Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

12.12 The Diels–Alder Reaction of Conjugated Dienes 549
If the mechanism is stepwise, it should lead to stereochemical scrambling of the
groups originally attached to the dienophile (Fig. 12.60).
.
.
H
H R
R
R
R
rotate
Diradical with trans R groups
cis Product
trans Product
Diradical with cis R groups
close
close
H
H
R
R
H
H
R
R
H
H
.
.
R
R
H
H
FIGURE 12.60 A two-step
mechanism predicts a mixture of
stereoisomers.
In fact, retention of stereochemistry is observed. The reaction is a concerted,
one-step process (Fig. 12.61).
WEB 3D
COOH
+
H
H
trans Alkene, the dienophile trans Product
cis Alkene, the dienophile cis Product
H
H
COOH
H
H
COOH
140 ⬚C
+
H
H
benzene
140 ⬚C
benzene
HOOC
FIGURE 12.61 The preservation of
stereochemistry in the Diels–Alder
reaction favors the concerted
mechanism.
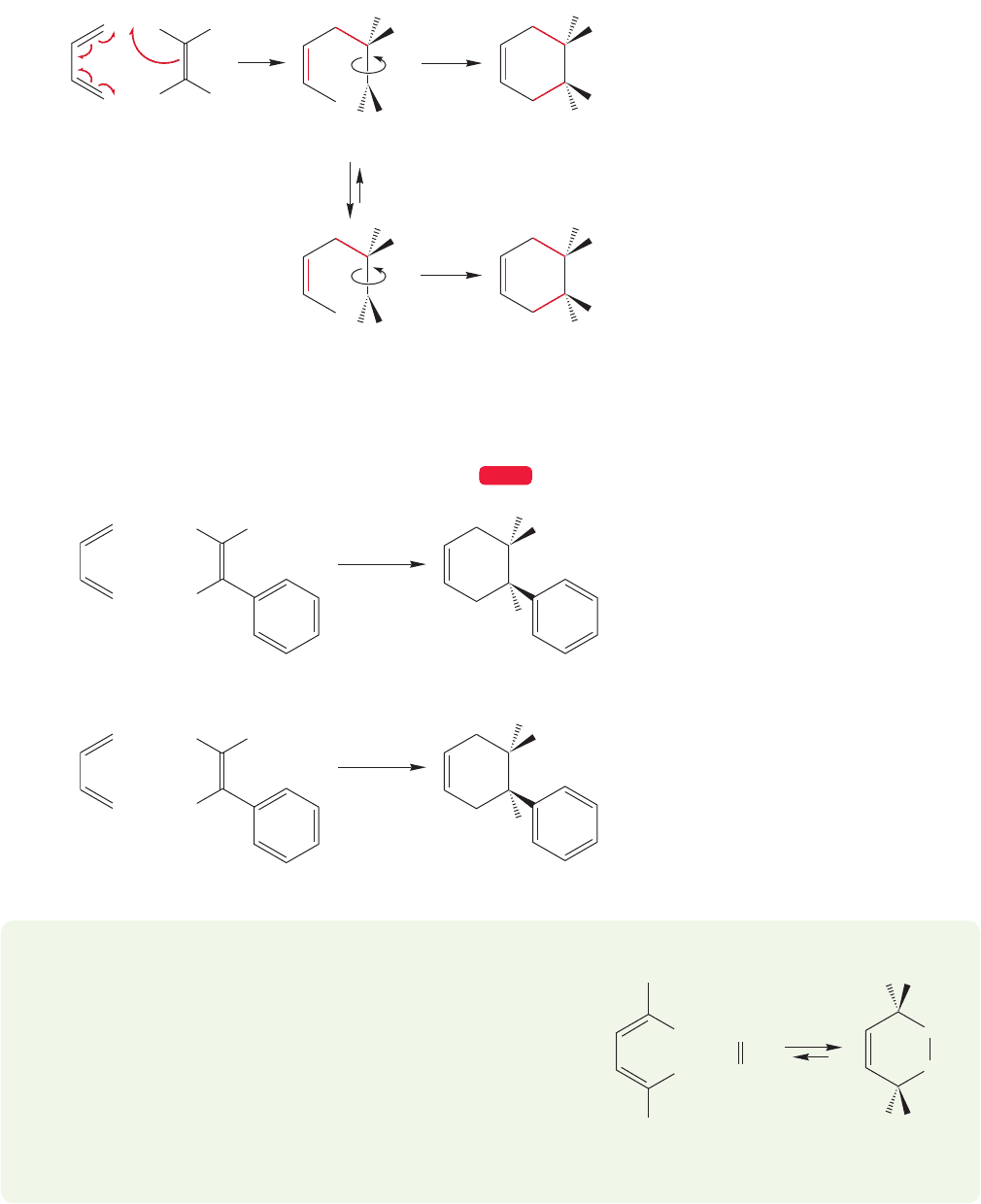
PROBLEM 12.28 The experiment described in Figures 12.59–12.61
is usually easy to understand, but there is another stereochemical
experiment possible. It tests the same thing, but is harder to see
without models. So, if this problem is difficult to follow, by all means
work it through with models. Let’s follow the stereochemical fate of
groups attached to the ends of the diene, not the alkene. Consider
the Diels–Alder reaction between a trans,trans-conjugated diene
and ethylene. Only a single cyclohexene product is formed and it
has the R groups cis. Explain how this result shows that the mecha-
nism of cycloaddition cannot be a two-step process.
