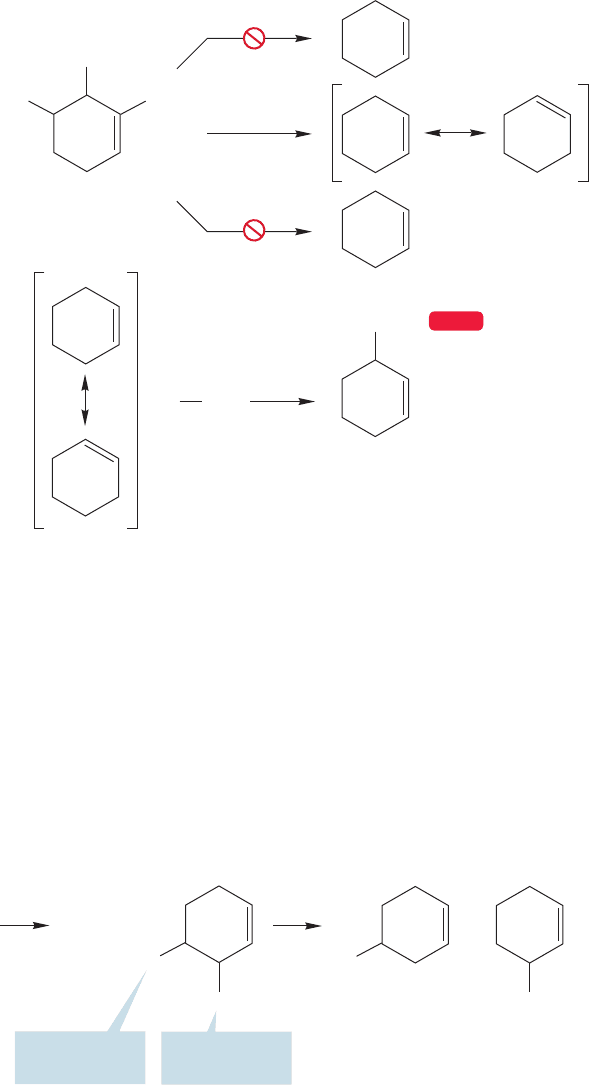
11.8 Allylic Halogenation: Synthetically Useful Reactions 499
Further reaction with bromine leads to the product of allylic bromination and a new
chain-carrying bromine atom (Fig. 11.54).
One might hope that the photohalogenation reaction would be general, that
photochlorination would be as useful as photobromination. Alas it is not, and Table
11.5 shows why. A chlorine atom is far less selective than a bromine atom. Utility
in synthesis comes from selectivity—from doing one reaction, not several.
Photobromination is useful because the bromine atom picks and chooses among
the various possible carbon–hydrogen bonds with fairly high discrimination. A
chlorine atom does not. For example, the allylic hydrogens in cyclohexene (H
a
)
are only slightly more reactive toward a chlorine atom than the H
m
methylene
hydrogens (Fig. 11.55).
Br
3-Bromocyclohexene
..
..
..
abstract H
v
Br
..
.
..
..
Br
..
..
..
Br
..
.
..
..
Br
..
..
..
H
a
H
m
H
v
abstract H
a
abstract H
m
hν
+
+
.
.
.
.
.
.
WEB 3D
FIGURE 11.54 Only the allylic
bromide is formed.
Cl
(37.5%)
H
m
abstracted
(62.5%)
H
a
abstracted
..
..
..
Cl
..
..
..
Cl
..
.
..
..
hν
Relative
reactivity = 0.6
Relative
reactivity = 1.0
+
+
Cl
2
H
m
H
a
2
FIGURE 11.55 Chlorine is much less
selective than bromine. The allylic
chloride is not the only product
formed in this reaction.
In photohalogenation reactions, keeping the concentration of X
2
low is essen-
tial to success. If there is too much halogen, polar addition to give vicinal dihalides
competes with formation of the allylic halide, and the desired selectivity is lost.
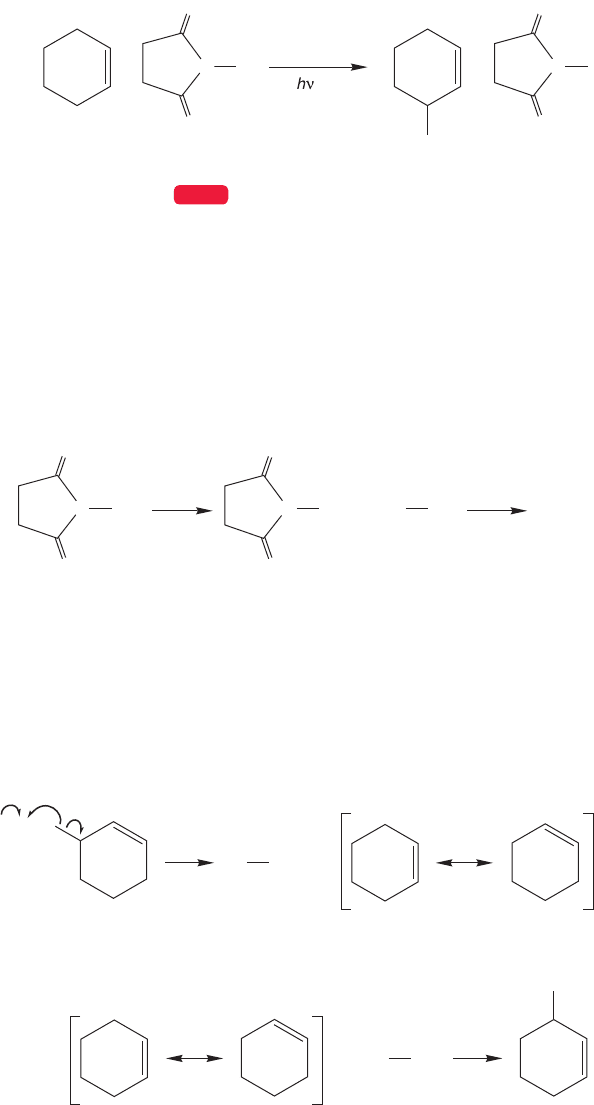
500 CHAPTER 11 Radical Reactions
..
