Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

15.6 NMR Measurements 739
15.6e Decoupled Spectra When we discussed the NMR spectra of alcohols
and amines, we encountered the phenomenon of chemical exchange of the OH and
NH hydrogens (Fig. 15.46). Rapid exchange of these hydrogens resulted in an aver-
aging of the coupling to adjacent hydrogens. Exchange effectively decoupled the
OH and NH hydrogens from hydrogens at adjacent positions. This same trick can
be accomplished electronically, and it can be very useful, or even essential in the
interpretation of NMR spectra.
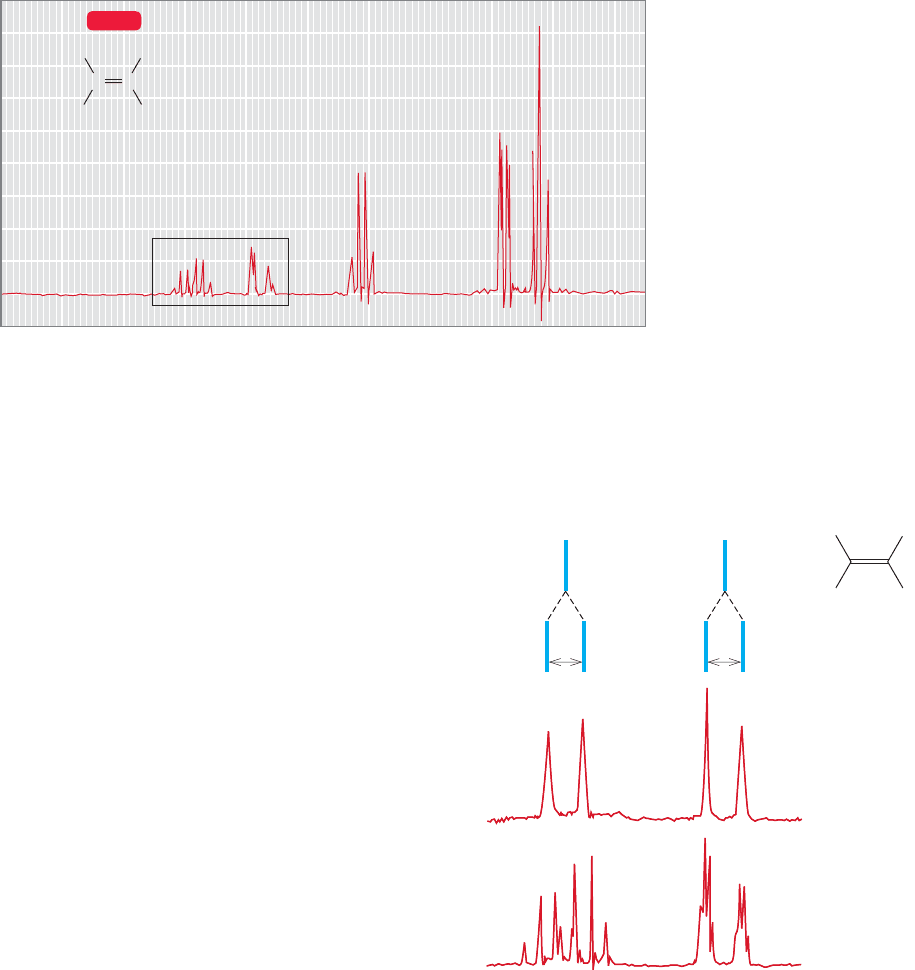
Suppose we are set the task of determining the stereochemistry of ethyl crotonate
from its
1
H NMR spectrum (Fig. 15.54). We might hope to make this distinction by
109876543210
(ppm)Chemical shift (δ)
H
a
H
b
H
a
H
b
Ethyl crotonate
CC
H
3
C
COOC
2
H
5
H
a
H
b
WEB 3D
FIGURE 15.54 The
1
H NMR
spectrum of ethyl crotonate.The
coupling between H
a
and H
b
is
obscured by the coupling to the
adjacent methyl groups.
measuring the coupling constant between the two vinyl hydrogens. If the compound
is trans, we would expect J
ab
to be 12–18 Hz; if it is cis, J
ab
would be smaller, 6–12 Hz
(Fig. 15.43). However, the rather complex spectrum makes the determination of J
ab
difficult.The problem is that both vinylic hydrogens are coupled to the methyl group,
and this coupling leads to the complex signals we see. If we
could somehow get rid of the coupling to the methyl group,
the spectrum would surely simplify, and it might become
possible to pick out the couplings between H
a
and H
b
. It is
possible to accomplish this simplification through electron-
ic decoupling. The spectrometer is modified to allow a sec-
ond application of radio-frequency waves during the NMR
experiment. While the spectrum is being measured, a radio
wave whose frequency matches the resonance frequency of
the methyl hydrogens is applied.The result is to induce rapid
transitions between the two possible spin states for each
methyl hydrogen. As in chemical exchange of ethyl alcohol,
the spin of the methyl hydrogens is averaged to zero and so
there is no observable coupling to H
a
or H
b
. Figure 15.55
shows a blow-up of the signals for the two vinyl hydrogens
with and without irradiation at the methyl group. It is now
easy to pick out the coupling between H
a
and H
b
and the
stereochemistry of the molecule can be determined with ease
from the observed J
ab
of 17 Hz. The compound is trans.
There are many similar applications,and modern NMR
spectrometers are all equipped to do decoupling experi-
ments routinely.
