Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

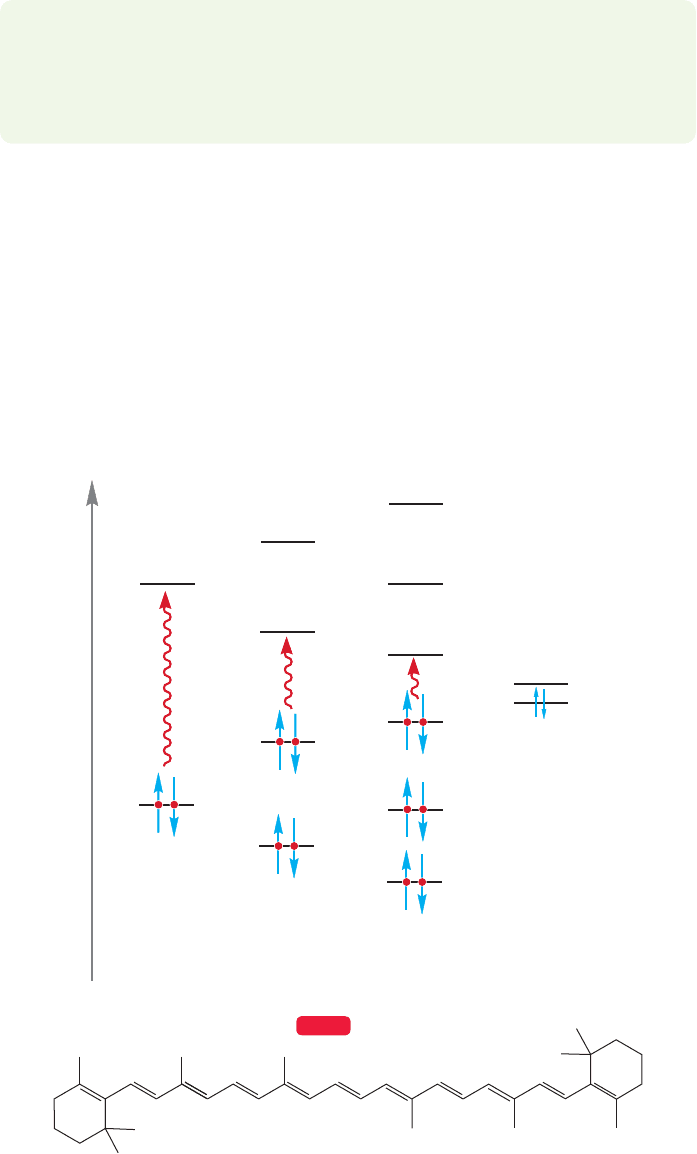
12.7 Molecular Orbitals and Ultraviolet Spectroscopy 529
molecular orbitals of ethylene and 1,3-butadiene. You can see that there is a much
lower energy ππ* (HOMO–LUMO) transition possible in the conjugated
molecule 1,3-butadiene than there is in ethylene. Spectroscopy bears this out, and
1,3-butadiene absorbs at 217 nm, which is a much longer wavelength (lower energy)
than the absorption in ethylene.
U
Further conjugation does two things. First, it reduces the energy gap between
the HOMO and LUMO, and this pushes the position of the lowest energy absorp-
tion to a longer wavelength (lower energy). Eventually, the HOMO–LUMO gap
becomes small enough so that absorption enters the visible range (400–700 nm),
and as a result we perceive highly conjugated polyenes as colored. The first
polyene to absorb in the visible range appears yellow, because it is violet light
(400–420 nm) that is first absorbed as the HOMO–LUMO gap shrinks.
Remember that perceived color is a result of reflected light, the wavelengths of
light that are not absorbed. β-Carotene (Fig. 12.29), an important precursor to
vitamin A, absorbs blue light at 453 and 483 nm, and appears orange.
WORKED PROBLEM 12.16 How much energy is contained in light of wavelength
217 nm?
ANSWER E Nhc/λ and the quantity Nhc 28.6 10
3
(p. 527). So,
E (28.6 10
3
)/217 132 kcal/mol at 217 nm.
WEB 3D
Energy
π Orbitals of
ethylene
HOMO
LUMO
LUMO
LUMO
LUMO
HOMO
HOMO
HOMO
π Orbitals of
1,3-butadiene
π Orbitals of
1,3,5-hexatriene
HOMO and LUMO
orbitals of
β-carotene
-Carotene
λ
max
= 453 and 483 nm
FIGURE 12.29 As conjugation
increases, the HOMO–LUMO gap
decreases and the position of the
ππ* absorption shifts to longer
wavelength (lower energy).
