Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

12.14 Special Topic: Steroid Biosynthesis 559
12.14 Special Topic: Steroid Biosynthesis
There is an extraordinarily important class of molecules called steroids. The carbon
framework common to all steroids is three cyclohexanes and a cyclopentane fused
together (Fig.12.77).This arrangement is often referred to as “three rooms and a bath.”
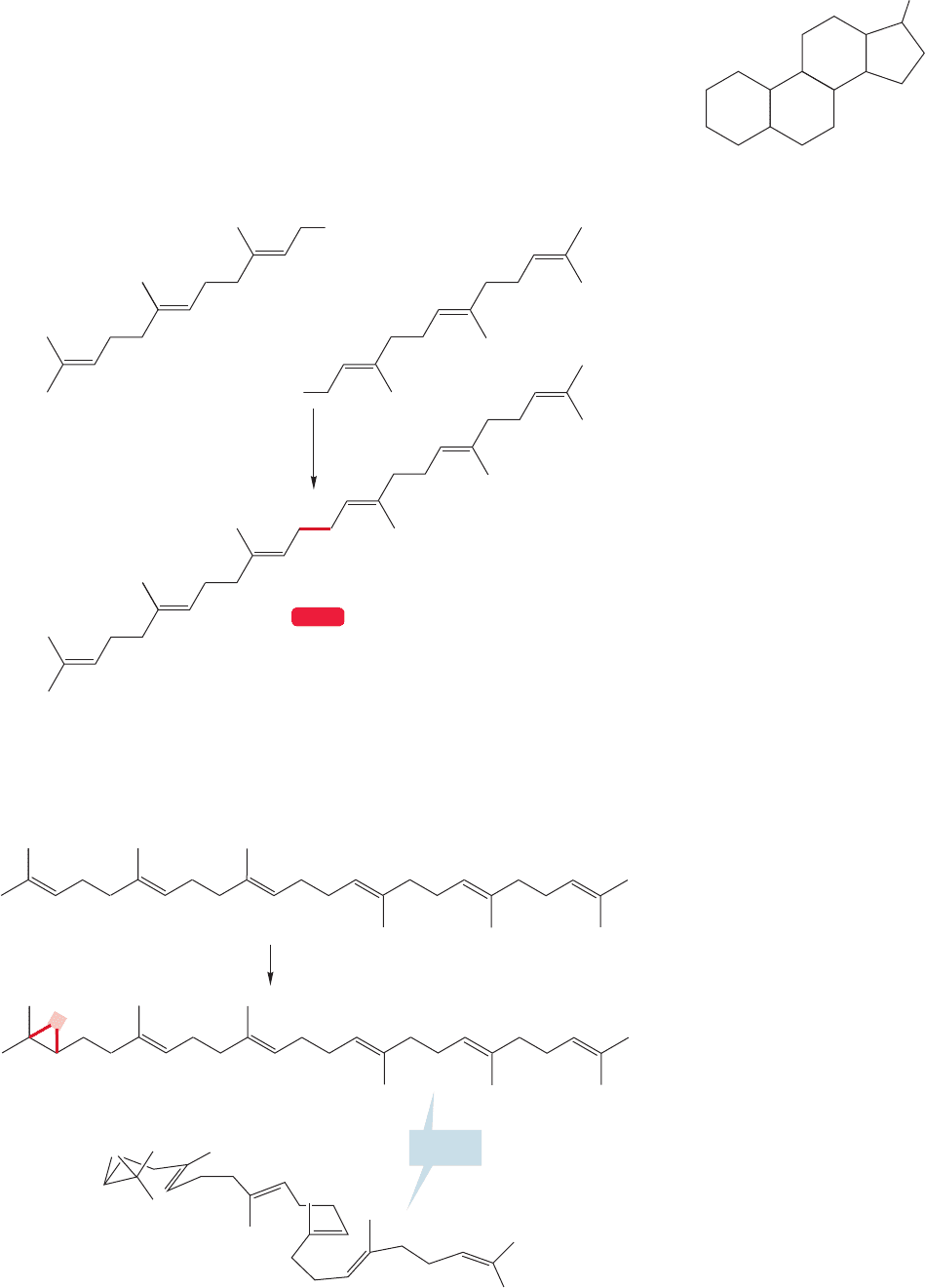
Steroids are formed in Nature from isoprene units.We will start our summary of the
biosynthesis with two units of farnesyl pyrophosphate, which can be enzymatically
coupled in a tail-to-tail fashion to give the symmetrical triterpene called squalene
(Fig. 12.78).
R
17
16
15
14
8
7
64
5
1
2
3
9
11
12
13
10
FIGURE 12.77 Basic steroid ring
system.
WEB 3D
Farnesyl–OPP Farnesyl–OPP
Squalene
OPP
PPO
coupling
FIGURE 12.78 Coupling of two
molecules of farnesyl pyrophosphate
gives squalene, a triterpene.
Squalene can be epoxidized to give squalene oxide, which is written in a sug-
gestive way in Figure 12.79. Protonation of squalene epoxide leads to a relatively
enzymatic
epoxidation
Squalene
Squalene
oxide
O
..
..
O
..
..
FIGURE 12.79 Enzymatic
epoxidation of squalene gives
squalene oxide, redrawn in a
suggestive fashion at the bottom.
