Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

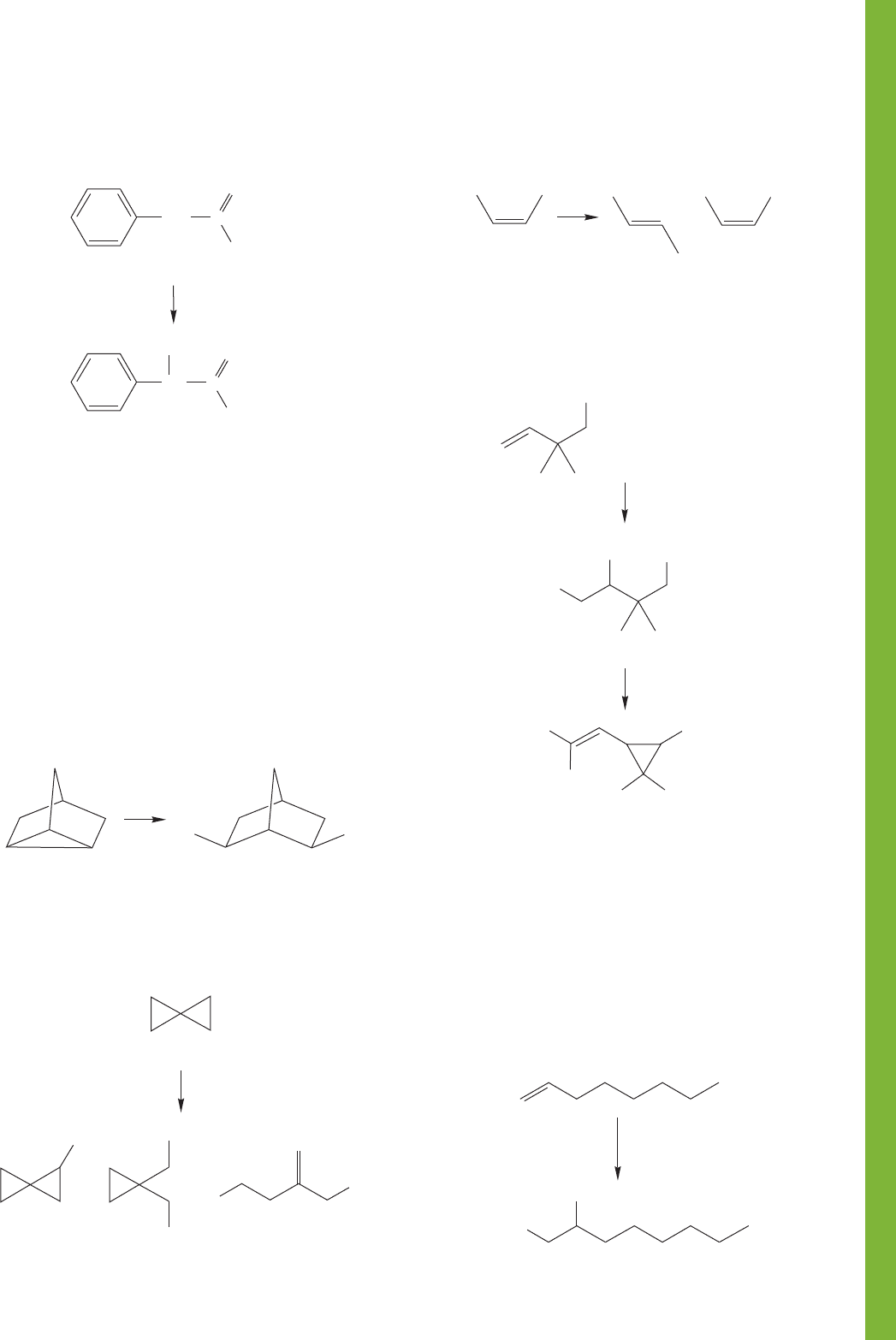
11.12 Additional Problems 509
CH
2
1
NBS, CCl
4
Δ
OCH
2
CH
3
C
O
CH
Br
OCH
2
CH
3
C
O
PROBLEM 11.47 Write a detailed mechanism for the bromina-
tion of ethyl phenylacetate (1) with NBS.
PROBLEM 11.48 Draw each of the resonance structures for the
radical intermediate formed in Problem 11.47. Focus on the
radical part.
PROBLEM 11.49 Predict the major product(s) for the reaction
between NBS (allylic halogenation) and the following alkenes:
(a) propene
(b) 1-butene
(c) 2-butene
(d) cyclopentene
(e) 1-methylcyclohexene
PROBLEM 11.50 Write a detailed mechanism for the
photochlorination of 1 to give 2. Why is compound 1 reactive
even though it contains no π bond?
PROBLEM 11.51 Here is a more complicated photochlorina-
tion of a compound containing a three-membered ring.
Explain the formation of the following products from
spiropentane (1).
Cl
2
Cl
21
Cl
h
ν
PROBLEM 11.52
Photolysis of iodine in the presence of
cis-2-butene leads to isomerization of the alkene to a mixture
of cis- and trans-2-butene. Explain.
+
+
Cl
Cl
Cl
Cl
2
1
Cl
Cl
h
ν
+
I
2
hν
PROBLEM 11.53
Because of their high-potency, low-mammalian
toxicity, and photostability, certain esters of halovinylcyclo-
propanecarboxylic acids (pyrethroids) are promising insecticides
(see Chapter 10, p. 456). An important precursor to this class of
insecticides is the carboxylic acid (1), prepared as follows:
(a) Write a mechanism for the formation of 2. Be sure to
account for the observed regiochemistry. Hint: The CuCl
can abstract a chlorine atom.
