Jones M., Fleming S.A. Organic Chemistry
Подождите немного. Документ загружается.

15.9 Special Topic: Dynamic NMR 749
Are the two methylene hydrogens equivalent? No. The Newman projections of
Figure 15.62 show the three rotational isomers and in all of them the two hydro-
gens, H
x
and H
y
are different. Never do they become equivalent. They are forev-
er different no matter how fast rotation about the carbon–carbon bond is, and so
must give rise to separate signals.These hydrogens are diastereotopic, and will give
rise to distinct signals. This point is particularly difficult, so let’s anticipate the
Common Errors section a little bit and deal with the problem right here. Is not
H
y
in Newman projection 2 of Figure 15.62 in the same position as H
x
in draw-
ing 1? Are the two not equivalent? Both are 120° from the hydroxyl group, and
both lie in between a methyl group and a hydrogen. The answer is no, and to see
why, we must look at the OH groups. A short demonstration shows that the two
OH groups in drawings 1 and 2 are not identical. One is between hydrogen
and chlorine, the other is between methyl and chlorine. Thus, H
x
and H
y
are 120°
from different OH groups in the two forms. A more elaborate demonstration
takes advantage of the thought process mentioned earlier (p. 721). Replace H
x
with a group X and determine the relationship between this compound and the
one produced by replacing H
y
with X (Fig. 15.63). These two compounds are dif-
ferent, neither identical nor enantiotopic. There is no way out, H
x
and H
y
are
diastereotopic.
OH
CH
3
Cl
H
y
X
H
OH
CH
3
Cl
H
y
X
H
OH
CH
3
Cl
H
y
H
x
replace H
x
with X
H
OH
CH
3
Cl
X
H
OH
CH
3
Cl
X
H
and
These two structures are neither
identical nor enantiomers; they are
diastereomers, and H
x
and H
y
are
diastereotopic hydrogens
replace H
y
with X
H
x
H
x
FIGURE 15.63 The two hydrogens H
x
and H
y
are diastereotopic and will give
different signals in an NMR spectrum.
PROBLEM 15.30 The methyl group of trans-1-tert-butyl-4-methylcyclohexane
appears as a doublet, but in the spectrum for cis-1-isopropyl-3-phenylcyclohexane
there are two methyl doublets. Explain.
CH
3
CH
3
H
3
C
C(CH
3
)
3
CH
Ph

750 CHAPTER 15 Analytical Chemistry: Spectroscopy
15.10 Summary
New Concepts
This chapter recapitulates the ideas introduced in Chapter 4 in
the discussion of column chromatography, and introduces the
much more versatile separation methods of gas chromatography
and high-performance liquid chromatography. All chromatogra-
phy works in much the same way: The components of a mixture
are forced to partition themselves between a moving phase and
a stationary phase.The more a given component exists in the
moving phase, the faster it moves through the column; the more
strongly it is absorbed by the stationary phase, the longer it
remains on the column.
In mass spectrometry, a molecule is first ionized by bom-
bardment with high-energy electrons. The molecular ion so
formed can undergo numerous fragmentation reactions before
it passes, along with the daughter ions formed through frag-
mentation, into a region between the poles of a magnet. Ions
are separated by mass, and focused on a detector. The mass
spectrometer gives the molecular weight of a molecule, provided
that the molecular ion can be detected, and high-resolution
mass spectrometry can determine the molecular formula of an
ion. Structural information can often be gained through an
analysis of the important daughter ions in the fragmentation
pattern. The presence of chlorine or bromine in a molecule can
be deduced from the appearance of signals from M 2 isotopes.
Infrared spectroscopy detects vibrational excitation of
chemical bonds. Functional groups have characteristic vibra-
tional frequencies in the IR region that depend on the masses
of the atoms in the bond and on the force constant of the bond.
Analysis of an IR spectrum gives information on the kinds of
functional groups present.
Nuclear magnetic resonance spectroscopy detects the energy
absorbed in the transition from a low-energy state in which the
nuclear spin is aligned with an external magnetic field to a slight-
ly higher energy state in which it is aligned against the external
field.The
1
H NMR spectra can be integrated to reveal the rela-
tive number of hydrogens in each peak. Hydrogens,
13
C, and
some other nuclei have characteristic absorptions (the chemical
shift, δ), which depend critically on the surrounding chemical
environment. Hydrogen nuclei are coupled to adjacent hydrogens
through a coupling constant, J. The number of lines observed for
a particular hydrogen depends on the number of other nuclei to
which it is coupled. For first-order spectra, the number of lines
equals n 1, where n is the number of equivalent coupled nuclei.
Key Terms
base peak (p. 702)
chemical shift (δ) (p. 718)
coupling constant (J) (p. 718)
daughter ion (p. 702)
decoupling (p. 739)
DEPT (distortionless enhancement with
polarization transfer) (p. 741)
diastereotopic (p. 721)
enantiotopic (p. 721)
first-order spectrum (p. 735)
force constant (p. 708)
fragmentation pattern (p. 702)
gas chromatography (GC) (p. 697)
GC/IR (p. 699)
GC/MS (p. 699)
high-performance liquid chromatography
(HPLC) (p. 697)
homotopic (p. 721)
infrared (IR) spectroscopy (p. 707)
integral (p. 718)
Karplus curve (p. 731)
long-range coupling (p. 731)
M 1 peak (p. 700)
mass spectrometry (MS) (p. 699)
molecular ion (p. 700)
n 1 rule (p. 729)
off-resonance decoupling (p. 740)
parent ion (p) (p. 702)
ppm scale (p. 716)
radical cation (p. 699)
tetramethylsilane (TMS) (p. 716)
vinylic hydrogen (p. 724)
wavenumber (p. 708)
Common Errors
Practice, practice, practice! The successful interpretation of
spectra requires it. The most common error is not to realize this
fact. The memorization of tables of data will not make you into
a spectroscopist, but working lots and lots of problems will. See
Section 15.8 for some hints on solving problems.
A central question in this chapter, and in all of chemistry, is,
What’s different? A common error is missing the symmetry of
a molecule and not noticing when two hydrogens are equivalent
or when they are not. This chapter offers a device that is useful
in making such distinctions.
15.11 Additional Problems 751
15.11 Additional Problems
General advice: Although this section is longer than most
problem sections, it cannot do more than illustrate some of
the difficulties involved in going from a set of lines on chart
paper, or a table of numbers, to a structure. Moreover, in the
real world of chemistry there is close interaction between the
reactions involved and the interpretation of spectra. The
chemist has some idea (usually!) of what is likely to be pres-
ent. Some of the later problems must be solved in an interac-
tive way. The chemistry involved will suggest structures, and
you will have to see if they are consistent with the data avail-
able. All of the following problems involve manipulating
spectral data. You should start well-equipped with a table of
IR frequencies and
1
H NMR and
13
C NMR correlation
charts for chemical shifts. A table of coupling constants will
also be essential, and a copy of the Karplus curve is often use-
ful. Do not be shy about looking values up. Most chemists
analyze their “real world” spectra that way, and everyone starts
that way.