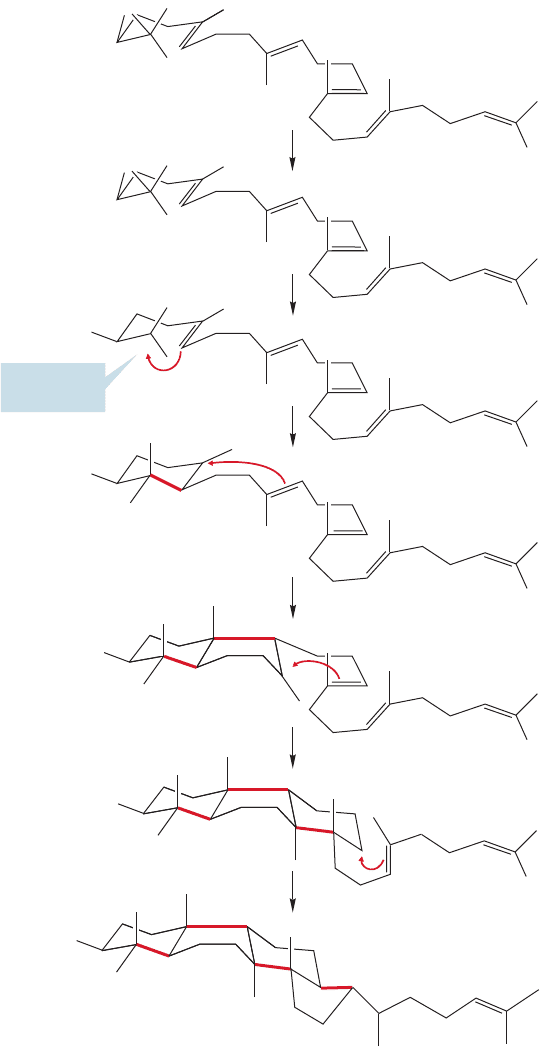
560 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
stable tertiary carbocation that is attacked by the proximate nucleophilic double
bond to give a new cation. This cation is attacked in turn by another nearby
alkene.The process continues until the molecule is sewn up in a four-ring steroid
skeleton as shown in Figure 12.80. The series of rearrangements summarized in
O
..
..
HO
..
HO
..
..
HO
..
..
HO
..
..
HO
..
..
+
+
+
+
+
+
HO
..
..
Tertiary
carbocation
The four-ring steroid skeleton is now constructed
FIGURE 12.80 Acid-catalyzed opening of the epoxide gives a tertiary cation that can
undergo a series of ring closings to give a new, tertiary carbocation in which the four-ring
steroid skeleton has been constructed.
12.14 Special Topic: Steroid Biosynthesis 561
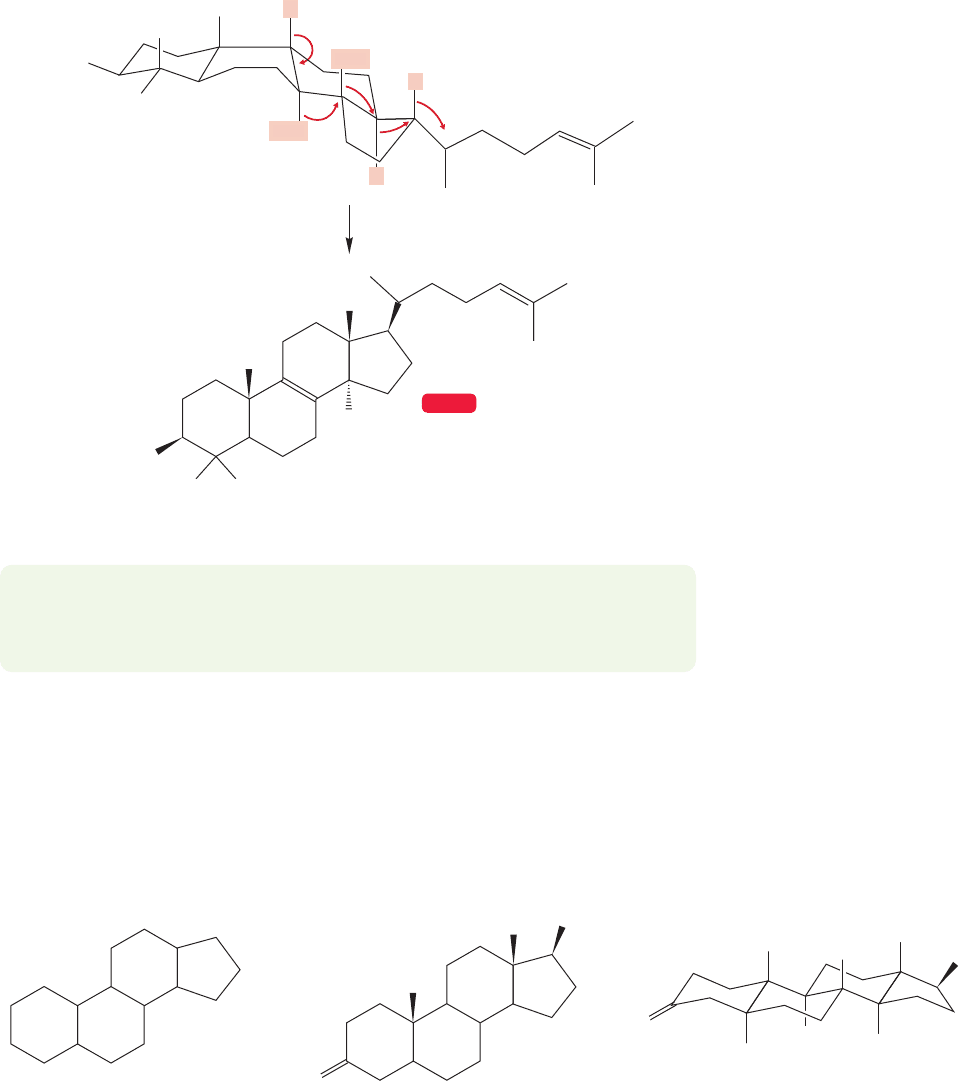
PROBLEM 12.36 Starting at the right-hand end of the top molecule in Figure
12.81, write a stepwise mechanism for the conversion to lanosterol. Take the sev-
eral rearrangements one at a time and draw out each reaction intermediate.