Decoupled spectrum
with irradiation at
the methyl group
Normal, coupled
spectrum
J JJ
ab
= 17 Hz
H
a
H
b
H
3
C
H
b
H
a
COOC
2
H
5
FIGURE 15.55 Irradiation at the resonance frequency of the
allylic methyl group averages the spins of the methyl hydrogens
and decouples them from H
a
and H
b
.The coupling constant J
between H
a
and H
b
is now easier to determine.
740 CHAPTER 15 Analytical Chemistry: Spectroscopy
15.7
13
C NMR Spectroscopy
Several other nuclei are of interest to the organic chemist, mainly
19
F,
15
N,
2
H
(deuterium),and
13
C,an isotope of carbon, which is present in 1.1% natural abundance.
Even though all organic compounds contain carbon, the natural abundance of
13
C is
low enough so that coupling to
13
C is not observed in hydrogen spectra. These other
nuclei can be used either in natural abundance or in enriched samples to provide their
own NMR spectra. Recall that the constant γ in Eq. 15.4 will be different for
19
F,
2
H,
15
N, or
13
C, and their NMR signals will not overlap with the hydrogen spectra.
For organic chemists, only
13
C is comparable in importance to
1
H. Like hydro-
gen,
13
C has a spin of
1
/
2
, and coupling between
13
C and
1
H will complicate
13
C spec-
tra.Normally,
13
C spectra are run using a broad-band decoupling so that all
coupling is removed. What about coupling between two
13
C atoms? Won’t this cou-
pling complicate the spectrum? The chance of finding a
13
C at any given position is
only 1.1% or 0.011.The chance of finding two
13
C atoms on adjacent positions is there-
fore 0.011 0.011 0.00012! At least for natural abundance spectra we can ignore
coupling. Accordingly,
13
C spectra are collections of sharp singlets. If the
broad-band decoupler is turned off, a spectrum can be obtained through a technique
called off-resonance decoupling, which allows the
13
C signals to be split according
to the n 1 rule, but only by the directly attached (not adjacent) hydrogens. Because
they have no attached hydrogens, quaternary carbons will still appear as singlets, but
methine carbons (one attached hydrogen) will be doublets, methylene carbons (two
attached hydrogens) triplets, and methyl carbons (three attached hydrogens) will appear
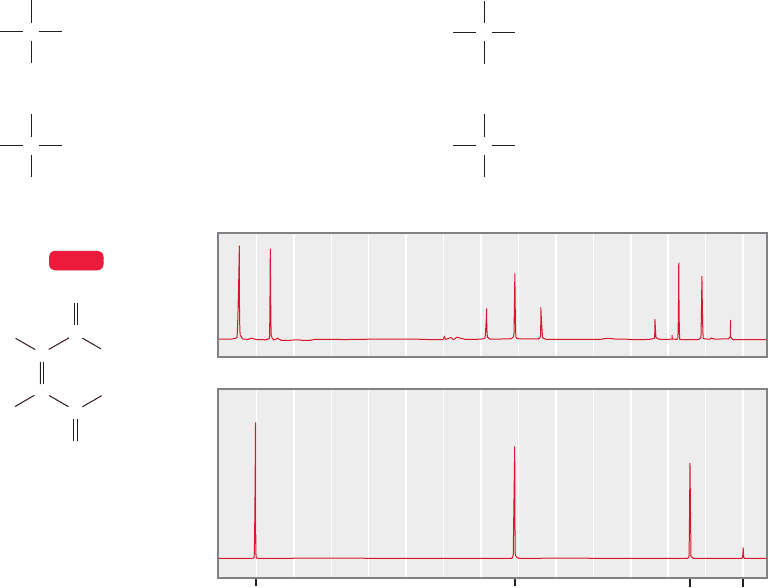
as quartets. Figure 15.56 summarizes this notion and shows the decoupled and off-
13
C
O
13
C
13
C
O
1
H
Carbonyl
carbon not
shown (165 ppm)
Broad-Band
Decoupled
Off-Resonance
Decoupled
Ethyl maleate
Broad-Band
Decoupled
Off-Resonance
Decoupled
This carbon is coupled
to three hydrogens and
will appear as a quartet
Singlet
CH
H
H
R
O
This carbon is coupled
to two hydrogens and
will appear as a triplet
Singlet
C
H
H
RR
This carbon is coupled
to one hydrogen and
will appear as a doublet
Singlet
C
R
R
HR
This carbon is coupled
to no hydrogens and
will appear as a singlet
Singlet
C
R
R
RR
C
C
C
H
C
O
OCH
2
CH
3
OCH
2
CH
3
H
Off-resonance decoupled spectrum
Chemical shift (δ)
61.45 14.15130.9 0
(ppm)
CH CH
2
CH
3
Decoupled spectrum
WEB 3D
FIGURE 15.56 Decoupled and off-resonance
decoupled
13
C NMR spectra of ethyl
maleate.The carbonyl carbon is not shown
in either spectrum. It appears as a singlet at
δ 165 ppm.
15.7
13
C NMR Spectroscopy 741
resonance decoupled spectra of ethyl
maleate.Notice that
13
C NMR spectra are
not integrated.Only heights of similar car-
bons (methyls, methylenes, or methines)
can be compared to determine the relative
number of carbons giving rise to a signal.
Several other techniques now allow
for the easy determination of the number
of hydrogens attached to a carbon. One
of the most convenient techniques goes
by the acronym DEPT (distortionless
enhancement with polarization trans-
fer). In this technique, several separate
NMR spectra are determined for a mol-
ecule under conditions that allow for the
appearance of only methine (CH), meth-
ylene (CH
2
), or methyl (CH
3
) carbons.
Quaternary carbons can be determined
by difference: Any signal in the full spec-
trum that does not appear in the separate
spectra for CH, CH
2
, and CH
3
carbons
must belong to a quaternary carbon.