Specific advice: In Chapter 3 we first encountered , the
number of “degrees of unsaturation.” In this chapter, we see
nitrogen-containing compounds and will have to modify our
formula a bit. Here is the protocol for nitrogen and some other
heteroatoms. Be sure to review in Chapter 3 (p. 131).
1. Oxygen and divalent sulfur can be ignored.
2. Halogens (F, Cl, Br, and I) are counted as if they were
hydrogens.
3. Treat nitrogen as if it were a carbon. However, as nitrogen
is trivalent, not tetravalent, subtract one hydrogen per
nitrogen from the total number of hydrogens computed
for the saturated hydrocarbon. A formula that can be
used is
We begin with some relatively straightforward problems
largely involving infrared spectroscopy and mass spectrometry,
and then move on.
PROBLEM 15.31 Here is a practical problem of no intellectual
content whatsoever. In doing problems involving IR, it is often
much easier to read the values on the chart paper in microns
(μ), rather than wavenumber ( ). Unfortunately, most of us
have learned to think in wavenumbers. There is a simple
conversion, lμ 10
4
cm. Convert 3.00, 5.00, 7.00, 9.00, and
11.00 μ into wavenumbers. Hint: See p. 708.
ν
[2(#C’s) + 2 - (#H’s and halogens) + (#N’s)]
2
=Æ
Æ
Æ
PROBLEM 15.33 The IR spectrum of a molecule known
to have structure A, B, C, or D is shown below. Which
structure is most consistent with the spectrum? Explain your
reasoning.
O
(a)
(e)
OH
(f)
OH
(b) (c)
(g)
NH
2
O Cl
(d)
N
NH
2
C
O
A
OCH
3
C CH
2
OH
O
CD
C
N
B
OH
NH
2
NO
2
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
PROBLEM 15.32 Calculate the degrees of unsaturation in the
following molecules:
It is often difficult to keep track of the interplay of the inte-
gration, the chemical shift, δ, and the coupling constant, J.A
common error is confusing a hydrogen’s signal with those of the
hydrogens that are vicinal to it. For example, if the signal for H
a
is a doublet then it “sees” one hydrogen. But H
a
itself might be
a 3H methyl, a 2H methylene, or a 1H methine.
752 CHAPTER 15 Analytical Chemistry: Spectroscopy
5
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
4
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
3
Wavenumber (cm
–1
)
Microns (
μ
)
0
100
10
20
30
40
50
90
60
70
80
0
100
10
20
30
40
50
90
60
70
80
1615141312111098765432.5
Transmittance (%)
62565070090010001100130015002000250030004000 800
2
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
1
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
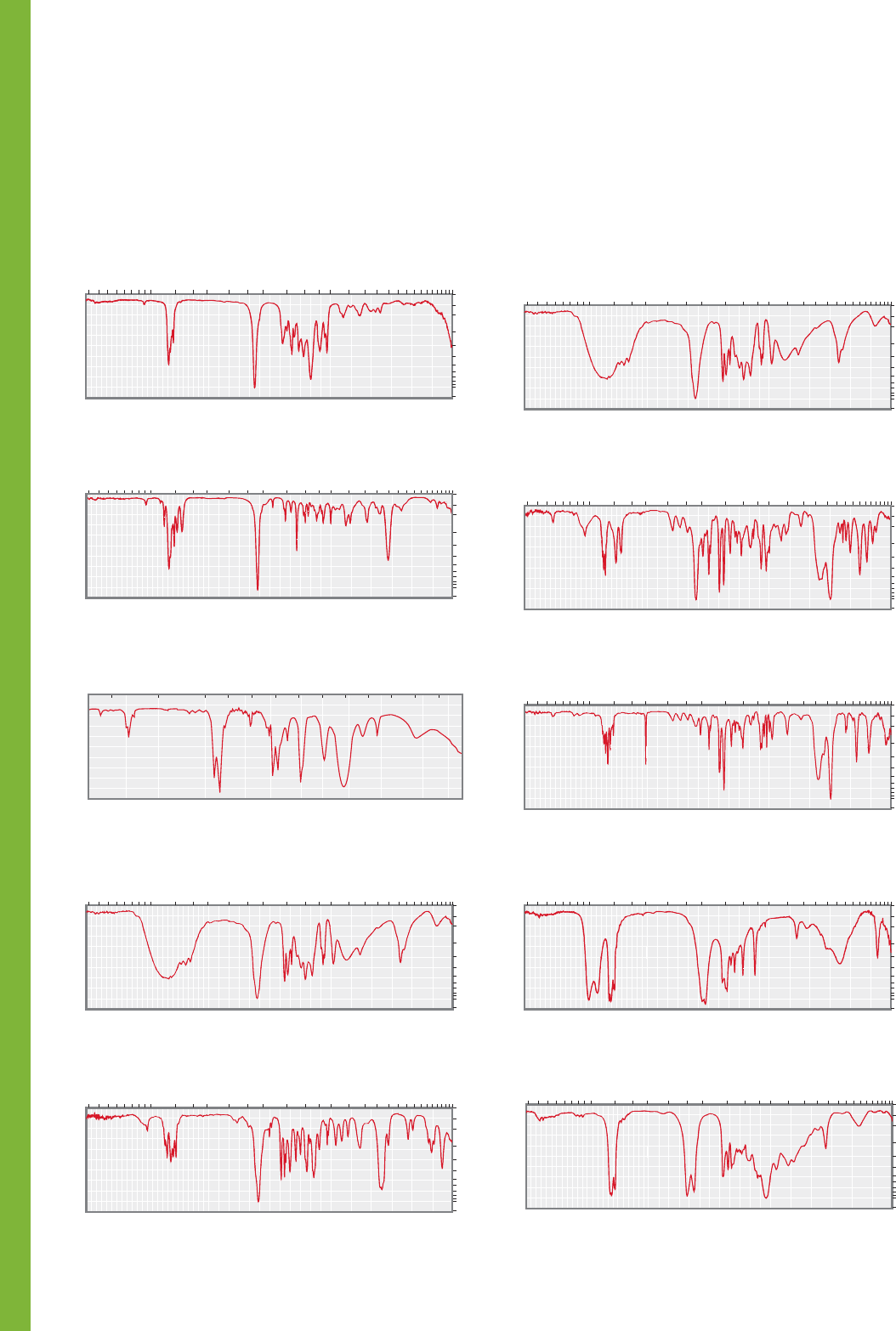
PROBLEM 15.35 For each of the following IR spectra (1–5)
there is a choice of three possible types of compounds. For each
spectrum, choose the most appropriate class of compound.
Explain your reasoning by noting the presence or absence of
characteristic bands in the spectrum.