+
trans,trans Diene cis Cycloalkene
R
R
R
R
H
H
H
H
CH
2
CH
2
CH
2
CH
2
550 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
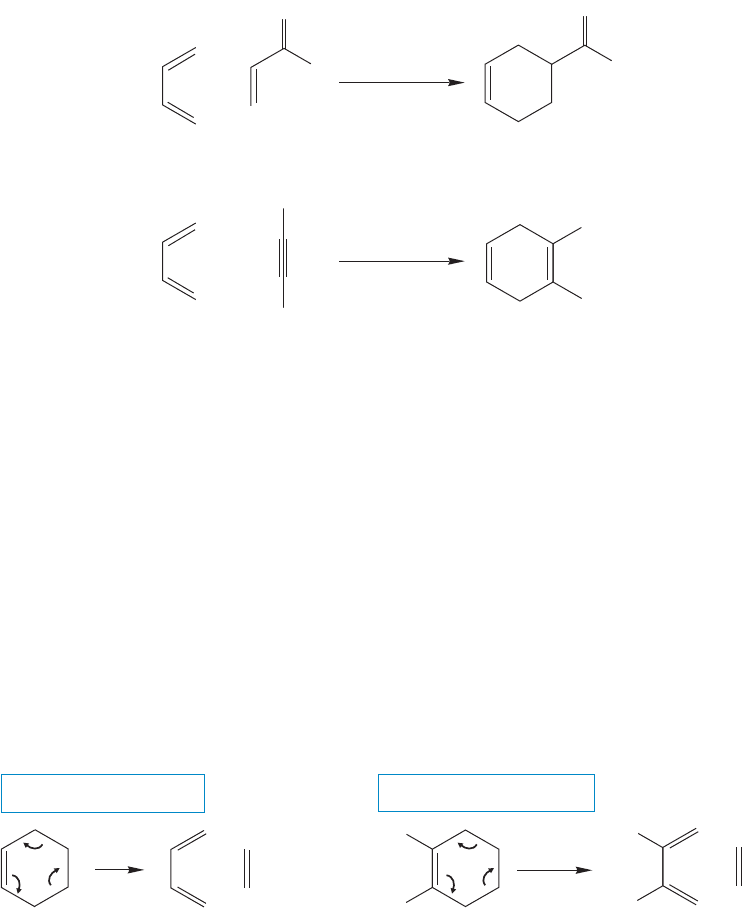
In the preceding pages, mechanistic points have been illustrated using very sim-
ple examples.Actually,the simplest Diels–Alder reaction,ethylene and 1,3-butadiene,
has a rather high activation enthalpy of about 27 kcal/mol and requires high pres-
sure and temperature for success. Even 200 °C and a reaction pressure of 4500 psi
yields only 18% cyclohexene after 17 hours. Other Diels–Alder reactions proceed
under milder conditions. Reactions are more facile when the dienophile bears one
or more electron-withdrawing groups and if the diene can easily achieve the required
s-cis conformation. Two examples of these relatively fast Diels–Alder reactions are
shown in Figure 12.62.
100 ⬚C
+
H
1 h
(100%)
O
..
..
H
O
..
..
(88%)
+
25 ⬚C
benzene, 15 h
CN
CN
CN
CN
FIGURE 12.62 Two efficient
Diels–Alder reactions.
Why is the Diels–Alder reaction so important and so useful? Six-membered rings
are very common, and the reaction builds six-membered rings from relatively sim-
ple building blocks. Moreover, the reaction is very general. Most dienes react with
most dienophiles to give Diels–Alder products.From now on,every cyclohexene you
see must trigger the thought “can be made by a Diels–Alder reaction” in your mind.
That’s an important concept for both synthesis and problem solving.
Like most reactions in organic chemistry, the Diels–Alder reaction is not a one-
way street. Reverse Diels–Alder reactions are common, especially at high tempera-
ture. Remember: ¢G ¢H T¢S (Eq. 8.6, p. 337), and at high temperature the
temperature-dependent ¢S term can overwhelm the temperature-independent ¢H
term. The reverse Diels–Alder reaction makes two molecules from one and there-
fore is favored by entropy, ¢S. Cyclohexenes are formed in a reaction of a diene and
a dienophile, but they can be used as sources of dienes through the reverse Diels–
Alder reaction (Fig. 12.63).
(
87%
)
⌬
800 ⬚C,
12 mm
0.02 s
+
NC
NC
NC
NC
+
THE GENERAL CASE A SPECIFIC EXAMPLE
FIGURE 12.63 The reverse Diels–Alder reaction.
Now let’s press on to look at other uses of the Diels–Alder reaction, which is
surely one of the most synthetically useful of all the reactions in organic chemistry.