(b) What transformations must be accomplished in the 21
conversion? Don’t worry about mechanisms.
(c) Given the stereochemistry about the carbon–carbon double
bond in 1, how many stereoisomers of 1 are possible? The
most potent insecticides are derived from the (1R,3S)
isomer of 1. Draw this isomer.
U
+
2
3
1
Cl
COOH
F
3
C
1
2
F
3
CCCl
3
COOCH
2
CH
3
Cl
two steps
F
3
CCCl
2
COOCH
2
CH
3
CuCl
Δ
PCl
3
ROOR
Cl
2
P
Cl
85 ⬚C
PROBLEM 11.54 Write a mechanism for the following reaction
of 1-octene:
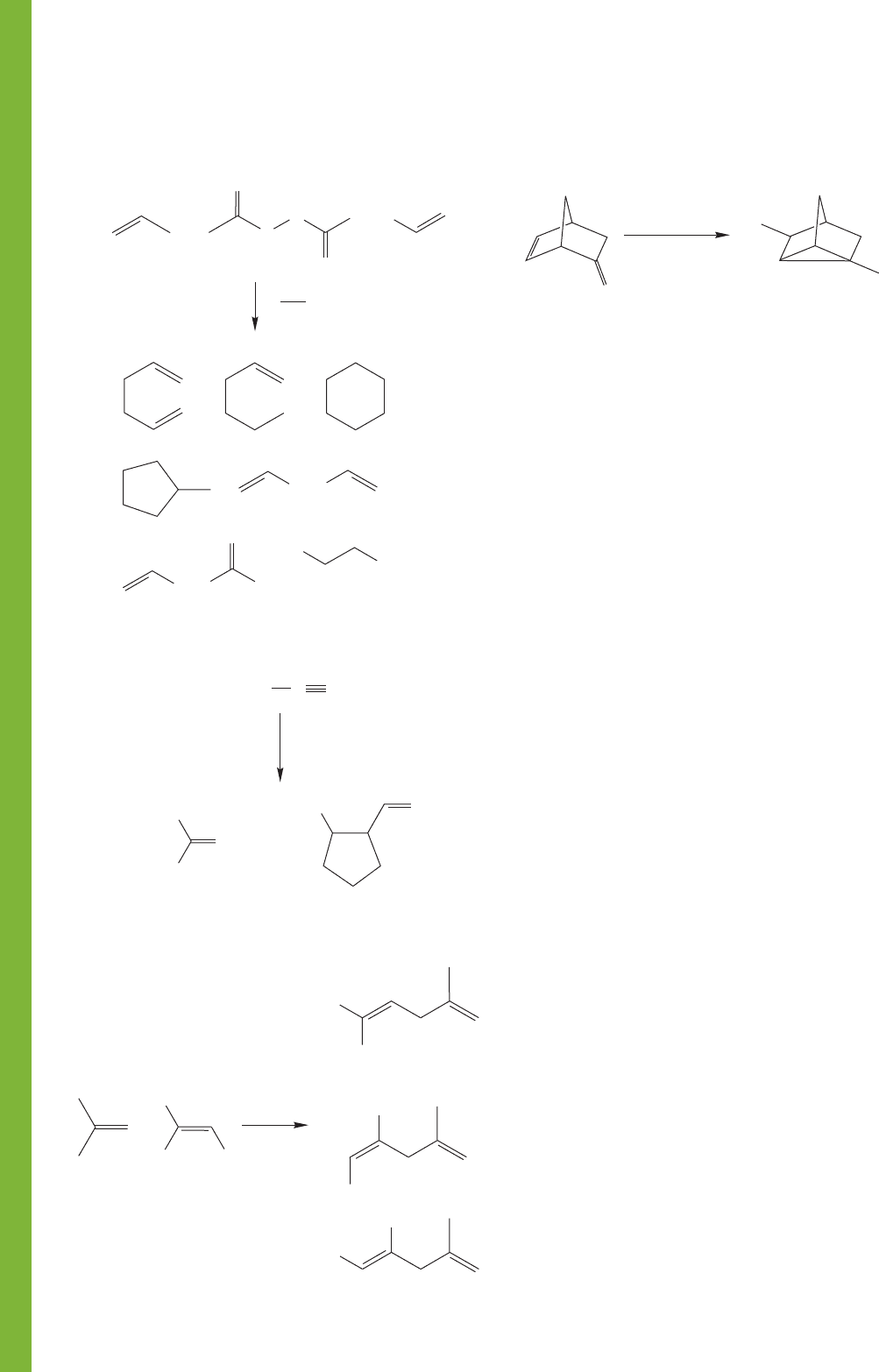
510 CHAPTER 11 Radical Reactions
PROBLEM 11.55 Provide mechanisms for the formation of the
following products:
PROBLEM 11.56 Provide mechanisms for the formation of the
following products:
Ph
CH
3
77 ⬚C
Ph
Ph
OH
O
O
O
O
O
(CH
2
)
4
(CH
2
)
4
(CH
2
)
4
(CH
2
)
8
CCl
4
ROOR,
77 ⬚C
Cl
CH
3
(CH
2
)
4
CH
3
(CH
2
)
4
CHCCl
3
CCH
+
CCl
2
H
3
C
PROBLEM 11.57 Propose a mechanism for the following reac-
tion. Be sure to rationalize the preferred formation of diene 1.
PROBLEM 11.58 Write a mechanism for the formation of
compound 1.
