Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.


12.10 Thermodynamic and Kinetic Control of Addition Reactions 539
The 1,2-addition product is favored under conditions of kinetic control
(short time, lower temperature), and the 1,4-product is favored under con-
ditions of thermodynamic control (long time, higher temperature). The
1,2-product is the kinetic product, and results from taking a pathway over a
lower-energy transition state.The 1,4-product results from conditions that favor
formation of the more stable product; it is the thermodynamic product in
this case.
Now we have a fourth question, and it’s a nice one. For both Cl
2
and HCl
addition, we know why thermodynamics favors the 1,4-product; a disubstituted
alkene is more stable than a monosubstituted alkene. But why does kinetic con-
trol favor the product of 1,2-addition?
Let’s look at HCl addition because the best experiments have been done in
this area.The simplest explanation focuses on distance. Where is the chloride ion
after the initial protonation at C(1)? At the moment of its birth, it is much closer
to C(2) than to C(4). Perhaps addition of chloride is faster at the 2-position than
at the 4-position by virtue of simple proximity (Fig. 12.44).
A second possibility looks at the two resonance forms for the allylic cation
(Fig. 12.44), which are not equivalent. The one on the left is a secondary carbo-
cation, the one on the right is a primary carbocation. The first will be weighted
more heavily than the second, and most of the positive charge in the ion will
be at the secondary position. Perhaps it is this more positive position that is
attacked by the anion under kinetic control to give the 1,2-addition product. It
is this factor that is almost always cited as the reason 1,2-addition is preferred
kinetically.
There is a brilliant experiment that settles the issue, done by Eric Nordlander
(1934–1984) at Case Western Reserve University. He studied the addition of deu-
terium chloride ( ), not to 1,3-butadiene, but to 1,3-pentadiene.In this mol-
ecule, addition of D
yields a resonance-stabilized diene in which both resonance
D
O
Cl
Chloride is closer to
C(2) than to C(4)
and perhaps this is
the reason that 1,2-
addition is favored
++
–
Cl
..
..
..
..
–
Cl
..
..
..
..
–
Cl
..
..
..
..
..
..
..
Cl
..
..
..
Cl
Cl
..
..
..
H
H
+
H
H
1,2-Product
C(2) addition C(4) addition
Addition to C(4)Addition to C(2)
1,4-Product
H
H
C(2)
C(4)
C(2)
C(4)
FIGURE 12.44 After protonation, the chloride ion is closer to C(2) than to C(4).
Perhaps this proximity accounts for the preference for 1,2-addition.

540 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
forms are secondary cations. Indeed these resonance forms are identical except for
the remote isotopic deuterium label (Fig. 12.45).
PROBLEM 12.19 Predict the kinetic and thermodynamic products for HCl addi-
tion to 4-methyl-1,3-pentadiene. Be careful, thermodynamic products are not
always a result of 1,4-addition.
++
–
Cl
..
..
..
..
–
Cl
..
..
..
..
–
Cl
..
..
..
..
..
..
..
Cl
..
..
..
Cl
Cl
..
..
..
D
D
+
D
D
If the proximity of chloride
to C(2) is the reason for
the kinetic preference for
1,2-addition, it should
still be preferred in this
electronically symmetrical
cation, and it is!
1,4-Addition (longer
path for chloride)
(24%)
1,2-Addition (shorter
path for chloride)
(75.5%)
D
D
+
FIGURE 12.46 1,2-Addition is still
preferred in 1,3-pentadiene. The
kinetic preference for 1,2-addition
is a simple proximity effect.
Summary
Under thermodynamic (relatively high energy) conditions, the more stable prod-
uct is the major compound formed in addition reactions with conjugated dienes.
Under kinetic conditions, the major product comes from 1,2-addition—even if
it is the less stable product.Why? Proximity! In the formation of the allyl cation,
the nucleophile is “born” closer to the 2-position than to the 4-position.
++
–
Cl
..
..
..
..
..
..
..
Cl
..
..
..
Cl
Cl
..
..
..
D
D
+
D
D
These resonance forms are equivalent (except for the D)
and the two positions must share the positive charge equall
y
D
D
+
FIGURE 12.45 Protonation (D
+
in
this case) of 1,3-pentadiene gives
a resonance-stabilized allylic
carbocation in which both
contributing resonance forms are
secondary. Addition of chloride at
the two positions must take place
to give equal amounts of 1,2- and
1,4-addition.
If the preference for 1,2-addition depends on the difference between a secondary
and a primary carbocation, that preference should disappear in the reaction with
1,3-pentadiene. If proximity is at the root of the effect, 1,2-addition should still be
favored.For once simplicity wins out, and 1,2-addition is still preferred (Fig. 12.46).