U
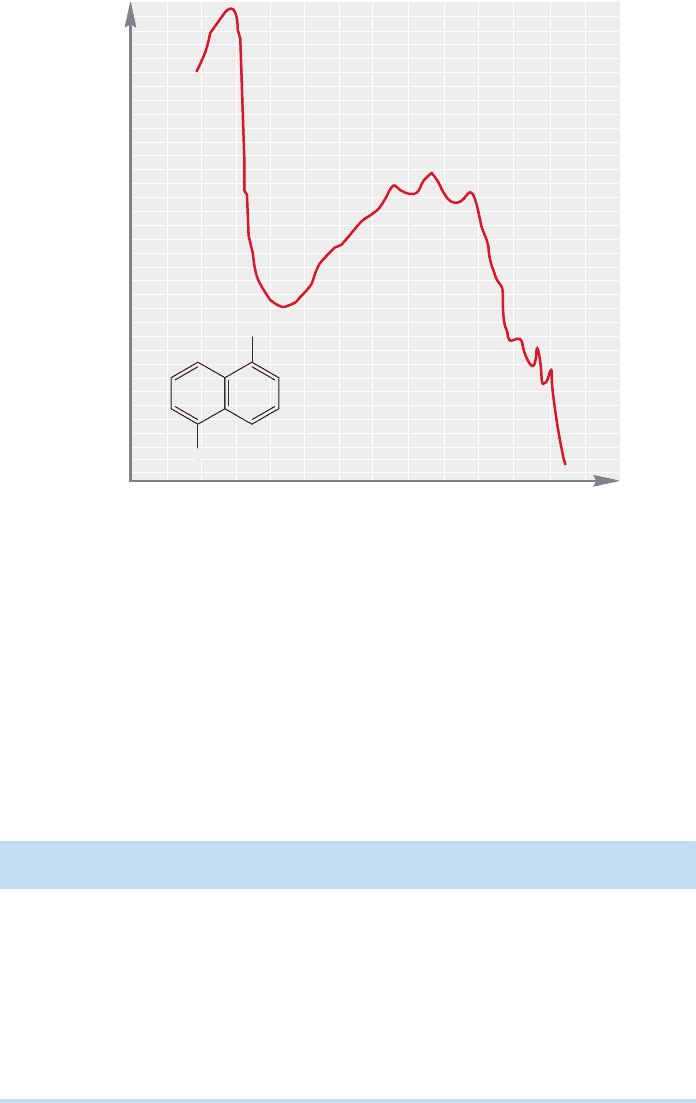
530 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
The second impact of increased conjugation is that the number of possible UV
transitions increases and,therefore,the spectra become complex. Figure 12.30 shows
the spectrum of one moderately complicated conjugated molecule,which surely will
resist a simple analysis, and which would be most difficult to predict in detail.
However, many important molecules contain simple diene or triene systems, either
acyclic or contained in rings, and UV spectroscopy remains a useful tool for
structure determination in such molecules.
5.0
4.5
4.0
3.5
3.0
2.5
2.0
200 220 240 280 300 400 320 340
log ε
Wavelen
g
th (nm)
Solvent:
isooctane
1,5-Dimethyl-
naphthalene
CH
3
CH
3
FIGURE 12.30 A complicated UV
spectrum.
The position of longest wavelength absorption (λ
max
) depends in a predictable way
on the substitution pattern of these simple molecules. A set of empirical correlations
was collated by Robert Burns Woodward (1917–1979) and was known initially as
“Woodward’s rules”and now sometimes as “Woodward’s first rules.”These rules were
extended by Louis Fieser (1899–1977) and Mary Fieser (1909–1997) to include
cyclic dienes in the polycyclic compounds called steroids. Table 12.2 summarizes
Woodward’s and the Fiesers’rules.Woodward’s very different second set of rules will
appear in Chapter 20.
TABLE 12.2 Woodward’s and Fieser’s Rules for Ultraviolet Spectra of Dienes
Woodward’s Rules for Fieser’s Rules for
Acyclic Dienes (nm) Steroid Dienes (nm)
Base diene value 217 Base diene with both double
bonds in the same ring 253
Each added alkyl group 5 Base diene with double bonds
in different rings 214
Each double-bond exocyclic Each additional conjugated
in a six-membered ring 5 double bond 30
Each alkyl substituent 5
Each exocyclic double bond 5
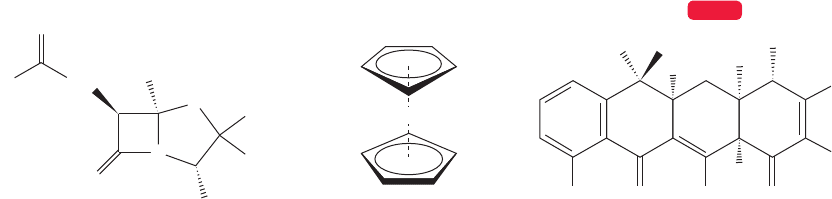
12.7 Molecular Orbitals and Ultraviolet Spectroscopy 531
R. B.Woodward was acknowledged as the greatest American organic chemist,
and was certainly one of the greatest chemists of all time.There is remarkable agree-
ment on the subjective evaluation of Woodward’s preeminence among organic
chemists, and it’s worth taking a little time to see what set this man apart from
his merely excellent colleagues. First of all, he was devoted to the subject and
worked unbelievably hard at it. Although the main themes of his professional life
were the elucidation of structure and the synthesis of molecules of ever more
breathtaking complexity, his work is also notable for the attention he paid to
mechanistic detail, and to the broad implications of the work. Working hard is not
a matter of mere hours, but of maintaining focus as well. Woodward was able to
concentrate and either had or developed a prodigious memory for minutiae. In
another person that might amount to mere obsession, but Woodward was able to
extract from the sea of detail the important facts that allowed him time and again
to turn his work from the specific to the general. Early on he was able to propose
correct structures for such disparate molecules as penicillin, tetracycline, and fer-
rocene (Fig. 12.31).
WEB 3D
COOH
CONH
2
N(CH
3
)
2
S
N
NH
O
O
Penicillin V
Ferrocene Tetrac
y
cline
O
O
H
H
OH
OH
OH
OH
OH
H
CH
3
CH
3
H
3
C
PhOCH
2
Fe
FIGURE 12.31 Some complex molecules whose structures were proposed by Woodward and
his co-workers.