+
Cl
Cl
Cl
Cl
Cl
1
(major product)
Cl
Cl
Cl
Cl
ROOR,
500 ⬚C
Δ
ROOR, CCl
4
Cl
1
CH
2
CCl
3
Use Organic Reaction Animations (ORA) to answer the
following questions:
PROBLEM 11.59 There are three radical processes included
in ORA. All three are on the second page of reactions.
Select the “Radical alkene hydrohalogenation” reaction. The
animation shows initiation steps and propagation steps for
the overall process. Write out the steps of the reaction and
indicate which are initiation steps and which are propagation
steps.
PROBLEM 11.60 No termination steps are shown in the
animation. Write out possible termination steps for the
reaction.
PROBLEM 11.61 The “Alkane halogenation” reaction is also
a radical process. Select this reaction and watch it several
times. What is the hybridization of the central carbon of
the intermediate? Select the SOMO (singly occupied
molecular orbital) track. Why has the calculation come up
with this hybridization? Is the hybridization fixed or does
it change?
PROBLEM 11.62 A third radical animation is “Benzylic
oxidation.”This reaction is shown using oxygen (O
2
) in the
initiation step. Why can oxygen remove a hydrogen from
isopropylbenzene? Is the benzylic hydrogen acidic? Is
oxygen basic? What is the hybridization of the benzylic
carbon in the intermediate? Is the hybridization of the
benzylic carbon fixed or does it change?

Dienes and the Allyl System:
2p Orbitals in Conjugation
511
12.1 Preview
12.2 Allenes
12.3 Related Systems: Ketenes and
Cumulenes
12.4 Allenes as Intermediates in the
Isomerization of Alkynes
12.5 Conjugated Dienes
12.6 The Physical Consequences
of Conjugation
12.7 Molecular Orbitals and
Ultraviolet Spectroscopy
12.8 Polyenes and Vision
12.9 The Chemical Consequences
of Conjugation: Addition
Reactions of Conjugated
Dienes
12.10 Thermodynamic and Kinetic
Control of Addition Reactions
12.11 The Allyl System: Three
Overlapping 2p Orbitals
12.12 The Diels–Alder Reaction
of Conjugated Dienes
12.13 Special Topic: Biosynthesis
of Terpenes
12.14 Special Topic: Steroid
Biosynthesis
12.15 Summary
12.16 Additional Problems
12
CONJUGATED ALKENES The different colors in fruits and vegetables arise from
the different conjugated alkenes that are produced by plants.
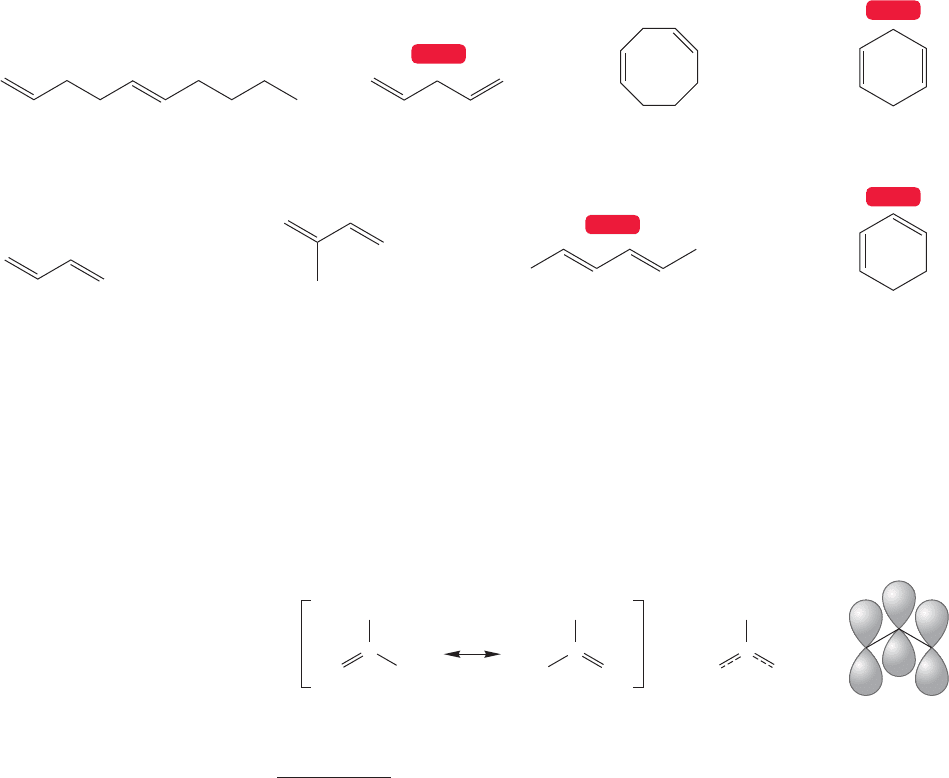
512 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
WEB 3D
WEB 3D
WEB 3D
WEB 3D
Unconjugated dienes
Conjugated dienes
(E)-1,5-Decadiene
1,3-Butadiene 2-Methyl-1,3-butadiene
(isoprene)
1,3-Cyclohexadiene
1,4-Pentadiene
(E,E
)-2,4-Hexadiene
1,4-Cyclohexadiene1,4-Cyclooctadiene
FIGURE 12.1 Some conjugated and unconjugated double bonds.
Since chemists are not physicians, we shall scarcely benefit by their art,
except by making the physician a chemist.
—GEORGE REES
1
12.1 Preview
Sometimes the whole really is greater than, or at least different from, the sum of the
parts. In this chapter,we see just such a situation. One cannot always generalize from
the properties of alkenes to those of molecules with multiple double bonds. Dienes,
containing two double bonds separated only by a single bond (Fig. 12.1), often have
properties that are quite different from those of isolated, individual alkenes. This
difference depends on a phenomenon called conjugation. Conjugated double bonds
are alkenes connected by a single bond. A molecule containing widely separated
double bonds will generally react as would the individual alkenes. Double bonds
in conjugation react quite differently. A conjugated diene constitutes a separate
functional group. Cumulated alkenes (cumulenes) are molecules that have three or
more atoms attached only by double bonds.