WEB 3D
Lanosterol
+
HO
..
..
HO
..
..
H
3
C
H
3
C
C
H
3
CH
3
CH
3
CH
3
CH
3
CH
3
H
H
H
H
3
C
CH
3
FIGURE 12.81 A series of skeletal
rearrangements, followed by removal
of a proton, gives lanosterol.
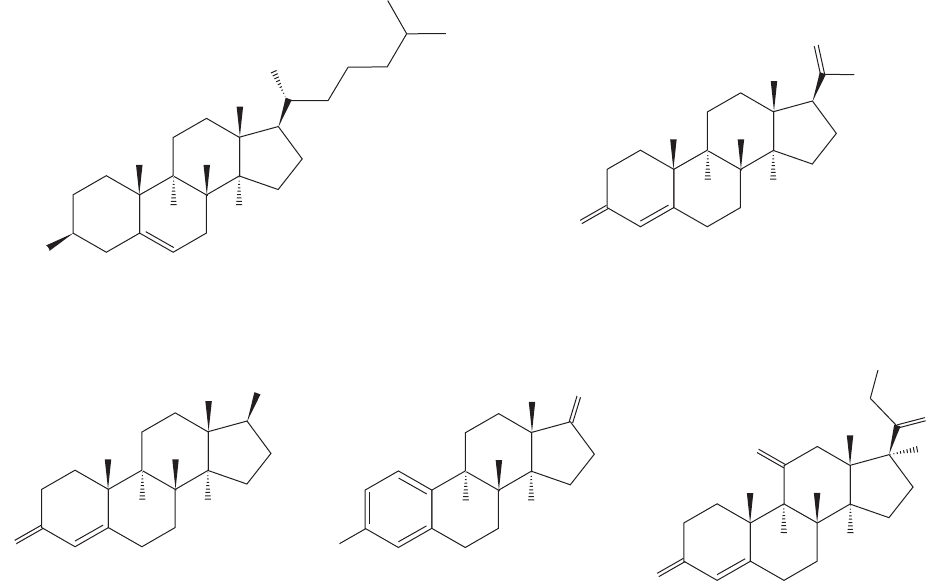
Steroids are commonly found in Nature and are considered important prima-
rily because of their powerful biological activities. They often function as regula-
tors of human biology. The four rings are designated A, B, C, and D, and the
carbons are numbered as shown in Figure 12.82. As in lanosterol, steroids usually
have axial methyl groups attached to C(10) and C(13), and often carry an oxygen
atom at C(3) and a carbon chain at C(17). The rings are usually, but not always,
fused in trans fashion.
AB
CD
2
3
3
6
7
8
9
10
10
11
12
13
13
14
15
16
18
19
17
17
4
1
5
O
CH
3
CH
3
H
O
H
H
R
R
H
FIGURE 12.82 The steroid ring system.
Steroids were, and still can be, isolated from natural sources. Such important bio-
molecules quickly became the subject of attempts at chemical modification and total
synthesis in the hopes of uncovering routes to new molecules of greater and different
Figure 12.81 ensues and a proton is finally lost to give the compound known as
lanosterol.
562 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
Cholesterol
Testosterone Estrone Cortisone
Progesterone
HO
H
3
C
H
3
C
H
3
C
O
HH
H
O
O
CH
3
H
3
C
H
3
C
HH
H
OH
O
H
3
C
H
3
C
HH
H
O
HO
H
3
C
HH
H
H
3
C
H
3
C
H
H
OH
H
O
O
OH
FIGURE 12.83 Some naturally occurring steroids.
Whole industries have been derived from the laboratory syntheses of steroids.
Consider, for example, the steroids used in our attempts to control human fertility.
“The pill”used by women to avoid pregnancy or to control hormonal imbalance con-
tains synthetic steroids that mimic the action of the body’s natural steroids,the estro-
gens and progesterone, which are involved in regulation of ovulation. The body is
essentially tricked into behaving as if it were pregnant, so ovulation does not take
place. It should be no surprise that it has been difficult to use such powerfully bio-
logically active molecules in a completely specific way. The goal of this work is to
interfere specifically with ovulation without doing anything else. Considering the
activity of the molecules involved and the complexity of the mechanisms we are try-
ing to regulate, it is remarkable that we have been so successful. This control is not
totally without cost, however. In fertility, those costs appear as the side effects of the
pill, which occur in some women. Perhaps the most difficult of human problems is
to find the balance between benefits and risks in cases such as this. Although such
problems have been with us throughout our history, questions of biology are partic-
ularly vexing, it seems. It is easier to judge that the benefits of the automobile out-
weigh the societal costs of the certain accidents than to make a decision regarding
the steroids used in the birth-control pill.This problem is certain to become much
worse as our ability to manipulate complicated molecules continues to grow.
bioactivities. Many naturally occurring steroids have been constructed in the labora-
tory and numerous “unnatural” steroids have been created as well. Figure 12.83 shows
a few important steroids found in Nature.