..
Br
..
..
..
Br
HBr
..
..
..
Br
..
..
..
Br2
..
..
..
.
+
N
NH
O
..
..
O
..
..
O
..
..
O
N
..
..
hν
FIGURE 11.57 The first two steps
in bromination of cyclohexene using
NBS.
Br
..
..
..
.
.
++
Br
..
..
..
Br
..
..
..
Br
..
..
..
.
.
.
Br
..
..
..
Br
..
..
..
.
+
H
H
FIGURE 11.58 The bromine radical
abstracts a hydrogen to form the
cyclohexenyl radical. Independently,
a molecule of bromine is formed
from the NBS. The Br
2
then reacts
with the cyclohexenyl radical to give
the product and a new bromine atom.
Methods have been developed to ensure that this low concentration is maintained
and to lower the temperature necessary for the initiation step. A common and very
effective method of allylic bromination involves the molecule N-bromosuccinimide,
generally known as NBS,in the solvent carbon tetrachloride (CCl
4
).The overall reac-
tion is shown in Figure 11.56.
++
NBr
H
trace HBr
NBSCyclohexene 3-Bromocyclohexene
N
Br
CCl
4
O
O
N
O
O
NHS
WEB 3D
FIGURE 11.56 Allylic bromination
with NBS.
The initiation steps in this reaction are formation of succinimide (NHS),
followed by formation of bromine atoms (Fig.11.57).The bromine radical abstracts
the allylic hydrogen to produce the cyclohexenyl radical,which in turn reacts with
the Br
2
to give the allylic bromide and a new, chain-carrying bromine atom. The
NBS is only slightly soluble in carbon tetrachloride, thus ensuring the low
concentration of bromine necessary for the success of this reaction (Fig. 11.58).
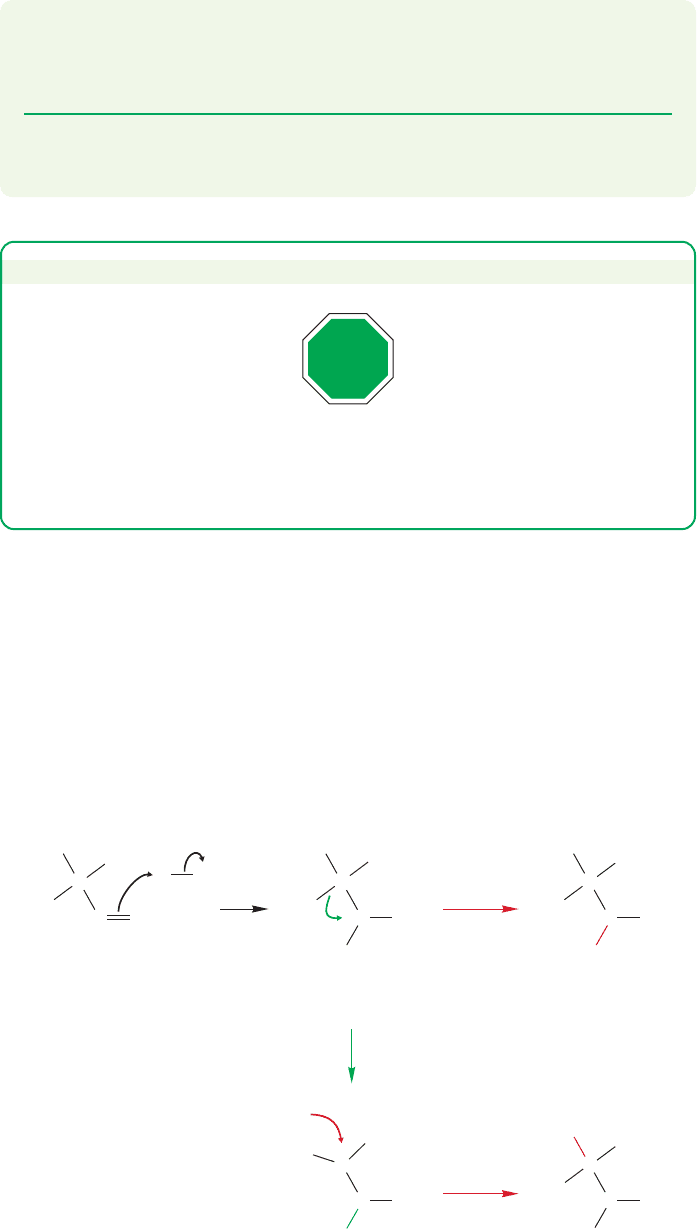
11.9 Special Topic: Rearrangements (and Nonrearrangements) of Radicals 501
PROBLEM SOLVING
11.9 Special Topic: Rearrangements (and
Nonrearrangements) of Radicals
We saw in Chapters 9 and 10 that polar additions to alkenes are bedeviled by
hydride or alkyl shifts when there is a possibility of forming a more stable cation
from a less stable one. A typical example, the polar addition of hydrogen chloride
to 3,3-dimethyl-1-butene, is shown again in Figure 11.59.Yet free radical additions
PROBLEM 11.28 Suggest a mechanism for the formation of bromine from NBS
and hydrogen bromide. Hint: The oxygen of a carbon–oxygen double bond is
a base.
