Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

12.5 Conjugated Dienes 519
WEB 3D
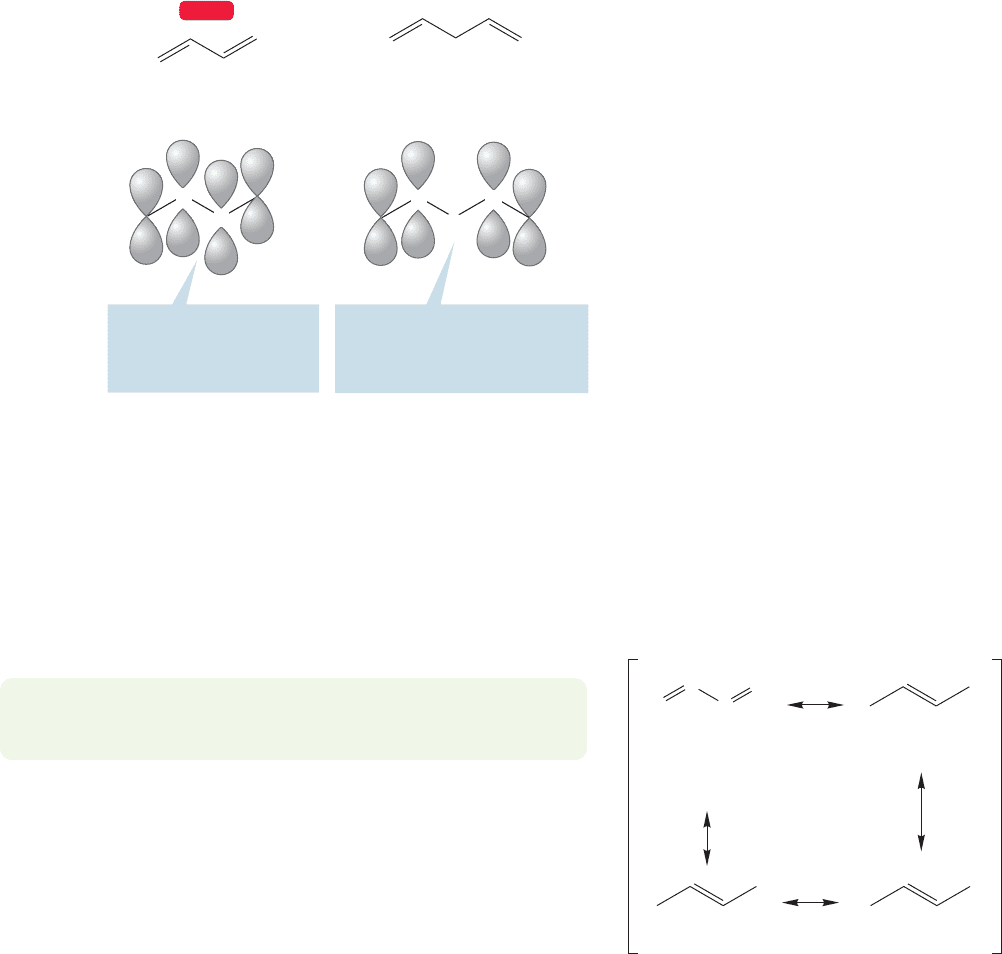
1,3-Butadiene
(conjugated)
1,4-Pentadiene
(unconjugated)
CH
2
2p/2p Overlap between
the C(2) and C(3) carbons
connects or “conjugates”
the double bonds
C
2
C
3
C
2
C
4
This methylene group insulates
the 2p orbitals of C(2) and C(4)
from each other; there is no
effective overlap
FIGURE 12.14 A conjugated and an
unconjugated diene.
In the conjugated diene, the two double bonds are connected through 2p/2p
overlap. By contrast, in the 1,4-diene the two double bonds are insulated by a
methylene group. These bonds are “not conjugated,” or “unconjugated,” and will
behave much like two separate alkenes. The question we now have to examine is
how the chemical behavior of a conjugated diene is influenced by the overlap between
the 2p orbitals on C(2) and C(3). To start, let’s take a closer look at the structure of
1,3-butadiene, giving special attention to the π system.
First, let’s look at 1,3-butadiene from a resonance point of view.
1,3-Butadiene does have resonance forms that contribute to the over-
all electronic structure of the molecule (Fig. 12.15).
The best resonance form, and therefore the best single descrip-
tion of the molecule, is the diene structure we conventionally write.
This structure has one more bond than the others and involves no
charge separation. Thus, it wins by almost all of our criteria for esti-
mating the relative energies of resonance forms (p.372). Nevertheless,
the three other resonance forms in Figure 12.15 show that there is
some “double-bond character” to the bond.C(2)
O
C(3)
12.5 Conjugated Dienes
The bonding in conjugated dienes is not so obviously exotic as it is in cumulated
dienes, such as the allenes. Nevertheless, there are some subtle effects with far-
reaching implications. The source of these effects can be seen from simple,
schematic orbital drawings of 1,3-butadiene and 1,4-pentadiene. In a conjugated
diene, the orbitals of the two double bonds overlap; in an unconjugated diene,they
do not (Fig. 12.14).
PROBLEM 12.8 Sketch the σ system of 1,3-butadiene.What orbitals
overlap to form the carbon–carbon and carbon–hydrogen bonds?
+
+
–
–
B
A
D
Note the double-bond character between C(2)
and C(3) as shown in resonance forms B, C, and D
The best
(lowest energy)
resonance form
..
..
.
.
C
2
C
C
1
C
3
C
4
FIGURE 12.15 Resonance forms contributing to
the structure of 1,3-butadiene.