12.15 Summary 563
12.15 Summary
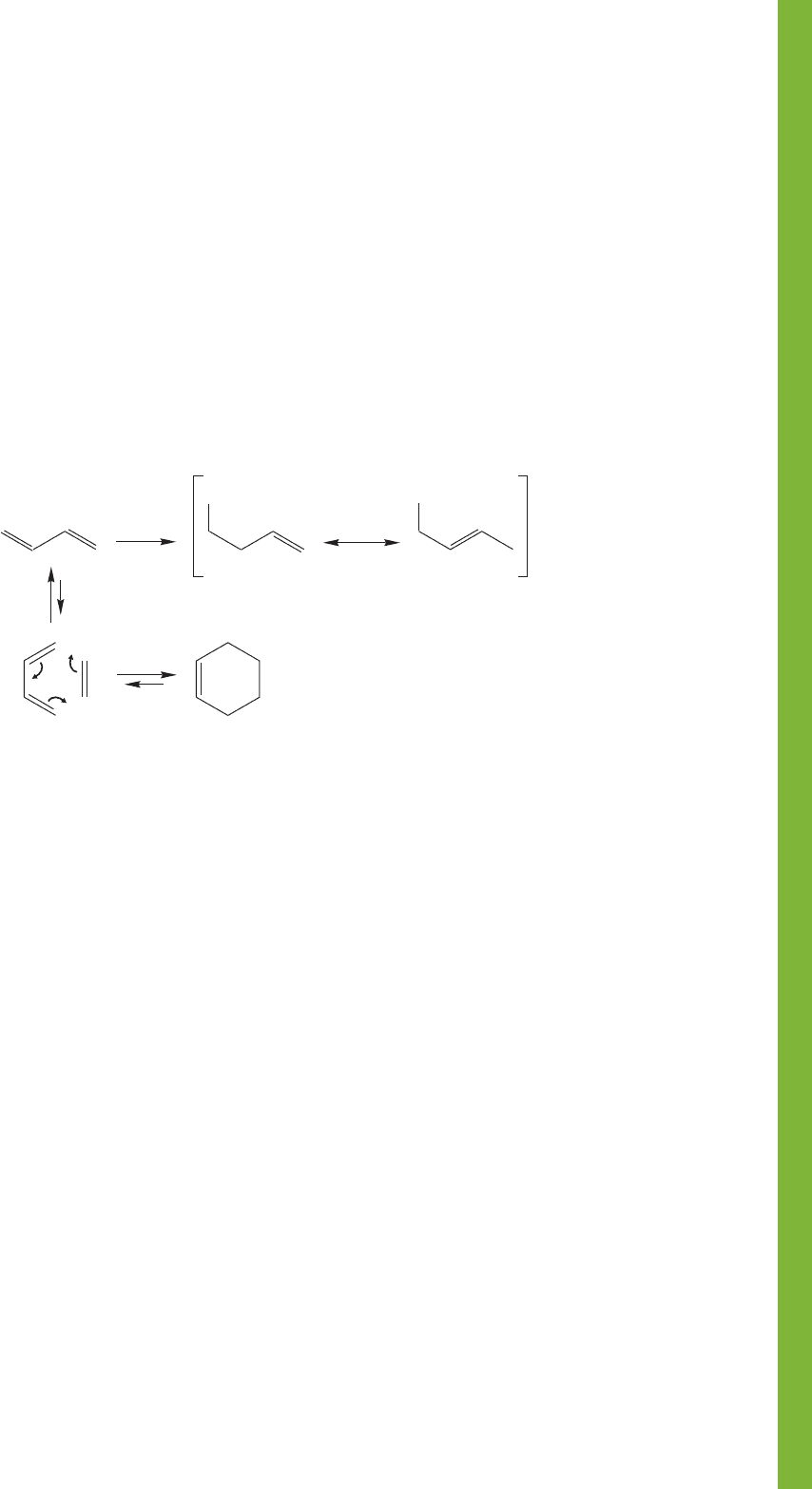
New Concepts
In this chapter, you learned about the cumulated dienes. These
molecules are characterized by at least one sp hybridized inter-
nal carbon atom.
The central topics of this chapter are the chemical and phys-
ical consequences of the overlap of 2p orbitals (conjugation). In
structural terms, conjugation appears as a short
bond, about which the barrier to rotation is small (Fig. 12.18).
Conjugation leads to the formation of allyl systems in
addition reactions to dienes, and is a requirement for the
Diels–Alder reaction (Fig. 12.84).
C(2)
O
C(3)
An important fundamental concept in this chapter
involves the difference between thermodynamic and kinetic
control of a reaction. Conjugated dienes will react with HX
or X
2
to give 1,2-addition products when kinetic conditions
are used. The use of thermodynamic conditions will favor
formation of the most stable product, usually the 1,4-addition
product.
In UV/vis spectroscopy, absorption of light results in
the promotion of an electron from the HOMO to the
LUMO.
H
H
2
O
..
..
+
H
An allylic cation
⌬
+
H
3
O
..
+
FIGURE 12.84 Two typical reactions of conjugated dienes: addition to
give an allyl cation and the Diels–Alder reaction.
Key Terms
s-cis (p. 523)
conjugated double bonds (p. 512)
cumulated alkene (cumulene) (p. 512)
Diels–Alder reaction (p. 544)
dienophile (p. 544)
electronic spectroscopy (p. 526)
endo (p. 551)
exo (p. 551)
extinction coefficient (p. 527)
isoprene (p. 555)
isoprene rule (p. 558)
ketene (p. 515)
steroid (p. 559)
terpenes (p. 554)
s-trans (p. 523)
ultraviolet/visible (UV/vis) spectroscopy
(p. 526)
Reactions, Mechanisms, and Tools
The base-catalyzed isomerization of disubstituted to terminal
alkynes takes place through allene intermediates (Fig. 12.13).
Addition reactions of HX or X
2
to conjugated dienes can
give products of both 1,2- and 1,4-additions (Fig. 12.38).
The Diels–Alder reaction forms cyclohexenes and cyclohexa-
dienes from the thermal reaction of conjugated dienes and
alkenes or alkynes. Stereochemical labeling experiments
show that both new σ bonds are formed simultaneously
(Figs. 12.61 and the figure in Problem 12.28). Many
natural products (terpenes, polyisoprene, steroids) are pro-
duced through the combination of various numbers of
isoprene units.
Syntheses
The most important synthetic methods described in
this chapter are a route to allylic halides through additions
to conjugated dienes and the Diels–Alder synthesis of
cyclohexenes and cyclohexadienes. Be especially careful
about the Diels–Alder reaction, because there are many
structural possibilities for the products depending on the
complexity of the diene and dienophile used as starting
materials. See the following page.