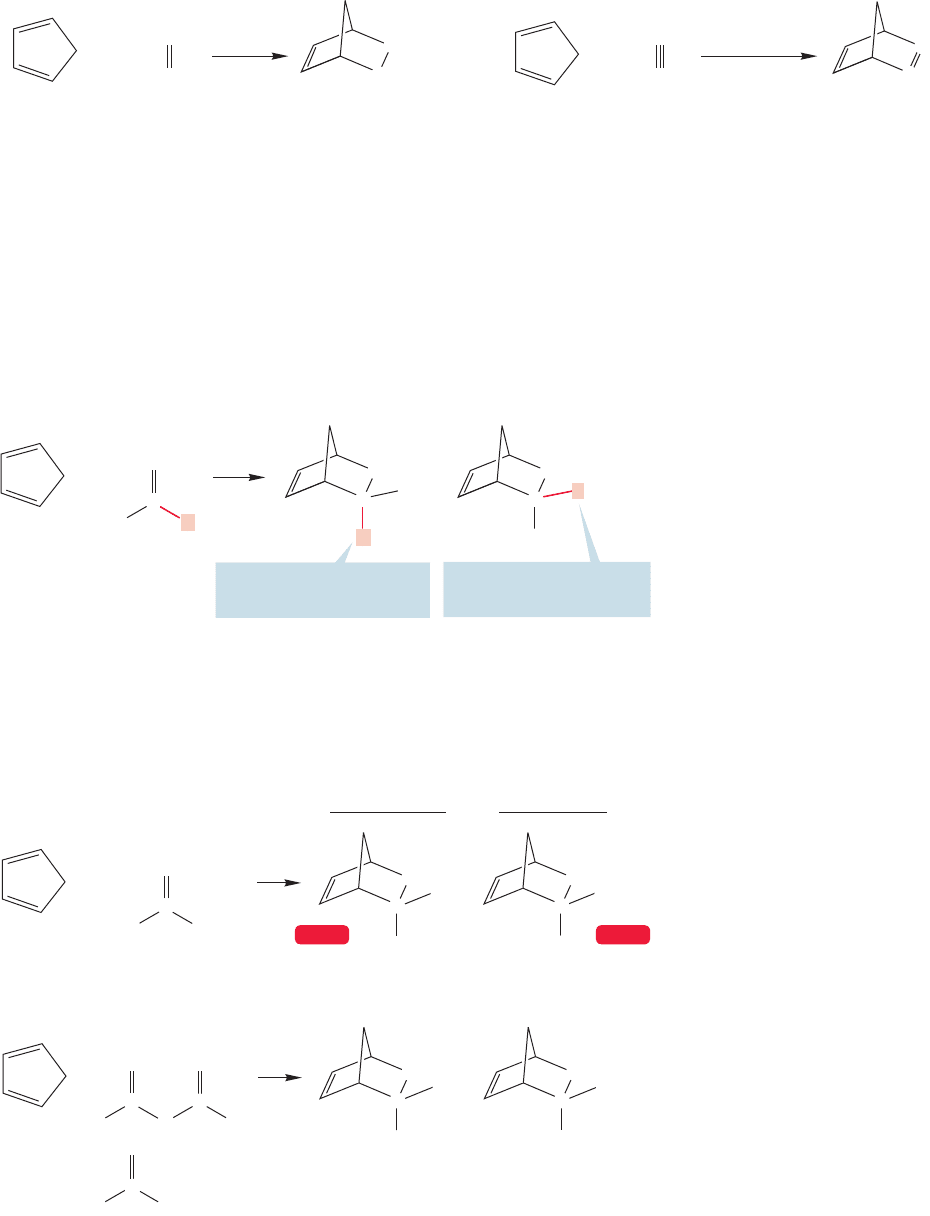
12.12 The Diels–Alder Reaction of Conjugated Dienes 551
+
Bicyclo[2.2.1]hept-2-ene
AcetyleneEthylene CyclopentadieneCyclopentadiene Bicyclo[2.2.1]hepta-
2,5-diene
200 ⬚C
23 h
+
280–300 ⬚C
10–12 atm
CH
2
CH
2
CH
2
CH
2
CH
CH
CH
CH
FIGURE 12.64 The Diels–Alder reaction between cyclic dienes and alkenes (or alkynes) gives
bicyclic molecules.
Chemists often use bicyclic molecules to investigate effects requiring a fixed geo-
metrical arrangement of orbitals or groups. Consider the simple Diels–Alder reaction
between ethylene or acetylene and a common cyclic diene, cyclopentadiene (Fig.12.64).
++
⌬
X pointing away from the
double bond (called exo)
X pointing toward the
double bond (called endo)
C
H
C
H
CH
2
C
CH
2
CH
2
X
X
H
X
FIGURE 12.65 In principle, this kind
of Diels–Alder reaction can give
either endo or exo products.
Presto! Bicyclic compounds! But this instant synthetic expertise is not without its price,
because now there is a new stereochemical effect to be considered.When a substitut-
ed alkene reacts with a cyclic diene,there are two possible stereoisomeric products even
though the two new σ bonds are formed simultaneously. The substituents on the
alkene must retain their stereochemical relationship during the reaction, but there are
still two possible products.The substituents on the alkene can point toward the newly
formed double bond or away from it (Fig. 12.65). In this molecule, the isomer with
the substituents aimed toward the double bond is called endo and that with the groups
aimed away from the double bond is called exo. More generally, a substituent that is
on the same side as the smaller bridge of a bicyclic system is exo.The exo isomers are
usually more stable,but the endo compounds are kinetically favored in the Diels–Alder
reaction (Fig. 12.66).
WEB 3DWEB 3D
+
+
⌬
⌬
C
H
C
H
CH
2
C
CH
2
CN
CN
(60%)
(81%)shorthand = (19%)
(40%)
CN
+
+
C
H
C
H
CH
2
C
CH
2
CH
2
OAc
H
H
OAc
O
O
CH
3
H
CH
2
OAc
endo Products exo Products
CH
2
C
C
FIGURE 12.66 It is the endo product
that is favored in most Diels–Alder
reactions. In each case, the percentage
of endo product is greater than the
percentage of exo product.