520 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
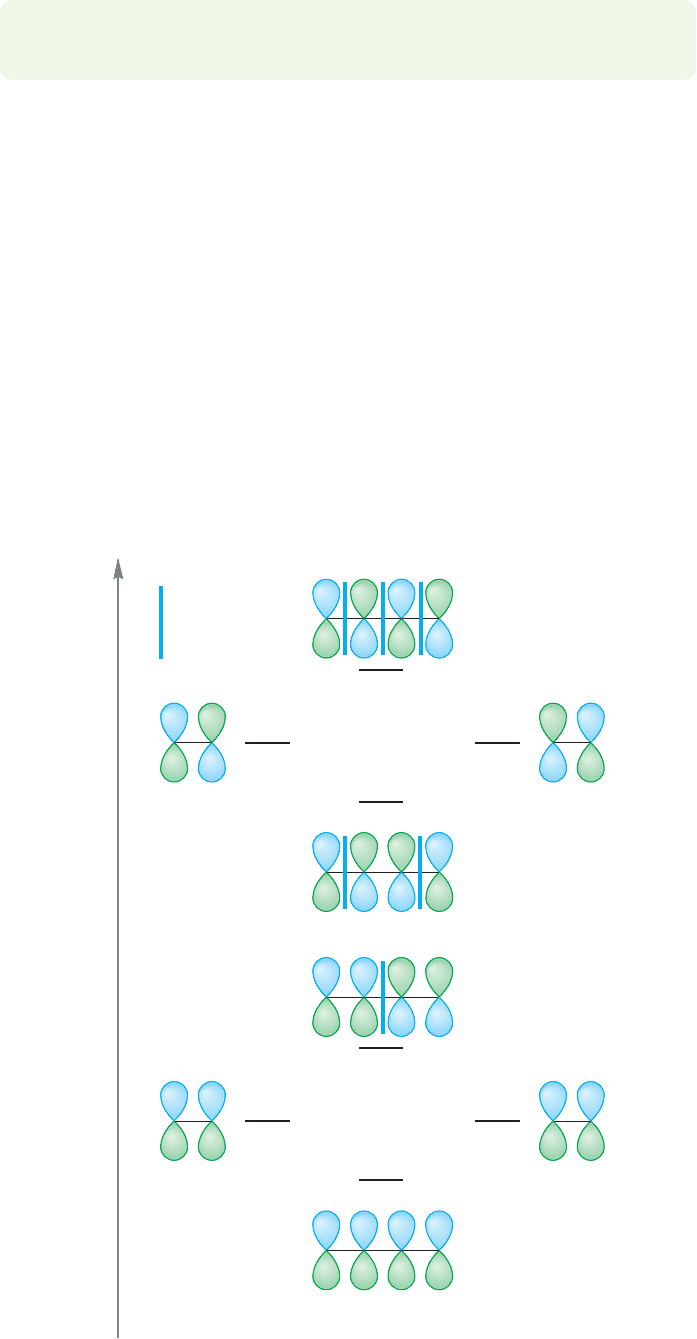
FIGURE 12.16 The schematic
formation of the π molecular orbitals
of 1,3-butadiene from the π
molecular orbitals of ethylene.
PROBLEM 12.9 For each of the resonance forms B, C, and D in Figure 12.15 indi-
cate what makes it less stable than the conventional structure, A.
Conjugation can be seen, perhaps even more clearly, if we examine the π molec-
ular orbitals of 1,3-butadiene. In butadiene, we have a system of four 2p orbitals on
four adjacent carbons. These four atomic 2p orbitals will overlap to produce four π
molecular orbitals.We can get the symmetries of the four molecular orbitals in a num-
ber of equivalent ways. It is always easiest to build up something new out of things
we know already, so it’s productive to construct the molecular orbitals of butadiene
out of two sets of the π molecular orbitals of ethylene, π and π*. One way to accom-
plish this is to allow the π molecular orbitals of ethylene to overlap side by side as
indicated in Figure 12.16. In producing the π molecular orbitals in this way, we have
made an important approximation. As often mentioned before, the closer in energy
two orbitals are, the stronger the stabilization resulting from their overlap. In prac-
tice, all orbital overlap except for the strongest interactions can be ignored, at least at
this level of theory.This approximation means that we need to look only at the result
of the ππand π* π* interactions and do not have to consider ππ*.
The symmetries of the butadiene molecular orbitals can be easily derived from
the symmetries of the starting ethylene orbitals (Fig. 12.16).
앐앐앐
Energy
Ethylene Ethylene
1,3-Butadiene
Φ
4
= π*– π*
Φ
3
= π*+ π*
Φ
2
= π–π
ππ
Φ
1
= π+ π
π* π*
Nodes
12.6 The Physical Consequences of Conjugation 521
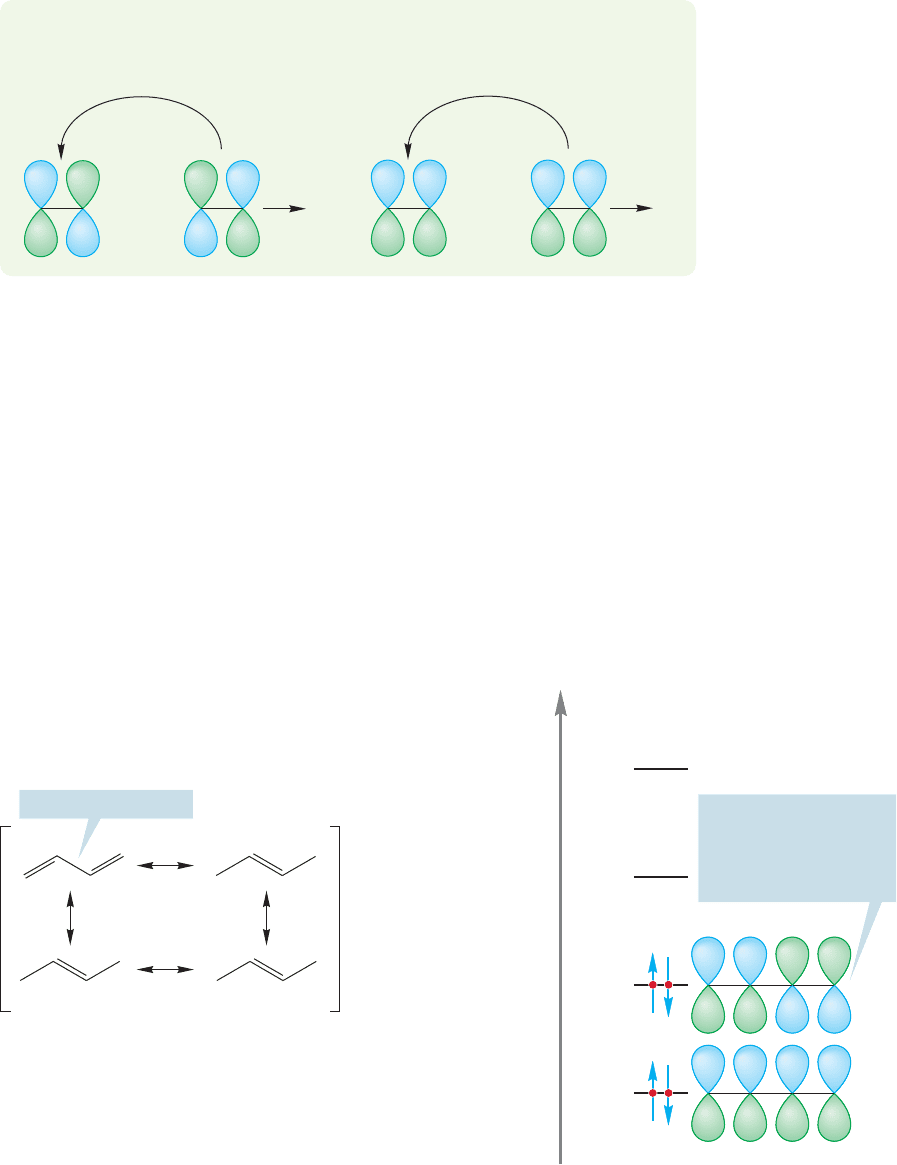
To order the butadiene molecular orbitals in terms of energy, count the new
nodes. Orbital Φ
1
in Figure 12.16 has no new nodes, Φ
2
has one, Φ
3
has two, and
Φ
4
has three. As the number of nodes in an orbital goes up, so does the energy of
the orbital (p. 32). Not all methods of construction will give the correct molecular
orbitals in the correct order of relative energy. So you must always go through the
procedure of counting nodes to do an energy ordering.