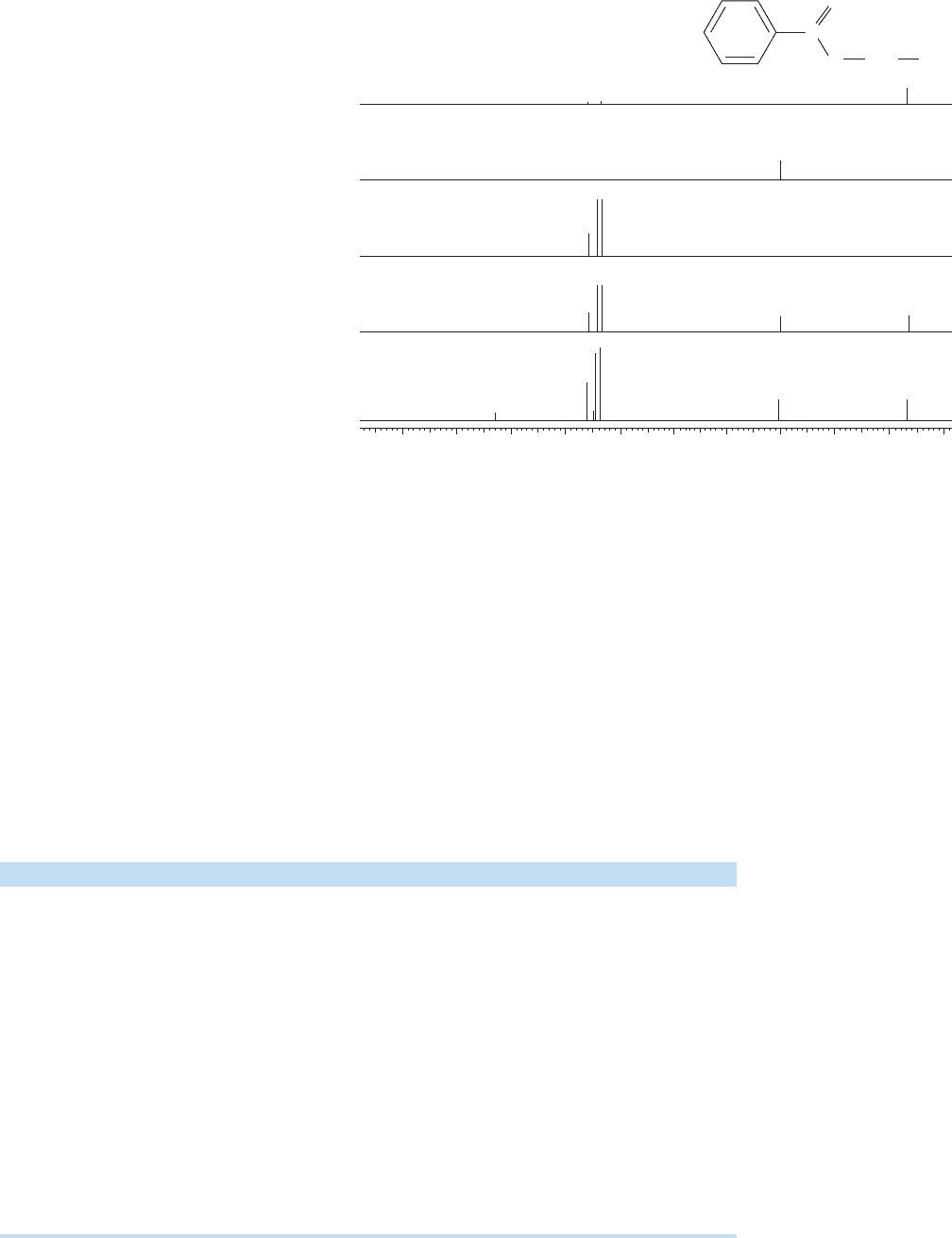
Figure 15.57 shows the DEPT technique applied by Kathryn Williams and Roy
W. King of the University of Florida to ethyl benzoate. The full
13
C spectrum is
shown on the bottom line,(e).The line (d) shows the signals for all carbons attached
to any number of hydrogens, note the absence of the signals for the quaternary
carbons at 130 and 166 ppm.The spectra on lines (c), (b), and (a) show the methine,
methylene, and methyl carbons, respectively.
Table 15.5 gives a series of chemical shift correlations for
13
C NMR. Notice the
greatly expanded range of chemical shifts extending over 200 ppm, as opposed to
the mere 10 ppm for
1
H (Table 15.4). The factors that give rise to chemical shifts
in
13
C NMR are the same as in
1
H NMR (electronegativity, hybridization,aromatic-
ity, and delocalization). Dispersion is much greater for
13
C NMR than for
1
H NMR.
(a) CH
3
(b) CH
2
(c) CH
(e) Unedited
(d) All CH
n
200 180 160 140 120 100 80 60 40 20
(ppm)
0
C
O
CH
2
CH
3
O
FIGURE 15.57 The DEPT technique applied to ethyl benzoate.
TABLE 15.5 Some
13
C Chemical Shifts
Type of Carbon Chemical Shift (␦)
a
Type of Carbon Chemical Shift (␦)
a
Alkanes Alcohols, ethers
Methyl 0–30 50–90
Methylene 15–55 Amines
Methine 25–55 40–60
Quaternary 30–40 Halogens
Alkenes 70–80
80–145 25–50
Alkynes 10–40
70–90 20–10
Aromatics 110–170 Carbonyls,
Benzene 128.7 190–220
150–180
a
The chemical shift δ is in parts per million (ppm) from TMS.