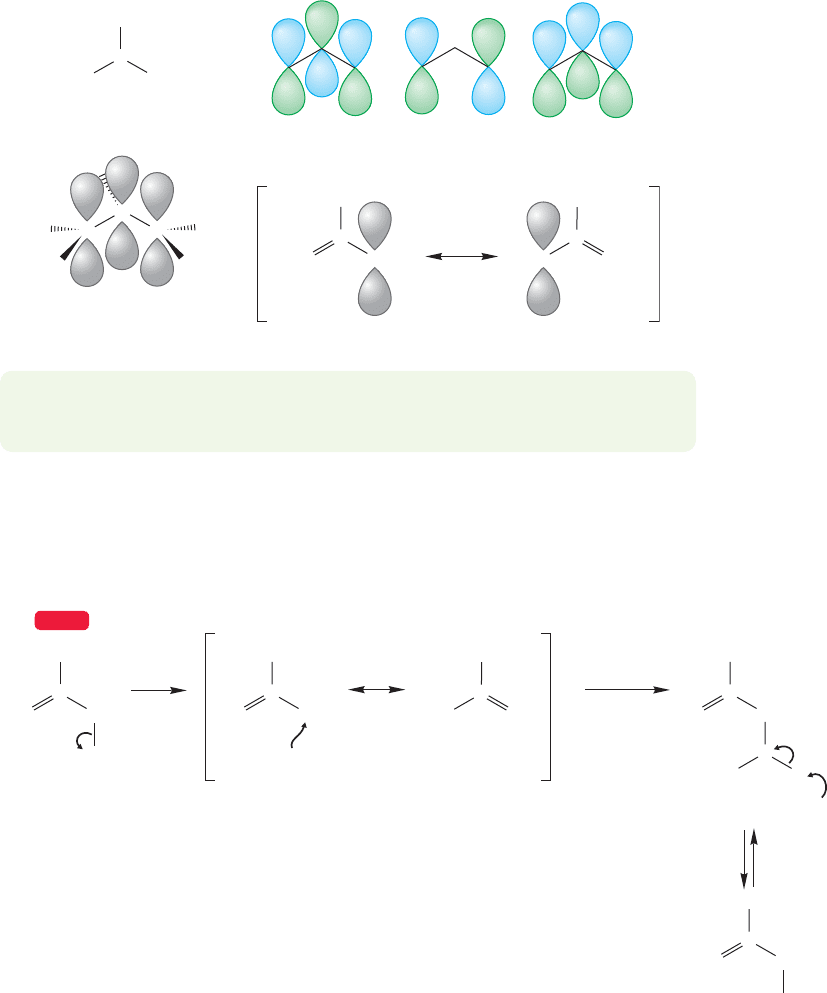
12.11 The Allyl System: Three Overlapping 2p Orbitals 541
H
2
C
CH
2
Φ
3
Φ
1
Φ
2
C
C
C
H
H
2
C
CH
2
H
C
H
2
C
CH
2
H
Allyl
H
H
H
H
H
CC
Molecular orbitals for allyl
Resonance forms for allyl
=
FIGURE 12.47 The resonance and
molecular orbital treatment of the
allyl system.
We will now continue with an examination of a few more reactions in which
allylic stabilization is important.
12.11 The Allyl System: Three Overlapping
2p Orbitals
We have met the structure of the allyl system several times already (Problems 1.61,9.3),
but Figure 12.47 again summarizes both the molecular orbital treatment and the
resonance treatment of this system of three contiguous 2p orbitals.
PROBLEM 12.20 Add electrons to both the resonance and molecular orbital
descriptions in Figure 12.47 to form the allyl anion, radical, and cation.
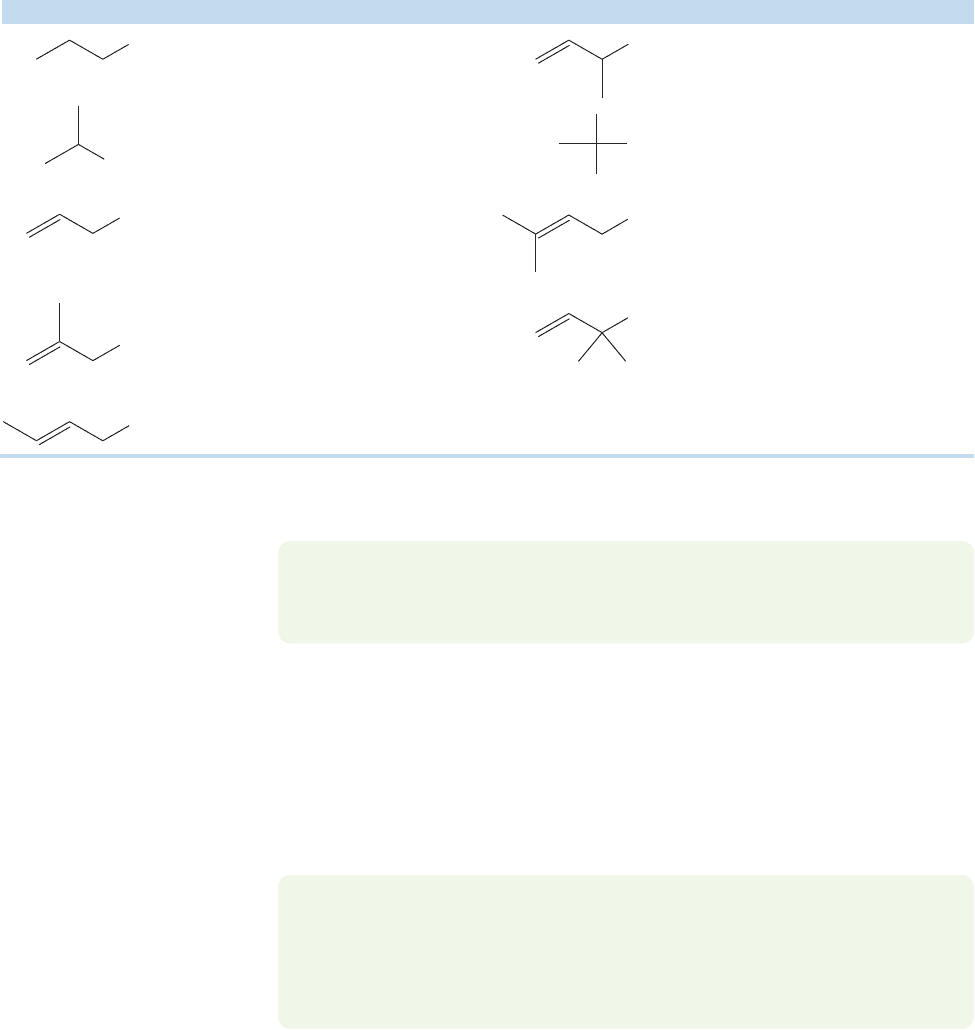
12.11a The Allylic Cation: S
N
1 Solvolysis of Allylic Halides The reso-
nance stabilization of the allylic cation makes allylic halides relatively reactive under
S
N
1 conditions (Fig. 12.48).