PROBLEM 11.29 Selectivity is not possible in a photochemical reaction of Br
2
with
a disubstituted alkene such as trans-2-pentene. Explain.
GO
NBS is a reagent that comes with its own pull-down menu that says, “watch out
for allylic bromination.” When you see NBS in a problem, allylic bromination is
very likely to be involved in the answer.
+
Cl
..
..
..
..
Cl
..
..
..
Cl
..
..
methyl
shift
More stable
tertiary carbocation
Less stable
secondary carbocation
Major product
Minor product
addition
–
Cl
..
..
..
..
..
–
Cl
..
..
..
H
C
HC
CH
2
CH
3
H
3
C
H
3
C
C
HC
CH
3
CH
3
H
3
C
H
3
C
C
HC
CH
3
CH
3
H
3
C
H
3
C
+
C
C
H
CH
3
CH
3
H
3
C
H
3
C
C
HC
CH
3
CH
3
H
3
C
H
3
C
addition
FIGURE 11.59 Rearrangements occur
in the polar addition of hydrogen
chloride to 3,3-dimethyl-1-butene.
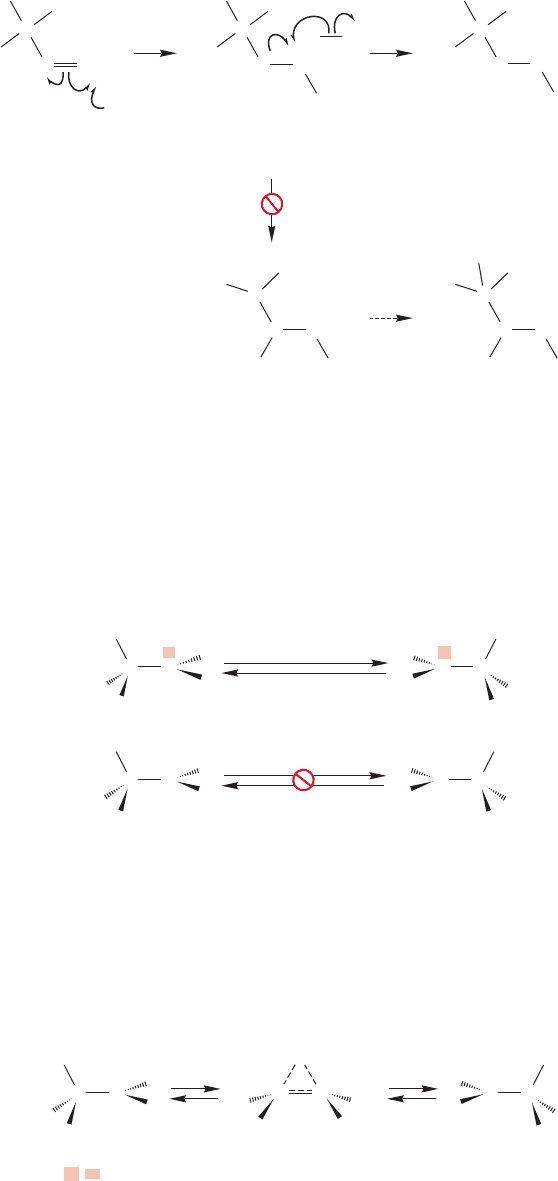
502 CHAPTER 11 Radical Reactions
We might have expected rearrangement through the shift of a methyl group to
give the more stable tertiary radical from the less stable secondary one, yet this reac-
tion doesn’t happen. In fact, the situation is quite dramatic: 1,2-Hydride shifts
(migration of ) are among the fastest known organic reactions but 1,2-shifts of
a hydrogen atom or an alkyl group are unknown (Fig. 11.61).
H
:
-
hydride shift
very common!
HH
C
C
C
C
..
..
..
..
..
..
hydrogen atom
shift unknown
HH
CC CC
..
..
..
..
..
..
.
.
+ +
FIGURE 11.61 The simple 1,2-shift
of a hydrogen atom ( ) is unknown.H
.