Woodward was able to pull obscure facts out of remote chemical hats and apply
them to the matter at hand in a way that often transformed the very nature of the
problem. The molecules he considered worthy of synthesis grew in complexity
throughout his career, but it was more than the difficulty of the target that made his
work so universally impressive.Woodward’s work led places—it had “legs.”For exam-
ple, Woodward, primarily a synthetic chemist, made the observations [with Roald
Hoffmann (b. 1937)] that led to the development and exploitation of what is arguably
the most important theoretical construct of recent times. Hoffmann and Kenichi
Fukui (1918–1998) of Kyoto University received the Nobel prize for this work in
1981. Many believe Woodward would have shared this Nobel prize had he lived (it
would have been his second; his first was awarded in 1965 for “his outstanding
achievements in the art of organic synthesis”). Some of the molecules synthesized
by Woodward and his co-workers are shown in Figure 12.32. Woodward said that
the synthesis of quinine was conceived when he was 12 years old.
Should you memorize Woodward’s rules? Frankly, we think not—although there
are those who strongly disagree, and some of these people write examination ques-
tions.These rules were an important part of structure determination but are restricted
in utility to relatively simple polyenes. You should know they exist, know what
systems they apply to, and look them up when you need them. If you wind up in
steroid research, you’ll learn them.
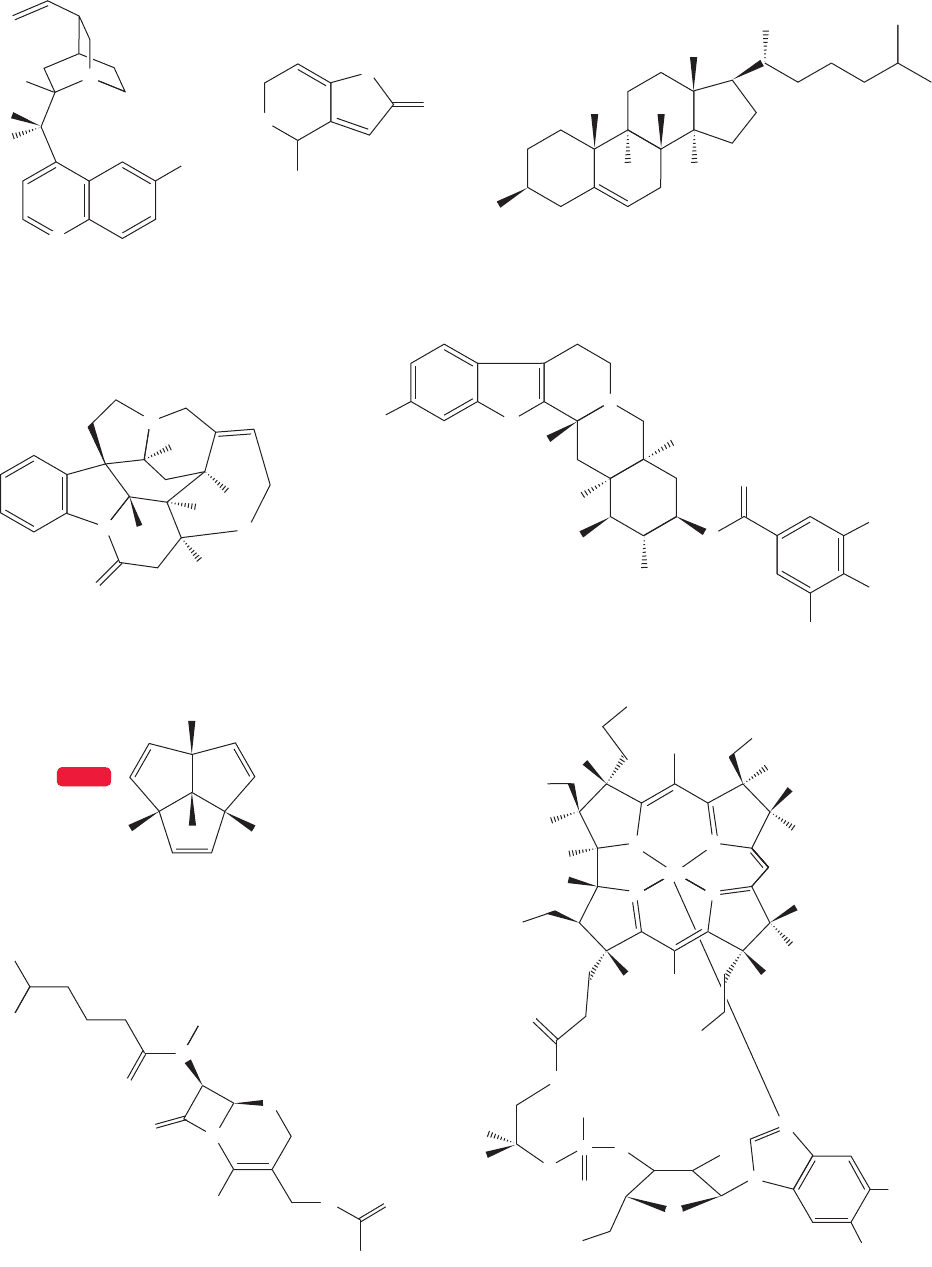
532 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
WEB 3D
Strychnine, 1954
Vitamin B
12
with A. Eschenmoser and a cast of thousands, 1972
Triquinacene, 1964
Reserpine, 1956
O
OH
HO
O
O
O
H
CH
3
OCH
3
OCH
3
OCH
3
OCH
3
CH
3
OOC
CH
3
O
N
H
N
H
H
H
H
H
N
N
O
Quinine,
with W. von E. Doering, 1944
Patulin, 1950 Cholesterol, 1951
H
H
H
H
H
H
H
H
H
H
HO
OCH
3
H
3
C
H
3
C
O
O
N
N
H
O
N
N
N
O
P
O
OH
O
O
N
NH
HO
H
3
C
H
3
C
H
3
C
CH
3
CH
3
H
H
H
H
H
CH
3
CH
3
CH
2
CH
2
CONH
2
N
O
H
O
COO
O
COOH
–
H
3
N
+
Co
CONH
2
CONH
2
CH
3
CH
3
CH
3
CH
3
H
2
NOC
H
2
NOC
H
2
NOC
+
–
N
N
CH
3
O
S
N
O
Cephalosporin C, 1966
FIGURE 12.32 Some molecules synthesized by the Woodward group.