WEB 3D
++
–
+
I
..
..
..
..
..
H
2
O
..
..
H
2
O
..
..
H
3
O
..
+
C
H
2
C
CH
2
H
C
H
2
C
CH
2
+
O
H
H
H
deprotonation
I
3-Iodopropene
(allyl iodide)
..
..
..
C
H
2
C
S
N
1
H
2
O
CH
2
H
OH
..
..
C
H
2
C
CH
2
H
C
H
2
C
CH
2
H
capture
by water
FIGURE 12.48 The S
N
1 solvolysis of allyl iodide in
water. This reaction proceeds much faster than the
solvolysis of propyl iodide.
542 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
As mentioned earlier, the allyl cation can be captured at either carbon sharing
the positive charge. Table 12.3 gives some average rates of S
N
1 solvolysis for a few
common structural types.Primary allylic halides react much faster than primary alkyl
halides.Secondary and tertiary allylic halides also ionize faster than their non-allylic
counterparts. Resonance stabilization does have an accelerating effect on the
ionizations.
TABLE 12.3 Relative Rates of S
N
1 Solvolysis of RCl in 50% Ethyl Alcohol at 45 ⬚C
Molecule
a
The value for the primary substrate is suspect, and may include some contribution from S
N
2 reactions.
Cl
Cl
Cl
Cl
Cl
Molecule
Cl
Cl
Cl
Cl
Ion
Primary
a
Secondary
Allyl: both resonance
forms are primary
Allyl: both resonance
forms are primary
Allyl: one resonance
form is primary,
one secondary
Relative Rate
1.0
a
1.7
14.3
21.4
1300
Ion
Allyl: one resonance
form is primary,
one secondary
Tertiary
Allyl: one resonance
form is primary,
one tertiary
Allyl: one resonance
form is primary,
one tertiary
Relative Rate
1157
3 ⫻ 10
4
1.9 ⫻ 10
6
7.9 ⫻ 10
6
PROBLEM 12.21 Explain why the last two entries in Table 12.3 react at nearly the
same rate and are so much faster than the others. Can you devise a molecule that
would react even faster than the last two entries? Draw it.
Remember: We are doing a dangerous thing here. We are again equating ther-
modynamic stability (a secondary allylic cation is more stable than a non-resonance-
stabilized secondary cation) with kinetics (therefore secondary allylic halides form
cations faster than do typical secondary halides). Although justified here, we must
be clear on the reason why thermodynamics and kinetics are closely related in this
example. Work Problem 12.22.
PROBLEM 12.22 Explain carefully, with the use of an energy diagram, why in an
S
N
1 reaction of an alkyl halide the thermodynamic stability of the intermediate
cation is related to the ease of ionization of the corresponding alkyl halide.
Remember the Hammond postulate (p. 351), and consider the transition state
for ionization.
12.11b S
N
2 Reactions of Allylic Halides Not only do allylic halides react
faster than non-allylic halides in the S
N
1 reaction, but they are accelerated in S
N
2
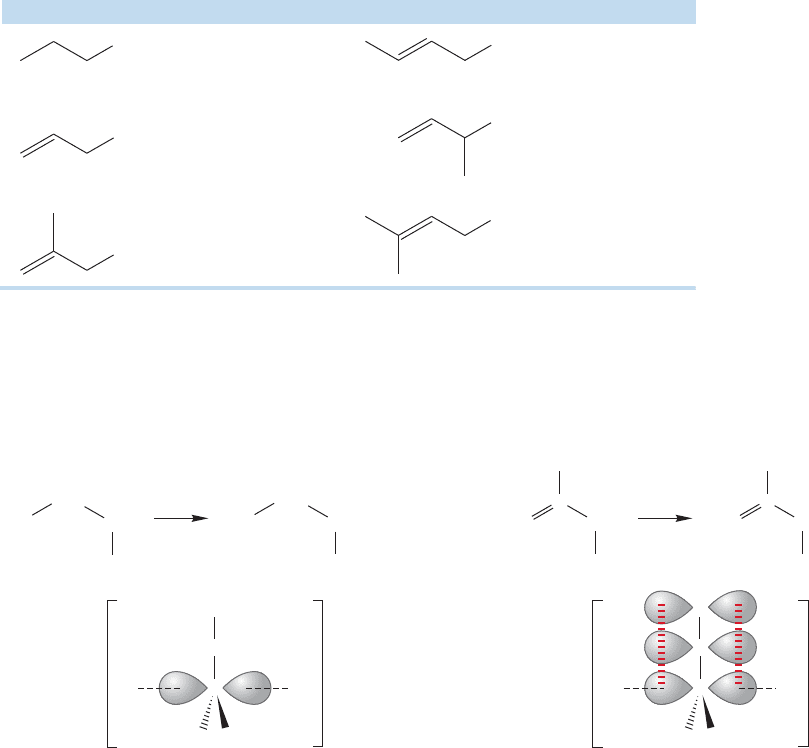
12.11 The Allyl System: Three Overlapping 2p Orbitals 543
reactions as well. Table 12.4 gives some average rates of S
N
2 reactions for a variety
of halides.