RXC
P
O (X = O or N)
R
2
C
P
O
C
P
O
C
O
IC
q
C
C
O
Br
C
O
ClC
P
C
C
O
F
C
O
N
C
O
O

Once again, the question of memorization arises. Use Table 15.5 to work problems,
and you will automatically memorize the information you really need (if you do
enough problems!).
742 CHAPTER 15 Analytical Chemistry: Spectroscopy
15.8 Problem Solving: How to Use
Spectroscopy to Determine Structure
The task set here is an almost impossible one—to show in a practical way how to
use the various kinds of spectral data to come up with a structure. Every compound
is different,of course, and so there can be no absolutely general method.Even worse,
there are many different approaches; what works for you may not be the optimal
way for others. Yet, there are some general techniques that are useful, and we’ll try
to set them out here.Remember, though, as the old lady in New York answered,when
asked for directions on how to get to Carnegie Hall, “practice, practice, practice.”
For you, that ancient joke means, “do lots and lots of problems.” Nowhere else is
that advice so essential.
First of all, when confronted by a set of spectral charts or data derived from
them, try to determine the molecular formula. Sometimes it will be given, some-
times you can work it out. Other times, it may be obvious or at least likely from
the chemical reactions involved. Sometimes you may have only a molecular weight,
from the molecular ion of a MS for example. Either way, having a formula or
molecular weight lets you determine the degrees of unsaturation (p. 131) and see
what’s left once you have determined part of the structure.
Next, use any available IR data, along with NMR chemical shift data, to get an
idea of what functional groups are present.The most useful data usually come from
the NMR spectrum.The convention for reporting signals is to list the chemical shift
followed by parenthetical listing of the coupling and the integration. So the NMR
spectrum of 1-bromobutane (Fig. 15.53) would be δ 0.9 (t, 3H), 1.5 (sextet, 2H),
1.8 (quintet, 2H), 3.4 (t, 2H). Coupling information is given by using s for singlet,
d for doublet, t for triplet, q for quartet, dd for doublet of doublets, and so on. If
the coupling is indecipherable, then we use m for multiplet (or mess, if you want).
There are many items in the Additional Problems section that let you practice this
technique.
Surprisingly, symmetry is often all that is needed to solve a structural problem—
a simple counting of signals may be sufficient to make a choice between several
alternatives, so you should always look hard at symmetry before starting on any
detailed analysis.
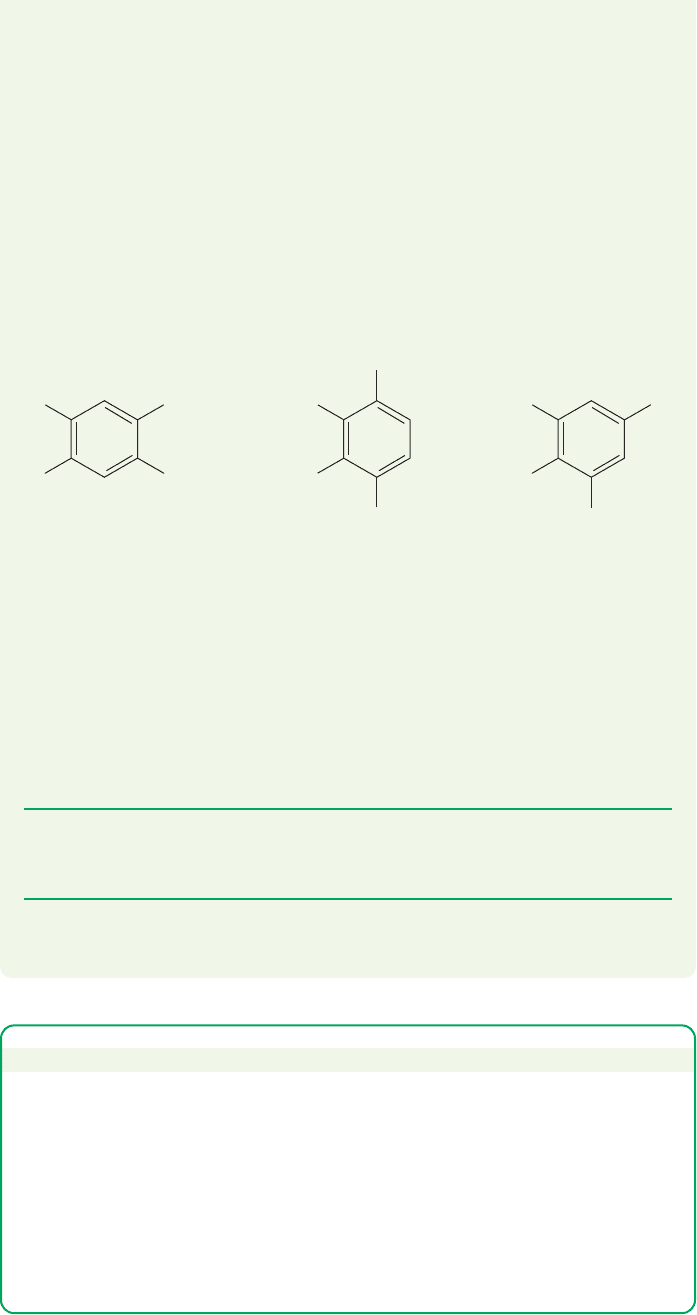
WORKED PROBLEM 15.23 You have bottles containing three isomeric compounds,
A, B, and C, each of the formula C
10
H
14
. The only significant bands in the
IR spectra of all three compounds are at about 3050, 2950, and 1610 cm
1
. The
1
H NMR spectrum of A shows signals at δ 2.13 (s, 6H) and 6.88 (s, 1H); B shows
signals at δ 2.18 (s, 3H), 2.25 (s, 3H), and 6.89 (s, 1H); and C shows signals at
δ 2.11 (s, 3H), 2.19 (s, 3H), 2.22 (s, 6H), and 6.80 (s, 2H). The
13
C NMR spec-
trum of A shows lines at δ 131.02, 133.52, and 19.04 ppm. Compound B shows
signals at δ 133.76, 133.79, 126.88, 20.65, and 15.78 ppm. Compound C shows
signals at δ 136.15, 134.43, 131.69, 128.29, 20.74, 20.20, and 14.86 ppm. What
are the structures?