π* π*
+
–
?
ππ
+
–
?
PROBLEM 12.10 Construct the π molecular orbitals of 1,3-butadiene from those
of ethylene by putting one ethylene “inside” the other.
12.6 The Physical Consequences of Conjugation
In 1,3-butadiene, or any other conjugated diene, there are four electrons to accom-
modate in the π molecular orbital system.The lowest two bonding molecular orbitals
are occupied and the two higher energy, antibonding molecular orbitals are empty.
The HOMO for butadiene is Φ
2
(see Fig. 12.17), and to a first approximation Φ
2
will control the reactivity of the molecule. It is Φ
2
that looks most like the tradition-
al representation of 1,3-butadiene. In the resonance formulation it is the traditional
picture of 1,3-butadiene that is surely the lowest-energy resonance form, and thus
the best representation of the molecule. So we see the usual (and necessary) conver-
gence between the resonance and molecular orbital way of looking at structures. Each
method generates a picture of 1,3-butadiene that looks mostly like a molecule con-
taining a pair of double bonds (Fig.12.17).Both methods warn us, however,that there
are likely to be some consequences of conjugation in this molecule. This is shown
+
+
–
–
The best (most
stable) resonance
form is the best
representation
for 1,3-butadiene
..
..
.
.
Energy
Molecular orbital
picture
HOMO
Best resonance form
The HOMO will control
chemical reactivity; this
molecular orbital looks
most like the traditional
picture of 1,3-butadiene
FIGURE 12.17 Both the resonance view and the molecular
orbital view of 1,3-butadiene lead to the conclusion that
1,3-butadiene is a molecule containing two traditional double bonds.
However, both pictures make it clear that there will be some
consequences of conjugation between the two double bonds.
522 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
by the connection of Φ
1
in
the molecular orbital picture of Figure 12.16, and by the
existence of resonance forms in which there is double-bond
character between C(2) and C(3).
What are the effects of the overlap on the
structure of 1,3-butadiene? Carbon–carbon single bonds are
relatively long; for example,in butane the carbon–carbon bond
is 1.53 Å.The carbon–carbon double bond in 2-butene is only
1.32 Å, reflecting the presence of two bonds between the car-
bons,and the carbon–carbon triple bond in acetylene,1.20 Å,
is even shorter.To the extent that there is double-bond character between C(2) and C(3)
in 1,3-butadiene,the bond should be shorter than an sp
3
/sp
3
carbon–carbon single bond.
And so it is; the bond distance is only 1.47 Å (Fig. 12.18). It has been
argued that the shortening is indicative of conjugation between the two double bonds.
C(2)
O
C(3)
C(2)
O
C(3)
C(1)
O
C(2)
O
C(3)
O
C(4)
+
+
–
–
..
..
.
.
1.53
1.47
=
1.32 A
⬚
A
⬚
A
⬚
FIGURE 12.18 The central
carbon–carbon bond length in
1,3-butadiene lies between that for
a single bond and double bond.
WORKED PROBLEM 12.11 Life is never simple. There is a persuasive counterargu-
ment that attributes the shortening of the butadiene bond to factors
other than conjugation. Can you come up with another explanation? Hint for this
tough question: We have ignored the σ system in this argument. What kind of a σ
bond joins C(2) and C(3)? So what? See Problem 12.8.
ANSWER The two inner carbons of 1,3-butadiene are joined by an sp
2
/sp
2
σ bond, in
contrast to the sp
3
/sp
3
σ bonds of butane. An sp
2
orbital has more s character than an
sp
3
orbital, and bonds made from sp
2
/sp
2
overlap are stronger and shorter than those
made from sp
3
/sp
3
overlap.Accordingly, some of the shortening of the central σ bond
in 1,3-butadiene may be attributed to the σ system and not to π orbital overlap.