This situation seems mysterious, but a look at the molecular orbitals involved in
the transition state for the reaction shows the reason. There is more than one way
to approach this problem, but we think an analysis of the transition state for the
rearrangement is the easiest. In the transition state for any symmetrical 1,2-shift
of hydrogen, the hydrogen atom is half-transferred from one carbon to the other
(Fig. 11.62).
The transition state
is exactly at the
halfway point in this
symmetrical reaction
H
*
CC
H
**
CC
CC
H
*
means:
+, –, or
.
FIGURE 11.62 In the transition
state for any symmetrical 1,2-shift,
the hydrogen is halfway between
the two carbons. Here the symbol
*
, , or .
.
are usually uncomplicated by similar rearrangements. For example, the peroxide-
initiated addition of hydrogen bromide to 3,3-dimethyl-1-butene gives only the
unrearranged product (Fig. 11.60).
Br
..
.
..
..
Br
..
.
..
..
Br
..
..
..
Br
..
.
..
..
Br
..
..
..
Br
..
..
..
methyl shift
does not occur
More stable
tertiar
y
radical
Less stable
secondary radical
.
H
C
HC
CH
2
CH
3
H
3
C
H
3
C
C
HC
CH
2
CH
3
H
3
C
H
3
C
C
HC
CH
2
CH
3
H
3
C
H
3
C
C
CH
2
CH
3
H
3
C
H
3
C
H
2
C
Br
Not formed
C
HC
CH
2
CH
3
H
3
C
H
3
C
+
H
FIGURE 11.60 The related radical
addition gives no rearranged product.
11.9 Special Topic: Rearrangements (and Nonrearrangements) of Radicals 503
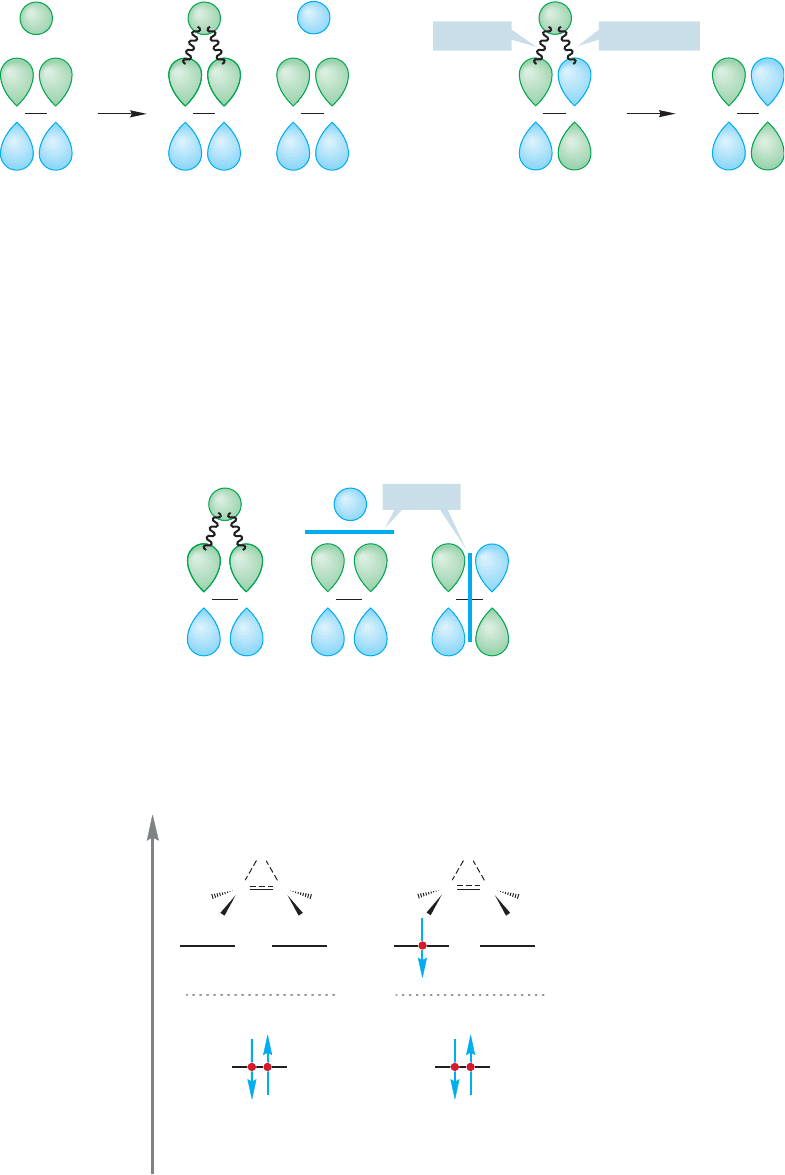
What do the molecular orbitals of the transition state look like? To answer this ques-
tion,we need only construct the molecular orbitals for the system of a hydrogen 1s orbital
interacting with the π and π* orbitals of an alkene. The combination of 1s, π, and π*
will yield three new molecular orbitals A,B,and C.They are constructed in Figure 11.63.
The 1s orbital interacts with the π orbital to form two new molecular orbitals
(A and B), but there is no interaction between 1s and π* in this geometry.
Accordingly, π* becomes C, the third molecular orbital for the transition state. To
order the energy of A, B, C, we need only count nodes. Molecular orbital A has no
new nodes, but B and C each have one additional node (Fig. 11.64).