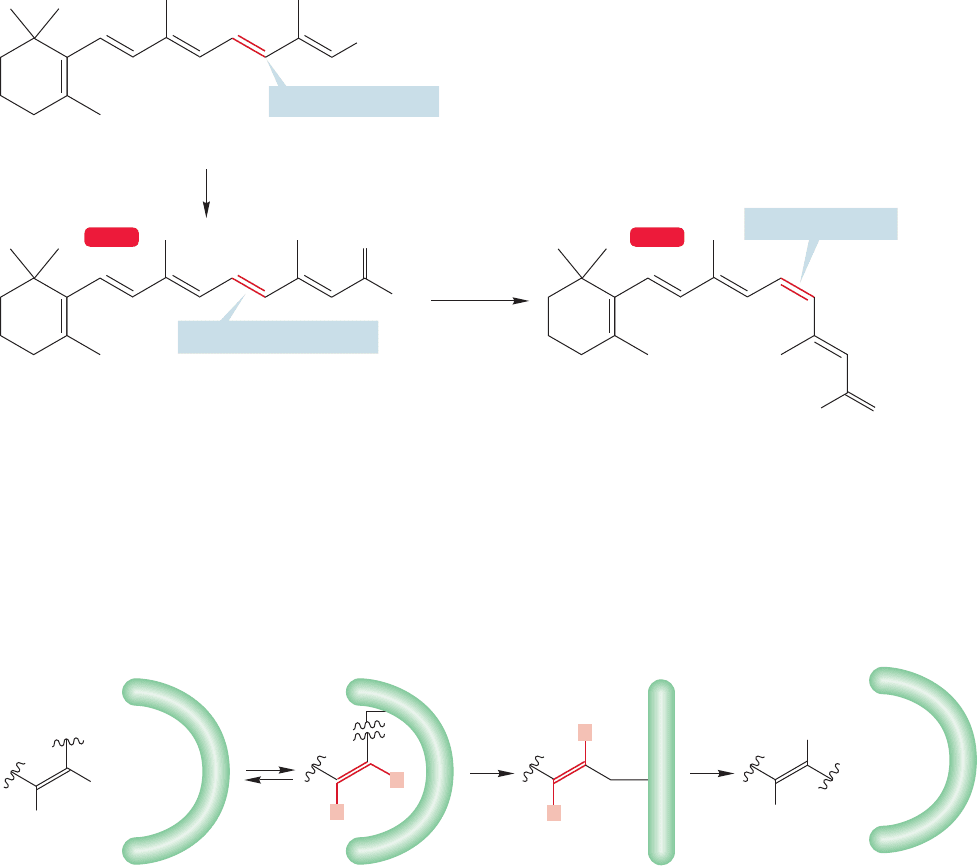
12.8 Polyenes and Vision 533
12.8 Polyenes and Vision
When we hear or read the word “spectroscopy,”we tend to imagine rooms full of expen-
sive machines measuring away. It’s quite an inhuman vision. Yet we are spectrometers
ourselves. All of our sensory experiences, most obviously vision, are examples of spectro-
scopic measurements. Those machines in our imaginations are mere extensions of our
biological,in-house equipment.When you see a red apple among a basket of green ones,
and decide that it is the ripe red one you will eat,you are acting on a spectroscopic meas-
urement. Let’s examine vision a little bit here, because it depends on polyene chemistry.
Many polyenes are important in biological processes, and as we might imagine,
polyenes that absorb in the visible spectra are important in human vision. Vitamin A
is trans-retinol,for example (Fig. 12.33).We have two enzymes,one of which, retinol
dehydrogenase, oxidizes trans-retinol to trans-retinal and another, retinal isomerase,
which isomerizes one double bond of trans-retinal to produce cis-retinal.
Vitamin A,
trans-retinol
trans -Retinal
cis-Retinal
CH
2
OH
retinal
A trans double bond
A cis double bond
Still a trans double bond
isomerase
retinol
dehydrogenase
H
O
H
O
WEB 3D WEB 3D
FIGURE 12.33 Vitamin A (trans-
retinol) is oxidized to trans-retinal
and then enzymatically isomerized to
cis-retinal.
cis-Retinal (but not trans-retinal) has the proper shape to react with a protein
called opsin to form rhodopsin (Fig. 12.34). Rhodopsin absorbs strongly in the visi-
ble spectrum, which results in isomerization of the cis double bond back to trans.