Nu
Transition state for S
N
2 displacement
of an all
y
l halide
(
lower ener
gy)
Transition state for S
N
2 displacement
of a
p
rimar
y
halide
(
hi
g
her ener
gy)
Nu
–
..
..
..
H
H
S
N
2
I
I
..
..
..
..
H
3
C
H
2
C
H
3
C
CH
2
C
CH
2
Nu
H
3
C
CH
2
CH
2
Nu
Nu
–
–
+
..
..
..
H
H
I
I
..
..
..
H
2
C
C
C
C
C
H
C
H
CH
2
H
2
C
CH
2
I
..
..
..
..
–
+
I
..
..
..
..
–
Nu
S
N
2
..
–
FIGURE 12.49 A comparison of the transition states for S
N
2 displacement of a primary iodide and allyl iodide.
TABLE 12.4 Relative Rates of S
N
2 Reaction with Ethoxide in Ethyl Alcohol at 45 ⬚C
Molecule
Cl
Cl
Cl
Molecule
Cl
Cl
Cl
Relative Rate
1.0
37.0
33.0
Relative Rate
97
1.85
556
When we are considering a question involving reaction rates, the answer can
always be found through an analysis of the transition states. The rate is dependent
on the activation energy for the reaction, which is the height of the transition state
relative to the starting halide. Let’s compare the transition state for the reaction of
a 1-propyl halide with that for the reaction of an allyl halide (Fig. 12.49).
In the transition state for the S
N
2 reaction of the allyl halide, there is overlap
between the 2p orbital developed on the carbon at which displacement occurs and
the 2p orbitals of the double bond (Fig. 12.49). Therefore, this transition state con-
tains an allylic system. It will benefit energetically from the delocalization of elec-
trons, and will lie at lower energy than the transition state for S
N
2 displacement in
the 1-propyl system, in which there is no delocalization.
12.11c The Allyl Radical It is much easier to form a resonance-stabilized
allylic radical than an undelocalized radical. We saw the effects of delocalization in
reactions involving allylic radicals in Chapter 11 (p. 497). For example, the regiospe-
cific bromination of the allylic position of alkenes with NBS is possible because of
the relative ease of forming the delocalized allyl radical (Fig. 11.56).
12.11d The Allyl Anion Now let’s look at the acidity of allylic hydrogens to
see if the formation of a resonance-stabilized allylic anion has any effect on that
acidity.We would surely expect it to.If delocalization of electrons is important, then
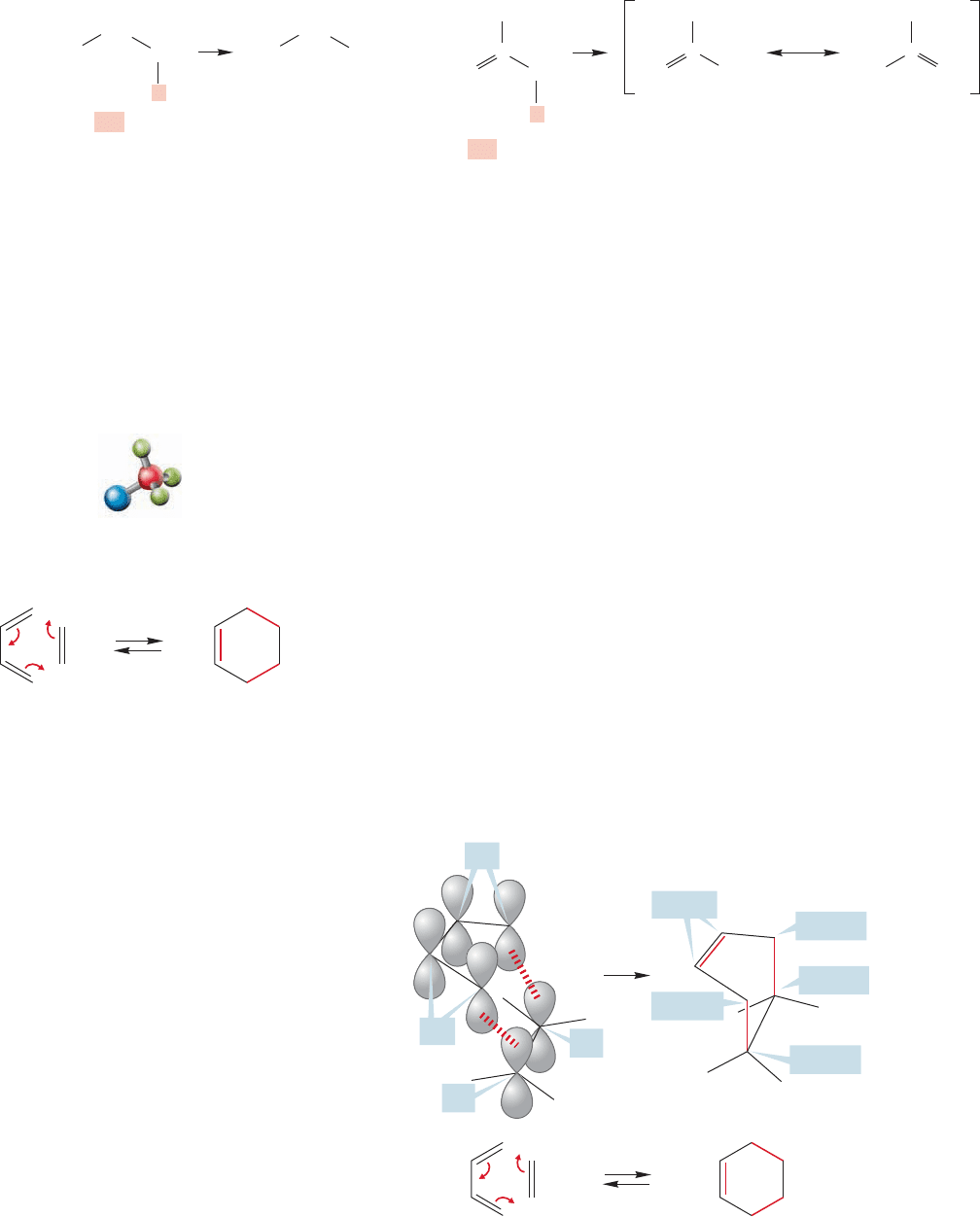
544 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
removal of a proton at the allylic position should be easier than at a position not
leading to a delocalized anion. A comparison of the acidities of propane and
propene should test this idea (Fig. 12.50).