1s
1s
1s
C
π
C
+
–
C
π*
C
+
–
C C
C
π
C
Antibonding
Bonding
There is no interaction
between
π* and 1s
AB C
+
1s
C
π
C
–
FIGURE 11.63 The three molecular orbitals for the transition state in this reaction can be easily
constructed from H (1s), π, and π*.
1s
C
π
C
AB
+
1s
C
π
C
C
C
C
–
Nodes
FIGURE 11.64 The new molecular
orbitals (A–C) can be ordered in
energy by counting nodes.
The ordering of the new molecular orbitals is shown in Figure 11.65. Now, how
many electrons do we need to put into the orbital system? For the cationic reaction
of Figure 11.61, there are only two and they will go nicely into the new stabilized
Energy
.
.
C
AA
CCBB
C
H
Nonbonding
Cation, two electrons
Radical, three electrons,
one in an antibonding orbital;
bad news
Nonbonding
CC
H
+
FIGURE 11.65 The transition state
will have a single bonding molecular
orbital (A) and two, equi-energetic
antibonding molecular orbitals
(B and C). For the cationic
rearrangement, the two electrons
involved nicely fill the bonding
molecular orbital (A). In the radical
case, there is another electron and it
must occupy an antibonding
molecular orbital (B or C) in the
transition state. Occupancy of this
antibonding orbital is unfavorable.
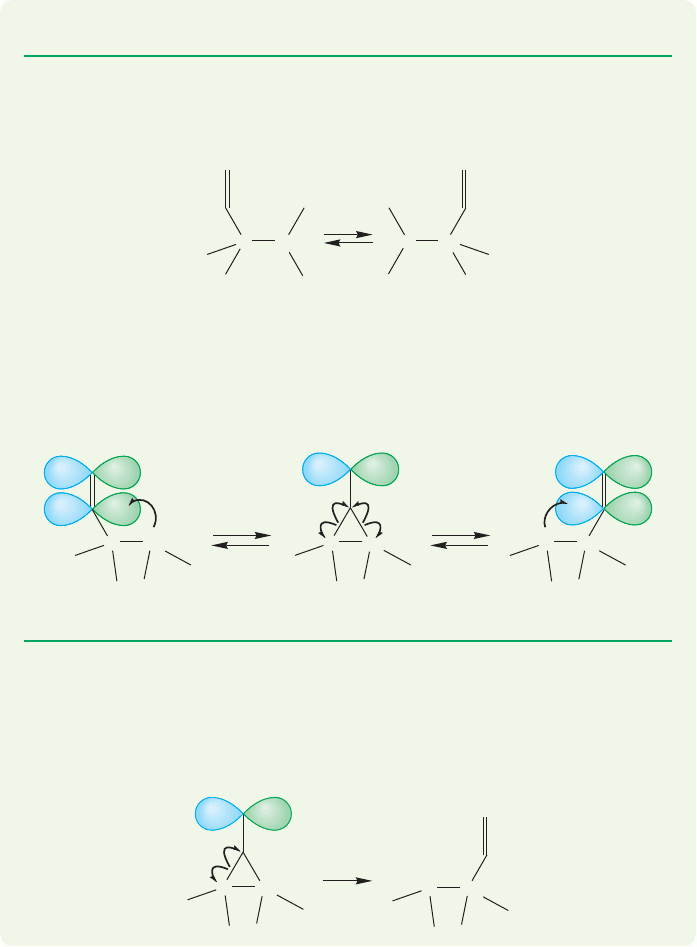
504 CHAPTER 11 Radical Reactions
ANSWER A vinyl group, or any other group with p orbitals, is not restricted to
using the σ bond in the migration. In this case, the radical on C(2) could add to
the vinyl group to give the symmetrical intermediate A. Opening of intermediate
A can occur in two directions (a and a′) either to regenerate the starting material
(a′) or complete the vinyl migration (a).
PROBLEM 11.30 Analyze the shift of hydrogen in the related anionic system.
WORKED PROBLEM 11.31 1,2-Shifts of vinyl groups are known in radicals.
Describe how this reaction might differ from the 1,2-shift of a hydrogen atom or
a methyl radical, both of which are unknown.
C
2
.
.
C
1
C
2
C
1
.
C
1
C
1
a
A
a
a'
a'
C
2
C
1
C
2
C
2
.
.
bonding molecular orbital A. However, for the radical reaction of Figure 11.61 there
is another electron, and it must be placed in one of the antibonding molecular
orbitals, B or C.This high-energy electron is evidently destabilizing enough to com-
pletely shut off the reaction (Fig. 11.65).
WORKED PROBLEM 11.32 We have already seen a reaction much like the opening
of the cyclic intermediate shown in the answer to Problem 11.31. What is the
name of this process?
ANSWER It is just a β cleavage (p. 472).
C
1
α
β
C
1
C
2
C
2
.
.
11.10 Special Topic: Radicals in Our Bodies;
Do Free Radicals Age Us?