This absorption of visible light changes the shape of the molecule severely, destroy-
ing the crucial fit of molecule and protein. The attachment point is exposed, and
the retinal is detached from the protein. As this process occurs, a nerve impulse is
generated and perceived by our brains as light.The chemistry involved in the attach-
ment and detachment of retinal and opsin is covered in Chapter 16.
cis -Retinal
Opsin
visible
light
+
+
H
H
H
H
Rhodopsin
H
H
Opsin
H
H
trans -Retinal
FIGURE 12.34 cis-Retinal has the correct shape to react with opsin to form rhodopsin.The squiggly lines indicate
the rest of the molecule.
534 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
12.9 The Chemical Consequences of Conjugation:
Addition Reactions of Conjugated Dienes
Now we move on to reactions (at last!). Conjugation affects not only the physi-
cal properties of molecules but their reactions as well. Although the basic chem-
istry of conjugated dienes does resemble that of isolated alkenes,there are detailed,
but important differences introduced by the presence of the pair of connected
double bonds.
12.9a Addition of HBr and HCl When hydrogen chloride adds to
1,3-butadiene at 78 °C, two major products of hydrohalogenation are formed,
3-chloro-1-butene and trans-1-chloro-2-butene (Fig. 12.35). There is a very small
amount of cis-1-chloro-2-butene also formed. The main product is produced
through a pathway of 1,2-addition and the minor products by a 1,4-addition.
H
1,2-Addition 1,4-Addition
–78 ⬚C
+
..
..
..
Cl
..
..
..
2
2
3
4
1
1
trans -1-Chloro-2-buten
e
(24%)
3-Chloro-1-butene
(75.5%)
H
Cl
..
..
..
1
4
H
Cl
FIGURE 12.35 Hydrohalogenation
of 1,3-butadiene with HCl
produces the products resulting
from a 1,2- and a 1,4-addition (red
numbers not IUPAC).
BOMBYKOL
receptor for bombykol also recognizes the other stereoiso-
mers, but the proper Z, E (cis, trans) isomer is far
more active than the Z, Z (cis, cis) or E, E (trans, trans)
versions.
As you might imagine, if bombykol is to function
effectively as an attractant in the open air, reception must
be very efficient; a little bombykol must go a long way if
the silkworm moths are to have a satisfactory love life.
In fact this is so; the male is staggeringly efficient at
detecting this molecule. It is effective in doses as small
as 1 10
12
μg/mL.
For a nice introduction to pheromones, see William
C. Agosta, Chemical Communication, Scientific American
Library, New York, 1992.
Most insects (and other animals,
including humans) “talk” to each
other by releasing chemicals that
carry meaning. Such molecules
are called “pheromones.” Some
are sexual attractants, others are trail markers, and still
others carry an alarm signal. Here is bombykol, a simple
cis, trans dienol that serves as the female silkworm moth’s
attractant for the male. The female of the species, Bombyx
mori, releases bombykol from two glands located at her
abdomen. The structure of bombykol was worked out by
the German chemist Adolph Butenandt over a long
period, ending in 1956. He collected 6.4 mg of the pure
pheromone from 500,000 moth abdomens. Such work was
extraordinarily difficult in those days, as he did not have the
advantage of modern spectroscopy to help with structure
determination.
Eventually, the correct structure was determined and
the molecule was made independently. The male moth’s
1,2-Hydrohalogenation of dienes
1,4-Hydrohalogenation of dienes
CH
3
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
OH
12.9 The Chemical Consequences of Conjugation 535
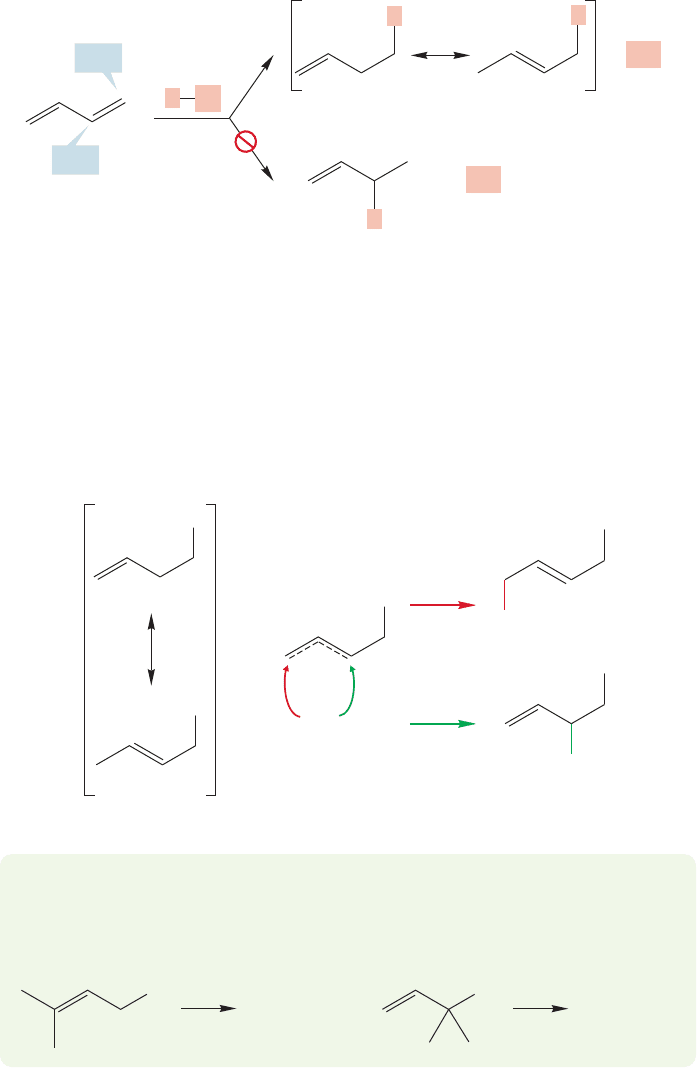
We saw this reaction briefly in the discussion of resonance in Chapter 9
(p. 369). The source of the two pathways becomes clear as we work through the
mechanism step by step. The first reaction must be protonation of butadiene by
hydrogen chloride (Fig. 12.36). There is a choice of two cations, one of which is
a resonance-stabilized allylic cation, and the other an unstabilized primary
carbocation. The stabilized cation is much lower in energy and its formation is
greatly preferred.