H
2
CH
2
C
CH
2
CH
2
==
C
H
C
H
.
.
H
2
C
CH
2
C
H
A summary structure
for the all
y
l radical
The allyl radical
.
.
.
.
FIGURE 12.2 Allyl has a 2p orbital on
each carbon atom.
First we will cover the cumulenes, then we will generalize our discussion of the
phenomenon of conjugation and its effect on chemical reactions. Next we will develop
further the chemistry of a molecule we already encountered,allyl.Figure 12.2 shows
the neutral allyl radical, a system of three parallel 2p orbitals.
1
George Owen Rees was a nineteenth-century clinician who specialized in the analysis of blood and urine.
A student of MJ’s, Jordan M. Cummins, found this nice quote in the book by Stanley J. Reiser, Medicine and
the Reign of Technology, Cambridge University Press, Cambridge, 1978.
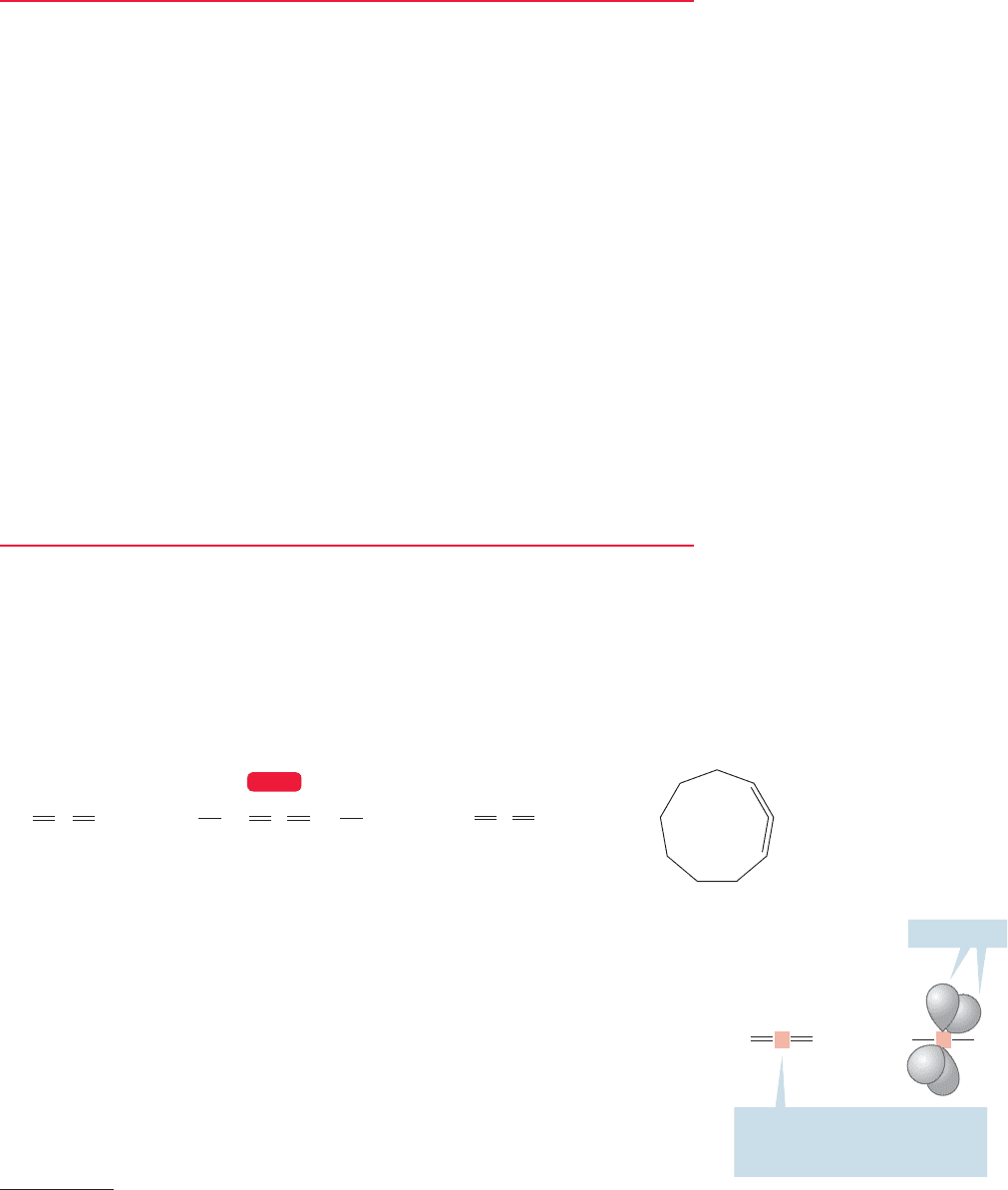
12.2 Allenes 513
Finally, we will see the outlines of how large molecules are constructed in Nature
from a rather simple building block, the diene isoprene (2-methyl-1,3-butadiene),
shown in Figure 12.1.
2
There is a chance for confusion here between the system of three parallel 2p orbitals called “allyl”and the “allene”
in which two double bonds are attached to one carbon. Be careful; these species are very different from each other!