Free radicals are strongly implicated in the aging process. Cell membranes, which
are made up of long-chain hydrocarbon carboxylic acids called fatty acids, are par-
ticularly vulnerable to oxidation through free radical attack. Once a cell’s membrane
has been oxidized, the cell is damaged. In time, the immune system comes to
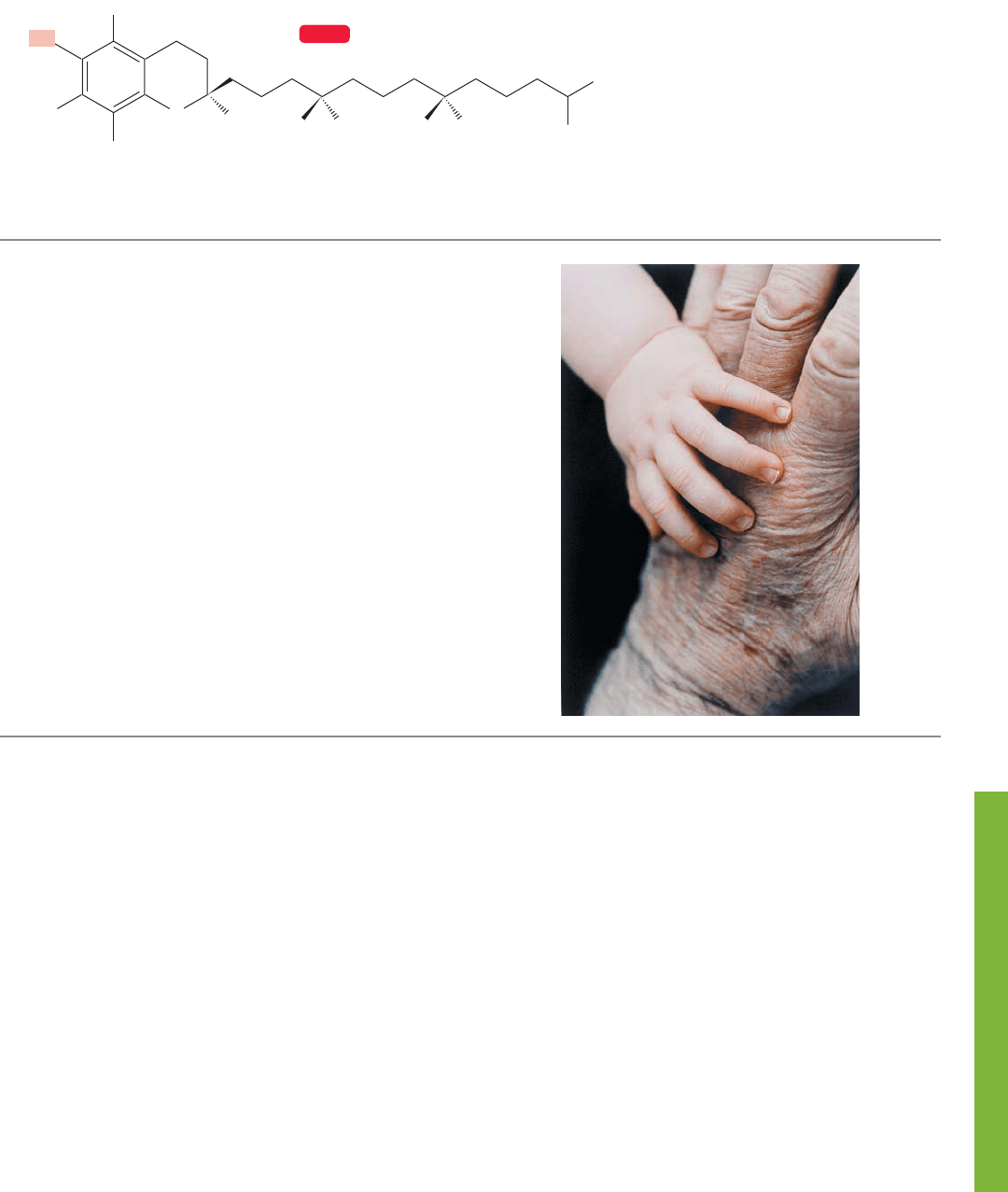
11.11 Summary 505
WEB 3D
Vitamin E
H
3
C
CH
3
CH
3
O
OH
H
3
C
H
3
C
H
3
C
H
H
3
C
H
3
C
H
FIGURE 11.66 Vitamin E.
recognize damaged cells as “foreign” and attacks them. This leads to cell death and
ages the organ that the cell is in. If radicals are involved in aging, then radical trap-
ping agents might be effective in arresting the aging process. One such potential
trapping agent is Vitamin E (Fig. 11.66):
VITAMIN E
In rats, vitamin E is essential for proper liver function as well
as nerve and muscle condition. At least one observer has
pointed out that because rats and humans are so alike, vita-
min E might be vital for humans as well. Despite the obvi-
ous truth of the observation, there is no proven need for
vitamin E in humans. Still, that lack is not sufficient to stop
speculation, and it has been suggested that the ability of
vitamin E to act as a free radical interceptor, an antioxidant,
might make it effective in delaying aging. Vitamin E traps
radicals through the hydroxyl group highlighted in red on
the six-membered ring (Fig. 11.66). A radical ( ) plucks
a hydrogen atom from this hydroxyl to make and
destroys itself.