1 Alcohol
Carboxylic acid
Phenol
2
Aldehyde
Ester
Ketone
5 Anhydride
Carboxylic acid
Ester
4 Primary amide
Primary amine
Nitro compound
3 1-Alkyne
Symmetrical disubstituted alkyne
Nitrile
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
PROBLEM 15.34 Each of compounds 1–5 is either an alde-
hyde, an anhydride, a carboxylic acid, an ester, or a ketone.
Identify to which class each compound belongs based on the IR
spectrum shown. Explain your reasoning by assigning character-
istic and/or distinguishing absorptions. Each class is represented
by one compound.
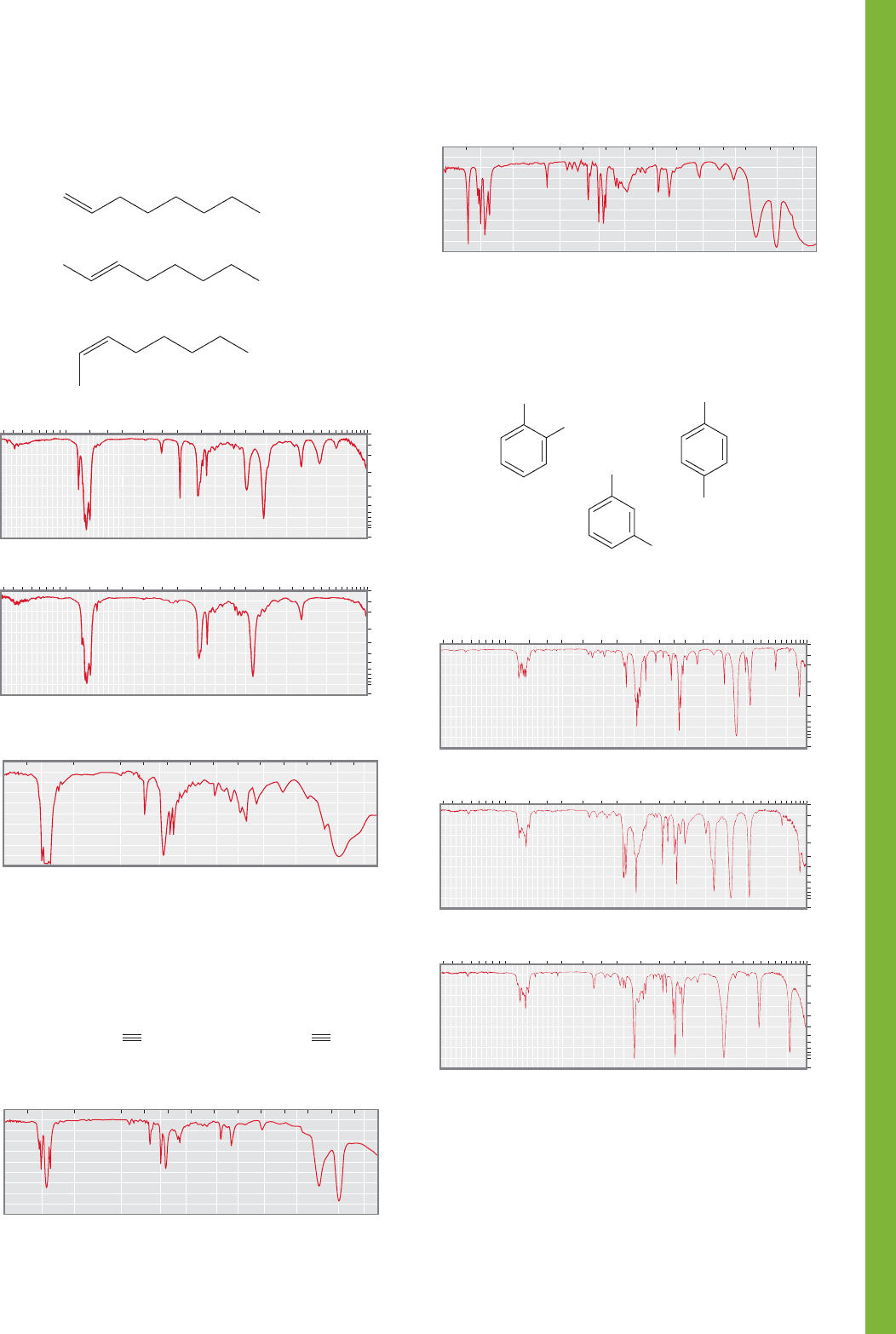
15.11 Additional Problems 753
PROBLEM 15.36 Assign the IR spectra below to the correct
octene isomer. Explain your reasoning.
1-Octene
trans -2-Octene
cis-2-Octene
1
3
2
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
Wavenumber (cm
–1
)
Microns (
μ
)
0
100
10
20
30
40
50
90
60
70
80
0
100
10
20
30
40
50
90
60
70
80
1615141312111098765432.5
Transmittance (%)
62565070090010001100130015002000250030004000 800
PROBLEM 15.37 The following IR spectra belong to
5-phenyl-1-pentyne and 6-phenyl-2-hexyne. Which is which?
Explain your reasoning.
PhCH
2
CH
2
CH
2
CCH PhCH
2
CH
2
CH
2
C CCH
3
1
Wavenumber (cm
–1
)
Microns (μ)
0
100
10
20
30
40
50
90
60
70
80
0
100
10
20
30
40
50
90
60
70
80
1615141312111098765432.5
Transmittance (%)
62565070090010001100130015002000250030004000 800
2
Wavenumber (cm
–1
)
Microns (μ)
0
100
10
20
30
40
50
90
60
70
80
0
100
10
20
30
40
50
90
60
70
80
1615141312111098765432.5
Transmittance (%)
62565070090010001100130015002000250030004000 800
PROBLEM 15.38 Assign the IR spectra below to the correct
chlorotoluene isomer.
1
3
2
CH
3
Cl
CH
3
Cl
CH
3
Cl
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
PROBLEM 15.39 Compound A has the formula C
8
H
7
BrO
2
.
The two most intense peaks in the mass spectrum are at
m/z 216 and 214, and they appear in almost equal amounts.
What ion gives rise to each of these peaks? Compound A is an
aldehyde. Explain why the next most intense peaks in the mass
spectrum come at m/z values of 215 and 213.