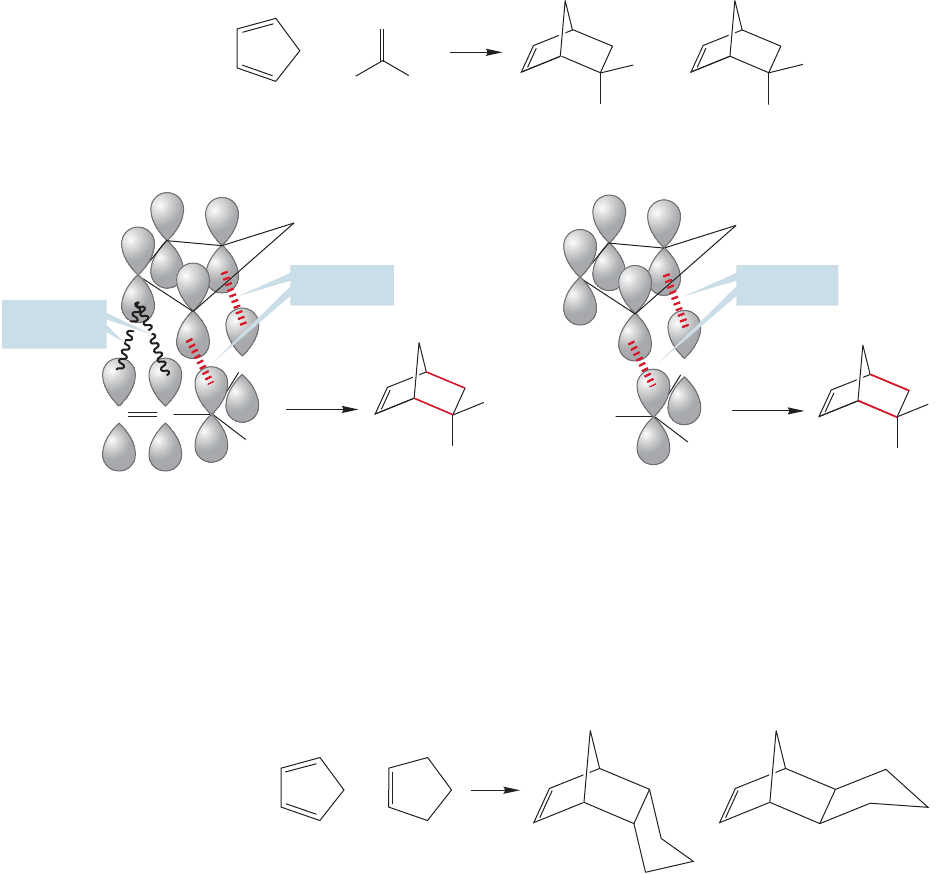
552 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
The exo compounds formed from a substituted dienophile are more stable for
steric reasons:The substituent points away from the more congested part of the mol-
ecule.Indeed, the transition state for exo product formation must also be less crowd-
ed than the transition state for endo product formation. But the transition state for
the endo product benefits from other factors that overwhelm steric considerations.
Chemists have performed high-level molecular orbital calculations and have found
that in the endo transition state, but not in the exo transition state, there can be
“secondary orbital overlap.” This fancy-sounding phrase simply means that there can
be interaction between the “back” diene orbitals and orbitals on the substituent X
only in the endo transition state. Figure 12.67 shows this effect for the first example
of Figure 12.66. Another explanation that has been suggested for the endo selectivity
relates to solvent interactions.Whatever the cause, the endo product is usually formed
preferentially and sometimes with very high selectivity (Fig. 12.68).
Even more complicated polycyclic structures can be made from the Diels–Alder
reaction between two ring compounds. In the simplest case, the reaction of a cyclic
1,3-diene with a cycloalkene (Fig. 12.68), notice that the endo addition is preferred.
++
H
CN
endo Product
endo Product
exo Product
exo Product
H
CN
H
CN
H
CN
endo Transition state exo Transition state has
no possible secondary
interactions
CN
H
H
CN
CH
2
CN
H
CH
2
New bonds New bonds
Secondary
interactions
FIGURE 12.67 Secondary orbital interactions stabilize the endo transition states.
endo Product
(98%)
exo Product
(2%)
⌬
++
FIGURE 12.68 The Diels–Alder
reaction between a cyclic diene and a
cyclic dienophile gives a polycyclic
product. Once again, it is the endo
form that is favored.
12.12 The Diels–Alder Reaction of Conjugated Dienes 553
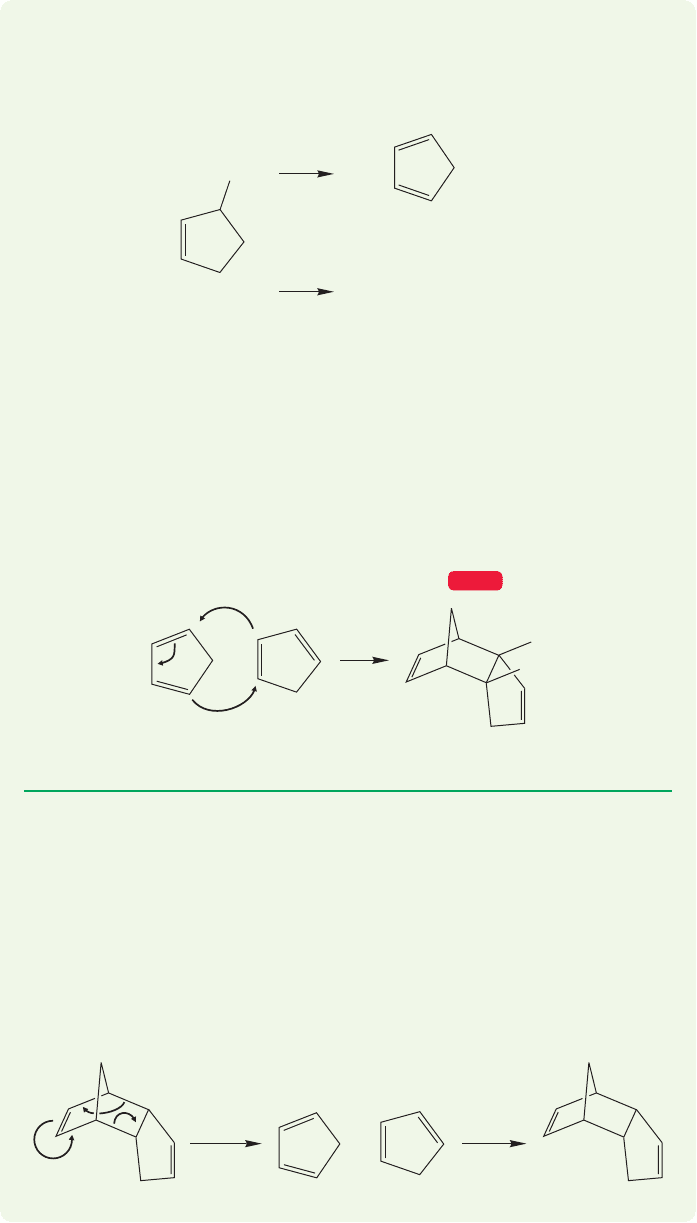
WORKED PROBLEM 12.29 You attempt to prepare 1,3-cyclopentadiene from the
base-catalyzed loss of hydrogen bromide (E2 reaction) from 3-bromocyclopen-
tene, but instead of isolating a compound having the formula C
5
H
6
, you get a
high yield of a compound with the formula C
10
H
12
.