+
+
+
C(1)
C(2)
+
–
+
Cl
..
..
..
..
Resonance-stabilized allylic cation
(more stable)
Unstabilized primary
carbocation
(very unstable)
H
–78 ⬚C
Cl
..
..
..
H
H
H
–
Cl
..
..
..
..
FIGURE 12.36 Formation of a
resonance-stabilized allylic cation
is preferred.
The resonance description of the allylic cation shows the source of the two prod-
ucts,because chloride can add to either of the two carbons sharing the positive charge
to give the two observed alkyl chloride products (Fig. 12.37). Hydrohalogenation of
conjugated dienes with HBr also results in 1,2- and 1,4-addition.
+
+
–
+
Cl
(b)(a)
path a
1,4-Addition
1,2-Addition
..
..
..
..
–
Cl
..
..
..
..
path b
–
Cl
..
..
..
..
H
H
H
H
=
..
..
..
Cl
H
..
..
..
Cl
FIGURE 12.37 Addition of the
nucleophilic chloride ion at the two
positions (a and b) sharing the
positive charge gives the products of
1,4- and 1,2-addition.
PROBLEM 12.17 When treated under S
N
1 conditions, the two allylic chlorides
shown here give the same pair of alcohols in the same ratio. Draw structures for
the two alcohols and show the mechanism leading to each product.
H
2
O
..
..
H
2
O
..
..
Two
alcohols
Same two
alcohols
in the same
ratio
..
..
..
Cl
..
..
..
Cl
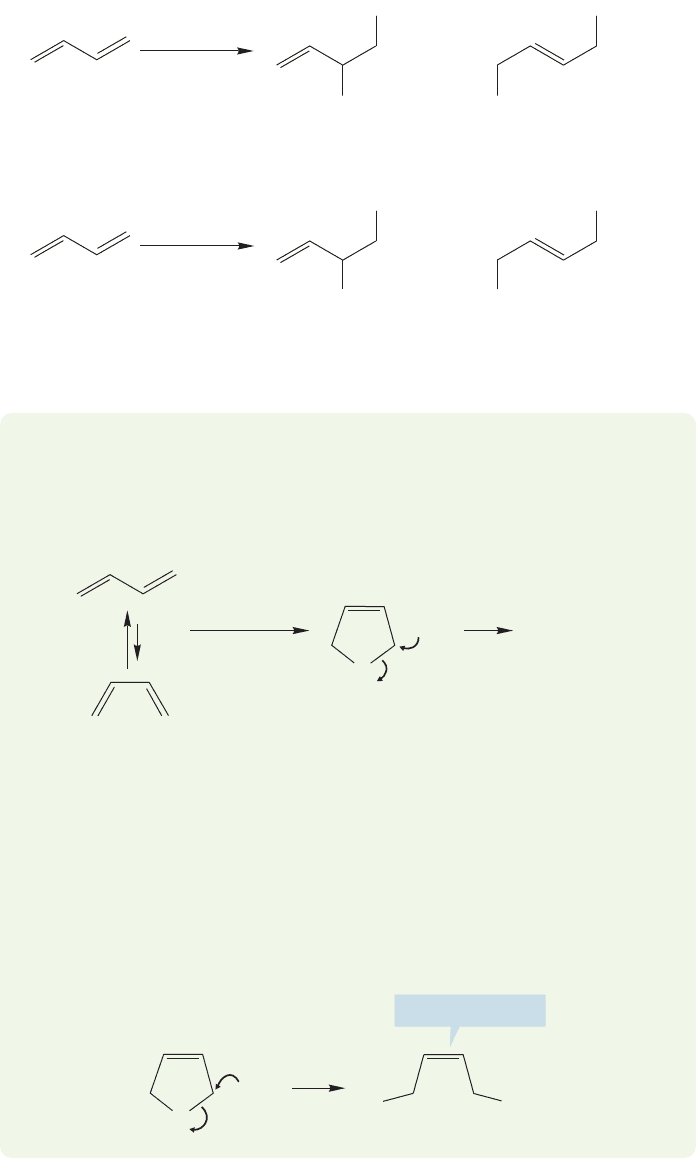
536 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
ANSWER Although there is nothing wrong structurally or electronically with the
five-membered ring bromonium ion, it cannot be involved in this reaction.
Opening of this ring must lead to an alkene containing a cis double bond, and the
1,4-product is,in fact, only trans (Fig. 12.38).The technique for solving this prob-
lem is to use the five-membered bromonium ion as the intermediate, see what the
structure of the product must be, and compare that to what is actually observed.