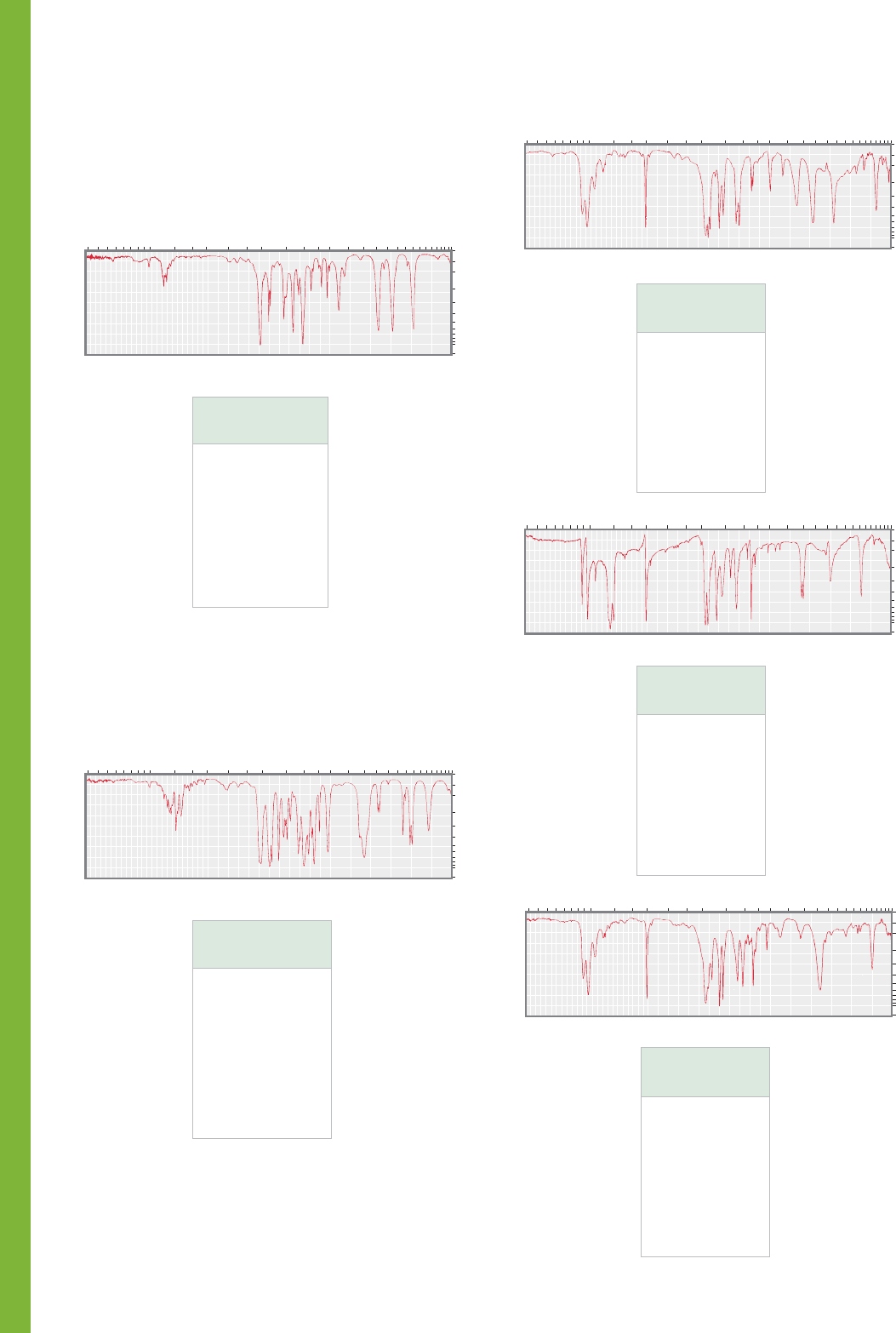
PROBLEM 15.40 Compound A, containing only C, H, and O,
gave 80.00% C and 6.70% H upon elemental analysis.
Summaries of the mass spectrum and the IR spectrum of
compound A are shown below. The molecular ion is at m/z 120.
Deduce the structure of A and explain your reasoning.
754 CHAPTER 15 Analytical Chemistry: Spectroscopy
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 2515141312111098755.563 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
m/z Relative
Abundance
100
88
40
29
21
17
10
9
105
77
51
120
50
43
78
39
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns (μ)
16 18 20 22 2515141312111098755.563 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
m/z Relative
Abundance
100
71
28
15
15
8
8
8
135
136
77
92
107
39
65
63
PROBLEM 15.41 Compound A, containing only C, H, and O,
gave 70.60% C and 5.90% H upon elemental analysis.
Summaries of the mass spectrum and the IR spectrum of
compound A are shown below. The molecular ion is at m/z 136.
Deduce the structure of A and explain your reasoning.
A
m/z Relative
Abundance
118
96
119
64
90
63
39
117
100
20
9
8
7
6
5
5
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns ()
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
100
35
12
7
7
7
6
4
118
96
119
64
90
63
39
117
m/z Relative
Abundance
C
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns ()
16 18 20 22 2515141312111098755.563 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
100
40
22
22
19
14
14
12
118
96
119
64
90
63
39
117
m/z Relative
Abundance
B
0
100
10
20
30
40
50
90
60
70
80
Transmittance (%)
Wavenumber (cm
–1
)
4006001200 100014001600180020003400 2800 220040004600 800
Microns ()
16 18 20 22 251514131211109875 5.5 63 3.5 4 4.52.4 2.6 2.82.2
0.0
0.4
0.5
0.6
0.8
1.0
2.0
0.3
0.05
0.2
0.1
Absorbance
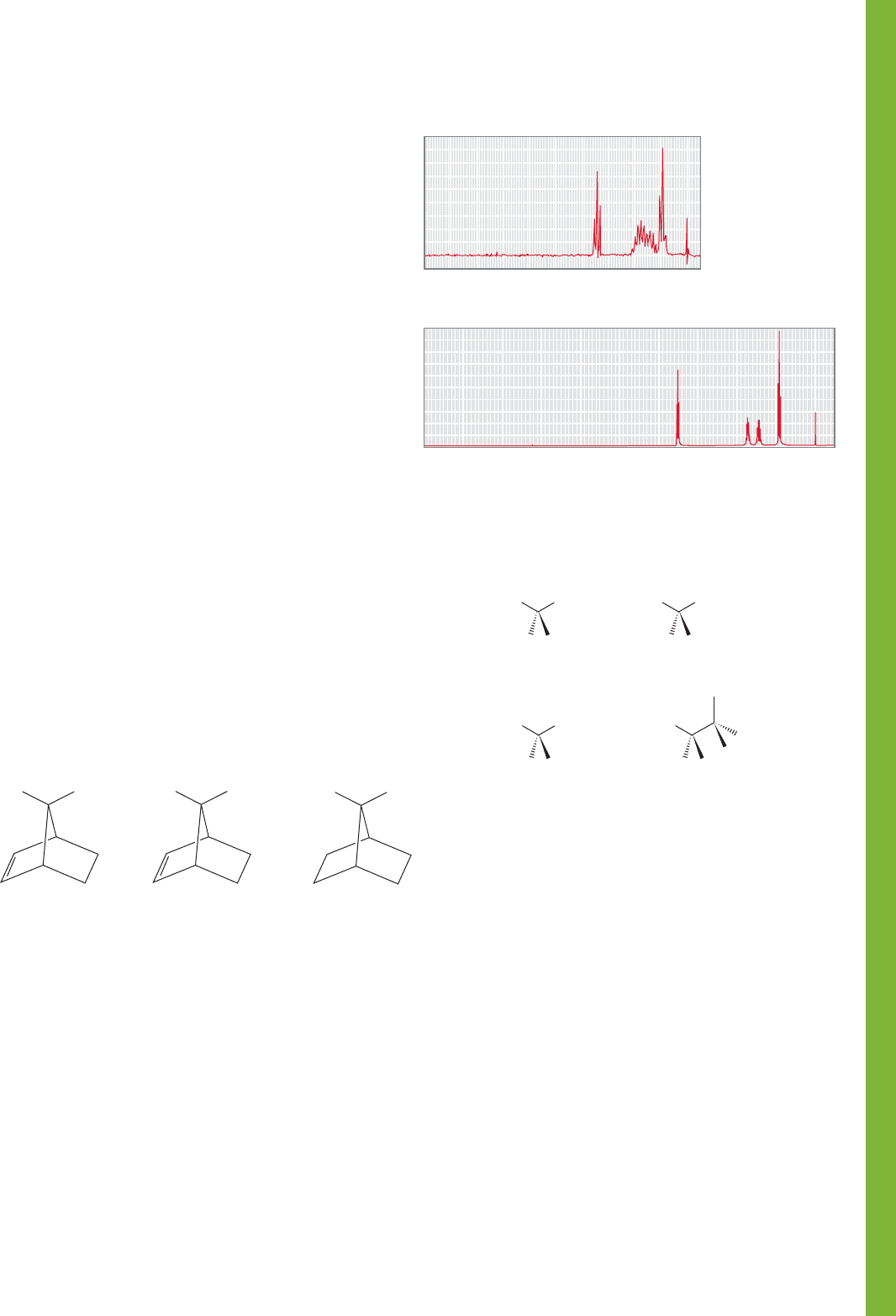
PROBLEM 15.42 Compounds A, B, and C all gave 71.17% C,
5.12% H, and 23.71% N upon elemental analysis. Summaries of
the mass spectra and the IR spectra of these compounds are shown
in the next column. Each one has a molecular ion of m/z 118.
Deduce the structures of A–C and explain your reasoning.
PROBLEM 15.43 Extraction of black Hemiptera bugs affords a
colorless oil A of the formula C
7
H
12
O possessing a sickening
odor. The IR spectrum of A displays peaks at 2833 (w),
2725 (w), 1725 (s), 1380 (m), and 975 (m) cm
1
. Upon catalytic
hydrogenation, the peak at 975 cm
1
disappears. Ozonolysis of
compound A, followed by an oxidative workup, gives succinic
acid ( ) and propionic acid
( ). Deduce a structure for A and explain
your reasoning.
PROBLEM 15.44 The portion of the IR spectrum