base
base
(C
5
H
6
)
The hoped-for product
(C
10
H
12
)
The isolated product
Br
Two cyclic dienes can also react in Diels–Alder fashion. The self-condensation
of cyclopentadiene is the classic example.We’ll illustrate this reaction with a pair of
problems.
Fame, fortune,and the Nobel prize await you if you can explain this result (at least
if you were explaining it in 1928). Be careful—although this question is obviously
set up in the text, it is not so easy to write a structure correct in every detail.
ANSWER The formula of C
10
H
12
is a clue. Clearly,two C
5
H
6
molecules have joined
to make the C
10
H
12
product.The reaction is, in fact, successful.The problem is that
cyclopentadiene dimerizes in a Diels–Alder reaction very quickly.The molecule you
isolate is the Diels–Alder dimer. Note formation (mainly) of the endo adduct.
Anytime you see a 1,3-diene, especially a cyclic one, think Diels–Alder.
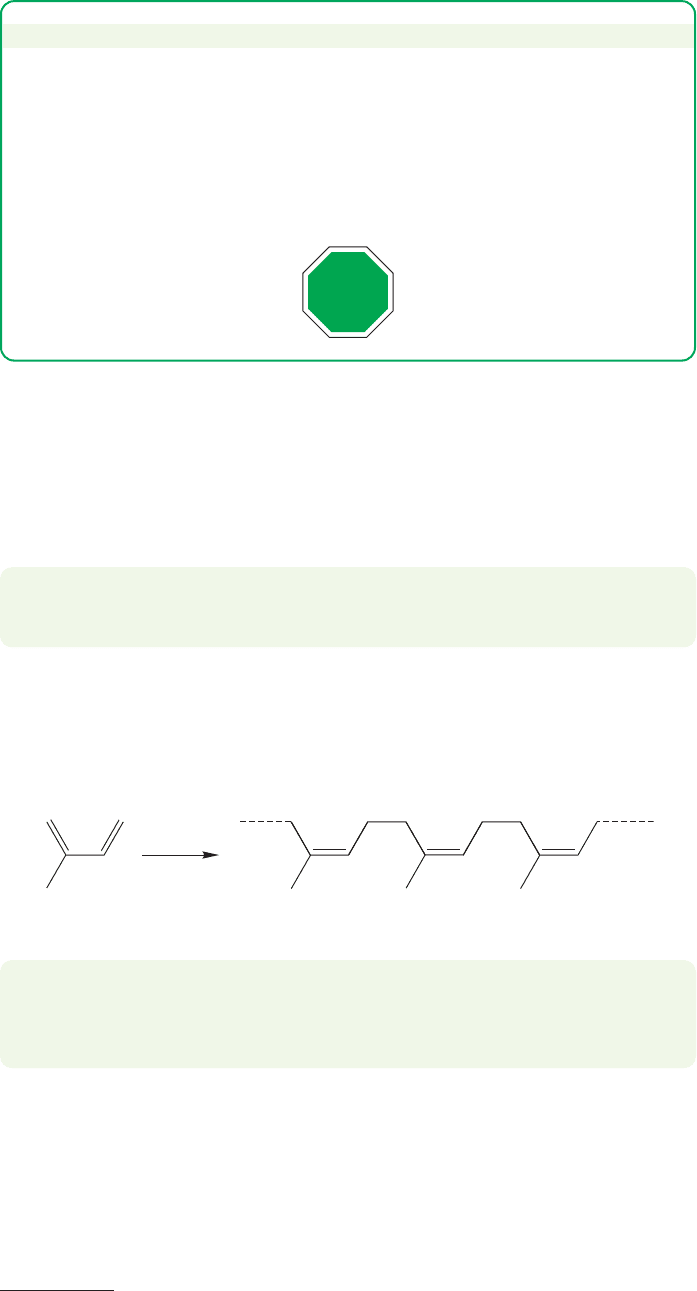
WORKED PROBLEM 12.30 In a fit of frustration or inspiration you decide to boil
(reflux) your unanticipated C
10
H
12
, and, in order to do that, you attach a long
condenser to a receiving vessel that is kept very cold. Your C
10
H
12
boils at about
165 °C, but you collect a product of the formula C
5
H
6
boiling at 40 °C. Moreover,
although this C
5
H
6
product is stable at low temperature for a long time, at room
temperature it rapidly reverts to your starting C
10
H
12
. Explain.