If we pay careful attention to stereochemistry, we can see that the five-membered
ring cannot be involved.
12.9b Addition of Br
2
and Cl
2
Other addition reactions of conjugated dienes
also produce two products. Like an isolated alkene, conjugated dienes react with
bromine or chlorine to give dihalides. As in hydrohalogenation addition, products
of both 1,2- and 1,4-addition are formed (Fig. 12.38).
Cl
2
1,2-Addition 1,4-Addition
–75 ⬚C
+
..
..
..
2
2
3
4
1
1
trans -1,4-Dichloro-2-butene
(50%)
3,4-Dichloro-1-butene
(50%)
Cl
..
..
..
1
4
Cl
Cl
..
..
..
Cl
..
..
..
Br
2
–15 ⬚C, CCl
4
+
..
..
..
2
2
3
4
1
1
trans -1,4-Dibromo-2-butene
(33%)
3,4-Dibromo-1-butene
(67%)
Br
..
..
..
1
4
Br
Br
..
..
..
Br
..
..
..
FIGURE 12.38 Addition of bromine
or chlorine to conjugated dienes gives
two products.
WORKED PROBLEM 12.18 One potential source of the 1,4-addition product in the
bromination of a conjugated diene is the bromonium ion shown below. Look
carefully at the products of the reaction of 1,3-butadiene and bromine in Figure
12.38 and then explain why the mechanism shown is certainly wrong.
+
–
Br
..
..
..
..
..
1,4-Produc
t
Br
2
–15 ⬚C, CCl
4
Br
..
+
–
S
N
2
Br
..
..
Br
..
..
..
..
Br
..
..
..
Br
..
..
..
A cis double bond
12.10 Thermodynamic and Kinetic Control of Addition Reactions 537
12.10 Thermodynamic and Kinetic Control
of Addition Reactions
The addition reactions in the previous sections allow us to look at general properties of
chemical reactions and to address fundamental issues of reactivity. Just what are the fac-
tors that influence the direction a reaction takes? Why is one product formed more than
another? Is it because of the energies of the products themselves, or something else?
There are curious effects of temperature and time on the addition reactions of
HX and X
2
with conjugated dienes. If the reactions are run at low temperature, it
is the product of 1,2-addition that is generally favored. The warmer the reaction
conditions, the more product of 1,4-addition is formed.At high temperature (25 °C)
the product of 1,4-addition is favored (Fig. 12.39).
..
..
..
+
H
–78 ⬚C
Br
..
..
..
H
25 ⬚C
Br
..
..
..
H H
Br
..
..
..
Br
1,2-Addition 1,4-Addition
(81%) (18%)
(44%) (56%)
trans -1-Bromo-2-buten
e
3-Bromo-1-butene
FIGURE 12.39 The ratio of 1,2-
to 1,4-addition is temperature
dependent.
The effect of time is seen in the observation that the products shown in Figure
12.39 will equilibrate if allowed to stand in solution.As Figure 12.40 shows,the same
mixture of 1,2- and 1,4-addition products is formed from each compound, and the
product of 1,4-addition is favored.
..
..
..
+
25 ⬚C
Cl
..
..
..
Cl
..
....
..
Cl
..
..
..
Cl
..
..
Cl
..
..
..
Cl
1,2-Addition
product
(
100%
)
1,2-Addition
product
(
30%
)
1,4-Addition
product
(
70%
)
1,4-Addition
product
(
100%
)
..
..
..
Cl
..
..
..
Cl
ZnCl
2
25 ⬚C
ZnCl
2
FIGURE 12.40 Equilibration of the
products of 1,2- and 1,4-addition at
25 °C.
Several questions now confront us: (1) Why is the product of 1,4-addition
favored? (2) How do the products of 1,2- and 1,4-additions equilibrate? (3) Why
is the 1,2-addition product favored at low temperatures?
The first question is easy. The product of 1,4-addition contains a disubstituted
double bond,whereas the 1,2-product has only a monosubstituted double bond, and
is therefore less stable (Fig. 12.41). Remember: The more substituted an alkene, the
more stable it is (p. 115).
Monosubstituted
double bond
(less stable)
Disubstituted
double bond
(more stable)
..
..
..
Cl
..
..
..
Cl
1,2-Addition
product
1,4-Addition
product
..
..
..
Cl
..
..
..
Cl
FIGURE 12.41 The compound
containing a disubstituted double
bond is more stable than the
compound with a monosubstituted
double bond.