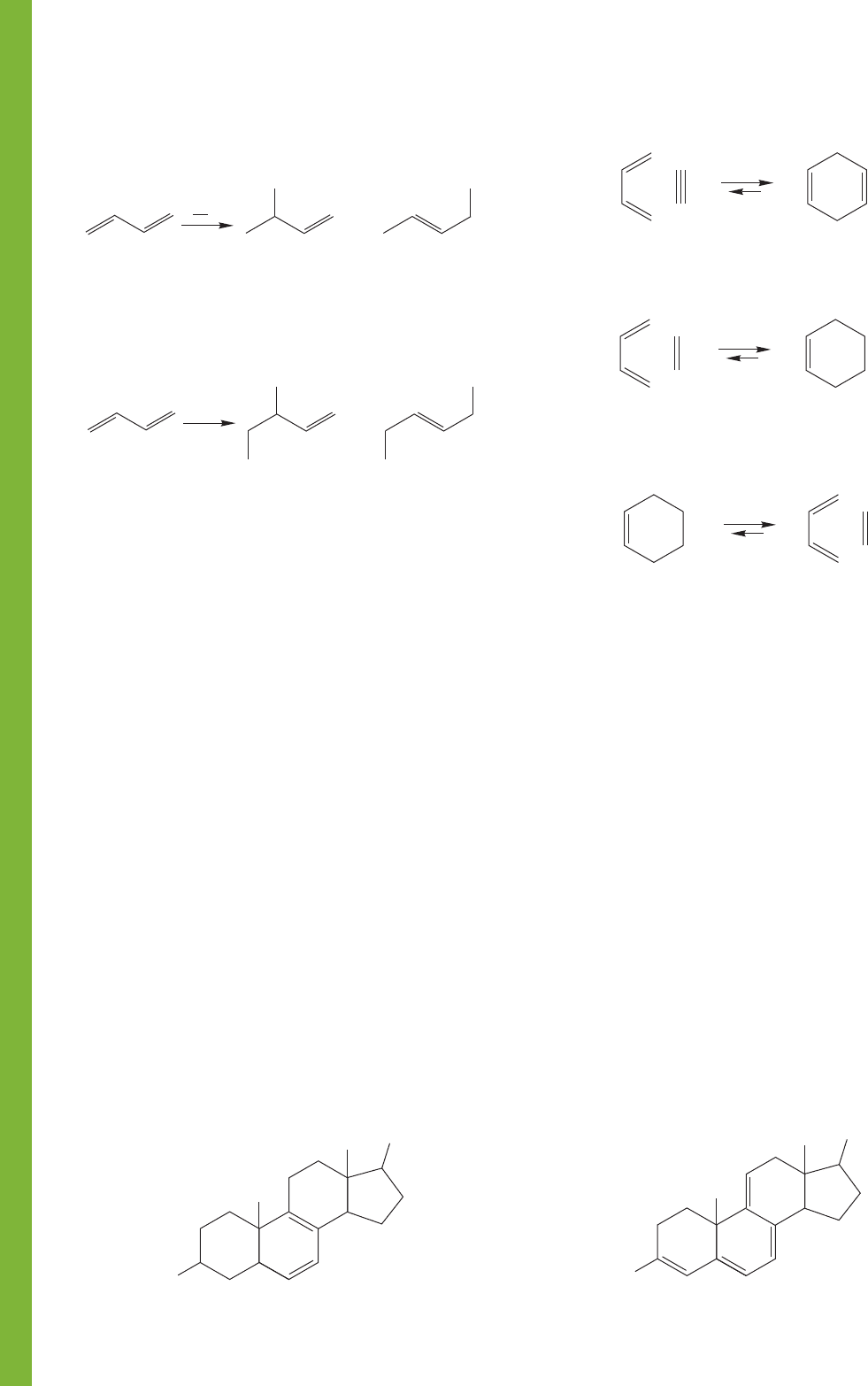
564 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
Common Errors
4. Dienes
3. Cyclohexenes
⌬
Diels–Alder reaction; watch out for structural variation; the
reverse reaction is a synthesis of 1,3-dienes and alkenes
from cyclohexenes
⌬
The reverse Diels–Alder reaction; watch out for structural
variation; this picture gives only the simplest version
Be sure to understand how the structures of allenes and cumu-
lenes can be derived from an analysis of the hybridizations of
the atoms involved. Although the structures all look flat on a
two-dimensional page, they are not all planar by any means.
Although the Diels–Alder reaction mechanism can be
quickly drawn using only three little curved arrows, and
although it is not difficult to see that all cyclohexenes can be
constructed on paper from the reaction of a diene and a
dienophile, very complex structures can be built very quickly
using Diels–Alder reactions. The simplicity can quickly disap-
pear in the wealth of detail! Try to see to the essence of the
problem; locate the cyclohexene, and then take it apart to find
the building-block diene and dienophile.
It is even harder to find a reverse Diels–Alder reaction in a
mechanism problem or a chemical synthesis. Not only can
cyclohexenes be constructed through Diels–Alder reactions, but
they can serve as sources of dienes and dienophiles as well.
It is time to mention again another very common conceptual
error that often traps students. Look, for example, at Figure 12.44.
Protonation of 1,3-butadiene leads to an allyl cation in which two
different carbons share the positive charge. Addition of chloride at
these two positions leads to the two products. It is very easy to
think of the resonance forms of the allyl cation as each having a
separate existence; of chloride adding to one resonance form to
give one product and addition to the other resonance form to give
the other product.This notion is absolutely wrong! The allyl
cation is a single species that has two partially positive carbon
atoms. Chloride adds to the allyl cation, not to one resonance form
or the other. The figure tries to help out by showing a summary
structure, but this is not always done, and you must be careful.