ANSWER At 165 °C, the Diels–Alder reaction reverses, and the low-boiling
cyclopentadiene can be collected and kept at low temperature. If it is allowed to
warm up, it again forms the Diels–Alder dimer.
⌬
+
(C
5
H
6
)(C
5
H
6
)
(C
10
H
12
)
H
H
WEB 3D
+
165 ⬚C
Cold
warm
554 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
PROBLEM SOLVING
Every cyclohexene that you see from now on—and there will be many—comes
with the messages: “Can be made through a Diels–Alder reaction,” and, “Think
reverse Diels–Alder.” Problem writers play the game called,“Hide the cyclohexene.”
Your job is to beat them at this game by recognizing all cyclohexenes, no matter
how well disguised. A very good way to become proficient at this game is to play
the other side, as it were. Set yourself the goal of disguising a cyclohexene so that it
is hard to see.
GO
12.13 Special Topic: Biosynthesis of Terpenes
When we discussed cationic addition reactions in Chapter 9, we included a section
on the polymerization reactions of alkenes (p. 384). Dienes form quite stable inter-
mediates in addition reactions and are especially prone to polymerization.
Nature!
PolyisopreneIsoprene
PROBLEM 12.32 Notice the Z configuration of the alkenes in natural rubber.
There is another, stereoisomeric form of natural rubber, also a polymer of iso-
prene, called by the wonderful name gutta percha.
3
What do you suppose it is?
3
Gutta percha comes from the Malay, getah percha (gum of the percha tree).
PROBLEM 12.31 Write a mechanism for the radical-induced polymerization of
1,3-butadiene.
Nature uses related polymerization reactions (the cart is way before the horse
here; we make approximations of Nature, not the other way round!). One form
of natural rubber is polyisoprene, which is polymerized isoprene (2-methyl-1,3-
butadiene).
Polyisoprene is a limiting case—a molecule composed of a vast number of iso-
prene units. Many important natural products called terpenes are constructed of
smaller numbers of isoprene units put together in various ways. Terpenes are wide-
spread in nature and are still often extracted from plants. The smaller terpenes are
widely used as flavors and fragrances. Indeed, terpene chemistry is the very basis of
the fragrance industry.

12.13 Special Topic: Biosynthesis of Terpenes 555
3-Methyl-3-buten-1-ol pyrophosphate
(isopentenyl pyrophosphate)
3-Methyl-3-buten-1-ol Pyrophosphoric acid
HO
P
P
O
..
..
..
..
..
..
O
..
..
O
..
..
OH
..
..
OH
OH
..
..
..
..
P
P
O
..
..
..
..
OH
..
..
OH
OH
..
..
..
..
+
O
OH
O
..
..
O
..
..
FIGURE 12.69 In the biosynthesis of terpenes, 3-methyl-3-buten-1-ol is first converted
into the corresponding pyrophosphate.
Recall the discussion of various ways to change OH, a very poor leaving group,
into much better leaving groups (p. 281). We saw examples of protonation to make
the leaving group water, and of formation of a sulfonate ester (reviewed in Fig. 12.70).
Formation of the pyrophosphate ester serves exactly the same purpose and is wide-
ly used in Nature to generate a good leaving group from a poor one.
Most of the reactions used to make terpenes from diene units can be largely under-
stood in terms of reaction mechanisms we know well.For example, 3-methyl-3-buten-
1-ol is converted first into the corresponding 3-methyl-3-buten-1-ol pyrophosphate
(isopentenyl pyrophosphate) by reaction with pyrophosphoric acid (Fig. 12.69).
Pyrophosphoric acid
Water (an excellent
leaving group)
Hydroxide (a very
poor leaving group)
Tosylate, OTs
(another excellent leaving group)
..
..
OH
PP
O
..
..
O
..
..
..
..
O
..
..
O
..
..
OH
..
..
OH
OH
..
..
CH
2
R
CH
2
R
CH
2
R
OH
2
..
H
3
O
..
+
..
..
OH
CH
2
SO
2
CH
3
R
..
..
OH
CH
2
R
CH
2
R
Cl
..
..
..
SO
2
CH
3
O
..
..
HO
PP
O
..
..
..
..
..
..
O
..
..
O
..
..
OH
..
..
OH
OH
..
..
Pyrophosphate, OPP
(still another excellent leaving group)
+
Tosyl chloride
FIGURE 12.70 Three ways of
converting OH, a poor leaving group,
into a good leaving group.
PROBLEM 12.33 Explain why the pyrophosphate ester in Figure 12.70 is an
excellent leaving group. The abbreviation OPP is used for the pyrophosphate
group.
556 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
3-Methyl-3-buten-1-ol pyrophosphate 3-Methyl-2-buten-1-ol pyrophosphate
P
P
O
..
..
..
..
O
..
..
O
..
..
OH
..
..
OH
OH
..
..
..
..
O
enzyme
P
P
O
..
..
..
..
O
..
..
O
..
..
OH
..
..
OH
OH
..
..
..
..
O
FIGURE 12.71 An enzyme
converts 3-methyl-3-buten-1-ol
pyrophosphate into a new,
allylic pyrophosphate.
3-Methyl-3-buten-1-ol
pyrophosphate
3-Methyl-2-buten-1-ol pyrophosphate
Geranyl pyrophosphate
(geranyl–OPP)
P
P
O
..