C(2)
O
C(3)
sp
2
/sp
2
σ bond 1.47 A
⬚
sp
3
/sp
3
σ bond 1.53 A
⬚
Now the question becomes: How strong is the overlap? What con-
sequences will this overlap have for the structure of conjugated dienes? Overlap
between 2p orbitals on adjacent atoms has profound consequences for the structure
of alkenes, for example, and the same might be true for dienes. It is the overlap
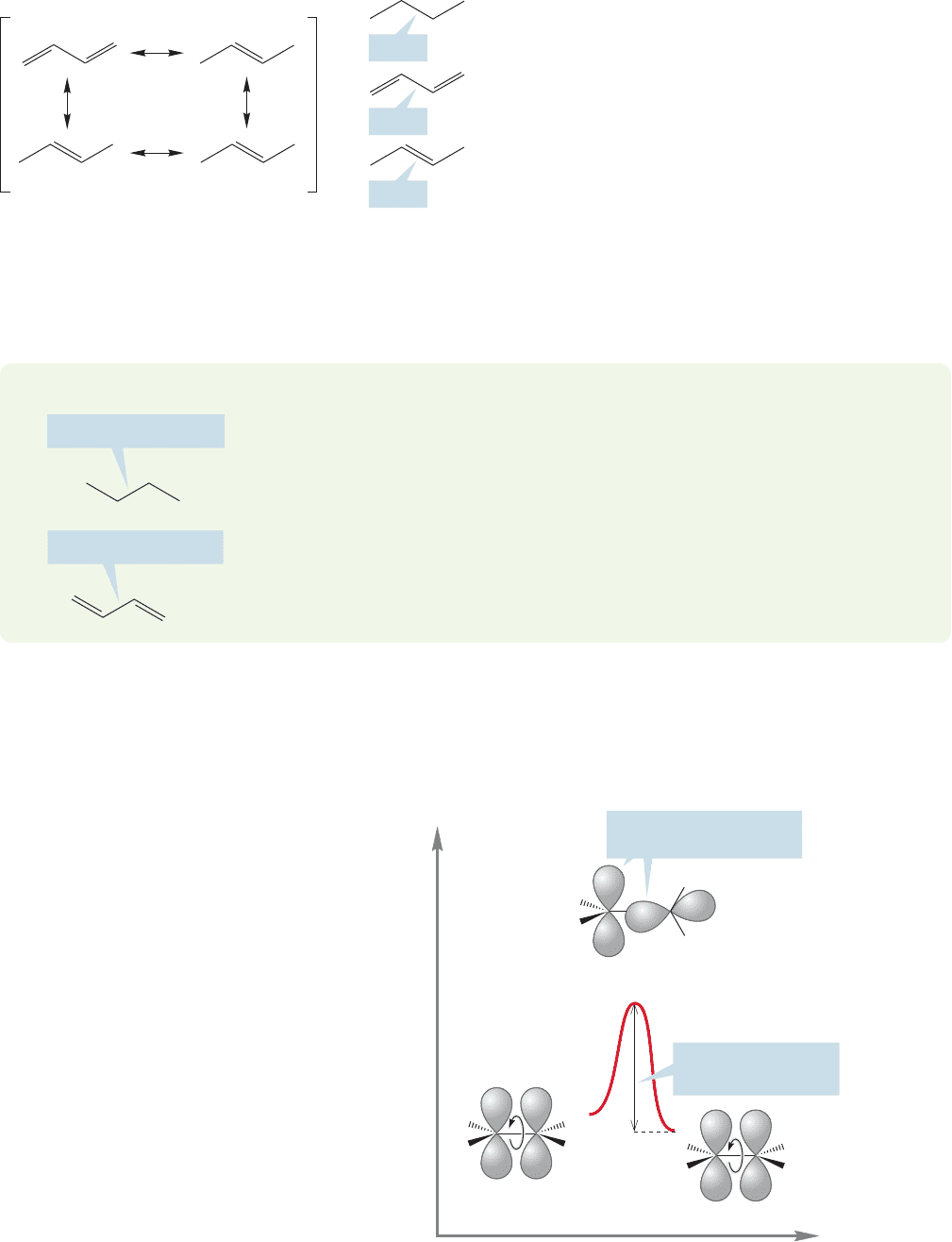
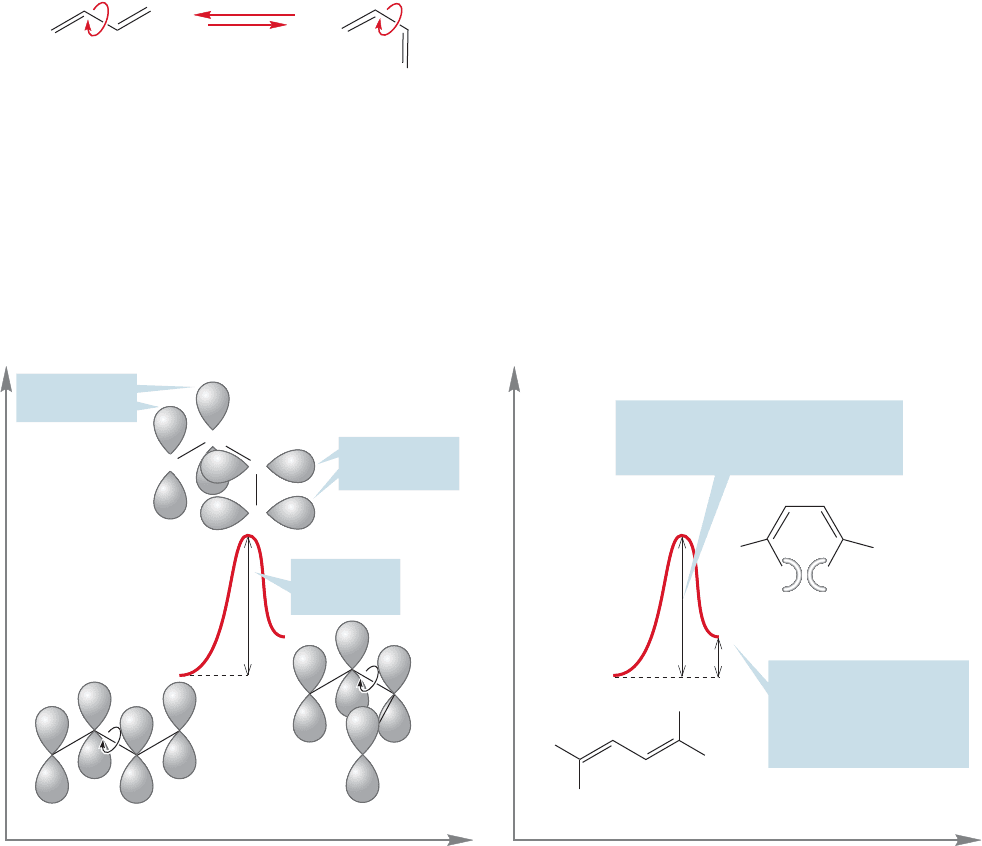
between 2p orbitals that makes cis and trans isomers of alkenes possible (Fig. 12.19).
C(2)
O
C(3)
FIGURE 12.19 In alkenes, rotation
about the carbon–carbon double
bond requires overcoming a barrier
of 66 kcal/mol.This barrier is too
high to be surmounted under most
conditions.