H
H
2
C
C
CH
2
H
3
C
pK
a
∼ 50–60
H
pK
a
= 43
CH
2
CH
2
H
3
C
CH
2
CH
2
H
2
C
C
H
C
H
H
2
C
–
..
CH
2
–
..
CH
2
–
..
H
FIGURE 12.50 Propene is far more
acidic than propane.
In fact,the pK
a
of propene is 43,some 7–17 pK
a
units lower than that for propane
(pK
a
50–60). The uncertainty arises from the difficulty of measuring the pK
a
of
an acid as weak as a saturated hydrocarbon. In any event, propene is a much stronger
acid than propane, and it is reasonable to suppose that the formation of the delo-
calized allyl anion is responsible for the increased acidity of the alkene.
12.12 The Diels–Alder Reaction of Conjugated Dienes
In 1950, Otto Diels (1876–1954) and his student Kurt Alder (1902–1958) received
the Nobel prize for their work on what has appropriately come to be known as the
Diels–Alder reaction, which they discovered in 1928. (So much for timeliness; at
least they lived long enough to outlast the Nobel committee’s renowned conser-
vatism.) We will have more to say about its mechanism in Chapter 20, but the
Diels–Alder reaction is characteristic of conjugated dienes and is of enormous syn-
thetic utility. Therefore we must outline it here.
When energy is supplied to a mixture of a conjugated diene and an alkene, a
ring-forming reaction takes place to produce a cyclohexene.The alkene in this reac-
tion is called a dienophile, because it has demonstrated an affinity for the diene.
The product of the reaction is often called the adduct, which is another word for
product in a reaction between two molecules. Our discussion of the Diels–Alder
mechanism begins with the arrow formalism,which points out which bonds are bro-
ken and shows where the new bonds are made in the forward reaction (Fig. 12.51).
Which orbitals are involved? The Diels–Alder reaction begins with the overlap
of the π systems of the dienophile and the diene.The two reaction partners approach
in parallel planes and as the bonds form,the end carbons of both the diene and alkene
rehybridize from sp
2
to sp
3
(Fig. 12.52).
'
sp
2
Now sp
3
Now sp
3
Now sp
3
Now sp
3
Still sp
2
⌬
sp
2
sp
2
sp
2
FIGURE 12.52 In the Diels–Alder
reaction, the two participants
approach each other in parallel planes.
As the reaction occurs, the end atoms
of the diene and the dienophile
rehybridize from sp
2
to sp
3
.
⌬
FIGURE 12.51 The arrow formalism
for the Diels–Alder reaction.
Diels–Alder reaction
12.12 The Diels–Alder Reaction of Conjugated Dienes 545
PROBLEM 12.23 Curiously, it matters just how energy is supplied to the molecules
in the Diels–Alder reaction. If we heat the reactants up, the reaction occurs, but
if we attempt to supply energy photochemically, it generally fails. We will exam-
ine such matters in detail in Chapter 20, but we might start to consider them now.
When we think about reactions between atoms, we do not consider every electron
in the atom, just those most loosely held, the valence electrons. Similarly, in
molecular reactions it is the most weakly held electrons, those in the HOMO,
that control reactivity. The electrons in the HOMO might well be thought of as
the valence electrons of molecules. Remember what Figure 12.25 tells us about
the interaction of light and molecules. First, consider the phase relationships in
the possible HOMO–LUMO interactions in the transition state for a simple
Diels–Alder reaction.Then think about how the absorption of a single light pho-
ton might change those interactions and offer an explanation for why light
energy does not promote the Diels–Alder reaction.
PROBLEM 12.24 Write an arrow formalism for the reverse Diels–Alder reaction.