changes with concentration. As the solution becomes more dilute,
the broad band centered at roughly 3300 cm
1
sharpens. Explain.
PROBLEM 15.45 The stretching IR band for tert-butyl
alcohol is much sharper than that for methyl alcohol at the
same concentration. Explain.
PROBLEM 15.46 Explain the difference between the IR spec-
tra of the starting material and the product that you would
expect to see in the reaction of benzyl bromide with NaOH.
PROBLEM 15.47 The IR stretching frequency for the
group in the syn unsaturated molecule shown here is sharp at
all concentrations and shows no change on dilution, whereas
that for the related saturated molecule or the anti unsaturated
molecule is broad and sharpens with dilution. Explain. Hint:
Oxygen is not the only base that can interact with an elec-
trophilic hydrogen through hydrogen bonding.
O
O
H
O
O
H
O
O
H
HOOC
O
CH
2
CH
3
HOOC
O
CH
2
CH
2
O
COOH
15.11 Additional Problems 755
syn-Unsaturated anti-Unsaturated Saturated
OH HO HO RR R
(b) Spectrum taken at 300 MHz
(a) Spectrum taken at 60 MHz
10 9 8 7 6 5 4 3 2 1 0
Chemical shift (δ)
109876543210
(ppm)
(ppm)
Chemical shift (δ)
CH
3
CH
2
CH
2
CH
2
Br
CH
3
CH
2
CH
2
CH
2
Br
Now we move on to NMR spectroscopy. Again, we start with
simple exercises:
PROBLEM 15.48 Explain why TMS appears so far upfield in
the
1
H NMR spectrum.
PROBLEM 15.49 List the factors that can influence the chemi-
cal shift of a hydrogen. Which of these factors do you suppose
accounts for the fact that cyclopropane hydrogens appear at
unusually high field, near δ 0.2 ppm.
PROBLEM 15.50 Explain carefully why the following two
1
H NMR spectra of 1-bromobutane look so different:
PROBLEM 15.52 Predict the
1
H NMR spectrum (chemical
shift and coupling for each signal) of 2-chloropropane.
PROBLEM 15.53 Predict the
1
H NMR spectrum (chemical
shift and coupling for each signal) of 1,2-dichloropropane.
PROBLEM 15.54 Assign the following chemical shifts to the
hydrogens shown in parts (a) through (d). Use the (n 1) rule
to predict the multiplicities of the peaks.
(a) (b)
δ (ppm) δ (ppm)
5.88 4.53
4.37 3.53
1.70 3.43
(c) (d) BrCH
2
CH
2
CH
2
Br
δ (ppm) δ (ppm)
3.16 3.59
1.72 2.36
1.03
PROBLEM 15.55 The two isotopes of bromine are
79
Br and
81
Br. These isotopes occur naturally in a 1:1 ratio. Based on this
information, predict the ratios of the molecular ion and related
isotopes of 1,1-dibromoethane.
(CH
3
)
2
CH
O
CH
2
I
ClCH
2
O
CH(OCH
3
)
2
Cl
2
CH
O
CHCl
O
CH
3
(a) (b)
(c) (d)
H
3
C
CH
3
HH
H
3
C
CH
2
CH
3
CH
2
CH
3
CH
3
H
3
C
CH(CH
3
)
2
H
3
C
––
HH
––
HH
––
HH
––
H
PROBLEM 15.51 Are the underlined hydrogens in the follow-
ing molecules homotopic, enantiotopic, or diastereotopic?
Justify your answers.