Energy
Reaction progress
H
H
R
R
cis Alkene
trans Alkene
H
H
R
R
H
R
Transition state for
cis
trans isomerization
No overlap at a rotational
angle of 90⬚
A 66-kcal/mol barrier
is too high to cross
H
R
→
→
12.6 The Physical Consequences of Conjugation 523
Might the same be true for conjugated dienes? Will overlap between the 2p orbitals
at the C(2) and C(3) positions create a large enough barrier so that isomers, called
the s-cis and s-trans conformations, can be isolated? The “s”nomenclature in dienes
stands for “single” because these are two conformations involving stereochemistry
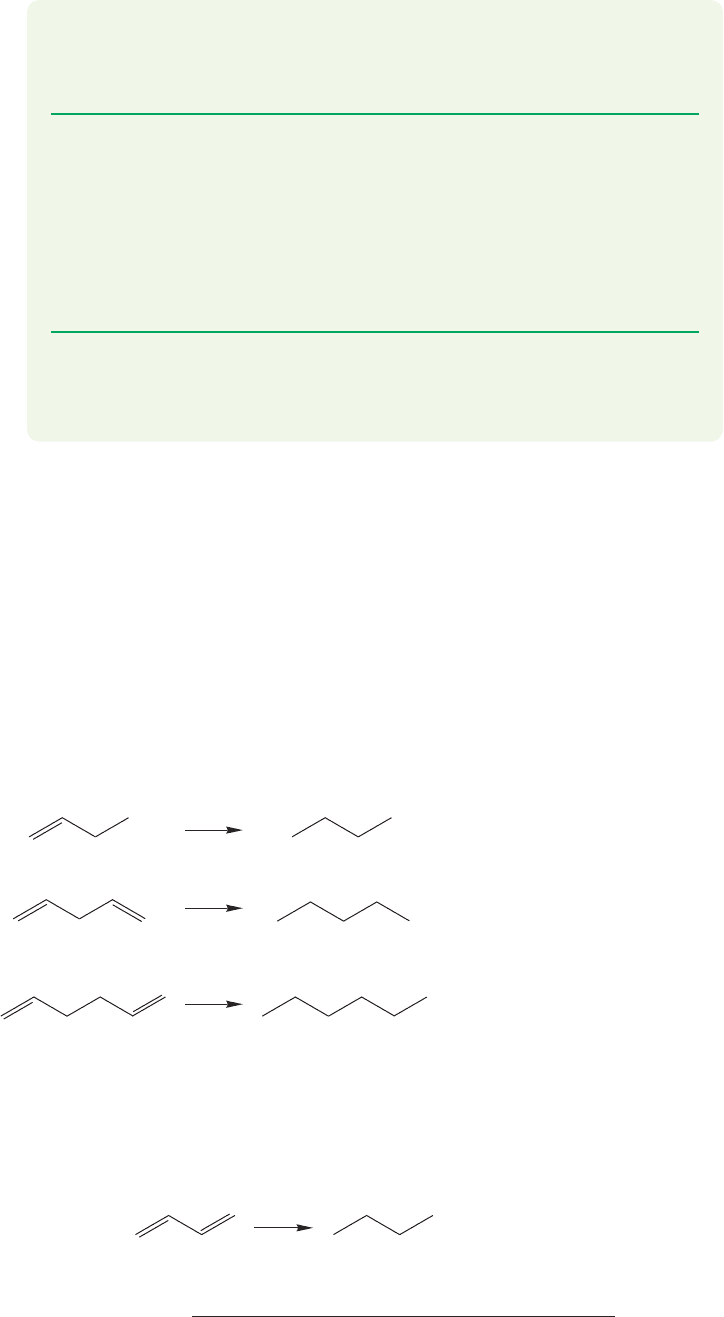
about the single bond of the diene (Fig. 12.20).
rotation
How easy is this
interconversion?
s-trans
conformation
s-cis
conformation
FIGURE 12.20 Interconversion of the
s-trans and s-cis conformations of
1,3-butadiene.
As we might guess, there is a barrier to the rotation, but it’s not very large.
Overlap of 2p orbitals between C(2) and C(3) is not nearly as important as that
between the 2p orbitals of the two adjacent carbons in ethylene. At the transition
state for the rotation of 1,3-butadiene about the central, bond, over-
lap between the 2p orbitals on C(2) and C(3) vanishes, but and
overlap is undisturbed (Fig. 12.21a).C(3)
O
C(4)
C(1)
O
C(2)
C(2)
O
C(3)
Reaction
p
ro
g
ress Reaction
p
ro
g
ress
Energy
C
2
C
1
C
3
C
4
How high is
this barrier ?
Good overlap
remains
Good overlap
remains
Transition state for
the s-trans–s-cis
interconversion
s -trans -1,3-Butadiene s -cis -1,3-Butadiene
Energy
The energy difference
between the s-trans
and s-cis conformations
of 1,3-butadiene is
2–3 kcal/mol
s -trans -1,3-Butadiene
s -cis -1,3-Butadiene
H
H
H
H
H H
H
H
(a) (b)
The height of the barrier separating
the s-trans and s-cis conformations
of 1,3-butadiene is 4–5 kcal/mol
FIGURE 12.21 The barrier for the interconversion of the s-trans and s-cis conformations of 1,3-butadiene.
The barrier to rotation in 1,3-butadiene turns out to be 4–5 kcal/mol, only a bit
larger than the barrier to rotation about the carbon–carbon bond in ethane. It is
the s-trans form that is favored at equilibrium, by 2–3 kcal/mol. There is a simple
steric effect favoring the s-trans conformation. In the s-cis conformation the two
inside hydrogens are quite close to each other (make a model!) and in the s-trans
conformation they are not. In order to make sense of these structures, we have to
watch out not only for the locations of the double bonds, but for the positions of
the hydrogens attached to them as well (Fig. 12.21b).
524 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
PROBLEM 12.12 Calculate the amount of s-cis-1,3-butadiene present at equilibrium
at 25 °C, assuming an energy difference between s-cis- and s-trans-1,3-butadiene
of 2.5 kcal/mol.
PROBLEM 12.13 There is another kind of rotation possible in 1,3-butadiene, that
about the or bonds. We might guess that the barrier to
this rotation would be little different from that for rotation in a typical double
bond but, as a former president of the United States once said, “that would be
wrong.” In 1,3-butadiene, it takes only 52 kcal/mol to do this rotation, some
14 kcal/mol less than the barrier of 66 kcal/mol for rotation in ethylene. Explain.
Hint: Look at the transition state for bond rotation in 1,3-butadiene.
PROBLEM 12.14 Design an experiment to determine whether rotation about the
bond in 1,3-butadiene is possible. Assume you have the ability to
make any labeled molecules you need.
C(1)
O
C(2)
C(1)
O
C(2)
C(3)
O
C(4)C(1)
O
C(2)
As both the resonance and molecular orbital pictures indicate, there is 2p/2p
overlap between C(2) and C(3), but this overlap does not dominate the structure of
1,3-butadiene the way 2p/2p overlap does for alkenes. We can get another idea of
the magnitude of this interaction from measuring the heats of hydrogenation (p.413)
of a series of dienes.