WEB 3D
CH
2
CCH
2
CH
2
C C(CH
3
)
2
CHCH
3
CCHCH
3
3-Methyl-1,2-butadiene
(1,1-dimethylallene)
2,3-Pentadiene
(1,3-dimethylallene)
1,2-Cyclononadiene
Propadiene
(allene)
FIGURE 12.3 Some allenes.
The first problem is to work out a structure for allene.The key position in this
cumulated diene is certainly the central carbon atom that is shared by the two
double bonds, and the bonding involved has important structural consequences.
The central carbon in any allene is attached to only two groups and, therefore, sp
hybridization is appropriate.The central carbon uses two sp hybrid orbitals to form
a pair of σ bonds with the flanking methylene groups, leaving the central carbon’s
remaining two p orbitals available for π bonding (Fig. 12.4).
CH
2
CCH
2
H
2
CCH
2
This carbon is attached to
only two other groups
(sp hybridization is appropriate)
2p Orbitals
=
C
FIGURE 12.4 The central carbon of
allene is sp hybridized.
ESSENTIAL SKILLS AND DETAILS
1. The overall skill that you will take away from this chapter is the ability to think about
the structural and chemical consequences of conjugation. Conjugation affects reactivity
markedly, and an ability to see how and why is essential before progressing to the
following chapters.
2. The difference between kinetic control and thermodynamic control must be understood.
Kinetic control means that the products are determined by the relative heights of the
transition states connecting starting materials and products, not by the energies of
the products themselves. Under conditions that supply enough energy to go over all
the transition states, thermodynamic control will operate. Under these conditions, the
product mixture is determined by the relative energies of the products themselves.
3. The Diels–Alder reaction has been characterized as the most important synthetic reaction
in organic chemistry. It is surely among the most important, because it makes the very
common six-membered rings in a single step. Every cyclohexene is the potential product
of a Diels–Alder reaction and the potential source of a diene and a dienophile through a
reverse Diels–Alder reaction. Be sure you can play the game of “find the cyclohexene”!
4. The number of sp hybridized carbons in an allene or cumulene has stereochemical
consequences: An even number of such carbons leads to cis/trans isomerism, whereas
an odd number produces a pair of enantiomers.
5. Allyl resonance is important in reactions of cations, radicals, and anions. Be sure you
can manipulate the allyl system easily.
12.2 Allenes
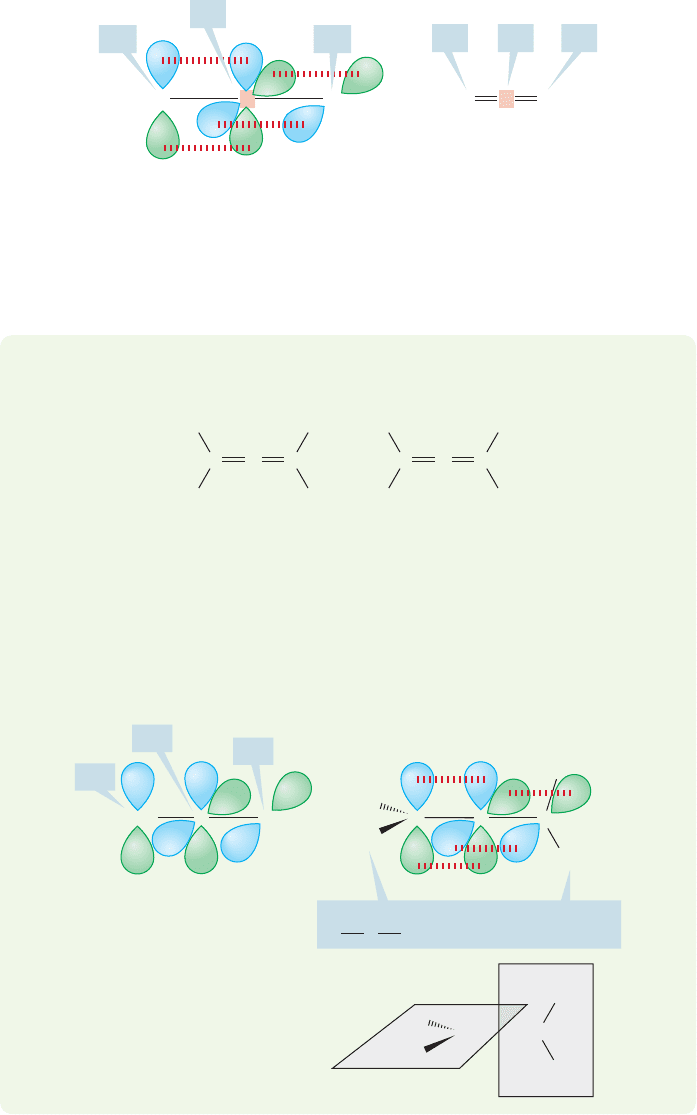
The simplest diene possible is propadiene,almost always known as allene (Fig.12.3).
2
Allene is the general term for any molecule containing two double bonds that share
a carbon atom. Allenes are systematically named as dienes or commonly named as
derivatives of allene (Fig. 12.3).
514 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
CH
2
CCH
2
H
2
C
Notice that the
π bonds
are in perpendicular planes
sp
2
sp sp
2
sp
2
=
sp
CH
2
sp
2
C
FIGURE 12.5 An orbital picture of
allene. Notice the perpendicular π
systems.
Each end carbon, though, is attached to three groups and is therefore hybridized
sp
2
.This easy approach leads immediately to a structure for allene (shown in Fig. 12.5).
Notice how each 2p orbital of the sp
2
hybridized end carbons overlaps perfectly with
one of the 2p orbitals of the central,sp hybridized carbon.The important thing is not
to be panicked by the odd central carbon and to keep in mind how we dealt with new
structures before.Assign an appropriate hybridization,be sure to pay attention to the
geometrical requirements of the hybrid, and the structure will always work out.