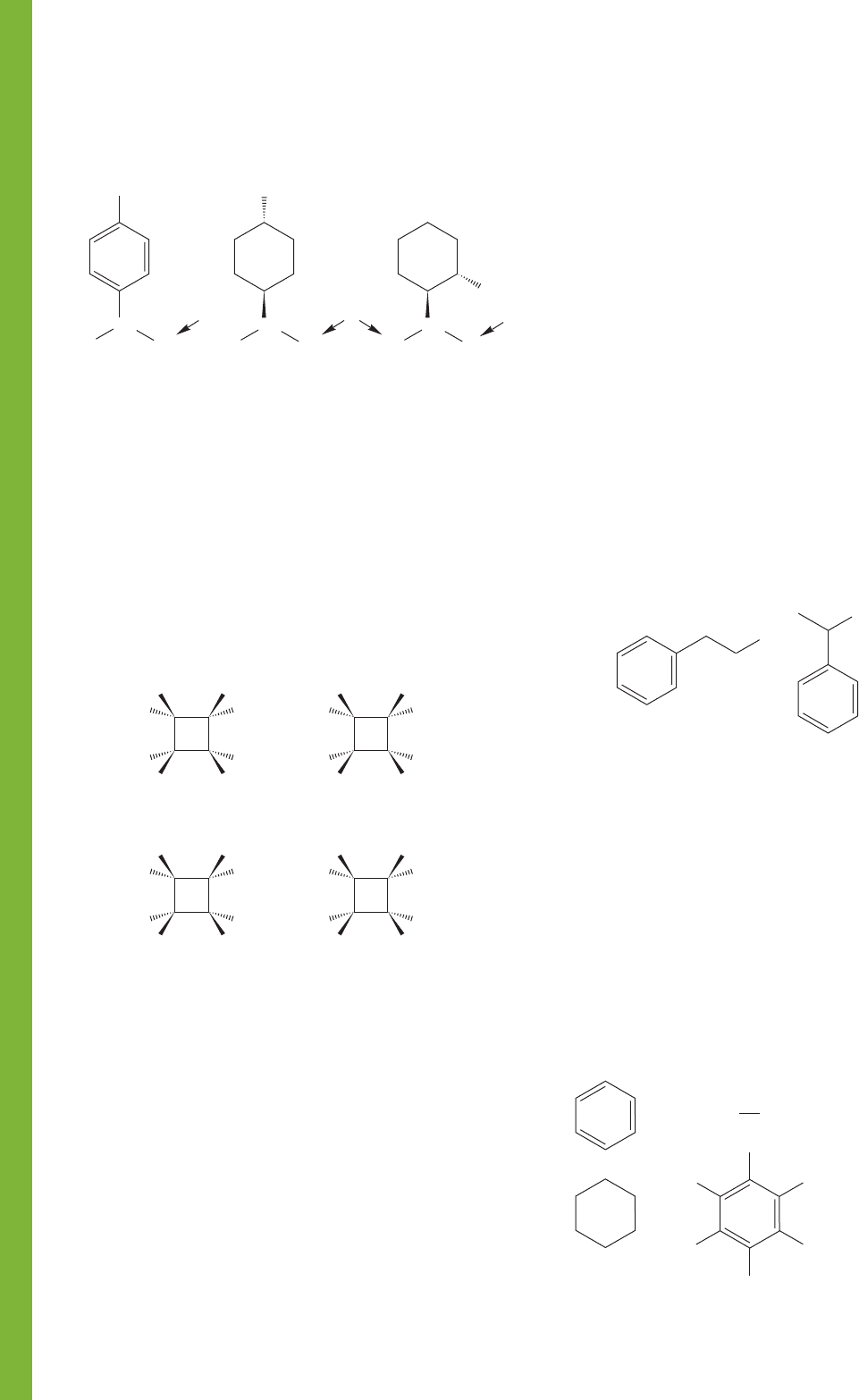
H
H
Ph
HO
CH
3
CH
3
Ph
HO
A
CH
3
CH
3
HO
HO
H
H
Ph
Ph
B
CH
3
H
Ph
Ph
CH
3
H
HO
HO
C
CH
3
H
HO
Ph
CH
3
H
HO
Ph
D
756 CHAPTER 15 Analytical Chemistry: Spectroscopy
PROBLEM 15.56 Predict the first-order spectra of the indicat-
ed hydrogens in the molecules shown in parts (a) through (c).
(H
3
C)
3
Si Si(CH
3
)
3
C(CH
3
)
4
EC
D
A
B
CH
3
CH
3
CH
3
CH
3
H
3
C
H
3
C
Compound 3: This compound is the second off the column.
1
H NMR: δ 7.4–7.0 (m, 5H)
3.40 (br s, 1H, signal shifts when D
2
O is added)
3.10 (m, 1H)
1.24 (d, J 8 Hz, 3H)
Compound 4: This compound is the third isomer off the column.
1
H NMR: δ 7.4–7.0 (m, 10H)
4.20 (br s, 2H, signal shifts when D
2
O is added)
2.58 (m, 1H)
2.39 (m, 1H)
1.38 (d, J 8 Hz, 3H)
0.71 (d, J 8 Hz, 3H)
PROBLEM 15.60 Figure 15.47 shows an enlarged view of the
multiplet centered at roughly δ 3.6 ppm in the
1
H NMR spec-
trum of pure ethyl alcohol. Use a tree diagram to analyze this
complex multiplet.
PROBLEM 15.61 The
1
H NMR spectra of the two scrupulously
dry alcohols shown below have signals (among others) at δ 2.60
(d, J 3.8 Hz, 1H) (A) and δ 2.26 (t, J 6.1 Hz, 1H) (B).
Assign the spectra, and explain your reasoning.
Compound 1: This diol is the least polar; it is the first isomer
eluted from a chromatography column.
1
H NMR: δ 7.6–7.2 (m, 10H)
3.78 (q, J 8 Hz, 1H)
3.20 (br s, 2H, signal shifts when D
2
O is added)
2.70 (q, J 8 Hz, 1H)
1.28 (d, J 8 Hz, 3H)
0.40 (d, J 8 Hz, 3H)
Compound 2: This diol is the most polar; it comes off the
column last.
1
H NMR: δ 7.5–7.2 (m, 5H)
3.07 (q, J 8 Hz, 1H)
2.62 (br s, 1H, signal shifts when D
2
O is added)
1.18 (d, J 8 Hz, 3H)
OH
OH
PROBLEM 15.62 When a drop of aqueous acid is added to the
samples described in Problem 15.61, the signals described col-
lapse to broad singlets. Explain.
PROBLEM 15.63 In this chapter, we claimed that the NMR
signals for
2
H (deuterium) and
13
C will not overlap
1
H spectra.
Verify that this statement is true. Show that the frequency (ν)
will be different for
1
H,
2
H, and
13
C at a field strength (B
0
)
of 4.7 T. The gyromagnetic ratios for
1
H,
2
H, and
13
C are
2.7 10
8
, 0.41 10
8
, and 0.67 10
8
rad T
1
s
1
,
respectively.
PROBLEM 15.64 Match the following
1
H NMR absorptions
to the compounds shown below, each of which has a one-signal
spectrum: δ 0.04, 0.90, 1.42, 2.25, 7.3 ppm.
CH
3
H
3
C
Cl
(a) (b) (c)
CH
CH
3
H
3
C
Cl
CH
CH
3
H
3
C
Cl
CH
PROBLEM 15.57 Nitrobenzene shows three signals in its
1
H NMR spectrum at δ 7.8 ppm (2H), δ 7.5 ppm (1H), and
δ 7.2 ppm (2H). Assign these signals and explain your reasoning.