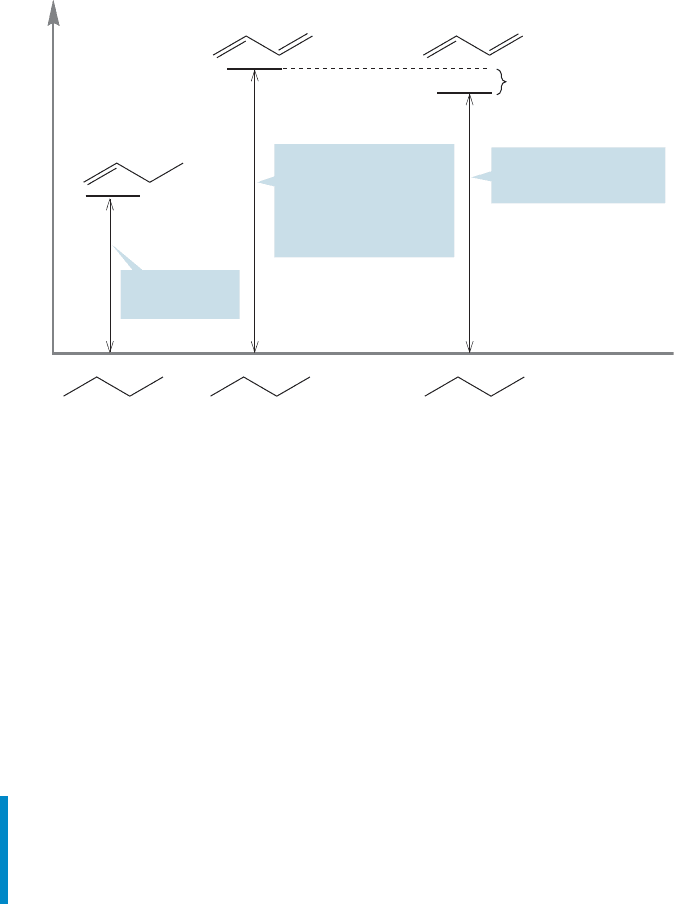
We begin with 1-butene. When it is hydrogenated to butane, the reaction is
exothermic by 30.3 kcal/mol (Fig. 12.22). A reasonable assumption would be that a
molecule containing two double bonds would be hydrogenated in a reaction twice
as exothermic as the hydrogenation of 1-butene. And this idea is just right; the heat
of hydrogenation is 60.8 kcal/mol for 1,4-pentadiene and 60.5 kcal/mol for
1,5-hexadiene.
H = –30.3 kcal/mol
H = –60.8 kcal/mol
H = –60.5 kcal/mol
Average H = –60.65 kcal/mol
2 –30.3 kcal/mol = –60.6 kcal/mol
H
2
PtO
2
Predict
Measured
Measured
Find
H
2
PtO
2
H
2
PtO
2
1,5-Hexadiene
1,4-Pentadiene
1-Butene
FIGURE 12.22 Hydrogenation of
1-butene is exothermic by
30.3 kcal/mol. Hydrogenation of
unconjugated dienes is exothermic
by almost exactly twice the heat of
hydrogenation of 1-butene.
Both of our sample dienes in Figure 12.22 were unconjugated. Now let’s look
at the heat of hydrogenation of the conjugated diene, 1,3-butadiene; is only
57.1 kcal/mol (Fig. 12.23).
¢H
⌬H = – 57.1 kcal/mol
Average ⌬H for unconjugated dienes = –60.7 kcal/mol
1,3-Butadiene = –57.1 kcal/mol
Difference = 3.6 kcal/mol
H
2
PtO
2
FIGURE 12.23 The heat of
hydrogenation of 1,3-butadiene is
57.1 kcal/mol, smaller than expected
by about 3.6 kcal/mol.
12.7 Molecular Orbitals and Ultraviolet Spectroscopy 525
The heat of hydrogenation of the conjugated diene is about 3.6 kcal/mol lower
than we would expect by analogy to the unconjugated dienes in Figure 12.22. This
amount of energy can be attributed to the energy-lowering effects of conjugation.
The situation is summarized in Figure 12.24.
⌬H
H
2
⌬H = – 30.3
kcal/mol
⌬H = – 57.1 kcal/mol
experimental value
⌬H = – 60.7 kcal/mol
Estimated value from
unconjugated dienes
or twice the value
for 1-butene
About 3.6 kcal/mol
stabilization owing
to conjugation
H
2
2 H
2
2 H
2
FIGURE 12.24 A summary of the
heat of hydrogenation data.
So now we have two measures of the energetic value of conjugation in
1,3-butadiene, and they are remarkably close to each other. The first is the height
of the barrier to rotation about the bond (4–5 kcal/mol), and the sec-
ond is the lowering of the heat of hydrogenation of a conjugated diene relative to
that of an unconjugated diene ( 3.6 kcal/mol).
Two conjugated double bonds are not utterly unconnected; the whole is some-
what different from the sum of the parts. Conjugation, the orbital overlap between
middle carbons in a conjugated diene, results in a shortening of the single bond and
in lower heats of hydrogenation for conjugated dienes than for unconjugated systems.
Now we move on to the consequences of further conjugation, and to an examination
of the reactivity of these molecules. Here, too, we will see that conjugation influences
the physical properties and chemical reactivity of these molecules.
'
C(2)
O
C(3)
Summary
Overlap between middle carbons of a conjugated diene has structural consequences.
The central sigma bond is slightly shorter than a normal carbon–carbon single bond.
The magnitude of the conjugative stabilization is small, about 4 kcal/mol.
12.7 Molecular Orbitals and Ultraviolet Spectroscopy
In the early days of organic chemistry, there were two extraordinarily difficult gen-
eral problems. One was to separate mixtures into pure compounds, and the other
was to determine the structures of the compounds once pure samples were obtained.
How simple these problems seem! Yet, much of the mechanistic and synthetic organ-
ic chemistry of the last decades would have been quite impossible without the
progress in solving these two old difficulties. We will have little to do with separa-
tion techniques in this book (your laboratory will take another approach), but we
must certainly spend some time here on structure determination.