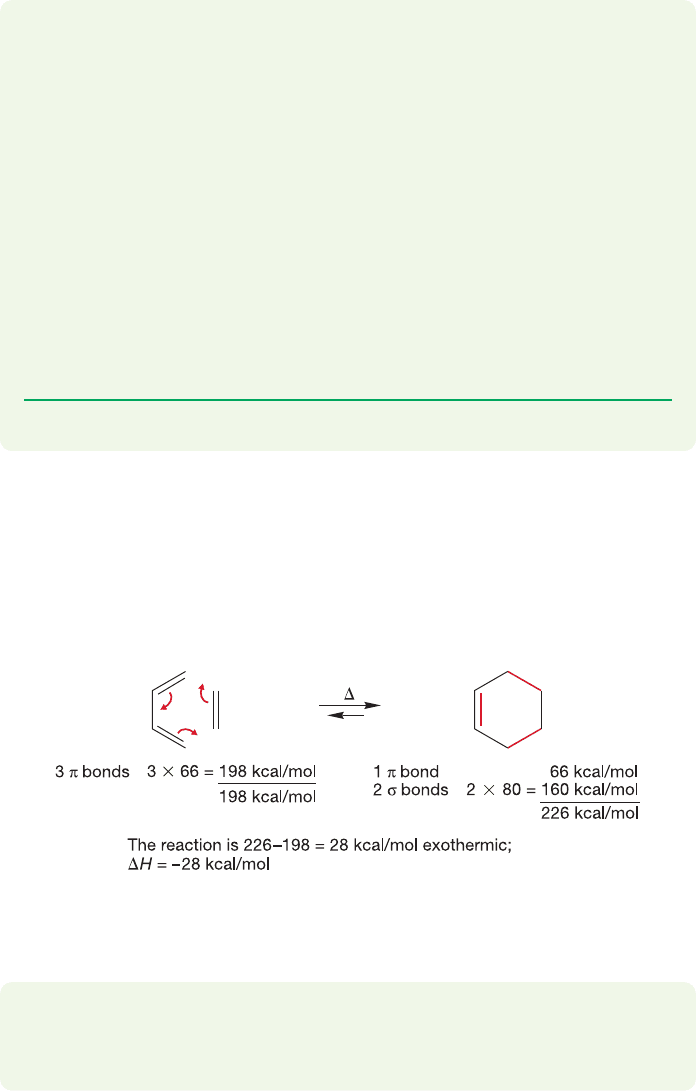
It is possible to estimate the thermochemistry in the Diels–Alder reaction by
comparing the bonds made and broken in the reaction.Three π bonds in the start-
ing material are converted into one π bond and two σ bonds in the product.
Accordingly, the reaction is calculated to be exothermic by approximately 28 kcal/mol
(Fig. 12.53).
FIGURE 12.53 The simplest Diels–Alder reaction is exothermic by about
28 kcal/mol.
PROBLEM 12.25 Why is the bond strength of the new σ bonds in cyclo-
hexene (80 kcal/mol each) used in Figure 12.53 lower than the bond in
ethane (90 kcal/mol)?
C
O
C
C
O
C
As shown in the previous figures, the Diels–Alder reaction requires the s-cis
form of the diene. However, it is the s-trans arrangement that is the more sta-
ble one, by approximately 3 kcal/mol (p. 523). If equilibrium favors the s-trans
form, why is the Diels–Alder reaction observed at all? Even though there is
little s-cis form at equilibrium, reaction with the dienophile disturbs the equi-
librium between the s-trans and s-cis form by depleting the s-cis partner.
Reestablishment of equilibrium generates more s-cis molecules, which can react
in turn. Eventually, all the diene can be converted into Diels–Alder product,
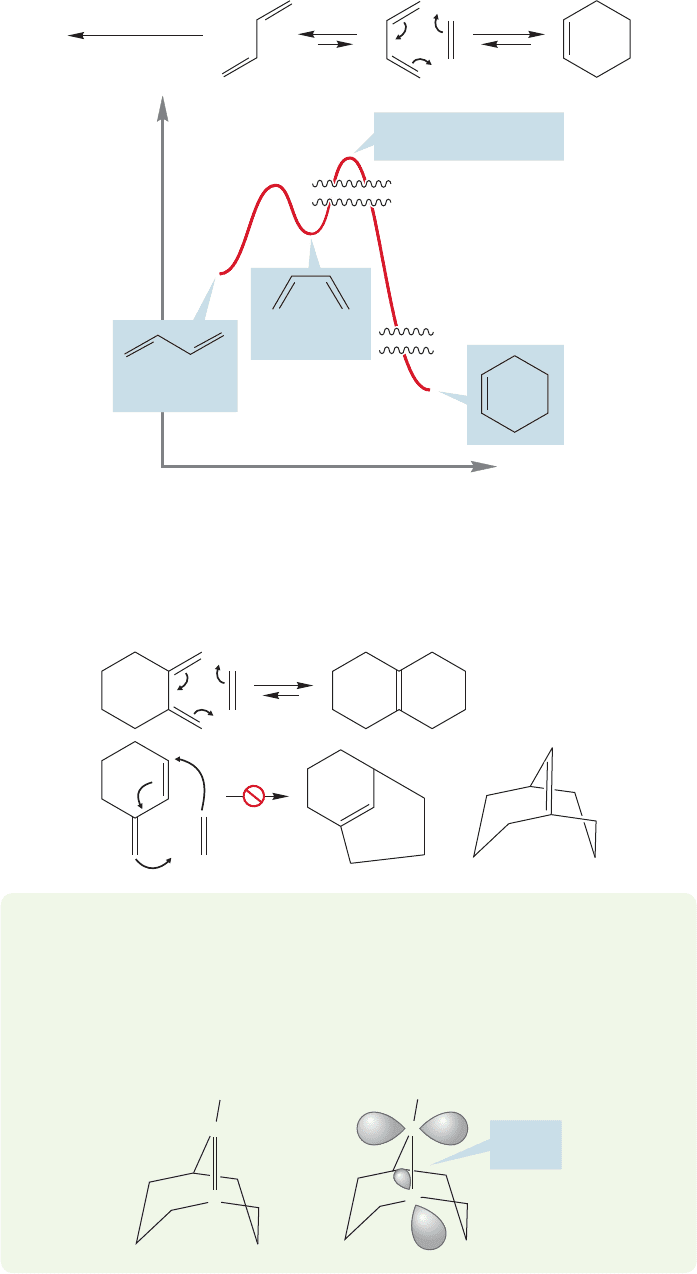
546 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
no reaction
with dienophile
Reaction
p
ro
g
ress
Energy
Transition state for the
Diels–Alder reaction
s-trans
Conformation
s-cis
Conformation
⌬
FIGURE 12.54 Even though
the unreactive s-trans form of
1,3-butadiene is favored at
equilibrium, the small amount of
the s-cis form present can lead to
product.The vertical axis of the
figure is not to scale.The squiggly
lines indicate gaps.