(continued)
15.8 Problem Solving: How to Use Spectroscopy to Determine Structure 743
ANSWER First of all, these compounds seem to be aromatic hydrocarbons. The
formula C
10
H
14
establishes that there are four degrees of unsaturation (p. 131),
and the IR spectra show both aromatic and aliphatic CH stretches, as well as a
band at 1610 cm
1
appropriate for an aromatic double-bond stretch. An aromatic
ring has three double bonds and a ring, which adds up to four degrees of unsatu-
ration. If six of the available carbons are tied up in an aromatic ring, the other
four must be attached in some way. A look at the
1
H NMR spectra shows only
singlets, and so the four remaining carbons are certainly methyl groups. The
chemical shift for each of the methyl groups is about δ 2.2, which is consistent
with a methyl group on an aromatic ring. Thus, putting the data together leads
to the idea that A, B, and C are the three possible tetramethylbenzenes. But
which is which?
CH
3
H
3
C
H
3
C
CH
3
A
CH
3
H
3
C
H
3
C
B
H
3
C
H
3
C
CH
3
CH
3
CH
3
C
To answer this question, there is no need to do anything more elaborate than
look at the number of signals.There is more than one way to do this, but let’s use
the methyl signals in the
1
H NMR spectrum. Compound A has only one methyl
signal, so all four methyl groups must be identical. This compound must be the
molecule on the left. Compound B has two methyl signals, and must be the struc-
ture in the middle. Of course, we now know that the remaining isomer must be
C, but it is gratifying that it has the appropriate three methyl signals. There was
no need to consider many details, only to use symmetry and count.
PROBLEM 15.24 Could we have used the aromatic CH signals in Problem 15.23
to determine the answer?
PROBLEM 15.25 Could we have used the
13
C NMR spectra in Problem 15.23 to
determine the answer?
PROBLEM SOLVING
In almost all spectroscopy problems in which NMR is used to distinguish
between various possible structures, the very first thing to do is count carbons
and/or hydrogens. Many otherwise quite tough problems can be shortcut, and
made much easier, by determining how many signals there will be. Problem
15.23 is a good example. Moreover, problem writers aren’t always aware that
simple counting can solve otherwise difficult problems. By counting, you may be
able to solve easily a problem that was meant to be very hard, and to require
specific detailed knowledge of spectroscopy. It happens all the time.
Chemical shift data are often decisive. After considering symmetry, that is what
we usually look at next. Table 15.4 will let you make many structural decisions.
WORKED PROBLEM 15.26 You have four bottles, each containing one of the four
compounds shown below, E, F,G, and H.From the spectral data given,determine
which bottle holds which compound.
CH
3
E
OCH
2
CH
3
O
OCH
3
F
CH
2
CH
3
O
CH
2
CH
3
G
OCH
3
O
OCH
2
CH
3
H
CH
3
O
All four compounds have signals for 4H in the aromatic region of the
1
H NMR
spectrum at δ 7–8 ppm.
Bottle 1 IR: 1720 cm
11
H NMR: δ 1.38 (t, 3H), 2.40 (s, 3H), 4.36 (q, 2H)
Bottle 2 IR: 1688 cm
11
H NMR: δ 1.12 (t, 3H), 2.81 (q, 2H), 3.76 (s, 3H)
Bottle 3 IR: 1725 cm
11
H NMR: δ 1.21 (t, 3H), 2.65 (q, 2H), 3.85 (s, 3H)
Bottle 4 IR: 1680 cm
11
H NMR: δ 1.40 (t, 3H), 2.50 (s, 3H), 4.07 (q, 2H)
ANSWER First, use Table 15.3 to determine that the two esters are in bottles 1 and
3 (IR: conjugated stretch at about 1720 cm
1
) and the two ketones are in
bottles 2 and 4 (IR: conjugated stretch much lower, at about 1680 cm
1
).
Now the chemical shift data allow a quick determination of the rest of the
problem. Again, there are several ways to do this last part, but the easiest is to
look at the chemical shift of the singlet methyl group (you could also use the
methylene quartet). Every signal has three pieces of data: integral,chemical shift,
and coupling. If we look at the chemical shift (δ 2.40) of the methyl (3H) sin-
glet in bottle 1, we see that it cannot be a methyl group attached to an oxygen.
Methyl groups on oxygen are at δ 3.2 or even further downfield. A methyl group
on an aromatic ring should be around δ 2.2 ppm. If the compound in bottle
1 must be an ester based on IR, then it must contain compound E. The ester
in bottle 3 has a 3H singlet at δ 3.85 ppm and is consistent with compound G.
A similar analysis of the ketones leads us to assign the 3H singlet at δ 2.50 ppm
in bottle 4 to the methyl ketone of compound H, and the 3H singlet at
δ 3.76 ppm in bottle 2 must be assigned to the methoxy group on the aromatic
ring in compound F.