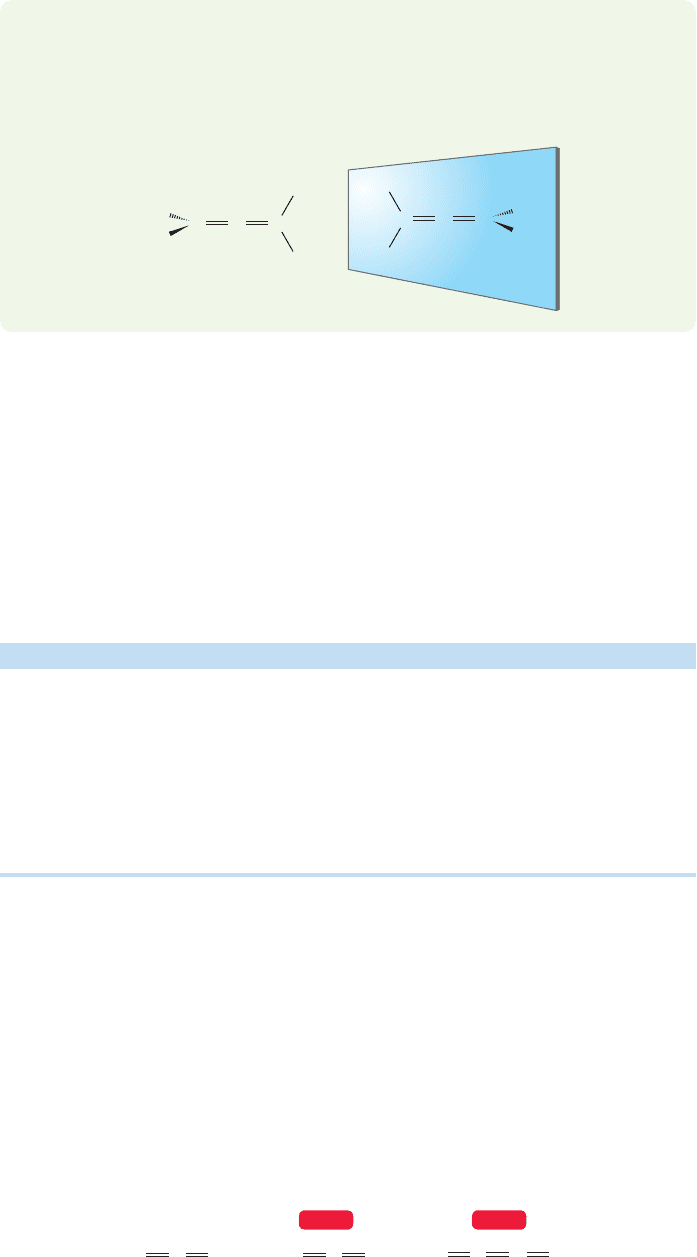
WORKED PROBLEM 12.1 It would appear that there could be cis and trans isomers
of 1,3-dimethylallene, but there aren’t. Explain.
CC
cis ???
H
3
C
C
CH
3
HH
CC
trans ???
H
3
C
C
CH
3
H
H
2,3-Pentadiene
(
1,3-dimeth
y
lallene
)
ANSWER The two-dimensional paper will fool us all the time if we do not think
in three dimensions. The end groups of an allene are not in the same plane, but
lie in perpendicular planes. The words cis (same side) and trans (opposite sides)
have absolutely no meaning for an allene.
C
sp
H
H
3
CCH =CHCH
3
sp
2
sp
2
C
H
3
C
H
C
H
H
3
C
C
The planes containing the two
H
C
CH
3
units are perpendicular
H
C
CH
3
CH
3
C
We have to be careful and pay attention to detail. Finding a reasonable structure
for allene is a classic example of a problem asked at this point in almost every course
in organic chemistry.
12.3 Related Systems: Ketenes and Cumulenes 515
WORKED PROBLEM 12.2 Two isomers of 1,3-dimethylallene do exist. What are they?
ANSWER There are two isomers of 1,3-dimethylallene, because it is a chiral mol-
ecule.The mirror image of 1,3-dimethylallene is not superimposable on the original;
therefore a pair of enantiomers exists.
Mirror
CCC
H
H
H
H
CH
3
CH
3
CCC
H
3
C
H
3
C
Allenes are high-energy molecules, just as are alkynes. The sp hybridization of
the central carbon of this functional group is not an efficient way of using orbitals
to make bonds, because π bonds are much weaker than σ bonds. The π bond energy
of about 66 kcal/mol is substantially lower than most σ bond energies. This ineffi-
ciency is reflected in relatively positive heats of formation ( values).Table 12.1
gives a few heats of formation for allenes and alkynes. Remember that heats of
formation are strictly comparable only among isomers. As you can see, allenes are
roughly of the same energy as alkynes. This similarity is not surprising because the
two molecules contain the same number of π bonds.