PROBLEM 15.58 In the nitration of toluene, two products are
formed. Predict the products and describe how you would use
1
H NMR spectral data to distinguish between the two.
PROBLEM 15.59 Cyclobutanes are relatively rigid molecules. As
a result, a cis 1,2-disubstituted cyclobutane has significant steric
interactions that translate into a much higher energy than the
trans isomer. Spectral data for four cyclobutanediol isomers are
given. Assign each set of
1
H NMR data to one of the isomers and
explain your reasoning. It might be helpful to use your model set.
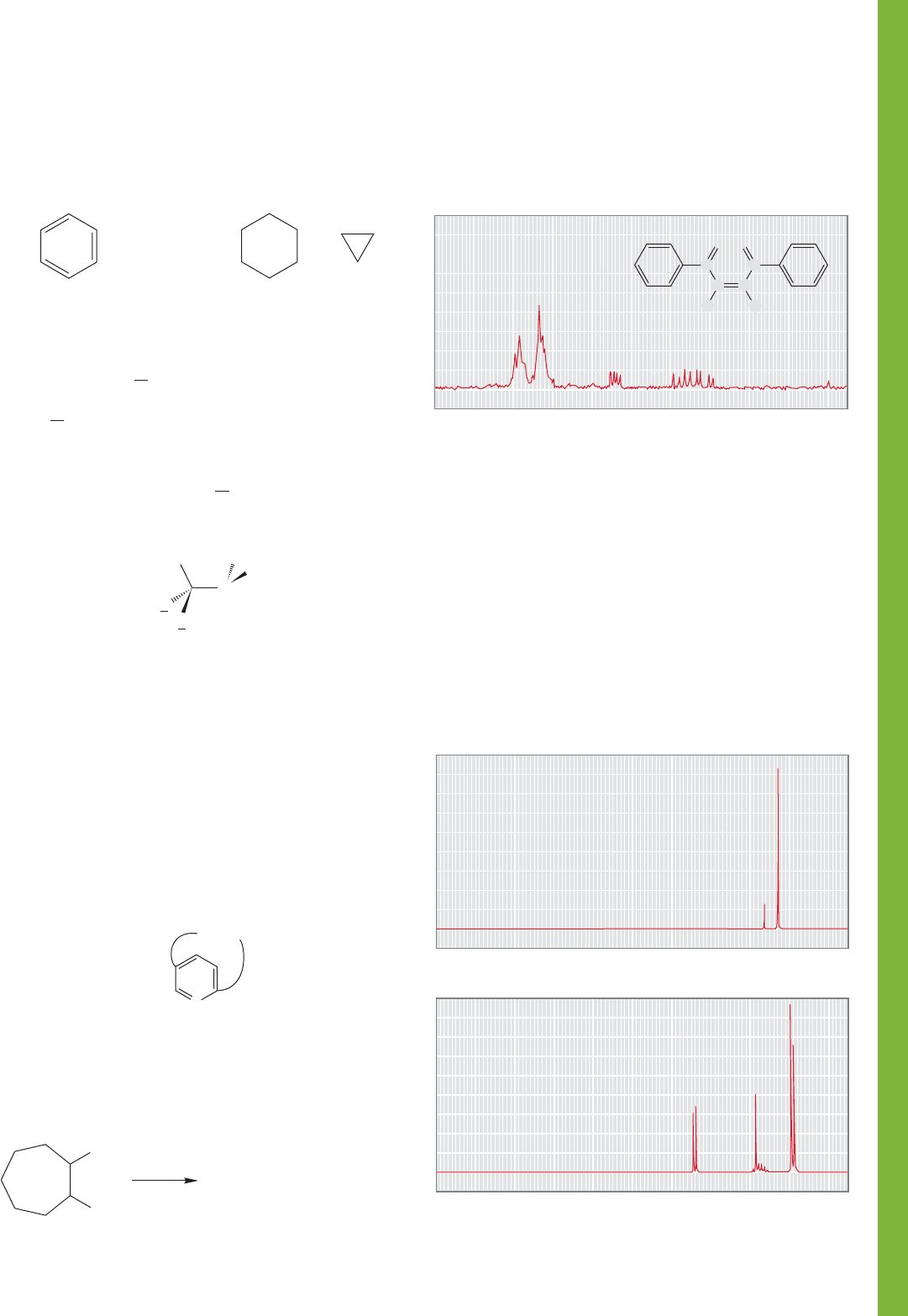
15.11 Additional Problems 757
CH
3
OCH
3
ABCD
1
H
3
C
H
H
H
N
Ph
PROBLEM 15.65 Match the following
13
C NMR absorptions
to the following compounds, each of which has a one-signal
spectrum: δ 2.9, 26.9, 59.7, 128.5 ppm.
N
..
(CH
2
)
8
PROBLEM 15.66 Explain why the hydrogens α to an
amino group ( ) appear further upfield in
the
1
H NMR spectrum than those α to a hydroxyl group
().
PROBLEM 15.67 Describe the signals for the methylene
hydrogens (underlined) of (1) in its
1
H NMR
spectrum. Analyze 1 exactly as drawn in the figure.
CH
3
CH
2
NHPh
R
O
CH
2
O
OH
R
O
CH
2
O
NH
2
(C
7
H
12
O)
H
3
O
+
H
2
O
IR: (CCl
4
) 1729 cm
–1
1
H NMR δ 9.5 (1H)
OH
OH
109876543210
(ppm)Chemical shift (δ)
O
O
C
HH
CC
C
2H
1H
2H
1H
PROBLEM 15.68 The carbon-bound hydrogens of pyrrole
appear at δ 6.05 and 6.62 ppm in the
1
H NMR spectrum,
upfield of the typical aromatic signal region. Does this
observation mean that pyrrole is not aromatic?
PROBLEM 15.69 At room temperature the pyridinophane
shown below has a signal in its
1
H NMR spectrum at
about δ 0.2 ppm. What relevance does this observation
have to the question of the aromaticity of pyridine? Hint:
See p. 720.
PROBLEM 15.70 Use the partial spectral data to predict a
structure for the product. Outline the steps in this reaction.
PROBLEM 15.71 The following spectrum appears in an old
catalog of
1
H NMR spectra of organic compounds. Is the
assigned structure correct? What’s wrong?
PROBLEM 15.72 In the synthesis of the compound described
in Problem 15.71, HCl is produced. Use this information to
postulate a reasonable structure for the compound whose
1
H NMR spectrum is shown above.
PROBLEM 15.73 2-Methylpropene can be hydrated in acid, or
under hydroboration–oxidation conditions to give two different
alcohols. Give structures for these alcohols and assign the peaks
in the following
1
H NMR spectra (1 and 2). Which peaks
would vanish if a drop of D
2
O were added?