C
P
O
C
P
O
744 CHAPTER 15 Analytical Chemistry: Spectroscopy
Sometimes the coupling constant J is the key to solving the puzzle, and often it
is the magnitude of J that is the key. The molecules in Problem 15.19 are a perfect
example of this use of J. More often, it is the splitting pattern that solves the prob-
lem. An examination of J reveals who is adjacent to whom in the hydrogen world,
and thus provides deep insight into structure.Here’s another problem, this time one
that requires you to think about J.
15.8 Problem Solving: How to Use Spectroscopy to Determine Structure 745
WORKED PROBLEM 15.27 Again you have bottles 1 and 2, this time containing I
and J. Explain how you would use
1
H NMR spectroscopy to tell which molecule
is in which bottle.
Cl
H
Cl
H
Ph
H
Ph
H
O
O
I
Cl
H
Ph
H
Cl
H
Ph
H
O
O
J
Now we pass on to some “bells and whistles” of NMR.
MAITOTOXIN
These days sophisticated NMR techniques seem capable of
determining any structure. This box is almost pure “gee-whiz.”
We are trying to dazzle you with the complexity of the molec-
ular structures that can be solved by the application of spec-
troscopy (and a lot of hard work). The example is maitotoxin.
Maitotoxin, a molecule for which one of your authors
maintains a certain affection, is extraordinarily lethal (for half
the mice treated the lethal dose is only 50 ng/kg) and is one
of the largest natural products known. In 1993, a group of
Japanese chemists reported a variety of complex NMR studies
that established the general structure, and in 1996, chemists at
Harvard used much spectroscopy, along with organic synthe-
sis, to establish the complete relative stereochemistry. That
same year the absolute stereochemistry was determined in
Japan by K.Tachibana (b. 1949) and his research group
at the University of Tokyo. How many stereogenic carbons are
there in maitotoxin? How many possible stereoisomers are
there? To solve this structure is a remarkable accomplishment.
Why show this molecule? No one is ever going to ask you
to reproduce the structure, we hope, and there are not likely to
be any immediate practical applications. We think that the
answer is simply that the sheer difficulty of the enterprise is
overwhelming. We humans can do some quite amazing things.
There are times when that gee-whiz factor must be confronted
directly, when we are allowed to sit back and marvel at some
accomplishment, and this is one of those moments.
OH
OH
OH
OSO
3
Na
HO
O
O
O
O
O
O
O
O
O
O
O
O
O
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
HO
OH
NaO
3
SO
OH
OH
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
OH
HO
OH
OH
OH
HO
HO
OH
OH
OH
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
HH
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
H
3
C
ANSWER In compound J, the hydrogen adjacent to the phenyl group and the
hydrogen adjacent to the chlorine will couple. Each will appear as a doublet, with a
small coupling constant of a few hertz (Hz). The hydrogen adjacent to the phenyl
ring and the hydrogen adjacent to the chlorine in compound I are too far from each
other to couple; they will appear as singlets because identical hydrogens do not cou-
ple. Be sure you see that there is no other easy way to make this determination.
746 CHAPTER 15 Analytical Chemistry: Spectroscopy
15.9 Special Topic: Dynamic NMR
We have already seen that rates of hydrogen exchange are important in NMR spec-
troscopy. Recall that fast exchange of hydroxyl and amine hydrogens generally results
in a decoupling of the OH and NH hydrogens (p.733). An adjacent hydrogen “feels”
only an averaged perturbation of the applied magnetic field if exchange is fast enough.
If exchange is slowed by careful removal of all acidic or basic catalysts, the coupling
can be detected. The rate of exchange is important to the spectrum. If it is fast, the
NMR experiment detects an averaged spectrum; if it is slow, it sees a static picture.
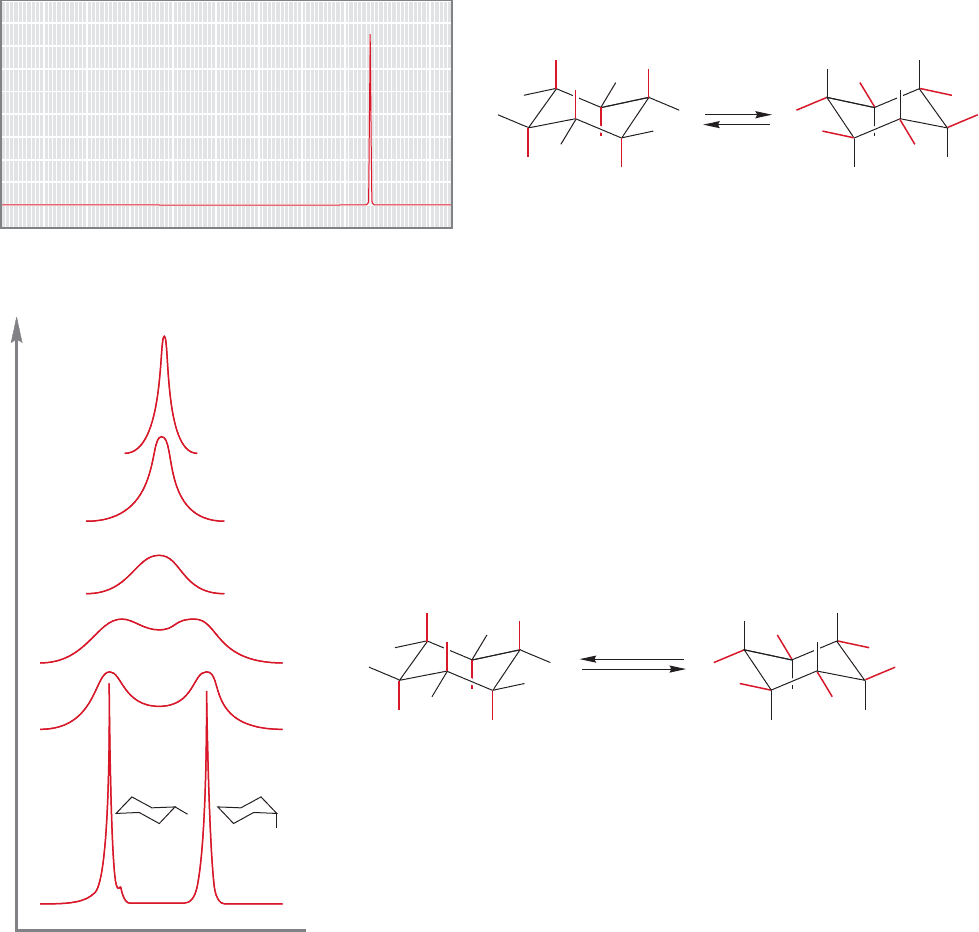
The classic example of this phenomenon involves the spectrum of cyclohexane.