The long hydrocarbon chain of vitamin E also has its
function. It aids in the delivery of vitamin E to the cell
membrane by making the molecule soluble in fatty acids,
which also contain long greasy hydrocarbon chains.
Remember: “Like dissolves like” (p. 239).
ROH
RO
.
11.11 Summary
New Concepts
One of the most important concepts of this chapter is that
of selectivity. It is not really new because you have already
seen many selective reactions. A simple example is the
protonation of isobutene to give the more stable tertiary
carbocation rather than the less stable primary carbocation
(Fig. 11.23).
In this chapter, you also see selectivity when a radical
abstracts one of several possible hydrogens from an alkane.
The ease of hydrogen abstraction is naturally determined by
the energies of the transition states leading to the products
(Figs. 11.49 and 50). A tertiary radical is more likely to be
formed than a primary radical. In the transition state for
radical formation, the factors that make a tertiary radical
more stable than a primary radical will operate to stabilize
the partially formed tertiary radical over the partially
formed primary radical, as noted in Figures 11.49 and
11.50.
What is more subtle, and at least somewhat new, is the
idea that the degree of selectivity in a reaction will be influ-
enced by how product-like the transition state is. The more the

506 CHAPTER 11 Radical Reactions
Key Terms
abstraction (p. 471)
acyl group ( ) (p. 476)
allylic halogenation (p. 497)
azo compounds (p. 476)
chain reaction (p. 468)
β cleavage (p. 472)
disproportionation (p. 471)
hydrocarbon cracking (p. 473)
inhibitors (p. 484)
initiation (p. 483)
propagation (p. 483)
pyrolysis (p. 470)
termination (p. 483)
thermolysis (p. 470)
R
O
C
P
O
transition state is like the product (the radical intermediate),
the more important the relative stabilities of two possible
products will be. Abstraction of a hydrogen by chlorine is
endothermic by about 2 kcal/mol. The same abstraction reac-
tion by bromine is endothermic by about 17 kcal/mol. The
transition state for the much more endothermic abstraction
reaction by bromine will be more product-like than that for
abstraction by chlorine. So we expect bromine to be more
selective than chlorine, because the carbon–hydrogen bond
will be more broken in the transition state. The stability of the
product radical will be more important in the transition state
for hydrogen abstraction by a bromine than it will in the tran-
sition state for abstraction by a chlorine.
There are a few other points to keep in mind. Radical sta-
bility parallels carbocation stability. Tertiary radicals are more
stable than secondary, secondary more stable than primary,
and primary more stable than methyl. The differences aren’t as
great for radicals as for cations. Delocalization helps stabilize
radicals by spreading the odd electron over two or more
atoms, so allylic and benzylic radicals are more stable than ter-
tiary radicals.
Radical reactions are often cyclic: Radical chain reactions
produce a chain-carrying radical in their last, product-making
step, and the new radical can begin another series of
propagation steps leading to another molecule of product.
Chain reactions only stop when starting material is exhausted
or a chain is terminated by bond formation by a pair of
radicals.
Chains can be started by small amounts of radicals
released by radical initiators. In this way, a small number of
chain-initiating radicals can have an impact far out of proportion
to their numbers.
The reactions in this chapter involve neutral free radicals, in
contrast to the ionic species of earlier chapters. Chain reactions
are characteristic of radical chemistry and consist of three steps:
(1) Initiation—a small number of radicals serves to start the
reaction; (2) Propagation—radical reactions are carried out that
generate a product molecule and a new, chain-carrying radical,
which recycles to the beginning of the propagating steps
and starts a new series of product-forming steps; and
(3) Termination—radical recombinations destroy chain-carrying
species and end the chain reaction. The success of a chain
reaction depends on the relative success of the chain-carrying
and termination steps. Figure 11.26 gives an example of a
typical chain reaction.
Important reactions of radicals include abstraction, addi-
tions to alkenes and alkynes, β cleavage, and disproportionation
(Fig. 11.9), but not simple 1,2-rearrangements.
Reactions, Mechanisms, and Tools
Syntheses
The new synthetic reactions of this chapter are shown below.
1. Alkanes
2. Alkenes
+2
.
Disproportionation gives both alkanes and alkenes;
this synthesis is not efficient
+2
.
Disproportionation gives both alkanes and alkenes;
this synthesis is not efficient
3. Alkyl Bromides
+
R
peroxides
Br
HBr
+
HBr
H
Br
Also works for Cl
2
; photobromination is more
selective than photochlorination
A chain reaction that works only for H
Br, not
other H
X molecules; note the anti-Markovnikov
regiochemistry
Br
2
+
RH
h
ν

11.12 Additional Problems 507
4. Allylic Halides
hν/CCl
4
NBS
Bromination is specifically at the allylic position
Br
5. Radicals
6. Vicinal Dihalides
7. Vinyl Bromides
R
Δ
R
NN
R
Δ
R
Pyrolysis of hydrocarbons is an unselective
method for making radicals; many different
radicals are typically produced
Pyrolysis of azo compounds
.
2 R
N
2
++
.
R
.