¢H
f
°
TABLE 12.1 Heats of Formation for Some Small Hydrocarbons
Alkynes (kcal/mol) Allenes (kcal/mol)
Propyne 44.6 Propadiene 47.7
1-Butyne 39.5 1,2-Butadiene 38.8
2-Butyne 34.7 1,2-Pentadiene 33.6
2,3-Pentadiene 31.8
H
3
C
O
CH
P
C
P
CH
O
CH
3
H
2
C
P
C
P
CH
O
CH
2
CH
3
H
2
C
P
C
P
CH
O
CH
3
H
2
C
P
C
P
CH
2
≤H °
f
≤H °
f
12.3 Related Systems: Ketenes and Cumulenes
In allenes, the double bonds share a central carbon atom. There are other cumulated
molecules in which heteroatoms (noncarbon atoms such as oxygen or nitrogen) take
the place of one or more carbons of an allene and/or in which there are more than
two double bonds in a row. When one end carbon of allene is replaced with an
oxygen, the compound is called ketene (Fig. 12.6). If both end atoms are oxygens,
the molecule is carbon dioxide. Cumulated hydrocarbons with three double bonds
attached in a row are called cumulenes, although they can also be named as
derivatives of 1,2,3-butatrienes (Fig. 12.6). Cumulenes are very reactive and most
difficult to handle.
OCO
CR
2
C O
C
R
2
C
CR
2
C
Carbon dioxide Ketenes Cumulenes
(butatrienes)
WEB 3D WEB 3D
FIGURE 12.6 Some molecules
containing cumulated double bonds.

516 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
Let’s look at this isomerization in detail. We do not begin with an allene, rather
we encounter one as an intermediate in this reaction.When 2-butyne is treated with
a very strong base such as an amide ion ( ), a proton can be removed from one
of the methyl groups to give a resonance-stabilized carbanion (Fig. 12.8).
-
NH
2
PROBLEM 12.3 Draw Lewis structures for the molecules in Figure 12.6 as well as for
the related molecules: carbon disulfide ( ),isocyanates
carbodiimides ( ), and carbon suboxide ( ).
PROBLEM 12.4 Unlike 1,3-dimethylallene, 2,3,4-hexatriene (1,4-dimethylbuta-
triene) exists as a pair of cis and trans isomers. Explain.
PROBLEM 12.5 Demonstrate that 2,3,4-hexatriene is achiral.
O
P
C
P
C
P
C
P
ORN
P
C
P
NR
(R
O
N
P
C
P
O),S
P
C
P
S
1. K
+
–
2. H
2
O
H
3
C
NH
2
/ NH
3
..
..
..
CC
CH
3
H
3
C
CHC
CH
2
2-Butyne 1-Butyne
FIGURE 12.7 The isomerization of
2-butyne to 1-butyne can be carried
out in strong base.
..
+
H
3
C
–
NH
2
..
..
–
–
NH
3
..
C
H
C CH
2
H
3
CCC CH
2
..
H
3
CCC CH
2
Resonance-stabilized carbanion
K
NH
3
–
+
NH
2
..
..
..
FIGURE 12.8 Deprotonation of
2-butyne to give a resonance-
stabilized anion.
Ammonia and the carbon–hydrogen bonds of the methyl groups of 2-butyne
both have pK
a
values of about 38. Therefore, we can expect reasonable amounts of
the carbanion to be produced on reaction with the amide ion. 2-Butyne is much more
acidic than the parent hydrocarbon butane because the anion formed from 2-butyne
is resonance stabilized, and that from butane is not (Fig. 12.8).
What can happen to this carbanion? It can be reprotonated by ammonia to regen-
erate 2-butyne, but this reaction surely gets us nowhere, even though it is reasonable and
must happen frequently. Suppose, however, that the anion reprotonates at the other car-
bon sharing the negative charge? Now the product is 1,2-butadiene,an allene (Fig.12.9).
..
H
3
CCC CH
3
H
3
CCC CH
2
..
–
–
H
3
CCC CH
2
K
NH
3
–
+
NH
2
..
..
..
H
3
C
H
CCCH
2
1,2-Butadiene
(meth
y
lallene)
K
–
+
NH
2
..
..
NH
3
..
FIGURE 12.9 This resonance-stabilized anion can reprotonate in two ways. Reattachment
of a proton at the end re-forms 2-butyne, but protonation at the other carbon sharing the
negative charge produces an allene.
12.4 Allenes as Intermediates in the Isomerization
of Alkynes
Now that we have the structures of allenes and cumulenes under control, it is time
to look at reactivity. Our brief look at allenes permits us to understand one of the
most astonishing reactions of alkynes: the base-catalyzed isomerization of an inter-
nal triple bond to the terminal position (Fig. 12.7).