As you know, there are two kinds of hydrogen in cyclohexane, axial and equatorial,
and these are interconverted by a ring flip, with an activation energy of about
11 kcal/mol (p. 198). Despite the existence of two kinds of hydrogen, the room-
temperature spectrum of cyclohexane shows only a single line at δ 1.4 ppm (Fig. 15.58).
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
H
ring
flip
H
H
H
H
H
H
H
109876543210
(ppm)Chemical shift (δ)
FIGURE 15.58 The
1
H NMR spectrum of cyclohexane at 25 °C.
ring flip
(ppm)
slow at
–89 ⬚C
–60 ⬚C
25 ⬚C
Each spectrum at higher temperature
–89 ⬚C
D
D
D
H
D
D
D
D
D
D
D
D
Equatorial
D
D
H
D
D
D
D
D
D
D
D
D
Axial
H
H
FIGURE 15.59 At low temperature, ring flipping is slowed and
the axial and equatorial hydrogens of cyclohexane can be
resolved in the NMR spectrum. As the temperature is raised, the
89 °C spectrum approaches the 25 °C spectrum.
This spectrum appears simple because the rate of interconversion of axial and equato-
rial hydrogens is so fast, with respect to the time necessary for the NMR measurement,
that the spectrum appears as an average. Suppose we decrease the energy available to
the system by lowering the temperature.If we can decrease the rate of the ring flip suf-
ficiently, the spectrometer will no longer detect an average of the two possible positions
but will be able to see both axial and equatorial hydrogens. Figure 15.59 shows the
1
H
NMR spectrum of undecadeuteriocyclohexane (cyclohexane-d
11
) at 89 °C.Two equal
signals appear, centered on the position of the room temperature, averaged signal.
15.9 Special Topic: Dynamic NMR 747
In practice, for a signal to be detectable for a given species, it must have a life-
time of about 1 s. As the temperature is raised to room temperature, the lifetime of
individual cyclohexanes decreases as the rate of ring flipping increases, and an aver-
aged spectrum appears.
Now, what happens at an intermediate temperature? Figure 15.59 shows you. A
common-sense answer would be correct here. If two signals are found at 89 °C,
and a single signal at 25 °C, at intermediate temperatures the two kinds of signal
must approach each other.The two sharp peaks of the 89 °C spectrum will slow-
ly merge into the single peak of the 25 °C spectrum. In fact, it is possible to derive
kinetic information from the temperature dependance of NMR spectra, and this is
still another use of NMR spectroscopy.
More molecules than you might at first expect give this kind of averaged spec-
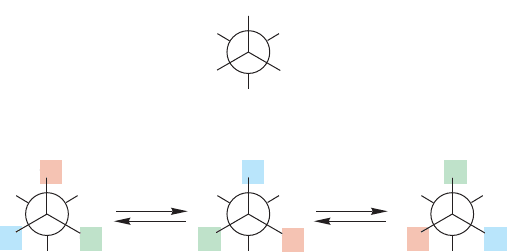
trum. It takes a detailed look at structure to see why. Consider ethyl alcohol. If we
ignore the OH signal, which we know to be dependent on concentration,pH, sol-
vent, and temperature, we expect to see only one signal for the three equivalent
methyl hydrogens and another for the two equivalent methylene hydrogens. But
wait—look at the Newman projection in Figure 15.60, which shows the most sta-
ble staggered conformation of ethyl alcohol.This perspective shows that there are
three kinds of hydrogen (H
a
,H
b
, and H
c
), not two. The three methyl hydrogens
are not all the same.The two that are gauche to the alcohol are in a different envi-
ronment from the hydrogen that is anti to the alcohol. Yet, we do not see sepa-
rate signals for these hydrogens. By now you should be able to see the answer to
this conundrum. As shown in Figure 15.60, rotation about the carbon–carbon bond
of ethyl alcohol interconverts the three methyl hydrogens, rendering them equiv-
alent. Moreover, we know that the low barrier for rotation, about 3 kcal/mol,
ensures a rapid rate for this simple process.In Figure 15.60, the hydrogens are indi-
vidualized with colored squares. Note how each methyl hydrogen occupies one of
the three possible positions as rotation occurs.The NMR spectrometer detects only
the averaged signal.
120⬚ 120⬚
OH
H
a
H
c
H
b
H
b
H
c
OH
H
c
H
a
H
b
H
b
H
c
OH
H
a
H
c
H
b
H
b
H
c
OH
H
c
H
c
H
b
H
b
H
a
FIGURE 15.60 In static ethyl alcohol,
there are three kinds of hydrogen
attached to carbon. However, rapid
rotation around the carbon–carbon
bond interconverts the methyl
hydrogens and makes them
equivalent.The two methylene
hydrogens are enantiotopic.