1
2
109876543210
(ppm)
Chemical shift (δ)
109876543210
(ppm)Chemical shift (δ)
2H 1H 1H
6H
2H 1H 1H
6H
1H
9H
1H
9H
758 CHAPTER 15 Analytical Chemistry: Spectroscopy
1
109876543210
(ppm)Chemical shift (δ)
2
109876543210
(ppm)Chemical shift (δ)
2H
1H
3H
6H
2H
1H
3H
6H
1H
3H
6H
1H
1H
1H
1H
1H
3H
6H
PROBLEM 15.74 2-Methyl-2-butene can be hydrated in acid,
or under hydroboration–oxidation conditions to give two differ-
ent alcohols. Give structures for these alcohols and assign the
following
1
H NMR spectra (1 and 2) to these isomers. Explain
your answers.
O
AB C
O
O
CH
3
CH
3
CH
3
CH
3
PROBLEM 15.75 On careful examination, it can be seen
that the upfield signal in spectrum 2 of Problem 15.74
(at δ 0.90 ppm) which appears to be a doublet, is actually two
doublets. Explain.
PROBLEM 15.76 The
1
H NMR spectra for the following
eight-membered ring compounds are summarized below
(remember m means multiplet). Assign structures, and explain
your reasoning. A: δ (ppm) 1.54 (s); B: δ (ppm) 5.74 (s);
C: δ (ppm) 2.39 (m, 8H), 5.60 (m, 4H);
D: δ (ppm) 1.20–1.70 (m, 4H), 1.85–2.50 (m, 4H),
5.35–5.94 (m, 4H).
(a) (b)
(c) (d)
OH
Cl
Cl
Cl
OH
Cl
Cl
OH
Cl
Cl
OH
Cl
Cl
Cl
Cl
Cl
1
1
09876543210
(ppm)
Chemical shift (δ)
PROBLEM 15.79 The three isomers of pentane, C
5
H
12
, have
the following
13
C NMR spectra. Assign the spectra of these
three compounds. A: δ (ppm) 13.9, 22.8, 34.7; B: δ (ppm) 22.2,
31.1, 32.0, 11.7; C: δ (ppm) 31.7, 28.1.
PROBLEM 15.80 Assign the following
1
H NMR spectra to
the isomers of trichlorophenol shown below and explain your
reasoning.
PROBLEM 15.78 Could looking at symmetry allow
you to assign the spectra of 4-methylcyclohexanone,
2-methylcyclohexanone, and 2,3-dimethylcyclopentanone,
A, B, and C? If not, suggest an experiment, involving
only
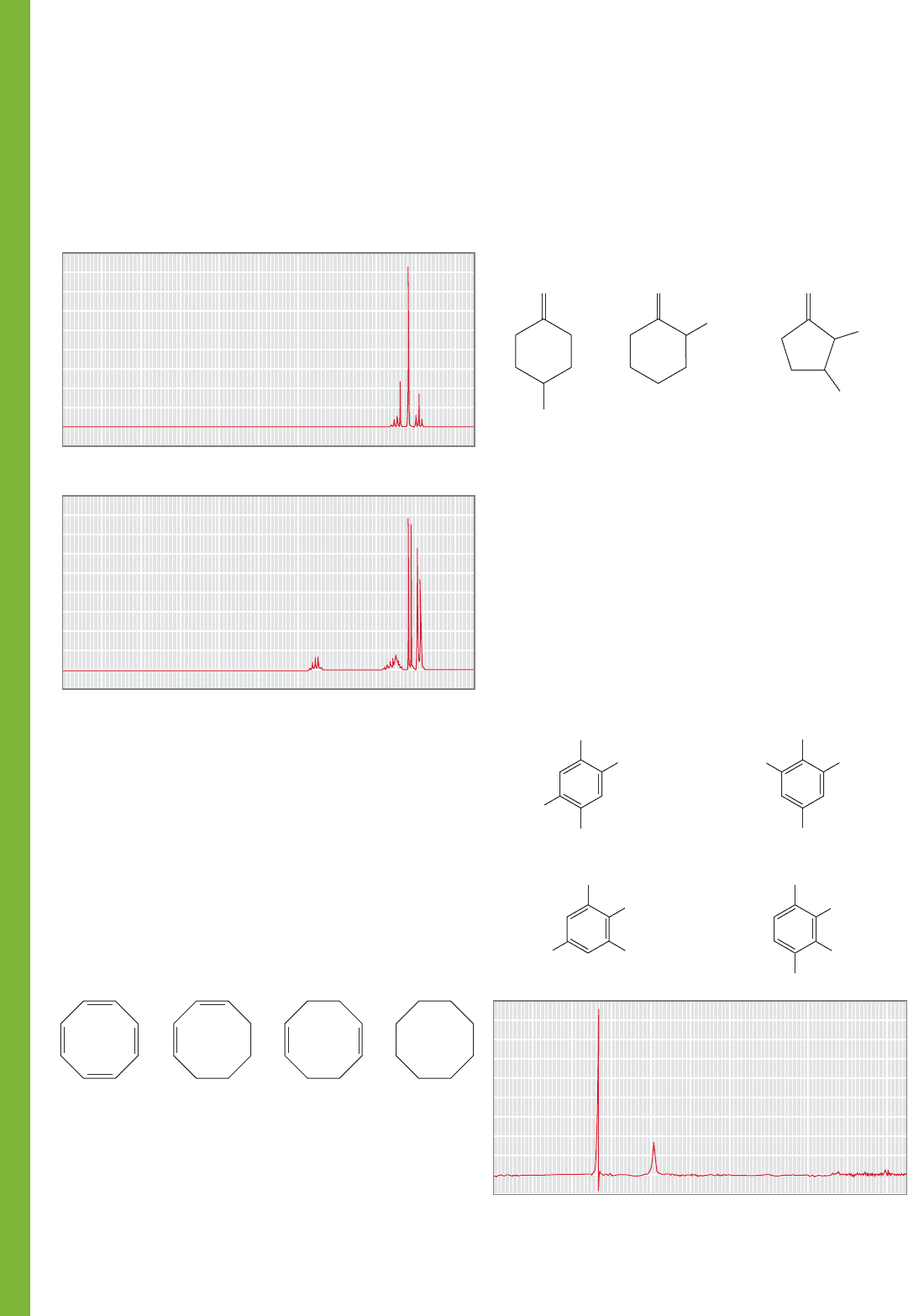
13
C NMR spectroscopy, that would let you finish the job.
(Hint: See p. 741.)
PROBLEM 15.77 You are given three bottles containing
o-dichlorobenzene, m-dichlorobenzene, and p-difluorobenzene,
along with broad-band decoupled
13
C NMR spectra of the three
isomers. Assign the three spectra to the three compounds and
explain your reasoning. A: δ (ppm) 127.0, 128.9, 130.6, 135.1.
B: δ (ppm) 127.7, 130.5, 132.6. C: δ (ppm) 116.5, 159.1.