HO
(a)
H
3
C
H
3
C
C
8
H
17
AcO
(b)
H
3
C
H
3
C
C
9
H
19
PROBLEM 12.37 Use the rules given in Table 12.2 (p. 530) to
calculate λ
max
for the following steroids:
12.16 Additional Problems
2. Cyclohexadienes
⌬
The dienophile is an alkyne; watch out for
structural variation and the reverse reaction
H X
+
The 1,2- and 1,4-addition; watch out, these allylic halides
can be transformed further though S
N
2 reactions
X
X
X
2
+
Both 1,2- and 1,4-addition take place
XX
X
X
1. Allylic Halides
12.16 Additional Problems 565
+
–
–
(a) (b)
(c) (d)
..
..
–
..
PROBLEM 12.39 Are any of the following compounds chiral?
Explain with good drawings.
PROBLEM 12.45 Suggest a plausible arrow formalism mecha-
nism to account for the products in the following reaction:
CC
C
C
H
3
C
H
3
C
CH
3
CH
3
CCC
H
3
C
CH
3
CH
2
CH
3
CH
2
CH
3
C
C
C
C
C
CH
3
H
3
C
PROBLEM 12.40 Show the reaction conditions you would
use to make 3-azido-1-butene if your only source of carbon is
1,3-butadiene.
PROBLEM 12.41 What are the possible nonhalogenated prod-
ucts in the reaction between cis-1,2-dibromocyclohexane and
excess strong base? Show the mechanism for the formation of
each product. Which one do you suppose would be the major
product? Explain why.
PROBLEM 12.42 Predict the major product for the hydrohalo-
genation reaction (HBr, cold, short time) with each of the
following dienes:
(a) 1,3-butadiene (b) 1,3-cyclopentadiene
(c) (Z)-1,3-pentadiene (d) (E)-2-ethyl-1,3-pentadiene
(e) 1-methyl-1,3-cyclohexadiene
PROBLEM 12.43 Predict the major product for the hydrohalo-
genation reaction (HBr, reflux, long time) with each of the
following dienes:
(a) 1,3-butadiene (b) 1,3-cyclopentadiene
(c) (Z)-1,3-pentadiene (d) (E)-3-ethyl-1,3-pentadiene
(e) 1-methyl-1,3-cyclohexadiene
PROBLEM 12.44 Show the main products of the reactions of
trans,trans-2,4-hexadiene under the following conditions. There
may be more than one product in some cases.
(a) HCl (b) H
2
/Pd (c) Cl
2
/CCl
4
(d) ⌬, H
3
COOC COOCH
3
CC
1
(63%)
2
(22%)
3
(10%)
4
(4%)
Br Br
BrBr
Br
Br
OCH
3
OCH
3
–15 ⬚C
Br
2
/CH
3
OH
PROBLEM 12.46 Predict the products of the reaction of hydro-
gen chloride with isoprene under kinetic and thermodynamic
control.
Isoprene
HCl
PROBLEM 12.47 Solvolysis of 3-chloro-4,4-dimethylcyclohex-
ene proceeds through an intermediate carbocation. Draw that ion.
Does an alkyl shift occur? Why or why not? Hint: Table 12.3.
PROBLEM 12.48 Show the reactions you would use to synthe-
size cis-4,5-dimethylcyclohexene if your only source of carbon is
cis-2-butene.
PROBLEM 12.49 Devise syntheses for the following molecules.
You may use 1-butyne and ethyl iodide as your only sources of
carbon, as well as any inorganic reagents you need.
CH
CC
CC
C
C
C
C
C
H
H
H
H
PROBLEM 12.38 Write resonance forms for the following ions:
566 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
PROBLEM 12.50 Conversion of 2-butyne into 1-butyne is
easy. The reverse reaction is more difficult. Suggest routes for
doing both these conversions. Mechanisms are not required.
H
3
CCC
?
CH
3
CH
3
CH
2
CC
H
H
3
CCC
?
CH
3
CH
3
CH
2
CC
H
PROBLEM 12.51 You have a bottle of C
6
H
10
. You know it
contains a terminal alkyne. Which alkyne does it need to be
in order to make 2,2-dimethyl-3-octyne most directly?