What about the two methylene hydrogens, H
b
? The Newman projection of
Figure 15.60 shows that even in the absence of rapid rotation they are equivalent,
and therefore must always give rise to one signal.These hydrogens are enantiotopic.
As we saw earlier, the origin of the name lies in the mirror-image relationship of
the two to each other.
So, as long as rotation is fast, neither the individual methyl hydrogens nor the
individual methylene hydrogens of ethyl alcohol are distinguishable by NMR
spectroscopy.
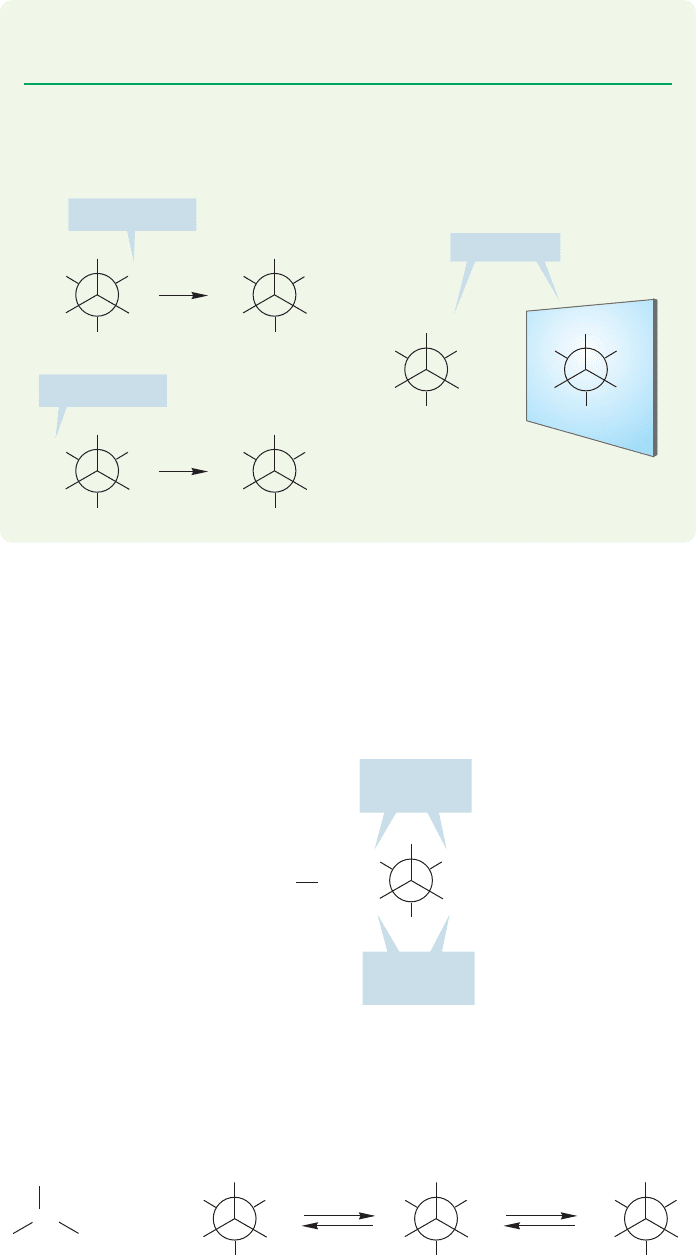
PROBLEM 15.28 In a solvent consisting of a single enantiomer, the two enantiotopic
hydrogens of ethyl alcohol (H
b
in Fig. 15.60) appear as separate signals. Explain.
WORKED PROBLEM 15.29 Use the device of replacing the methylene hydrogens
(H
b
in Fig. 15.60) with an X group to show that they are enantiotopic (p. 721).
ANSWER
748 CHAPTER 15 Analytical Chemistry: Spectroscopy
Mirror
OH
H
a
H
c
H
b
H
b
H
c
OH
H
a
H
c
X H
b
H
c
OH
H
a
H
c
X
H
b
H
c
OH
H
a
H
c
H
b
X
H
c
Replace with X
OH
H
a
H
c
H
b
H
b
H
c
OH
H
a
H
c
H
b
X
H
c
Replace with X
Enantiomers
Now let’s change some things about ethyl alcohol. Let’s first convert it into
1-propanol by replacing one methyl hydrogen with a methyl group (Fig. 15.61).
There are now three sets of hydrogens: the methyl hydrogens and two different sets
of methylene hydrogens. Rapid rotation ensures that the three methyl hydrogens will
be equivalent, and each pair of methylene hydrogens consists of two magnetically
equivalent, enantiotopic hydrogens.
OH
H
HH
OH
H
CH
3
CH
3
CH
2
CH
2
Enantiotopic
hydrogens
Newman projection
Enantiotopic
hydrogens
FIGURE 15.61 In propyl alcohol, the
methyl hydrogens are homotopic;
there are two pairs of enantiotopic
methylene hydrogens.
Next let’s replace a hydrogen of 1-propanol with a chlorine to make 2-chloro-
1-propanol. Look at the Newman projection of this molecule in Figure 15.62.
120⬚ 120⬚
OH
Is H
x
equivalent to H
y
?
H
CH
3
H
y
H
x
Cl
OH
CH
3
Cl
H
y
H
x
H
OH
Cl
H
H
y
H
x
H
3
C
H
3
C
CH
2
OH
=
CH
2-Chloro-1-propanol
12 3
Cl
FIGURE 15.62 In 2-chloro-
1-propanol, the two methylene
hydrogens, H
x
and H
y
, are neither
equivalent (homotopic) nor
enantiotopic.