=
⌬
FIGURE 12.55 When there can be no
s-cis form of the diene, the
Diels–Alder reaction fails.
WORKED PROBLEM 12.26 Identify the source of strain in the putative product
shown for Diels–Alder addition to 3-methylenecyclohexene in Figure 12.55. Use
your models.
ANSWER Although the paper puts up with the double bond drawn in Figure
12.55, Nature won’t.The orbitals making up this bridgehead double bond do not,
in reality, overlap.
H
=
C
C
Poor
overlap
H
C
C
even though at any moment there is very little of the active s-cis form present
(Fig. 12.54).
When there is no possibility of any s-cis form, there can be no Diels–Alder reac-
tion. For example, although 1,2-dimethylenecyclohexane reacts normally with
dienophiles, 3-methylenecyclohexene does not. The product of this reaction would
contain a double bond far too strained to be formed (Fig. 12.55).
12.12 The Diels–Alder Reaction of Conjugated Dienes 547
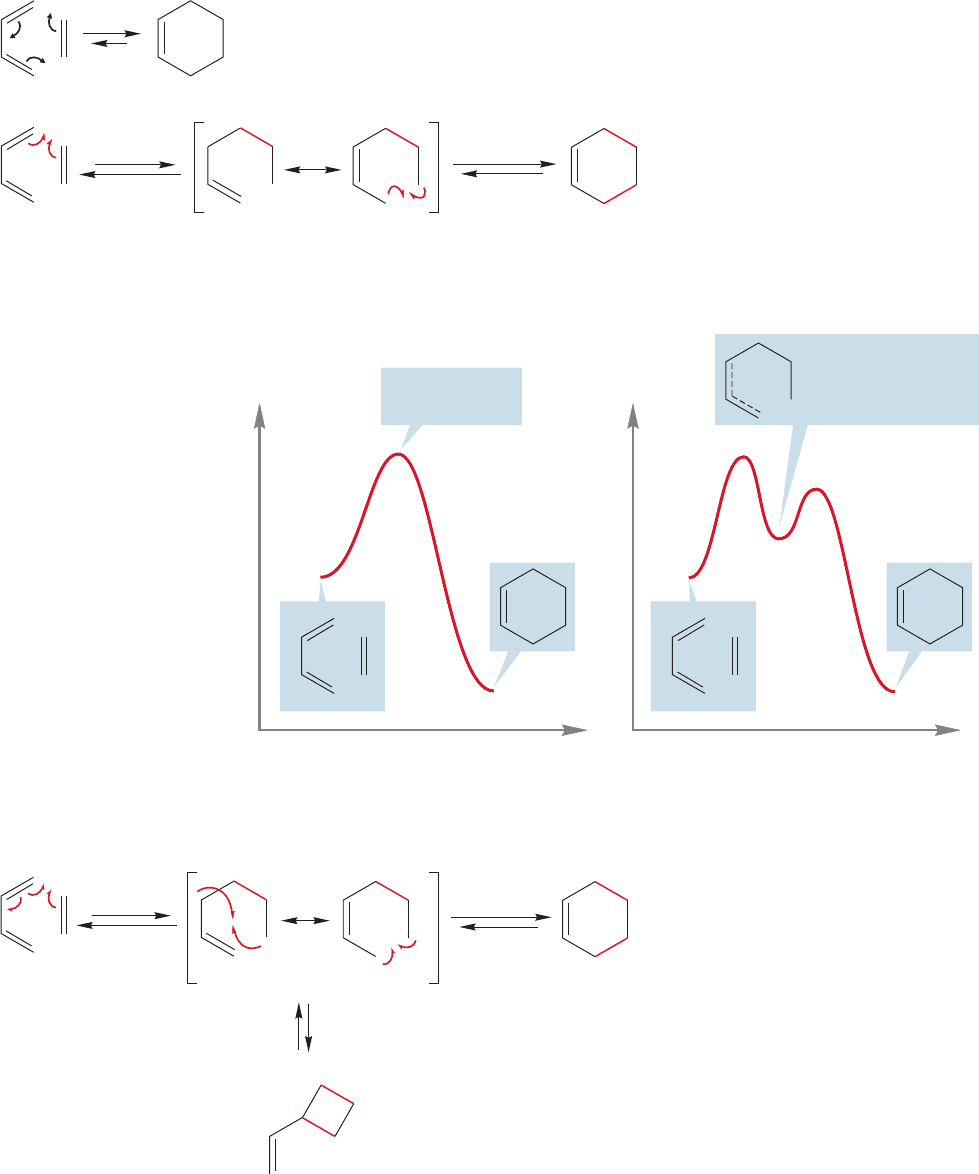
Another serious mechanistic question involves the timing of the formation of the
two new σ bonds in the cyclohexene. Are they made simultaneously in a concerted
fashion, or are they formed in two separate steps? In principle, the mechanism of the
Diels–Alder reaction could either be concerted or involve two steps (Fig. 12.56).
step two
step one
In this nonconcerted, two-step
mechanism, the two
σ bonds
are formed in two separate steps
A concerted mechanism;
both new
σ bonds are
formed simultaneously
.