PROBLEM 12.52 Predict the major product for the Diels–Alder
reaction (toluene, reflux) between 2-methyl-1,3-butadiene and
the following dienophiles:
(a) cyclopentene
(b) diphenylacetylene
(c) (E)-4-octene
(d) (Z)-4-octene
PROBLEM 12.53 Predict the products of the following
Diels–Alder reactions:
(b)
(a)
(c)
(d)
0–25 ⬚C
ether
150 ⬚C
toluene
0 ⬚C
alcohol
dichlorobenzene
O
O
+
+
+
+
⌬
Ph
Ph
O
Ph
Ph
Ph =
COOCH
3
COOCH
3
COOCH
3
COOCH
3
C
10
H
6
O
4
Ph
4
COOCH
3
(f)
benzene
⌬, 9 h
CH
3
O
O
O
+
H
3
C
C
8
H
17
(g)
N
N
N
N
25 ⬚C
dioxane
COOCH
3
COOCH
3
+
C
8
H
8
N
2
O
4
OCH
2
CH
3
(e)
toluene
O
O
O
+
⌬,10 h
CH
3
H
3
C
C
8
H
17
PROBLEM 12.54 Although simple alkyl and aryl nitriles
are poor dienophiles, nitriles substituted with electron-
withdrawing groups are more reactive. Provide mechanisms
for the formations of the two products in the following
reaction:
NC
Δ
CH
3
CH
2
OOC
O
+
+
CH
3
Ph
Ph
Ph
N
Ph
Ph
Ph
CH
3
COOCH
2
CH
3
N
Ph
Ph
COOCH
2
CH
3
H
3
C
Ph
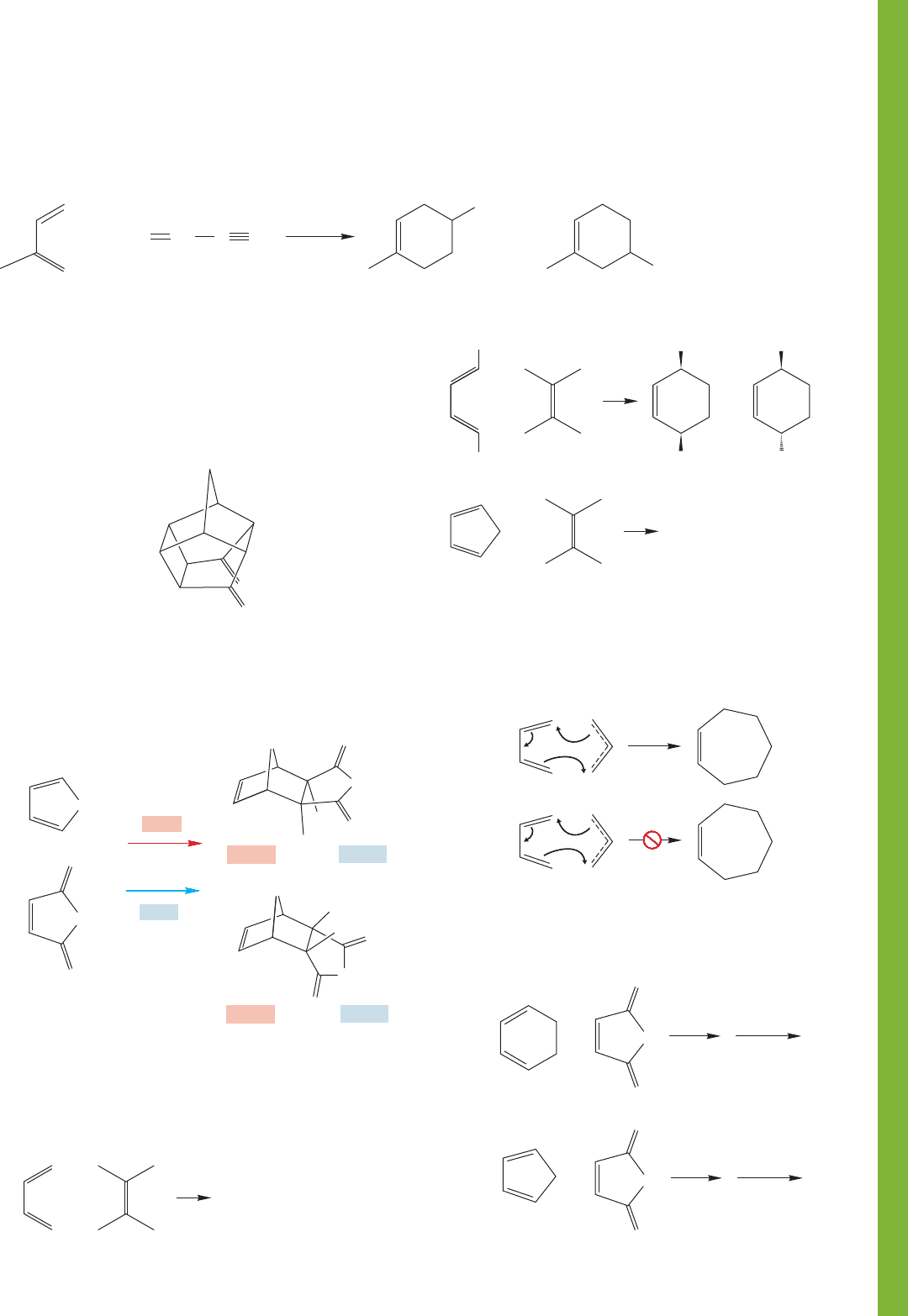
12.16 Additional Problems 567
PROBLEM 12.55 Although the Diels–Alder reaction is con-
certed, an analysis of a polar, stepwise mechanism can explain
the regiochemical preferences often observed. Use such
an analysis to explain the following data:
PROBLEM 12.58 In each of the following three reactions, two
products are possible. Could
13
C NMR spectroscopy distinguish
between the isomeric products formed? Explain. Can you
remember or predict which products are actually formed in
these reactions?
PROBLEM 12.56 In Problem 12.53c, the initially formed
adduct can be closed in a photochemical reaction to give a com-
plex cage structure (shown below). Write an arrow formalism
for this process and explain how the observation of this reaction
was helpful in determining the stereochemistry (exo or endo) of
the original Diels–Alder adduct.
++
H
2
C
CH
2
Cl
2
CH C N
70% 30%
CN
CN
O
O
PROBLEM 12.57 Use a carefully constructed Energy versus
Reaction progress diagram to rationalize the following data. Be
sure to explain why the product at low temperature is mainly
endo and why the product at high temperature is mainly exo.
+
O
O
O
O
O
O
O
O
O
H
NH
NH
exo
endo
NH
H
H
H
90 ⬚C
(minor)
(major)
25 ⬚C
(minor)
(major)
+
Cyclohexene or
vinylcyclobutane
(a)
HH
HH
+
(b)
HH
HH
+
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
or
(c)
HH
HCN
+
endo or exo Product
PROBLEM 12.59 Here are two reactions of allyl systems that
are related to the Diels–Alder reaction. Use a HOMO–LUMO
molecular orbital analysis to show why reaction a succeeds
whereas reaction b fails.
PROBLEM 12.60 In principle, one of the following reaction
sequences could give two products, while the other must pro-
duce only one. Explain.