..
..
..
O
..
..
O
..
..
O
..
..
O
..
..
OH
..
..
OH
OH
=
..
..
..
..
O
P
P
O
..
..
..
..
OH
..
..
OH
OH
..
..
..
..
O
+
+
S
N
1
OPP
OPP
+
E1
OPP
H
New bond
FIGURE 12.72 Loss of the pyrophosphate gives an allylic cation that can be
attacked by a molecule of 3-methyl-3-buten-1-ol pyrophosphate to give a
new cation. Deprotonation gives geranyl pyrophosphate.
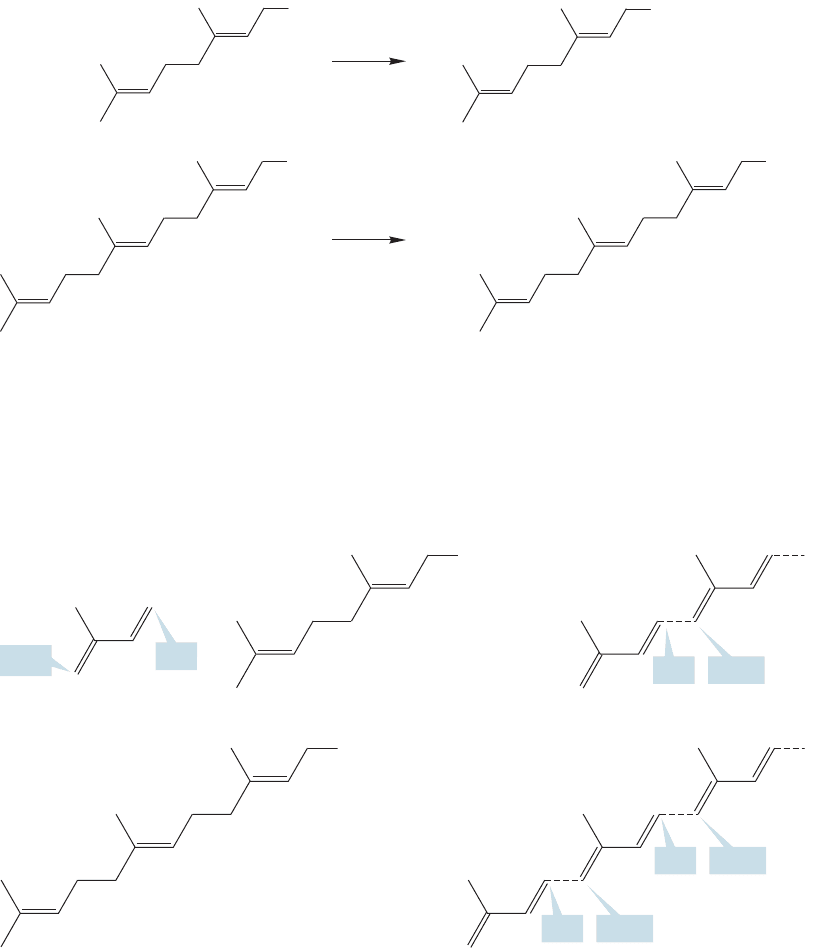
There is an enzyme that equilibrates 3-methyl-3-buten-1-ol pyrophosphate with
an isomeric pyrophosphate, 3-methyl-2-buten-1-ol pyrophosphate (Fig. 12.71).
Geranyl–OPP
+
+
OPP
H
OPP
New bond
OPP
Farnesyl pyrophosphate
(farnesyl–OPP)
OPP
E1
FIGURE 12.73 The addition process can be repeated to give farnesyl pyrophosphate.
Note that this new pyrophosphate now has a good leaving group in an allylic posi-
tion.The combination of a good leaving group and the allylic position makes ioniza-
tion to form an allylic cation easy. The cation is attacked by 3-methyl-3-buten-1-ol
pyrophosphate to produce ultimately the C
10
compound, geranyl pyrophosphate
(Fig. 12.72).
Because geranyl pyrophosphate has a good leaving group in an allylic position,
this process can be repeated to produce the C
15
compound, farnesyl pyrophosphate
(Fig. 12.73).
12.13 Special Topic: Biosynthesis of Terpenes 557
Geranyl–OPP Geraniol
Farnesyl–OPP
OPP
OPP
Farnesol
OH
..
..
H
3
O
..
+
H
2
O
..
..
H
3
O
..
+
H
2
O
..
..
OH
..
..
FIGURE 12.74 The alcohols geraniol
and farnesol are formed by hydrolysis
of the related pyrophosphates.
Hydrolysis of geranyl pyrophosphate and farnesyl pyrophosphate produces the
terpenes geraniol and farnesol, respectively (Fig. 12.74).
Isoprene units can be characterized as having a head and a tail as shown in
Figure 12.75. Notice that both geranyl and farnesyl pyrophosphate are formed by
head-to-tail connections of isoprenes.
Geranyl–OPP
Farnesyl–OPP
OPP
OPP
Isoprene
OPP
OPP
comes from
comes from
HeadTail
Head
Tail
HeadTail
HeadTail
FIGURE 12.75 Head-to-tail attachment of isoprene units gives geranyl and farnesyl pyrophosphates.
The limit of head-to-tail combining is polyisoprene,natural rubber (p.554).Your
drawing of the polymer of isoprene called gutta percha (Problem 12.32) should also
have head-to-tail connections.
Molecules formed from isoprene units are made of multiples of five carbons. Dif-
ferent names are used corresponding to the number of isoprene units in the molecule.