526 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
About 70 years ago, structure determination was an entire branch of chem-
istry. People spent whole careers working out the structures of complicated
molecules by running chemical reactions. The products of many reactions of an
unknown compound were analyzed and inferences were made regarding the
structure of the unknown. This task was time-consuming and extraordinarily
demanding of experimental technique, intellectual rigor, and the development,
over years of work, of an intuitive sense of what was right, of what a given exper-
iment might mean. In a way, this work had all the satisfactions and charms of
handwork, of a craft. Nowadays, the work of years can usually be done in hours,
and chemists attack structural problems that would have been beyond any chem-
ical determination. Spectroscopy has made the difference. First came the devel-
opment of ultraviolet/visible (UV/vis) spectroscopy. We will deal with that
subject in this chapter. Then followed infrared (IR) spectroscopy and finally
nuclear magnetic resonance (NMR). The power of NMR is so great that this
technique has largely eclipsed all other forms of structure determination. We
introduced NMR spectroscopy in Section 2.14, and we will devote much of
Chapter 15 to this subject.
The development of spectroscopy certainly brought progress, and no reasonable
person would want to turn back the clock to the days of hand-crafted structure deter-
mination. Yet, one can sympathize with the feeling that there has been some loss
with the progress. We are somehow not as close to the molecules as we once were,
even though we know much more about them. There is a psychological distance
involved here. One tends to believe what spectrometers say and to read too much
into the data. In chemistry, nothing is more important than honing one’s critical
sense.It is somehow easier to ignore some small, but ultimately vital glitch on a piece
of chart paper than to ignore the results of an experiment gone in an unexpected
direction. The former somehow melts into the noise of the baseline, while the lat-
ter teases the imagination.
One can also sympathize with those practitioners of the art of structure
determination who saw the future when the first NMR machine was delivered,
and realized that for them, “NMR” meant “No More Research.” They were
right.
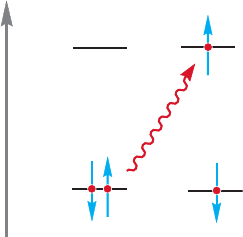
Ultraviolet/visible spectroscopy is called electronic spectroscopy because it deals
with the absorption of energy by electrons in molecules. The energy absorbed (¢E)
is used to promote an electron from one orbital to a higher-energy orbital (Fig. 12.25).
The magnitude of ¢E is proportional to the frequency of the light, ν, and inversely
proportional to wavelength of the light, λ, as shown by
¢E hν hc/λ (12.1)
where h is Planck’s constant and c is the speed of light. Because electrons are restrict-
ed to orbitals of specific energy, ¢E is also quantized and may have only certain values.
The distinction between ultraviolet and visible spectroscopy is an artificial one and
is based solely on the fact that the detectors built into our bodies, our eyes, are sensi-
tive only to radiation of wavelength 400–800 nm (nm nanometers; 1 nm 10
9
m),
which we call the visible spectrum. Spectrometers are less limited in this respect than
we are, and UV spectrometers easily detect radiation from 200–400 nm, as well as the
entire visible range and beyond. At higher energy, below 200 nm, the atmosphere begins
to absorb strongly, and spectroscopy in this range is more difficult, although by no
means impossible.
Energy
LUMO
HOMO
⌬E =h
ν
FIGURE 12.25 Absorption of UV
light promotes an electron from the
HOMO to the LUMO.
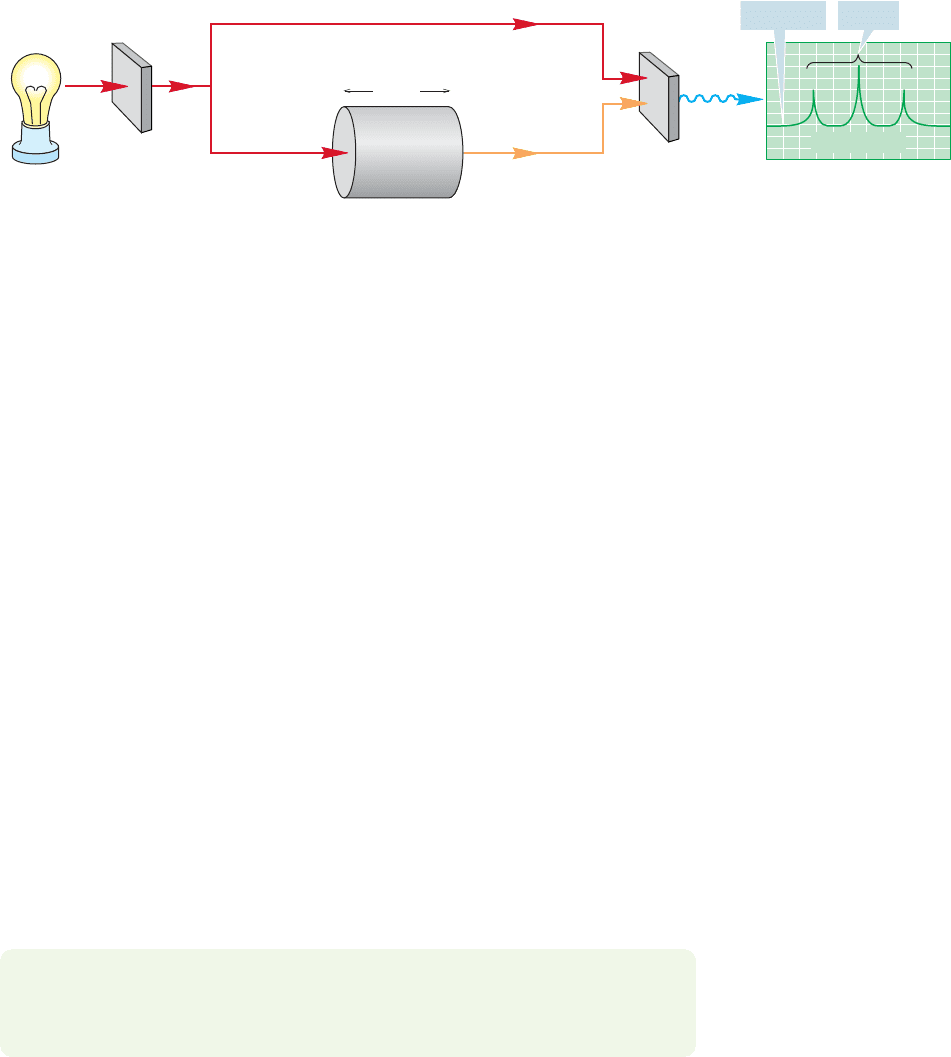
12.7 Molecular Orbitals and Ultraviolet Spectroscopy 527
A schematic spectrometer is shown in Figure 12.26. It consists of a source of
radiation, a monochromator that is capable of scanning the range of light emitted
by the source, a sample cell, and a detector.