538 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
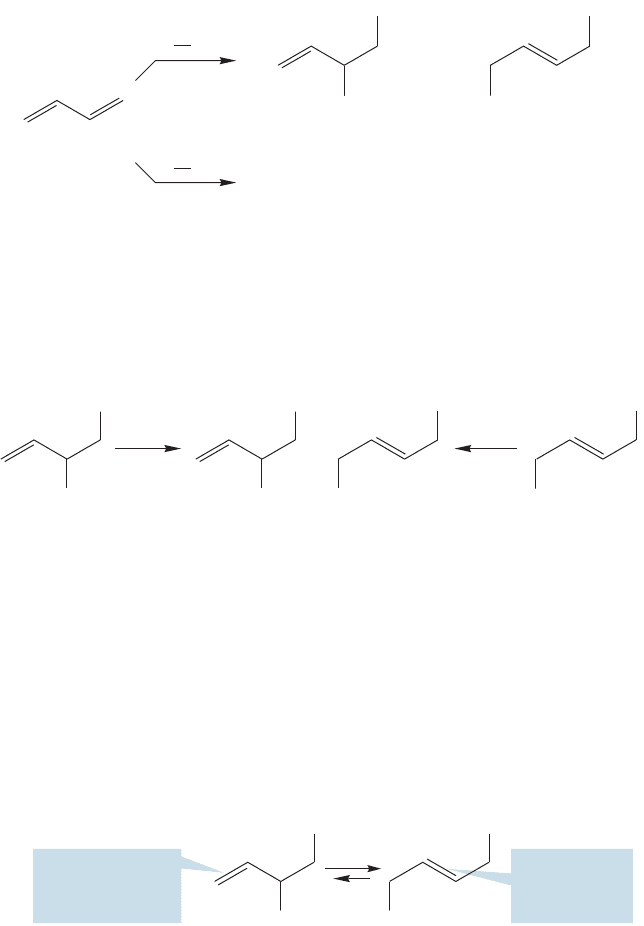
It is also not difficult to see how the two products equilibrate. For example, S
N
1
solvolysis of either of the two products in Figure 12.40 leads to the same,resonance-
stabilized allylic cation (Fig. 12.42). The chloride ion can then add at either of the
two positions sharing the positive charge and at equilibrium it will be the more sta-
ble product of 1,4-addition that dominates.
+
–
(a)
(a)
(b)
(b)
S
N
1
S
N
1
+
Cl
..
..
..
..
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
..
..
..
Cl
FIGURE 12.42 The 1,2- and
1,4-addition products equilibrate
through the formation of a
resonance-stabilized allylic cation.
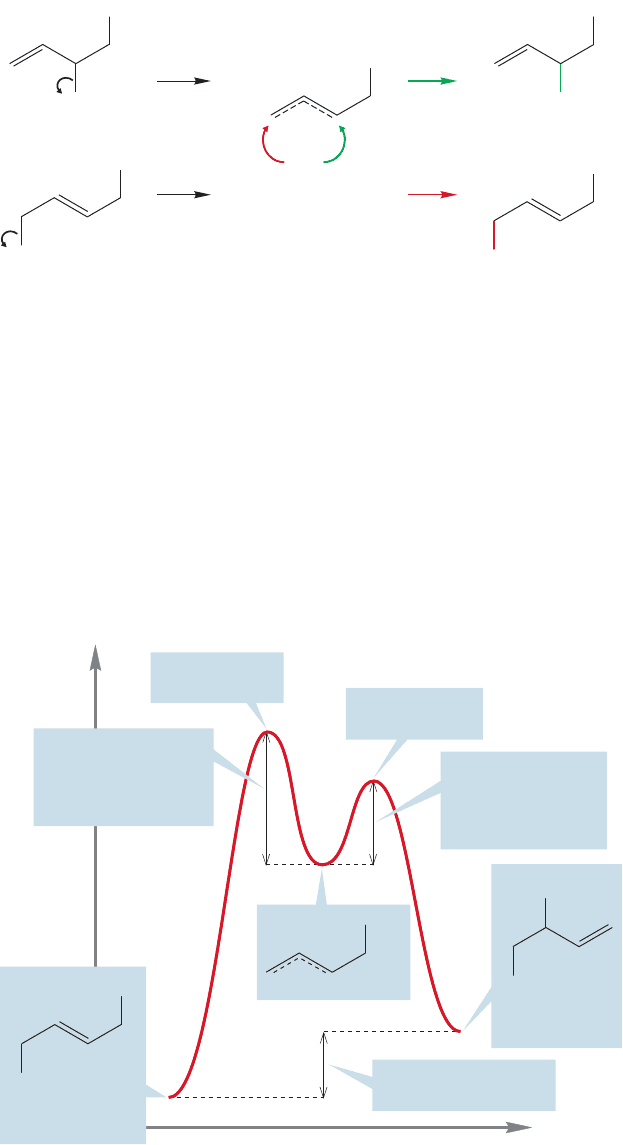
The last question is tougher. Why should one product be favored at low tempera-
ture and another at higher temperature and at longer reaction times? With longer
reaction times and higher temperatures, thermodynamics takes over and the more
stable product prevails. At shorter reaction time,or under mild conditions, it is not the
more stable 1,4-compound that is favored, but the less stable 1,2-addition product.This
change can be explained if the 1,2-addition product is formed more easily.The rate of
formation of the 1,2-product is then by definition faster than that of the 1,4-product.
Therefore, the transition state for formation of the less stable 1,2-product must be lower
in energy than the transition state for formation of the more stable 1,4-product.The
lower energy product is formed by passing over the higher energy transition state and
the higher energy product is formed by passing over the lower energy transition state
(Fig. 12.43). In Chapter 8, you were warned that such cases would occur; here is one.
Reaction progress
Energy
Transition state
for 1,4-addition
Transition state
for 1,2-addition
..
..
..
Cl
..
..
..
Cl
Cl
..
..
..
Cl
..
..
..
Cl
+
+
Cl
–
1,2-Product
(less stable)
1,4-Product
(more stable)
Activation energy for
formation of the more
stable product
(formed slower)
Activation energy
for formation of the
less stable product
(formed faster)
The energy difference
between the products
FIGURE 12.43 An energy diagram
that summarizes formation of
1,2- and 1,4-addition products from
a common intermediate.