+
+
(a)
–
–
(b)
(a)
(b)
O
O
O
+
⌬
H
2
/Pd
O
O
O
+
⌬
H
2
/Pd
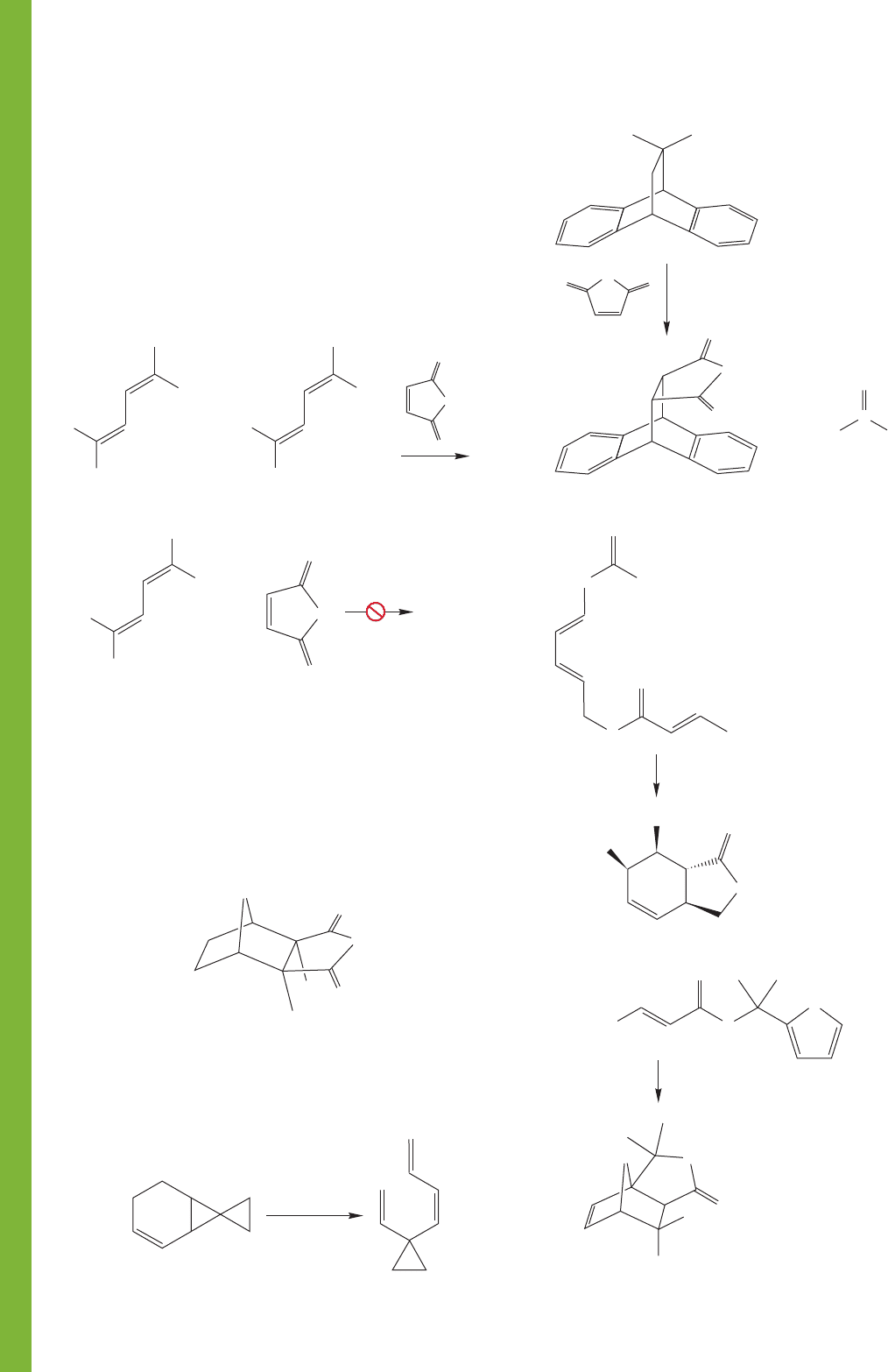
568 CHAPTER 12 Dienes and the Allyl System: 2p Orbitals in Conjugation
PROBLEM 12.61 In 1951, the great Kurt Alder himself exam-
ined the reactions of a mixture of dienes 1 and 2 with maleic
anhydride. He discovered that diene 1 reacted at 35 °C to give a
single adduct (A). Diene 2 reacted only at 150 °C to give a
single, different, adduct (B). Write structures for A and B and
explain the difference in the ease of reaction. Why is reaction of
1 so much easier than reaction with 2? Alder never actually
looked at the reaction with diene 3, but if he had, we predict
that no reaction would have occurred at 150 °C. Explain.
PROBLEM 12.62 Propose a synthesis of the powerful vesicant
(blister inducer) cantharidin, the active ingredient in the puta-
tive aphrodisiac “Spanish fly.” You may assume that organic
starting materials containing no more than six carbon atoms are
available, along with any inorganic materials you may need.
Hint: The thermodynamically more stable exo Diels–Alder
adducts of furan are generally isolable.
O
O
O
Δ
No
reaction
O
O
150 ⬚C
O
+
+
H
H
H
H
CH
3
1
3
2
A
+
B
CH
3
CH
3
H
3
C
H
H
CH
3
H
3
C
Cantharidin
O
O
O
O
CH
3
CH
3
PROBLEM 12.63 Write arrow formalism mechanisms for the
following transformations:
(a)
188–214 ⬚C
(b)
OO
200–250 ⬚C
+
C
O
COOCH
3
H
3
COOC COOCH
3
CH
2
O
O
O
H
3
COOC
(c)
140 ⬚C
O
O
PhCOO
O
O
O
Ph
O
CH
3
CH
2
OOC
COOCH
2
CH
3
(d)
25 ⬚C
O
O
H
O
O
O
COOCH
3
H
3
COOC
O