Two units of isoprene combine to form terpenes,C
10
compounds such as geraniol.Three
isoprene units form sesquiterpenes,C
15
compounds such as farnesol.Diterpenes are C
20
compounds, triterpenes are C
30
compounds, and so on. Myriad terpenoid compounds
are known, and most can be traced to derivatives of isoprene as starting material. Most
terpenes follow the isoprene rule, which dictates the head-to-tail formation described
earlier. All of the terpenes shown in Figure 12.76 do not strictly follow the pattern, but
each has at least one head-to-tail connection.
558 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
WEB 3D
Limonene Menthol
Thujone
(1S )-(
–)-β-Pinene
(1R)-(
+)-Camphor
(1R)-(
+)-α-Pinene
(1S)-(
–)-Camphor
OH
O
O
O
O
O
=
=
=
=
FIGURE 12.76 Some terpenes.
If you have experienced the rich odor
of a pine tree forest, then this photo
will remind you of that pleasant
smell. It comes from volatile terpenes
that fill the air.
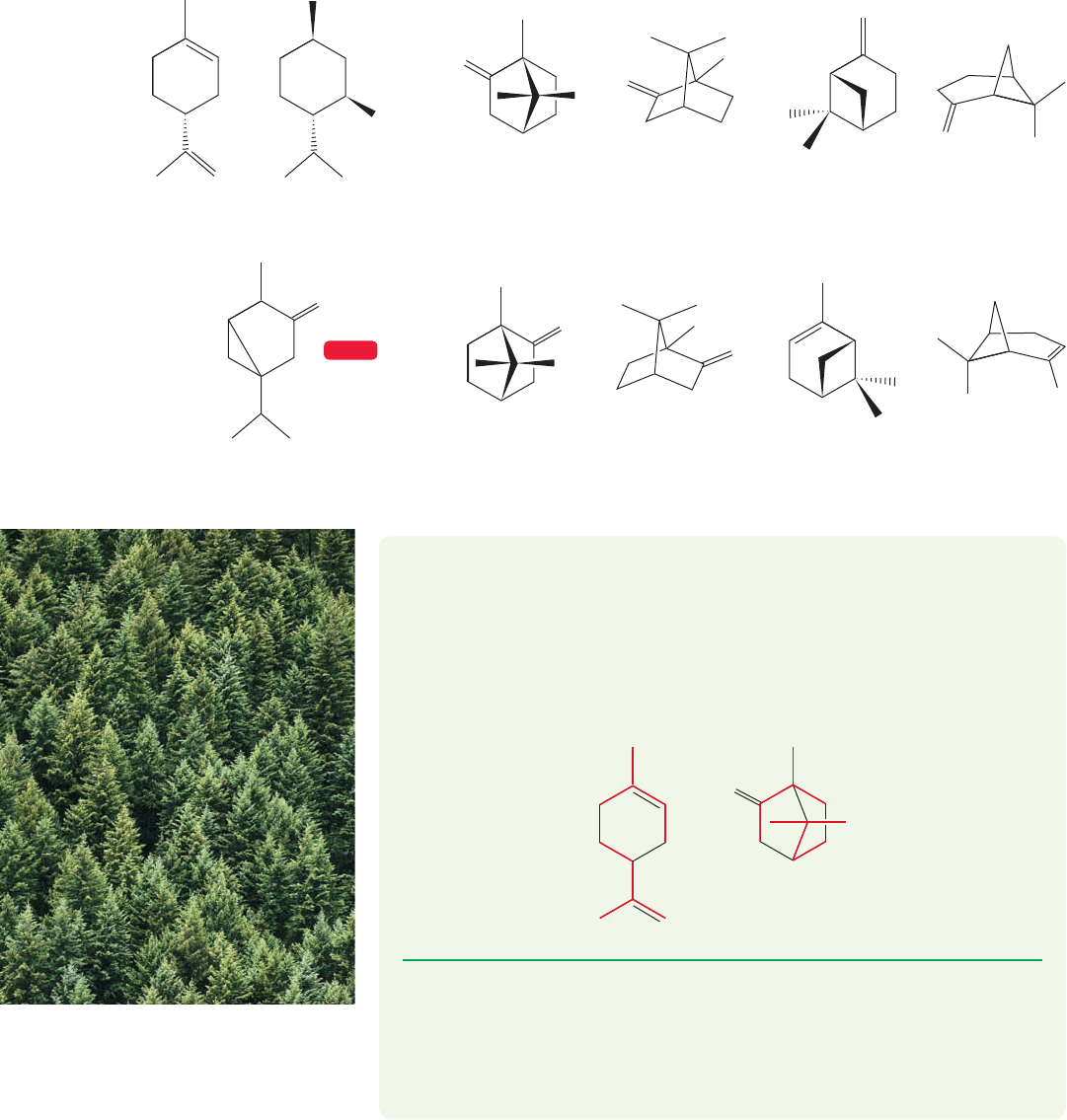
WORKED PROBLEM 12.34 Dissect limonene and (1R)-()-camphor (Fig. 12.76)
into isoprene units.
ANSWER The isoprene units can usually be found by searching for the methyl
groups first. One of these problems is easy, the other is more complicated. Don’t
worry about oxygen atoms or double bonds, just look for carbon framework. The
two isoprenes for each compound are shown in red.
O
PROBLEM 12.35 Devise a mechanism for converting geranyl pyrophosphate into
racemic limonene (Fig. 12.76). Such processes are usually enzyme-mediated in
Nature. When you try this problem you should encounter a roadblock, because
the process can’t really be done without a cis/trans isomerization, very likely
carried out in Nature by the enzyme.