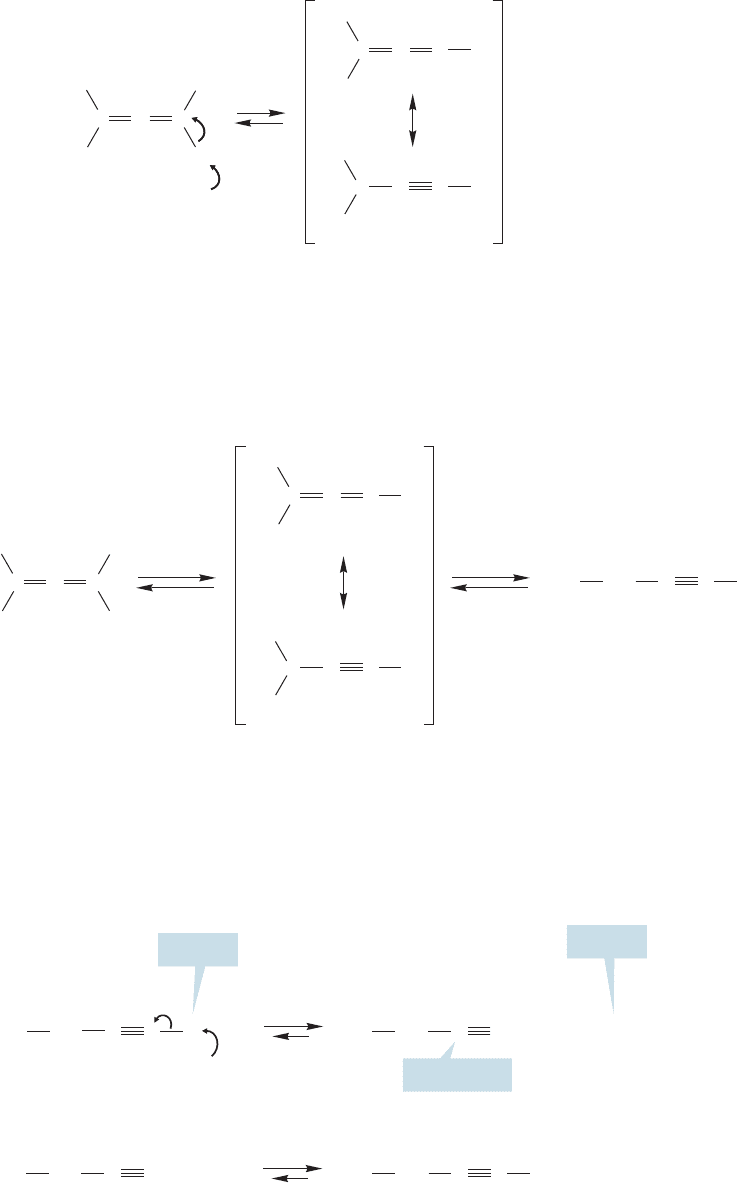
12.4 Allenes as Intermediates in the Isomerization of Alkynes 517
negative charge regenerates the allene, but reprotonation at the other carbon yields
a new, terminal alkyne, 1-butyne (Fig. 12.11).
NH
3
..
H
3
C
HH
H
..
–
CH
CCC
H
3
C
H
CC
C
–
CH
H
3
C
H
C
–
NH
2
–
..
..
..
+
Resonance-stabilized anion
FIGURE 12.10 Deprotonation of the
allene at the terminal position leads
to a new resonance-stabilized anion.
H
3
C
HH
H
CCC
H
H
3
CCH
2
C
C
–
NH
2
..
....
NH
3
....
–
NH
2
..
....
NH
3
....
1-Butyne
1,2-Butadiene
..
–
CH
H
3
C
H
CC
C
–
CH
H
3
C
H
C
..
FIGURE 12.11 Reprotonation either
re-forms the allene or gives the
isomerized alkyne, 1-butyne.
H
3
CCH
2
CC
NH
2
..
..
–
+
..
..
–
H
3
CCH
2
CHC
..
C
–
..
NH
3
....
1-Butyne
Na
Na
+
+
+
+
H
3
CCH
2
C
An acetylide
when water is added
CH
H
3
CCH
2
C
H
2
O
..
..
–
OH
..
..
..
pK
a
= 38
pK
a
= 25
FIGURE 12.12 1-Butyne is by far the
strongest acid in this equilibrating
system. Removal of the acetylenic
hydrogen gives an acetylide, the
thermodynamic energy minimum in
this reaction. When the reaction
mixture is quenched by the addition
of water, the acetylide protonates
to give 1-butyne. It is critical to
understand why it is not possible to
re-enter the equilibrium at this point.
After acidification there is no base
present strong enough to remove the
acetylenic hydrogen!
The pK
a
of the allene at the terminal methylene position is also about 38, so the
amide ion is strong enough to remove the terminal proton to generate a new, reso-
nance-stabilized anion (Fig. 12.10).Reprotonation at one of the carbons sharing the
1-Butyne (pK
a
25) is a much stronger acid than any other molecule present in the
mixture and will be rapidly and, in a practical sense,irreversibly deprotonated to give the
acetylide (Section 3.14).This deprotonation is the key step in this reaction (Fig. 12.12).

518 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
H
2
O
C
–
..
..
NH
3
....
+
+
H
3
CCH
2
C
CH
H
3
CCH
2
C
–
OH
..
..
..
..
..
–
C
..
–
..
–
H
H
3
C
HH
H
CCC
H
3
C
H
CC
C
..
–
CH
H
3
C
H
C
H
H
3
CCH
2
C
C
NH
2
..
..
–
..
..
NH
3
....
NH
2
..
..
–
..
..
NH
3
....
NH
2
..
..
–
..
..
NH
3
....
–
NH
2
..
..
..
..
NH
3
....
H
3
C
CH
3
C
C
H
3
C
CH
2
C
C
H
3
C
CH
2
C
C
NH
2
..
..
–
..
..
NH
3
....
FIGURE 12.13 The full mechanism for the isomerization of 2-butyne to 1-butyne in strong base.
No matter where the equilibria in the earlier steps of this reaction settle out,the ther-
modynamic stability of the acetylide anion will drag the overall equilibrium far to
the right. When water is added to neutralize the solution and end the reaction, the
acetylide will be protonated and 1-butyne can be isolated. The overall reaction is
summarized in Figure 12.13.
–
CC
H
C
C
C
CC
–
..
..
..
NH
2
NH
3
..
..
NH
3
There are no hydrogens on this
carbon to remove and carry the
isomerization further!
–
CCC
..
H
PROBLEM 12.6 Write a mechanism for the isomerization of 3-hexyne to 1-hexyne
in the presence of sodium amide (NaNH
2
) and NH
3
.
WORKED PROBLEM 12.7 Di-sec-butylacetylene (3,6-dimethyl-4-octyne) fails to
isomerize to another alkyne when treated with NaNH
2
/NH
3
. Explain.
ANSWER Isomerization is blocked by the alkyl groups. An allene can be formed,
but the reaction can go no further because there is no allenic hydrogen that can
be removed to produce a new resonance-stabilized anion.