Mono-
chromator
Sample cell
Reference beam
Incident beam
Source of
radiation
Transmitted beam
Spectrum
(drawn by recorder)
Detector
Intensity
Wavelength
Base line Peaks
b(cm)
FIGURE 12.26 A schematic picture of a UV/vis spectrometer.
Generally, the spectrometer determines A, the absorbance, and plots A versus
wavelength (λ in nm). The absorbance is calculated according to the Beer–
Lambert law
(12.2)
where the intensity of the light entering the sample cell is I
0
, the intensity of the
light exiting the sample cell is I, the concentration in moles per liter is c (not to
be confused with the c for speed of light), the length of the sample cell in cen-
timeters is b, and ε is a proportionality constant known as the extinction
coefficient. Every compound has a characteristic ε, which depends not only on
A, b, and c as shown in Equation 12.2, but also on wavelength, solvent, and
temperature.
It is important to have a feel for the magnitudes of the energies involved in
spectroscopy. Equation 12.1 shows the familiar relationship between energy, Planck’s
constant
h, and the frequency of light. Because ν c/λ, and because we are
interested in the energy per mole, we obtain
E Nhc/λ (12.3)
where N 6.02 10
23
molecules/mol, h 1.583 10
37
and
c 3.00 nm/s. The quantity N
hc is equal to 28.6 (nm kcal/mol).
#
10
3
10
17
kcal
#
s/molecule,
A = log I
0
>I = εbc
PROBLEM 12.15 The wavelength of light used in UV/vis spectroscopy ranges from
200 nm to 800 nm. Calculate the energy (in kcal/mol) corresponding to these two
wavelengths.
For a given molecule there will be many possible UV (or vis) transitions,because there
are many different energies of electrons even in a simple molecule. For ethylene there
are several possible absorptions, as σ or π electrons are promoted to antibonding σ* or
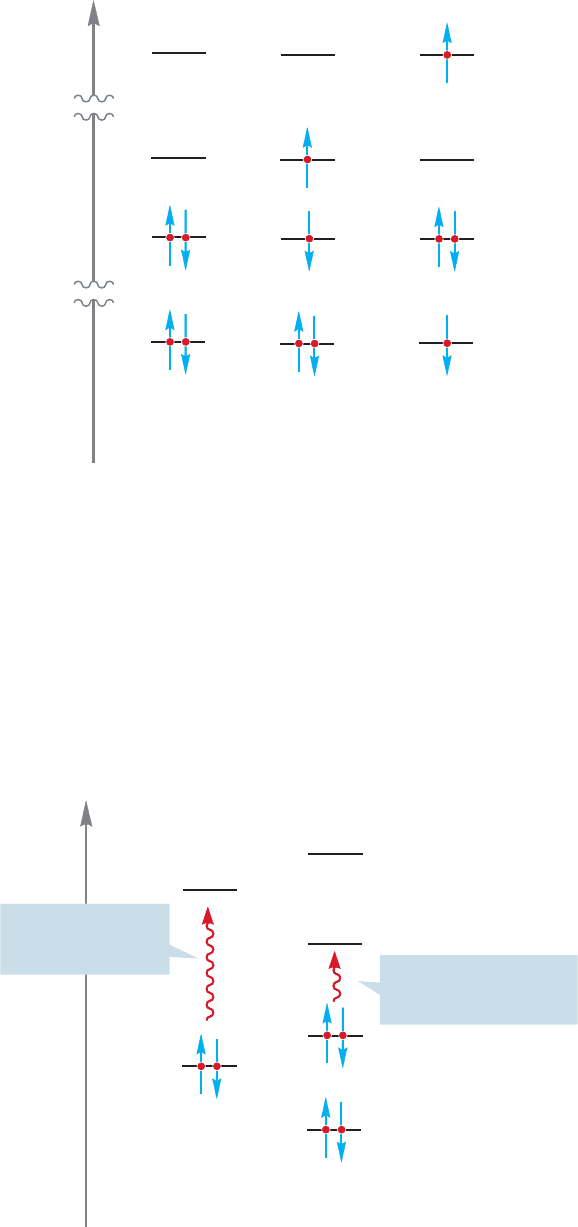
528 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
Energy
π* π* π*
ππ π
σσ σ
σ* σ* σ*
Electronic
configuration of
ethylene before
irradiation
Ethylene after a
π→π* transition
Ethylene after a
σ→σ* transition
FIGURE 12.27 A ππ* and σσ*
transition for ethylene.
UU
π* orbitals. Figure 12.27 ignores the carbon–hydrogen bonds and shows only σσ*
and ππ* transitions.There are other possible absorptions,even without including the
carbon–hydrogen σ and σ* orbitals: σπ* and πσ* are two examples. Such tran-
sitions between orbitals of different symmetry are usually relatively weak, however.
UU
U
U
The starting point and final destination of the electron are used to denote the tran-
sition; hence σσ*,σπ*,ππ*,and πσ* in this case.The energies, and there-
fore the wavelengths of light involved, depend on the energy separating the orbitals. For
ethylene, even the ππ* separation is great enough so that detection is difficult with
common spectrometers. The ππ* absorption occurs at about 165 nm, which corre-
sponds to 173 kcal/mol, a high energy indeed.Little change is induced by alkyl substitu-
tion, and neither simple alkenes nor unconjugated dienes absorb strongly above 200 nm.
When a diene is conjugated, however, substantial changes occur in orbital ener-
gies, and therefore in the possible UV transitions. In general, the lowest energy
(longest wavelength λ) transition possible for a polyene, a molecule containing mul-
tiple double bonds, will be from the HOMO to the LUMO. In ethylene or butadi-
ene, this transition is ππ*. Figure 12.28 shows the relative energies of the π
U
U
U
UUUU
Energy
π
π*
π
Orbitals of
ethylene
HOMO
LUMO
HOMO
LUMO
π Orbitals of
1,3-butadiene
Φ
1
Φ
2
Φ
3
Φ
4
π→π* For 1,3-butadiene
is lower energy
(longer wavelength)
π→π* For ethylene
is higher energy
(shorter wavelength)
FIGURE 12.28 The HOMO–LUMO
transition in ethylene is higher in
energy (at shorter wavelength) than
the HOMO–LUMO transition in
1,3-butadiene.