R
C
H
R
peroxides
BrHC
HBr
V
inyl halides (see 7) can add a second molecule of
HBr to
g
ive vicinal
(
not
g
eminal
)
dihalides
HR
Br
H
C
Br
H
C
peroxides
CH
R
BrHC
R
HBr
Radical-induced addition to alkynes gives
anti-Markovnikov addition; cis and trans
products are formed
HC C
A very common mistake is to confuse the propagation step in a
chain reaction with the termination step. In the photobromina-
tion of an alkane such as methane, the product is formed in a
chain reaction in which bromine and methyl radicals carry the
chain (Fig. 11.45). It is all too tempting to imagine that the
product, methyl bromide, is formed by the combination of one
methyl radical and one bromine radical! In fact, although this
step does produce a molecule of product, it destroys two chain-
carrying radicals. This reaction would terminate the chain
process and so we know it does not contribute significantly to
product formation.
Common Errors
PROBLEM 11.33 Let’s build right away on Problem 11.30 and
Section 11.9. Suppose you are walking down the street, and a
stranger comes up to you and asks, Which is more stable, linear
or triangular H
3
? We maintain that you should be able to give
him or her a quick answer to this seemingly bizarre question.
Obvious hint: Look at Section 11.9.
Here’s an outline of how to do this problem: First, build the
molecular orbitals for linear and cyclic H
3
. You did the linear
molecule long ago in Chapter 1 (Problem 1.60, p. 48). Next,
put the appropriate number of electrons in. Finally, look at the
orbital containing those electrons in each species. Which is
lower in energy?
PROBLEM 11.34 Show the reactions you would use to synthe-
size propene if your only source of carbon is propane.
PROBLEM 11.35 Show how you would make 2-butene if your
only source of carbon is butane.
PROBLEM 11.36 Show how you would make trans-1,2-dibro-
mocyclohexane starting with cyclohexane.
PROBLEM 11.37 Predict the major product(s) for the radical
hydrohalogenation reaction (HBr, peroxides, heat) with each
of the following alkenes:
(a) 1-pentene
(b) 2-pentene
(c) cyclopentene
(d) cis-3-hexene
(e) trans-3-hexene
PROBLEM 11.38 Draw the monochlorinated products formed
by radical chlorination of the following compounds:
(a) cyclobutane
(b) cyclopentane
(c) methylcyclopentane
(d) pentane
PROBLEM 11.39 Draw four dibromo products that could be
obtained by radical bromination of propane. Show the reaction
pathway for the compound you think would be the major product.
PROBLEM 11.40 Which of the products in the previous prob-
lem are chiral and which are achiral?
11.12 Additional Problems
(a)
(b)
HBr
ROOR
HBr
CH
3
CH
2
CH
3
CH
2
CCH
CCH
508 CHAPTER 11 Radical Reactions
PROBLEM 11.41 Give the major product(s) expected for
each of the following reactions. Pay attention to regiochemistry
and stereochemistry where appropriate. Mechanisms are
not required, although they may be useful in finding the
answers.
PROBLEM 11.42 Predict the products of hydrogen bromide
addition to 1-butyne. Consider both ionic (a) and radical-
initiated (b) reactions. Rationalize your predictions with arrow
formalism descriptions. Remember that a second molecule
of hydrogen bromide can add to the initial product(s) in
both cases.
PROBLEM 11.43 The relative rates of recombination of methyl
and isopropyl radicals are quite different. Give two possible
reasons for this difference.
(a)
HBr
ROOR
+
(b)
HBr
CH
3
(c)
(d)
(e)
(f)
(g)
HCl
ROOR
HBr
ROOR
HBr
hν
hν
CH
3
CH
2
CCl
4
NBS, CCl
4
COOCH
3
H
CCH
H
H
3
C
H
3
C
H
3
C
CH
3
CCH
CH
3
2 H
3
CH
3
C
Relative
Rate
22
1
H
3
C
H
3
C
CH CH
H
3
C
H
3
C
CH
3
CH
3
CH2
.
.
PROBLEM 11.44 Decomposition of the following azo
compounds at 300 °C occurs at quite different rates. Give two
possible explanations for these differences.
R
2
R
2
R
1
R
3
C
R
1
R
3
C
NN
300 ⬚C
.
CH
3
, CH
3
, CH
3
CH
3
, CH
3
, H
CH
3
, H, H
H, H, H
N
2
2
13,000
111
11
1
+
C
R
1
R
2
R
3
R
2
R
1
R
3
Relative Rate
PROBLEM 11.45 Predict the stereochemical results of the
photochlorination of the optically active compound 1.The (
*
)
indicates a single enantiomer, here (R). It is the tertiary
carbon–hydrogen bond that is chlorinated.
H
1
C
Cl
CH
3
No stereochemistry implied
in this drawing
C
Cl
2
hν
CH
2
Cl
CH
2
Cl
H
3
C
CH
3
CH
2
CH
3
CH
2
*
PROBLEM 11.46 In Problem 11.25, we examine the pho-
tochlorination of ethane to give ethyl chloride. As was the
case in the photochlorination of methane (p. 490), products
containing more than one chlorine are also isolated in the
photochlorination of ethane. What is the structure of the
major dichloroethane produced in this reaction? Rationalize
your prediction with an analysis of the mechanism of the
reaction.