.
.
.
⌬
FIGURE 12.56 Arrow formalisms for
concerted and two-step mechanisms
for the Diels–Alder reaction.
This question asks if there
is an intermediate in the
reaction or not. Figure
12.56 shows the two arrow
formalisms and Figure
12.57 gives Energy versus
Reaction progress diagrams
for both the concerted and
two-step processes.
We might immediately
be suspicious of the two-
step mechanism. As it
involves an intermediate
allylic radical (Figs. 12.56
and 12.57), shouldn’t we
observe closure of the
diradical at both possible
positions? Should there not
be a vinylcyclobutane pro-
duced as well as the cyclo-
hexene (Fig. 12.58)?
One step
Reaction progress
Energy
Transition state
(no intermediate)
Reaction progress
Two step
Energy
.
.
Allylic radical and a
primary radical within
the same molecule (a
diradical intermediate)
(b)
path b
(a)
path a
step two
step two
step one
Vinylcyclobutane
(not observed)
Normal
Diels–Alder
product
.
.
.
.
FIGURE 12.58 The two-step reaction
could lead to vinylcyclobutane.This
product is not observed.
FIGURE 12.57 Energy versus Reaction progress diagrams for the concerted and two-step mechanisms.
548 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
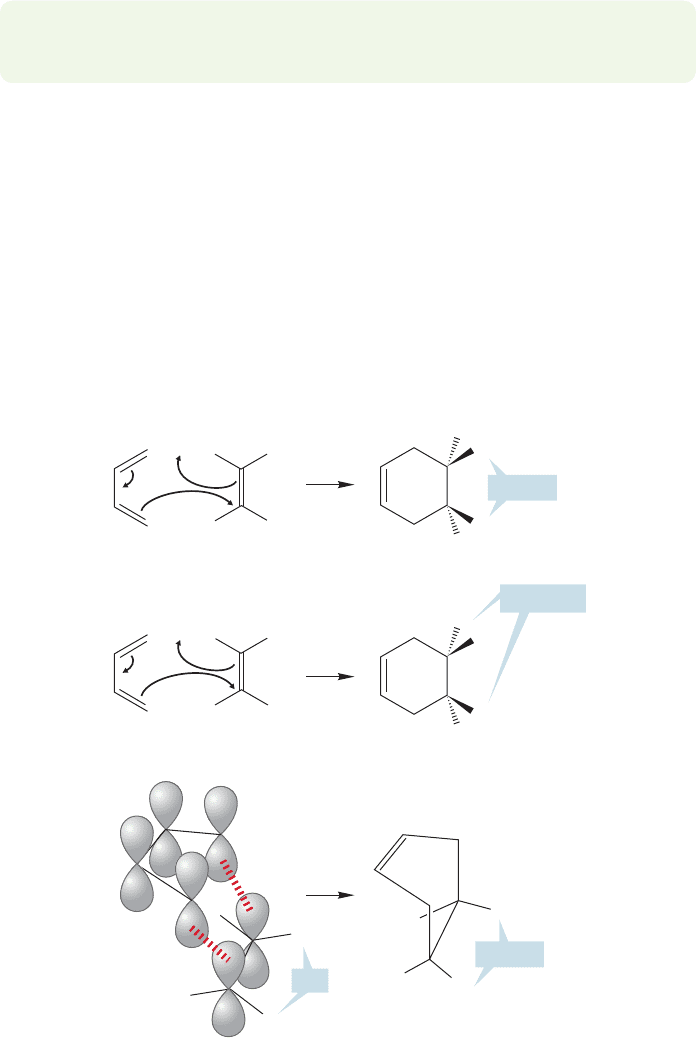
PROBLEM 12.27 Write an Energy versus Reaction progress diagram for the two-
step Diels–Alder reaction that includes formation of the vinylcyclobutane.
H
H R
R
R
R
cis
cis
Diene
Dienophile
A one-step, concerted mechanism must
retain stereochemistry
A cyclohexene
trans
Still trans
Still cis
Still cis
H
H
H
R
H
R
H
H
R
R
R
R
R
H
H
R
H
H
FIGURE 12.59 A concerted mechanism must preserve the
stereochemical relationships of the groups on the dienophile.
In fact, Diels–Alder reactions do not lead to vinylcyclobutanes, but give
cyclohexenes exclusively. Although this argument would seem at first to favor
the concerted mechanism, which can only give cyclohexenes, it could be
countered that the transition state for closing the four-membered ring will
contain much of the strain present in all cyclobutanes, and will therefore be high
in energy.
As is so often the case, the mechanistic question of the timing of new bond
formation is finally answered with a stereochemical experiment. A one-step reac-
tion must preserve the stereochemical relationships present in the original alkene.
For example, in a concerted reaction, a cis alkene must give a cis disubstituted
cyclohexene. If the reaction is concerted,which means the two new σ bonds form
simultaneously, there can be no change in the stereochemical relationship of
groups on the alkene (Fig. 12.59). If the bonds are formed in two steps, there
should be time for rotation about the carbon–carbon σ bond in the dienophile.
